Abstract
The present study evaluated whether flurbiprofen increased the naturally circulating dendritic cells (DCs) subsets in patients with esophageal squamous cell carcinoma (ESCC) undergoing esophageal resection. Compared to healthy donors (n=20), the significantly depressed percentages of plasmacytoid DCs (pDCs), CD1c+ myeloid DCs (mDCs), and CD141+ mDCs among ESCC patients (n=60) were confirmed. Flurbiprofen was administered before skin incision and at the end of operation in group F (n=30), as well as placebo in group C (n=30). The postoperative suppressed percentages of pDCs, CD1c+ mDCs, and CD141+ mDCs increased significantly following the perioperative treatment with flurbiprofen. Flurbiprofen also significantly stimulated the postoperative IFN-f and IL-17 production, but inhibited the immunosuppressive IL-10 and TGF-β levels. Furthermore, flurbiprofen exerted a similar analgesic effect and brought a significantly less sufentanil consumption compared to group C. Taken together, flurbiprofen provided a short-term increase of postoperative naturally circulating DCs in ESCC patients.
Keywords: dendritic cells, flurbiprofen, PGE2, esophageal carcinoma, esophageal resection surgery
INTRODUCTION
Despite rare occurrence among peripheral blood mononuclear cells (PBMCs), naturally circulating dendritic cells (DCs) display a strikingly strong ability in taking up, processing, and presenting pathogens or tumor-associated antigens (TAAs) to stimulate naive T cells and to induce cytotoxic T lymphocytes (CTLs) or helper 1 T cells (Th1)/Th17 polarization [1, 2]. Recently, a novel DCs-based vaccination strategy, which employed enriched naturally circulating DCs directly isolated from PBMCs, showed a impressive clinical efficacy in patients with prostate cancer and melanoma, strongly suggesting a potent ability of naturally circulating DCs to induce the specific anti-cancer CTLs and Th1/Th17 response [3, 4]. The perioperative treatment with cyclooxygenase (COX) inhibitors, such as non-steroidal anti-inflammatory drugs (NSAIDs), not only alleviate postoperative pain and reduce opioid analgesics consumption, but also enhance anti-tumor immunity, particularly in T cell subsets and associated cytokines-dependent anti-tumor immunity [5, 6]. In the present study, we investigated whether flurbiprofen increased postoperative naturally circulating DCs subsets in patients with esophageal squamous cell carcinoma (ESCC) undergoing esophageal radical resection.
RESULTS
Naturally circulating DCs subsets in healthy donors and ESCC patients
Traditionally, circulating DCs among PBMCs was detected by characterization of negative for lineage (Lin) markers (CD3, CD14, CD19, CD20, CD56) and positive for HLA-DR. The Lin− HLA-DR+ cells can be further subdivided into mDCs and pDCs by the expression of CD11c and CD123, respectively. Recently, some novel and potent circulating DCs subtypes, including CD1c+ mDCs (Lin− CD11c+ CD1c+), CD16+ mDCs (Lin− CD11c+ CD16+), and CD141+ mDCs (Lin− CD11c+ CD141+), among naturally circulating mDCs (Lin− HLA-DR+ CD11c+) were reported [1, 2]. The percentages of CD1c+ mDCs, CD16+ mDCs, CD141+ mDCs and CD123+ pDCs among PBMCs in healthy donors and ESCC patients were preoperatively (pre-OP) evaluated by fluorescence activated cell sorting (FACS) and depicted as the histogram (Figure 1a). Specifically, CD1c+ mDCs (Figure 1c), CD141+ mDCs (Figure 1e) and CD123+ pDCs (Figure 1f) were significantly suppressed compared to the healthy controls. But except for CD16+ mDCs, CD16+ mDCs percentage in ESCC patients was similar with healthy control (Figure 1d).
Figure 1. The percentages of circulating DCs subsets among PBMCs in healthy donors and ESCC patients.
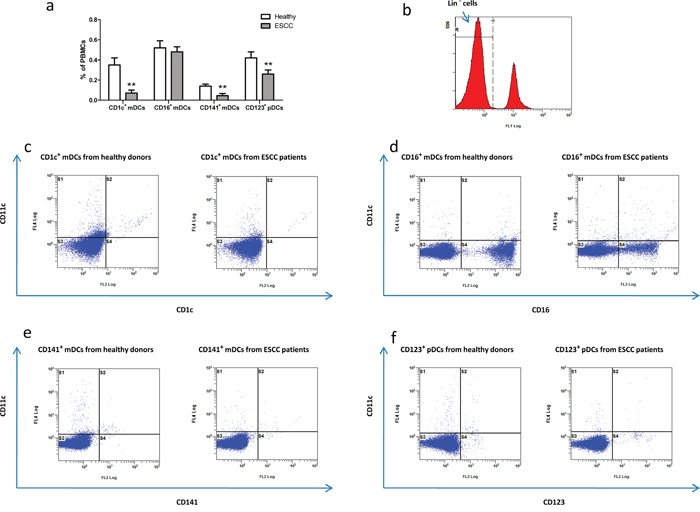
The percentages of circulating DCs subtypes depicted in the histogram in a. Lin− PBMCs as a gating strategy in b. Specifically, the representative FACS plots of CD11c+ CD1c+ in c. CD11c+ CD16+ in d. CD11c+ CD141+ in e. CD11c− CD123+ in f. 10,000 events were acquired for each sample. ** P<0.01 versus healthy controls.
Flurbiprofen increased postoperative circulating DCs subsets in patients
The percentages of pDCs and mDCs subtypes among PBMCs from patients were determined by FACS at postoperatively (post-OP) 24h and 48h, respectively. In group C, the percentages of pDCs and three mDCs subtypes at post-OP 24h were significantly depressed compared to pre-OP baseline levels, and no significant difference in the four DCs subsets percentage between post-OP 48h and pre-OP baseline levels. Comparing to group C, the percentages of CD1c+ mDCs (Figure 2e), CD141+ mDCs (Figure 2g) and CD123+ pDCs (Figure 2h) at post-OP 24h were up-regulated significantly in group F after perioperative treatment with flurbiprofen, except for CD16+ mDCs (Figure 2f). At following post-OP 48h, the four DCs subsets percentage between group F and group C did not show a significant change. In group F, the percentages of CD1c+ mDCs (Figure 2a) and CD141+ mDCs (Figure 2c) at post-OP 24h were significantly increased compared to pre-OP baseline levels, and the percentages of CD16+ mDCs (Figure 2b) and CD123+ pDCs (Figure 2d) at post-OP 24h were significantly suppressed compared to pre-OP baseline levels, and the four DCs subsets percentage returned to pre-OP baseline levels at post-OP 48h.
Figure 2. The percentages of pDCs and three mDCs subtypes among PBMCs at pre-OP, post-OP 24h and 48h from patients in group F and C, respectively.

a-d. Representative FACS plots of DCs subtypes at post-OP 24h e-h. **P<0.01 versus pre-OP baseline levels. ##P<0.01 versus patients received placebo in group C.
Flurbiprofen promoted the postoperative anti-tumor cytokine profile
In group C, the cancer-inhibitory cytokine IFN-γ and Th17-associated IL-17 production were significantly suppressed at post-OP 48h compared to pre-OP baseline levels (Figure 3a, 3c). Yet the immunosuppressive cytokines IL-10 and TGF-β levels at post-OP 48h were significantly higher than pre-OP baseline levels in group C (Figure 3b, 3d). Notably, flurbiprofen significantly increased anti-cancer IFN-γ and IL-17 levels at post-OP 48h in group F (Figure 3a, 3c). Accordingly, IL-10 and TGF-β levels at post-OP 48h were depressed significantly in patients from group F following perioperative treatment with flurbiprofen (Figure 3b, 3d).
Figure 3. IFN-γ and IL-17 levels in group F and group C.
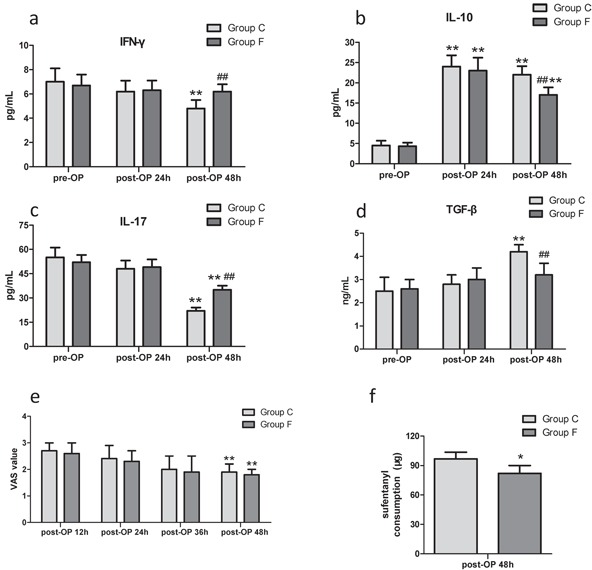
in a and c. IL-10 and TGF-β levels in group F and group C in b and d. **P<0.01 versus pre-OP baseline level. ##P<0.01 versus patients received placebo in group C. No significant difference in VAS scores between groups in e. **P<0.01 versus post-OP 12h. Sufentanil consumption during post-OP 24h in group F was significantly less in f. *P<0.05 versus patients received placebo in group C.
Flurbiprofen brought a similar analgesia and less sufentanil consumption
During post-OP 48h patient-controlled intravenous analgesia (PCIA), there was no significant difference in visual analogue scale (VAS) scores between group C and group F (Figure 3e). The opioid analgesics sufentanil consumption during post-OP 24h in group F was significantly less than group C (Figure 3f). The incidence of nausea/vomiting between group C and group F did not show a significant change (data not shown).
DISCUSSION
Previously, not only numerical defective of circulating DCs and tumor-infiltrating DCs in tissue specimen, but also functional impairment of monocyte-derived DCs (moDCs) from the peripheral blood was reported in patients with esophageal and other tumors [7–10]. In the present study, FACS results showed that CD1c+ mDCs represented by far the most predominant mDCs subset (0.35±0.07%, Figure 1c) in the healthy donors (except for CD16+ mDCs), whereas CD141+ mDCs (0.14±0.02%, Figure 1e) formed a minute population. Specifically, CD1c+ mDCs and CD141+ mDCs can be activated by a distinct set of toll-like receptors (TLRs), and subsequently secrete large amounts of cytokines IFN-γ and IL-12 which allow the highly effective induction of CTLs and Th1 responses against tumor [11, 12]. In line with the defective quantification of DCs in esophageal carcinoma, FACS results in the present study displayed that the percentages of CD1c+ mDCs, and CD141+ mDCs among PBMCs in ESCC patients were significantly lower than healthy controls. However, the similar percentages of CD16+ mDCs subset, considered to be more monocyte-like, among PBMCs were identified in healthy donors and ESCC patients. Besides mDCs, pDCs are specialized in the detection and control of viral infections, as well as prime both Th1 and CTLs by the strong release of IFN-γ in an IL-12-independent Th1 polarization [13, 14]. Moreover, several studies demonstrated that despite lower antigen uptake and limited phagocytosis than mDCs subsets, pDCs isolated from blood and spleen were shown to efficiently cross-present antigens and prime potent tumor-specific CTLs [15–17]. Thus, pDCs, as well as CD1c+ mDCs, represent a promising target in DCs-based vaccine immunotherapy for cancer. But unfortunately, similar to CD1c+ mDCs, the FACS results showed that pDCs percentage (0.26±0.04%, Figure 1f) among PBMCs from ESCC patients was also significantly depressed compared to healthy control (0.42±0.06%, Figure 1f).
The COX-derived prostaglandin E2 (PGE2), a well-known tumor-sustaining inflammatory mediator, greatly contributes to tumorigenesis, angiogenesis, metastasis in malignances through successful evasion of tumor immune surveillance and resistance to cancer immunotherapy [18–20]. A growing amount of information supports that tumor-derived PGE2 specifically exerts a suppressive effect on DCs biology through a paracrine and/or autocrine manner, which reduces the maturation of DCs and their expression of MHC class II molecules, ability to present TAAs and prime anti-tumor T cells, possibly via enhancing the production of immunosuppressive IL-10 [21–23]. Although data not shown in the present study, the previous researches demonstrated that the surgery operations significantly induced the over-production of PGE2 perioperatively, which greatly contributed to further disruption of the impaired immune function of cancer patients [24, 25]. In the present study, FACS and ELISA results showed that the postoperative naturally circulating DCs subtypes and anti-tumor cytokines profile were markedly decreased mainly due to the surgical trauma and pain-induced the immune suppression via immunosuppressive mediators release, such as PGE2, IL-10, TGF-β. Subsequently, these naturally circulating DCs subtypes returned to pre-OP baseline levels shortly after post-OP 48h, possibly related to opioid analgesics usage for alleviating the postoperative pain and/or limited life expectancy of circulating DCs. Given the immunosuppressive role of PGE2 in cancer progression, a series of researches have provided evidence on the clinical beneficial of NSAIDs which inhibit increased COX activity and PGE2 production on tumor progression, particularly in a remarkable synergy between NSAIDs and CTLA-4 or PD-1 blockade immunotherapy results in tumor eradication [20, 26, 27]. Accordingly, research found that the preoperative treatment with NSAIDs (indomethacin or celecoxib) was enough to increase tumor infiltration and peripheral blood by seemingly activated immune cells in patients with colorectal carcinoma and gastric cancer [6, 20, 28]. The tumor-specific COX ablation stimulated tumor-infiltrating CD11c+ MHC-II+ DCs displayed higher levels of co-stimulatory molecules, and over-production of PGE2 impaired accumulation of DCs within melanoma and suppress their activation, including IL-12-producing activity [20]. In the present study, our results focused on perioperative treatment with flurbiprofen significantly up-regulated the postoperative percentages of CD1c+ mDCs, CD141+ mDCs and CD123+ pDCs, as well as anti-tumor cytokine profile, but this short-term increase of the postoperative naturally circulating DCs subsets in flurbiprofen-treated patients didn't last for post-OP 48h. This might be explained by the short elimination half-life time of flurbiprofen in plasma (approximately 5.8 h) [29, 30].
In the present study, we preliminarily observed the perioperative prevalence of naturally circulating DCs among PBMCs, and assessed the impact of flurbiprofen on the postoperative naturally circulating DCs percentages in the ESCC patients. Although, we found that the postoperative 3-month survive rate of patients between group F and group C did not show a significant change after esophageal resection (data not shown), whether the short-term increase of the circulating DCs subsets in flurbiprofen-treated patients effectively provides an improved long-term prognosis with ESCC patients still needs consistent follow-up. What's more, we are currently investigating the effect of PGE2/flurbiprofen on the function of enriched naturally circulating DCs ex vivo, such as phagocytic activity, co-stimulatory molecule expression, antigen presenting ability and cytokines production. In summary, the present study provided a better understanding of the anti-tumor role of naturally circulating DCs and promotion of PGE2 on tumor immune evasion in esophageal cancer, and suggested that NSAIDs could be useful additions to potential circulating DCs-based immune therapy or conventional treatment of cancer patients.
MATERIALS AND METHODS
Patients
The protocol of this study was approved by the Ethics Committee of Anhui Medical University and the Chinese Clinical Trial Registry (No.ChiCTR-IPR-15006482). Written informed consent was obtained from all subjects. 60 patients with ESCC undergoing esophageal resection with an American Society of Anesthesiologists (ASA) status of I-II were enrolled, as well as 20 age-matched healthy donors. The enrolled patients, whom met the inclusion criteria in the present study, ranged in age from 50 to 75 years and weighed from 45 to 80kg (summarized in Table 1). The exclusion criteria were as follows: (1) allergy to NSAIDs, (2) blood coagulation disorder, (3) hepatic or renal dysfunction, (4) autoimmune disease or acute inflammation, (5) perioperative blood transfusion, (6) bronchial asthma, (7) preoperative treatment with radiotherapy, chemotherapy, immunodepressant, or glucocorticoid.
Table 1. Characteristics of patients enrolled in the present study.
| Patients in group F (n=30) | Patients in group C (n=30) | Healthy donors (n=20) | |
|---|---|---|---|
| Age (year) | 56.45±5.29 | 57.12±5.58 | 56.82±5.37 |
| Gender (male/female) | 27/3 | 28/2 | 17/3 |
| Weight(kg) | 64.95±8.26 | 63.35±8.72 | 62.97±6.95 |
| ASA (I/II) | 27/3 | 26/4 | |
| Surgery time (min) | 303.26±29.59 | 298.65±28.65 | |
| Infusion fluid (ml) | 1945.00±250.21 | 1935.00±279.61 | |
| Urine output (ml) | 493.00±75.26 | 485.00±63.04 | |
| Blood loss (ml) | 281.50±63.27 | 297.00±49.96 |
Group and treatments
The ESCC patients were randomly assigned to either the flurbiprofen treatment group (group F, n=30) or the control group (group C, n=30). For patients in group F, flurbiprofen axetil (1mg/kg, i.v., 50mg/5ml, Beijing Tide Pharmaceutical, China) was administered 15 min before skin incision and at the end of surgery, respectively. The placebo (intralipid, Chengdu Huarui Pharmaceutical, China) was administered at the same time points in patients from group C. Both two groups were equally received postoperative PCIA using sufentanil (100μg).
Anesthesia and analgesia
General anesthesia was induced with 0.05mg/kg midazolam, 2mg/kg propofol, 0.4μg/kg sufentanil, and 1.0mg/kg rocuronium. A left double-lumen endobronchial tube (Mallinckrodt, Ireland) was inserted and confirmed by fiberoptic bronchoscopy. Mechanical ventilation was performed using an anesthesia machine (S/5 Avance, Datex-Ohmeda, USA) with tidal volume (VT) ranged in 6-8 ml/kg and positive end-expiratory pressure (PEEP) 5cmH2O, and was adjusted to maintain the PETCO2 at 35-45mmHg. The inspiratory to expiratory time (I/E) ratio was 1:2. During the surgery, 50μg/kg/min propofol and 0.1-0.2μg/kg/min remifentanil were target-controlled infused to maintain anesthesia, and cis-atracurium was simultaneously given to muscle relaxation.
Sample collection and cytokines measurement
Patients blood sample were collected from the central venous catheters before anesthesia, as well as post-OP 24h and 48h. Plasma from venous blood was obtained by centrifugation (1000×g, 4°C, 5 min) and stored at −80°C for further analyses. Cytokines IFN-γ, IL-10, IL-17, TGF-β levels in plasma were determined using the ELISA kits (R&D, CA, USA) according to the manufacturer's instructions. PBMCs were separated from peripheral blood by Ficoll-Hypaque density gradient centrifugation and re-suspended in 0.2 ml PBS (4×106/100μl) for further FACS study.
Circulating DCs subsets measured by FACS
As shown in Figure 1b detection of naturally circulating DCs subsets was achieved by CD1c+ mDCs (CD11c+ CD1c+), CD16+ mDCs (CD11c+ CD16+), CD141+ mDCs (CD11c+ CD141+), and CD123+ pDCs (CD11c− CD123+) within a Lin− PBMCs gate. Following mouse monoclonal antibodies (mAbs) were used in the present study, including FITC anti-human Lin cocktail (Biolegend, US), the anti-human CD11c-APC, anti-human CD1c-PE, anti-human CD16-PE, anti-human CD141-PE, anti-human CD123-PE, and respective mouse IgG isotype controls (Miltenyi, Germany). The absolute counts of pDCs and three mDC subsets were quantitated as the percentages of them among PBMCs measuring by Cytomics FC500 Dual Laser System (Beckman Coulter, US).
PCIA clinical assessment
PCIA efficacy was evaluated at post-OP 12h, 24h, 36h, and 48h according to the VAS scores, including: 0 (painless), 1-4 (mild pain), 5-8 (moderate pain), and 9-10 (severe pain). Sufentanil consumption and incidence of nausea/vomiting during post-OP 48h were recorded.
Statistical analysis
Statistics were analyzed with the SPSS software (version 16.0). Quantitative data are presented as means ± SD. Significant differences were evaluated by Student's t-test or ANOVA. P values less than 0.05 were considered statistically significant.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
GRANT SUPPORT
This study was supported by the Nation Natural Science Foundation of China (No.81503080), the Anhui Provincial Natural Science Foundation (No.1408085MH187, No.1608085QH210), the Scientific Research Foundation of Anhui Provincial Health Department (No.13zc002, No.13zc027).
REFERENCES
- 1.Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, Lambeck AJ, Figdor CG, de Vries IJ. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood. 2013;121:459–467. doi: 10.1182/blood-2012-06-435644. [DOI] [PubMed] [Google Scholar]
- 2.Wimmers F, Schreibelt G, Sköld AE, Figdor CG, De Vries IJ. Paradigm Shift in Dendritic Cell -Based Immunotherapy: From in vitro Generated Monocyte-Derived DCs to Naturally Circulating DC Subsets. Front Immunol. 2014;5:165. doi: 10.3389/fimmu.2014.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson R, Kassianos AJ, Swindle P, Hart DN, Radford KJ. Numerical and functional assessment of blood dendritic cells in prostate cancer patients. Prostate. 2006;66:180–192. doi: 10.1002/pros.20333. [DOI] [PubMed] [Google Scholar]
- 4.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, Winkels G, Coulie PG, Punt CJ, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [DOI] [PubMed] [Google Scholar]
- 5.Lönnroth C, Andersson M, Nordgren S, Lundholm K. Downregulation of Prominin 1/CD133 expression in colorectal cancer by NSAIDs following short-term preoperative treatment. Int J Oncol. 2012;41:15–23. doi: 10.3892/ijo.2012.1460. [DOI] [PubMed] [Google Scholar]
- 6.Lönnroth C, Andersson M, Arvidsson A, Nordgren S, Brevinge H, Lagerstedt K, Lundholm K. Preoperative treatment with a non-steroidal anti-inflammatory drug (NSAID) increases tumor tissue infiltration of seemingly activated immune cells in colorectal cancer. Cancer Immun. 2008;8:5. [PMC free article] [PubMed] [Google Scholar]
- 7.Somja J, Demoulin S, Roncarati P, Herfs M, Bletard N, Delvenne P, Hubert P. Dendritic cells in Barrett's esophagus carcinogenesis: an inadequate microenvironment for antitumor immunity? Am J Pathol. 2013;182:2168–2179. doi: 10.1016/j.ajpath.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Song N, Liu Y, Liu Y, Li J, Ding J, Tong Z. Efficient induction of anti-tumor immune response in esophageal squamous cell carcinoma via dendritic cells expressing MAGE-A3 and CALR antigens. Cell Immunol. 2015;295:77–82. doi: 10.1016/j.cellimm.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Vo MC, Lee HJ, Kim JS, Hoang MD, Choi NR, Rhee JH, Lakshmanan VK, Shin SJ, Lee JJ. Dendritic cell vaccination with a toll-like receptor agonist derived from mycobacteria enhances anti-tumor immunity. Oncotarget. 2015;6:33781–33790. doi: 10.18632/oncotarget.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chistiakov DA, Orekhov AN, Bobryshev YV. Dendritic Cells in Esophageal Adenocarcinoma: The Currently Available Information and Possibilities to use Dendritic Cells for Immunotherapeutic Approaches. Curr Pharm Des. 2016;22:307–311. doi: 10.2174/1381612822666151112153620. [DOI] [PubMed] [Google Scholar]
- 11.Hémont C, Neel A, Heslan M, Braudeau C, Josien R. Human blood mDC subsets exhibit distinct TLR repertoire and responsiveness. J Leukoc Biol. 2013;93:599–609. doi: 10.1189/jlb.0912452. [DOI] [PubMed] [Google Scholar]
- 12.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, de Vries IJ. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tel J, Smits EL, Anguille S, Joshi RN, Figdor CG, de Vries IJ. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood. 2012;120:3936–3944. doi: 10.1182/blood-2012-06-435941. [DOI] [PubMed] [Google Scholar]
- 14.Schlecht G, Garcia S, Escriou N, Freitas AA, Leclerc C, Dadaglio G. Murine plasmacytoid dendritic cells induce effector/memory CD8+ T-cell responses in vivo after viral stimulation. Blood. 2004;104:1808–1815. doi: 10.1182/blood-2004-02-0426. [DOI] [PubMed] [Google Scholar]
- 15.Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, Lambeck AJ, Figdor CG, de Vries IJ. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood. 2013;121:459–467. doi: 10.1182/blood-2012-06-435644. [DOI] [PubMed] [Google Scholar]
- 16.Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 17.Segura E, Durand M, Amigorena S. Similar antigen cross-presentation capacity and phagocytic functions in all freshly isolated human lymphoid organresident dendritic cells. J Exp Med. 2013;210:1035–1047. doi: 10.1084/jem.20121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa F, Amano H, Eshima K, Ito Y, Matsui Y, Hosono K, Kitasato H, Iyoda A, Iwabuchi K, Kumagai Y, Satoh Y, Narumiya S, Majima M. Prostanoid induces premetastatic niche in regional lymph nodes. J Clin Invest. 2014;124:4882–4894. doi: 10.1172/JCI73530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zelenay S, van der Veen AG, Böttcher JP, Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais R, Quezada SA, Sahai E, Reise Sousa C. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e (2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–2263. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 22.Gualde N, Harizi H. Prostanoids and their receptors that modulate dendritic cell-mediated immunity. Immunol Cell Biol. 2004;82:353–360. doi: 10.1111/j.0818-9641.2004.01251.x. [DOI] [PubMed] [Google Scholar]
- 23.Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, Magy L, Balaji KN, Kaveri SV, Bayry J. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase -2-dependent prostaglandin E2 in human dendritic cells. Blood. 2013;122:1419–1427. doi: 10.1182/blood-2012-11-468264. [DOI] [PubMed] [Google Scholar]
- 24.Buvanendran A, Kroin JS, Berger RA, Hallab NJ, Saha C, Negrescu C, Moric M, Caicedo MS, Tuman KJ. Upregulation of prostaglandin E2 and interleukins in the central nervous system and peripheral tissue during and after surgery in humans. Anesthesiology. 2006;104:403–410. doi: 10.1097/00000542-200603000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Aida T, Furukawa K, Suzuki D, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Miyazaki M. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery. 2014;155:124–133. doi: 10.1016/j.surg.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 26.Takada M, Fukusaki M, Terao Y, Yamashita K, Inadomi C, Takada M, Sumikawa K. Preadministration of flurbiprofen suppresses prostaglandin production and postoperative pain in orthopedic patients undergoing tourniquet inflation. J Clin Anesth. 2007;19:97–100. doi: 10.1016/j.jclinane.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Eberstål S, Badn W, Fritzell S, Esbjörnsson M, Darabi A, Visse E, Siesjö P. Inhibition of cyclooxygenase-2 enhances immunotherapy against experimental brain tumors. Cancer Immunol Immunother. 2012;61:1191–1199. doi: 10.1007/s00262-011-1196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen JC, Sun HL, Zhang MQ, Liu XY, Wang Z, Yang JJ. Flurbiprofen improves dysfunction of T-lymphocyte subsets and natural killer cells in cancer patients receiving post-operative morphine analgesia. Int J Clin Pharmacol Ther. 2014;52:669–675. doi: 10.5414/CP202027. [DOI] [PubMed] [Google Scholar]
- 29.Liu ZF, Chai XQ, Chen KZ. Flurbiprofen axetil enhances analgesic effect of fentanyl associated with increase in β-endorphin levels. J Anesth. 2011;25:679–684. doi: 10.1007/s00540-011-1192-6. [DOI] [PubMed] [Google Scholar]
- 30.Chai XQ, Ma J, Xie YH, Wang D, Chen KZ. Flurbiprofen axetil increases arterial oxygen partial pressure by decreasing intrapulmonary shunt in patients undergoing one-lung ventilation. J Anesth. 2015;29:881–888. doi: 10.1007/s00540-015-2060-6. [DOI] [PubMed] [Google Scholar]


