Abstract
Objective
To investigate the factors related to upper extremity functional improvement following inhibitory repetitive transcranial magnetic stimulation (rTMS) in stroke patients.
Methods
Forty-one stroke patients received low-frequency rTMS over the contralesional hemisphere according to a standard protocol, in addition to conventional physical and occupational therapy. The rTMS-treated patients were divided into two groups according to their responsiveness to rTMS measured by the self-care score of the Korean version of Modified Barthel Index (K-MBI): responded group (n=19) and non-responded group (n=22). Forty-one age-matched stroke patients who had not received rTMS served as controls. Neurological, cognitive and functional assessments were performed before rTMS and 4 weeks after rTMS treatment.
Results
Among the rTMS-treated patients, the responded group was significantly younger than the non-responded group (51.6±10.5 years and 65.5±13.7 years, respectively; p=0.001). Four weeks after rTMS, the National Institutes of Health Stroke Scale, the Brunnstrom recovery stage and upper extremity muscle power scores were significantly more improved in the responded group than in the control group. Besides the self-care score, the mobility score of the K-MBI was also more improved in the responded group than in the non-responded group or controls.
Conclusion
Age is the most obvious factor determining upper extremity functional responsiveness to low-frequency rTMS in stroke patients.
Keywords: Stroke, Transcranial magnetic stimulation, Recovery of function, Age factors, Upper extremity
INTRODUCTION
Stroke is the leading cause of adult disability worldwide. Between 55% and 75% of the stroke survivors have some limitations in functional use of the upper extremity [1]. Motor recovery of the upper extremity is one of the major concerns after stroke, given the marked impact on the ability to independently perform activities of daily living (ADL) [2]. Repetitive transcranial magnetic stimulation (rTMS), a noninvasive tool for induction of electric current in the brain, is widely utilized for achieving recovery of function of the involved upper extremity following stroke [3,4,5,6]. The frequencies of rTMS, which are typically categorized into low (≤1 Hz) and high (>1 Hz) ranges, are known to be related to the control of cerebral cortex activation. For example, it has been reported that low-frequency rTMS reduces the excitability of the contralesional hemisphere, while high-frequency rTMS increases the cortical excitability of the ipsilesional hemisphere [7,8]. Some studies have reported that high-frequency rTMS is more effective for visuospatial neglect than low-frequency rTMS [9]. In the early phase, high-frequency rTMS has been determined to be more beneficial for motor improvement of the affected upper extremity than low-frequency rTMS [10]; however, in the late phase, the difference was found to be insignificant [8]. Although several studies have compared the effects of stimulatory and inhibitory rTMS on post-stroke motor recovery [8,10], it is still unclear which type of rTMS has a greater effect on upper extremity functional improvement.
In fact, in the clinical setting, the effects of rTMS on both motor and functional recovery are quite variable. Several studies have sought to identify the characteristics determining individual responses to high-frequency rTMS treatment [3,11,12]. One important factor influencing the inter- and intra-individual variability of rTMS effects, reportedly, is the level of cortical excitability in the subject prior to stimulation [3,13]. Other studies have reported that subcortical stroke, presence of motor-evoked potentials (MEPs), mild paresis and higher cognitive status are related to positive effects of high-frequency rTMS [11,12]. However, several factors were shown to have different effects following rTMS treatment. Although some researchers have suggested that aging can modify the clinical effects of rTMS [3,13], other researchers did not find any age-related differences after high-frequency rTMS [9,12]. One study reported that ipsilesional stimulatory rTMS caused a significant improvement in patients with and without cortical involvement, whereas the beneficial effect of contralesional inhibitory rTMS was more marked in subcortical stroke than in cortical stroke [12]. To date, the causes of inter-individual variability of inhibitory rTMS effects compared with stimulatory rTMS effects remain unclear. The aim of our current study was to delineate the variables related to stroke patients' upper extremity functional improvement following low-frequency rTMS.
MATERIALS AND METHODS
Subjects
A total of 337 patients in the subacute stage of stroke were reviewed retrospectively in this study. All of the subjects met the following inclusion criteria: (1) hemorrhagic or ischemic stroke within the last 3 months; (2) Brunnstrom's hand recovery stage 1–5. Patients with any of the following conditions were excluded: (1) prior history of seizure; (2) severe physical or mental illness requiring medical management; (3) contra-indications to rTMS (e.g., cardiac pacemakers, intracranial implants, implanted medication pumps, pregnancy); (4) severe cognitive impairment (Korean version of Mini-Mental Status Examination [K-MMSE]<5). Finally, 41 patients in whom low-frequency rTMS was performed were selected for the study. Additionally, 41 age-matched subjects in whom rTMS had not been performed were assigned to the control group for comparing their neurological and functional changes with those in the stroke patients. The subjects underwent neurologic, cognitive and functional evaluation, first on admission to an inpatient rehabilitation unit and then again 4 weeks after treatment. All of the stroke patients also received conventional physical and occupational therapy until discharge. We used the self-care score on the Korean version of the Modified Barthel Index (K-MBI) to evaluate upper extremity functional status as the primary outcome of rTMS. The K-MBI consists of two categories: self-care and mobility. The self-care component largely depends on upper extremity function, while the mobility score indicates lower-extremity function [14]. The criterion was determined based on the mean change in the K-MBI self-care score in the control group, which did not undergo rTMS treatment: 17.5 (mean+2 SD; 7.24+2×5.13). The rTMS-treated patients were divided into two groups according to this criterion, which represented their treatment responsiveness as reflected in their upper extremity functional status: the responded group (19 patients; self-care score of K-MBI≥17.5) and the non-responded group (22 patients; self-care score of K-MBI<17.5).
Methods
Intervention
All of the subjects undertook, for 1 hour twice a day, conventional physical and occupational therapy including range of motion training, strengthening exercises, gait training, ADL training, and manual dexterity exercises.
In the rTMS-treated group, transcranial magnetic stimulation was used to assess the functional integrity of the corticospinal tract [15]. Stimulation of the primary motor cortex (M1) induced a response in the abductor pollicis brevis (APB) muscle, which manifested as a muscle twitch that was recorded as a MEP using surface electromyography for the affected upper extremity. Each intervention consisted of 10 sessions of 20-minute rTMS applied to the contralesional hemisphere. The rTMS was delivered with a figure-of-eight coil and a Magstim Super Rapid2 magnetic stimulator (Magstim, Carmarthenshire, UK). In each session, 1,200 pulses of 1 Hz rTMS were applied over the hand area of the motor cortex (F8c) in the contralesional hemisphere, at the site that elicited the largest MEPs in the APB muscle of the unaffected upper extremity. The intensity of the stimulation was set to 100% motor threshold, which was defined as the lowest intensity necessary for evoking an MEP response of 50 µV in 50% of the trials.
Functional assessment
The severity of impairment in performance of ADL was evaluated twice by a rehabilitation physician using the K-MBI, first on admission to the rehabilitation unit and then again 4 weeks after the initial assessment. The K-MBI, which includes self-care and mobility components, has 10 subscales with scores ranging from 0 (completely dependent) to 100 (independent in basic ADL). The score for the self-care component of the K-MBI, which consists of personal hygiene, bathing, feeding, toileting, going up and down stairs, dressing, defecation and voiding categories, ranges from 0 to 70; the score for the mobility component, which consists of ambulation and bed transfer categories, ranges from 0 to 30 [14].
Neurological assessment
On admission to the rehabilitation unit, the controls and rTMS-treated stroke patients underwent a neurological assessment scored using the National Institutes of Health Stroke Scale (NIHSS) [16]. The Brunnstrom recovery stage was used to discern whether the upper extremity neurologic function had improved or not [17]. The type of stroke was classified as hemorrhagic or ischemic. Additionally, the laterality of stroke was evaluated by magnetic resonance imaging. The site of the lesion was categorized as cortical or subcortical based on the presence or absence of cortical involvement, respectively.
The degree of motor impairment is known as the simplest prognostic indicator, with greater initial impairment predicting worse functional recovery [18,19,20]. Voluntary shoulder abduction and finger extension within 72 hours of stroke and 4 weeks after the initial assessment were assessed in this study. In each case, the strength was graded on a scale of 0 to 5 using the Medical Research Council criteria, and it was then summed to determine the SAFE (Shoulder Abduction, Finger Extension) score on a 0–10 scale.
Cognitive assessment
All of the subjects were cognitively assessed twice using the K-MMSE, first on admission to the rehabilitation unit and then again 4 weeks after the initial assessment. The K-MMSE consists of five subscales including orientation, registration recall, attention and calculation, language, and complex commands. The total scores ranges from 0 to 30 [21].
Statistics
Statistical analysis was performed using PASW Statistics ver. 18 for Windows (IBM Corp., Armonk, NY, USA). The Pearson chi-square test was used to analyze the baseline categorical data in the initial assessment according to the gender, type, laterality of stroke and site of lesion, among the other parameters. The numerical data on age, time since stroke and length of stay were compared between the controls and rTMS-treated patients using the Kruskal-Wallis test. The Wilcoxon signed-rank test was run to compare the changes in the NIHSS, Brunnstrom recovery stage, muscle power, K-MBI and K-MMSE between admission and follow-up among the controls and rTMS-treated patients with or without responsiveness. Then, the Kruskal-Wallis test was conducted once again, this time to compare the NIHSS, Brunnstrom recovery stage, muscle power, K-MBI and K-MMSE baseline and changed scores among the controls and rTMS-treated patients with or without responsiveness. After the Kruskal-Wallis test, Bonferroni correction was applied to pair the groups. Finally, Spearman correlation analysis was performed to delineate the relationships between the K-MBI self-care and mobility scores. The significance level was set at p≤0.05.
RESULTS
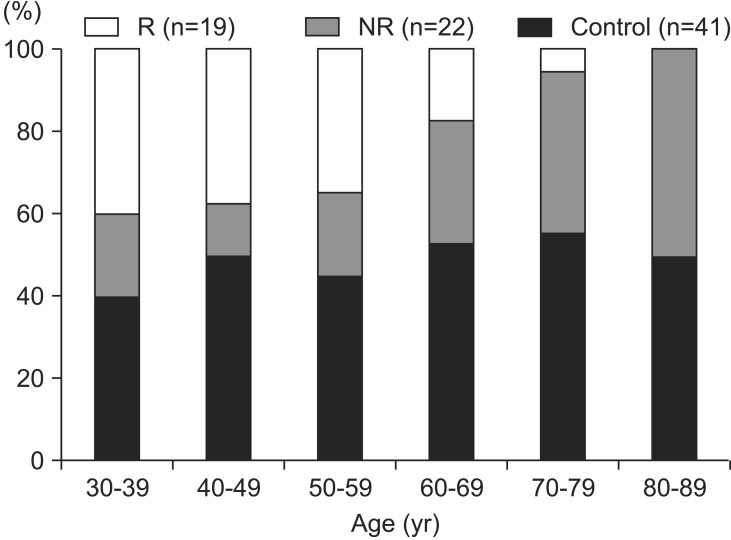
Forty-one patients (24 men and 17 women) in whom low-frequency rTMS was performed were divided into two groups according to their K-MBI self-care scores: non-responded group (n=22; 53.7%) and responded group (n=19; 46.3%). The mean age of the subjects was 60.0 years (range, 32–83 years). There was a significant age difference between the rTMS-treated patients with and without responsiveness (Table 1): the responded group was markedly younger (51.6±10.5 years vs. 65.5±13.7 years, respectively; p=0.001). The age distribution plotted in Fig. 1 shows the relative youth of the responded group. To delineate the influence of age factor on functional improvement following rTMS application, control and rTMS-treated groups were divided into two groups (younger and older groups), and the cutoff value was set to the mean age of subjects (60 years). The gain scores of K-MBI self-care were improved more significantly in the younger rTMS-treated group than in the younger control group (21.5±8.8 and 0.9±4.7, respectively; p<0.001). None of the other clinical characteristics, including gender, cause, laterality of stroke, site of lesion, onset, and length of stay, differed significantly among the three groups.
Table 1. Baseline characteristics of controls and rTMS-treated patients.
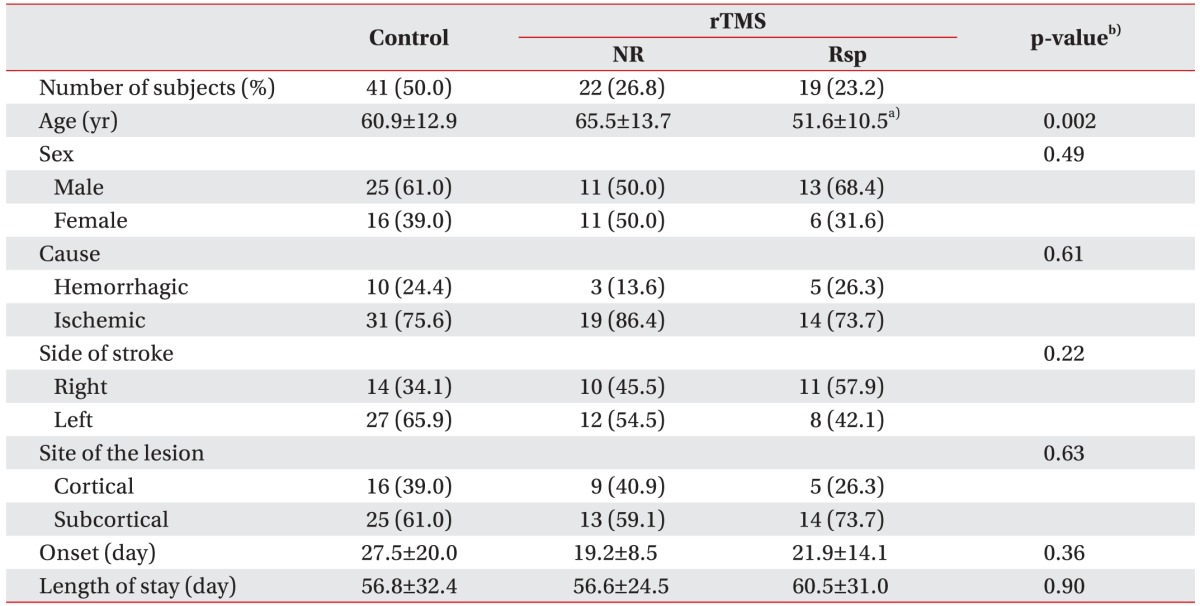
Values are presented as number (%) or mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; NR, non-responding stroke patients; Rsp, responding stroke patients.
a)p<0.05 compared with control and NR, b)Kruskal-Wallis test with Bonferroni correction for continuous variables, or Pearson chi-square test for categorical variables.
Fig. 1. Age distribution of controls and rTMS-treated stroke patients. rTMS, repetitive transcranial magnetic stimulation; NR, non-responding stroke patients; R, responding stroke patients.
On admission, none of the functional scores were significantly different among the groups (Table 2). After 4 weeks, the follow-up and gain scores for self-care and mobility as well as the total K-MBI scores were higher in the rTMS-treated group than in the controls. Moreover, the K-MBI self-care, mobility and total scores were significantly more improved in the responded group than in the non-responded group. We found that the gain scores for self-care on the K-MBI were positively correlated with those for mobility and the total score (Spearman correlation coefficient=0.591, p<0.001).
Table 2. Initial and follow-up results for the two categories of the K-MBI and total scores in controls and rTMS-treated stroke patients.
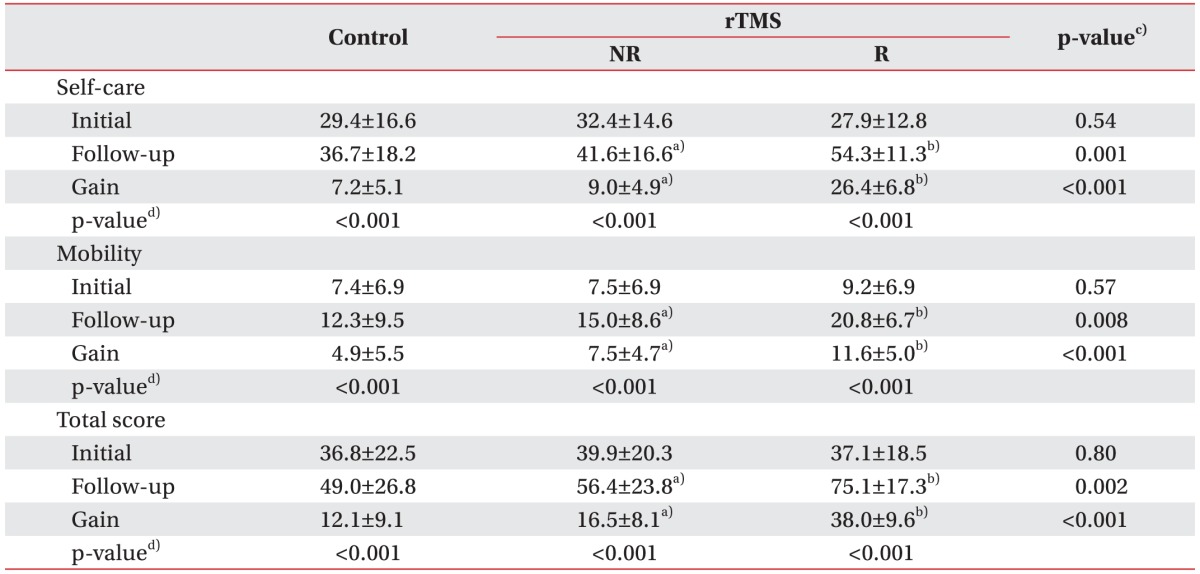
Values are presented as mean±standard deviation.
K-MBI, Korean version of Modified Barthel Index; rTMS, repetitive transcranial magnetic stimulation; NR, non-responding stroke patients; R, responding stroke patients.
a)p<0.05 compared with control, b)p<0.05 compared with NR, c)Kruskal-Wallis test with Bonferroni correction, d)Wilcoxon sum rank test.
The initial NIHSS score and the Brunnstrom recovery stage did not statistically differ between the controls and rTMS-treated patients, although all of the scores were increased at 4 weeks after the initial assessment. Also, the gains in the NIHSS score and Brunnstrom recovery stage in the responded group were superior to those in the controls and the non-responded group (Table 3). Shoulder abduction, finger extension and SAFE score were also significantly improved in all the three groups. Additionally, the follow-up and gain scores for the upper extremity muscle power showed statistically significant differences between the controls and rTMS-treated patients. For example, the shoulder abduction test showed a significant improvement in the responded group relative to the control group and non-responded group (gains: 1.2±1.1 vs. 0.4±0.7 and 0.8±0.8, respectively; p=0.007). Moreover, the finger extension test and SAFE score were also significantly more improved in the responded group than in the control group and the non-responded group (p<0.001) (Table 4).
Table 3. Initial and follow-up results for NIHSS and Brunnstrom stage in controls and rTMS-treated stroke patients.
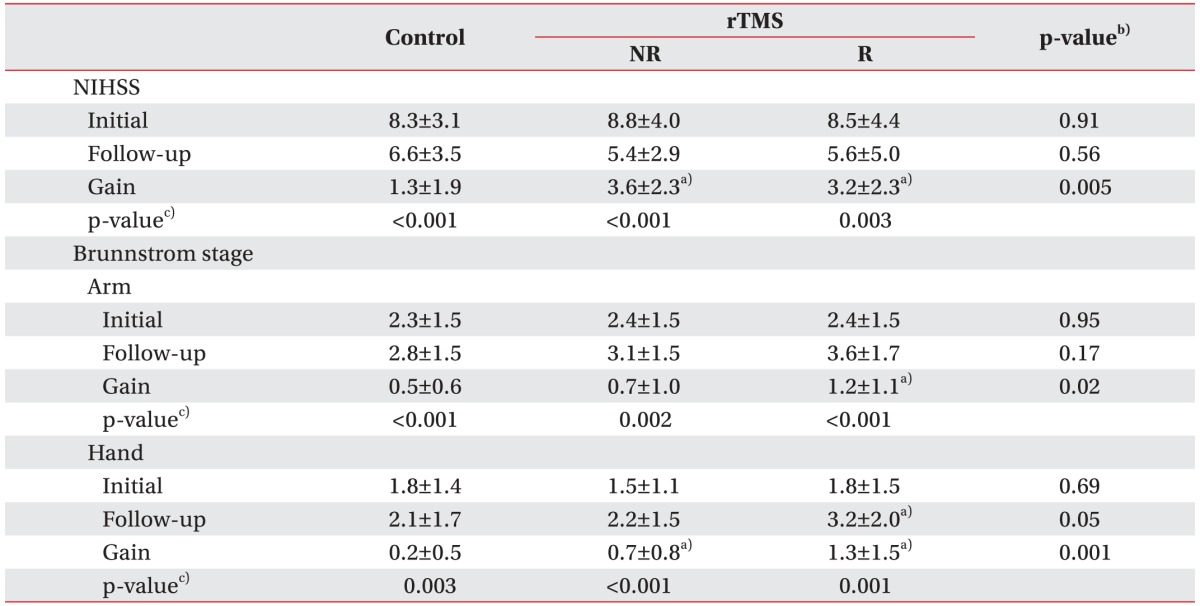
Values are presented as mean±standard deviation.
NIHSS, National Institutes of Health Stroke Scale; rTMS, repetitive transcranial magnetic stimulation; NR, non-responding stroke patients; R, responding stroke patients.
a)p<0.05 compared with control, b)Kruskal-Wallis test with Bonferroni correction, c)Wilcoxon sum rank test.
Table 4. Initial and follow-up results for upper extremity muscle power in controls and rTMS-treated stroke patients.
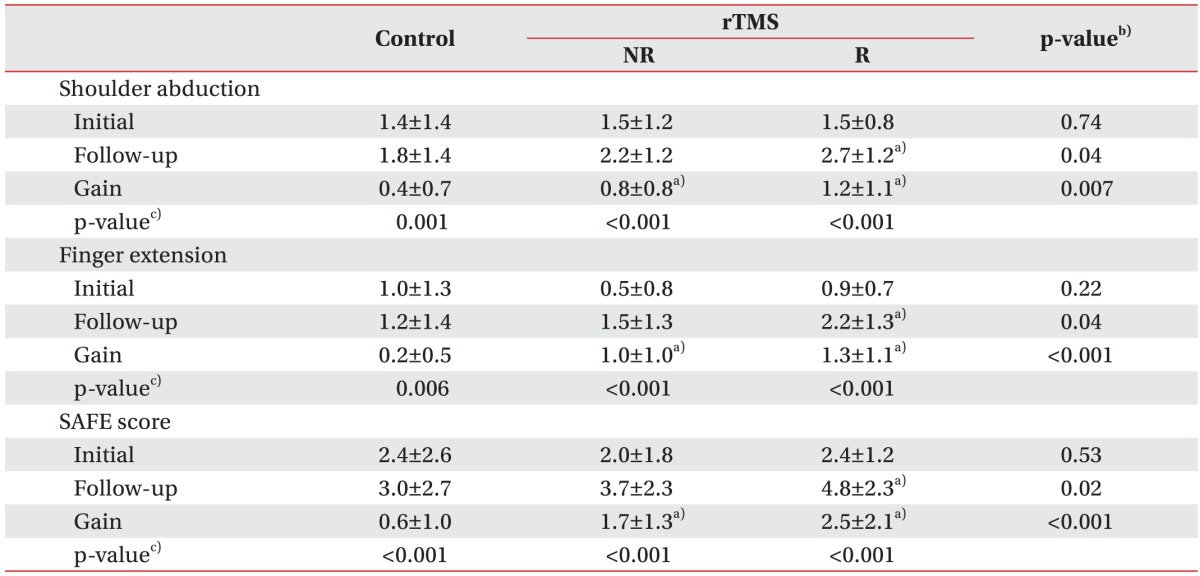
Values are presented as mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; NR, non-responding stroke patients; R, responding stroke patients; SAFE score, shoulder abduction and finger extension score.
a)p<0.05 compared with control, b)Kruskal-Wallis test with Bonferroni correction, c)Wilcoxon sum rank test.
In all three groups, the K-MMSE score showed a significant improvement between admission and 4 weeks later (Table 5). Although the difference in the mean changes between the control group and the responded group was not statistically significant, the follow-up K-MMSE score in the responded group was superior to that in the control group.
Table 5. Initial and follow-up results of K-MMSE in controls and rTMS-treated stroke patients.
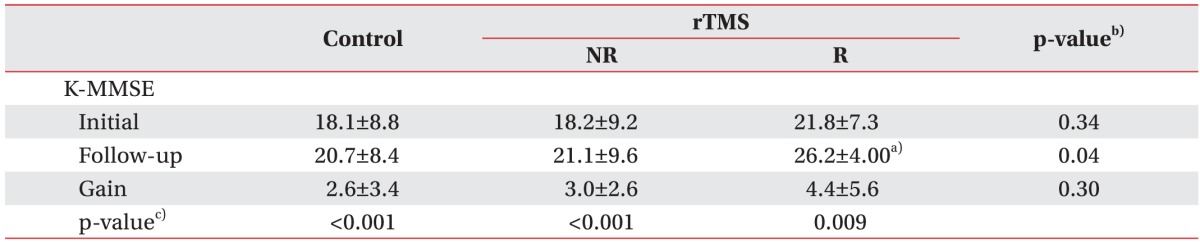
Values are presented as mean±standard deviation.
K-MMSE, Korean version of Mini-Mental Status Examination; rTMS, repetitive transcranial magnetic stimulation; NR, non-responding stroke patients; R, responding stroke patients.
a)p<0.05 compared with control, b)Kruskal-Wallis test with Bonferroni correction, c)Wilcoxon sum rank test.
DISCUSSION
Many studies have suggested that rTMS has an effect on the excitability of the brain that can enhance the motor function recovery of paretic upper extremities [3,4,5,6,7,8]. Although rTMS can improve functional impairment in selected patients, the overall clinical results have been variable. Several studies have demonstrated that specific characteristics, including disease-related plasticity, age, gender, lesion location and cognitive status, influence the effects of rTMS [3,11,12]. For example, age-dependency of motor cortical plasticity has been implicated in low-frequency brain stimulation [3,13] but not in high-frequency brain stimulation [11,12].
Inhibitory rTMS has shown a therapeutic potential in restoring the balance of inter-hemispheric inhibition after stroke [3]. Such an effect occurs in an intact ipsilesional cerebral cortex, which is not the target site of stimulatory rTMS [12]. Several investigators have demonstrated age-related differences in inter-limb coordination and control of corticospinal inhibitory processes [22,23]. Older individuals exhibiting motor deterioration and diminished ability to coordinate movement appear to show a reduced capability to modulate GABA-mediated inhibitory processes [24]. However, these studies were either conducted in normal individuals [22,23] or they used paired associative stimulation [13]. In other words, these studies did not find a direct relation between aging and functional impairment following rTMS. One of the most remarkable findings of our present study was the age of the patients in the responded group: 9.3 years lesser than the age of the patients in the control group on average, which is a significant difference. Indeed, younger patients, compared to older patients, have a greater potential for enhanced post-stroke motor and functional recovery following inhibitory rTMS. We hypothesized that with increasing age there would be less extra activation in the inhibitory circuits targeting the contralesional motor cortex. When the criterion for the responded group was set according to Brunnstrom recovery stage and responsiveness to rTMS was determined based on improvements of more than 2 Brunnstrom hand recovery stages, the responded group was significantly younger than the non-responded group (53.8±13.7 years vs. 64.1±13.3 years, respectively; p=0.018).
It has been assumed that upper extremity motor recovery after inhibitory rTMS is also influenced by lesion location, especially the presence or absence of cortical involvement [12]. However, that study included only 20 stroke patients. Contrastingly, in our present study, there were no significant differences in the respective proportions of patients with and without cortical involvement. It remains unclear to what extent stroke location and distribution determine the individual response to rTMS treatment, especially to inhibitory rTMS [25,26]. Clearly, additional, large-cohort studies focusing on lesion size are necessary.
In the responded group in the present study, the functional improvements were more prominent than those in the controls or the non-responded group at 4 weeks following rTMS. The most plausible explanation is that the follow-up scores for Brunnstrom's recovery of hand function and upper extremity muscle power, as well as the gain scores, were significantly more increased in the responded group than in the other groups. Notably, increases in the K-MBI self-care and mobility scores were also larger in the responded group. Moreover, the mobility score was positively correlated with the self-care score on the K-MBI (Spearman correlation coefficient=0.591, p<0.001). It seems that application of rTMS over the motor cortex of the hand area can indirectly contribute to improvements in lower-extremity function. Some researchers have evaluated the effect of rTMS on the walking ability [27,28]; however, in those studies, rTMS was applied over the leg area of the motor cortex. Studies to determine whether application of rTMS over the hand area has any influence on lower-extremity function are required.
Several studies have demonstrated that rTMS can influence learning, but they did not provide the precise mechanism by which that particular effect was achieved [3,11,29]. In our current study, the follow-up K-MMSE score in the responded group was higher than that in the control group. However, the gain scores among the three groups were not significantly different, probably due to the ceiling effect.
It is already well established that down-regulation of the excitability of the unaffected motor cortex, in concert with up-regulation of the excitability of the affected side, can improve motor skills [30]. However, it is still unclear which type of stimulation has a greater effect on upper extremity motor function [8]. Given the unproven efficacy of treatment and the differential excitability capacities, the variables influencing the respective effects of stimulatory and inhibitory rTMS on upper extremity motor recovery might differ. For the purpose of predicting the difference in responsiveness with respect to the two rTMS modes, future studies should employ a specific neuroimaging technique (e.g., diffusion-tensor imaging) for evaluation of white-matter integrity and connectivity.
There are several limitations to our study. First, the population size was relatively small, and the 4-week follow-up period was too short to reveal the long-term effect of rTMS treatment on functional improvement. The subjects were not blinded, and the study lacked a sham treatment group. Also, the main limitation of our study is that we did not compare the changes in MEP amplitude and resting motor threshold following rTMS treatment between the responded group and the non-responded group, which could more clearly represent the effect of rTMS treatment on cortical plasticity. Finally, it has been established that upper extremity functional status can be influenced by the unaffected upper extremity as well as by the affected upper extremity. However, application of rTMS over the contralesional hemisphere has an influence on the dexterity of the affected hand only, and not on the dexterity of the unaffected hand [31]. The gain scores for K-MBI self-care in the rTMS-treated group were significantly higher than those in controls, in both the responded group and the non-responded group (26.4±6.8 and 9.0±4.9 vs. 7.2±5.1, respectively; p<0.001). If upper extremity functional status was largely influenced by the unaffected upper extremity, there would be no significant differences between the rTMS-treated group and the control group. Studies are needed to determine the extent to which patients might be able to use the unaffected upper extremity. However, this study sheds light on proper prognosis and rehabilitation of stroke patients according to the responsiveness to rTMS treatment. More accurate prognosis as well as the ability to predict the potential for functional upper extremity recovery would enable more realistic rehabilitation goal setting and more efficient resource allocation [32].
In conclusion, younger stroke patients showed better upper extremity functional outcomes following low-frequency rTMS. Also, the functionally responding patients demonstrated greater upper extremity muscle power, more independent gait, and higher cognitive status. The mechanism underlying the changes in functional outcome that correlate with advancing age and changes in corticospinal excitability requires further investigation.
ACKNOWLEDGMENTS
This work was carried out with the support of Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ01024403) in Rural Development Administration, Republic of Korea.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Aprile I, Rabuffetti M, Padua L, Di Sipio E, Simbolotti C, Ferrarin M. Kinematic analysis of the upper limb motor strategies in stroke patients as a tool towards advanced neurorehabilitation strategies: a preliminary study. Biomed Res Int. 2014;2014:636123. doi: 10.1155/2014/636123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen TS. Arm and leg paresis as outcome predictors in stroke rehabilitation. Stroke. 1990;21:247–251. doi: 10.1161/01.str.21.2.247. [DOI] [PubMed] [Google Scholar]
- 3.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Rose DK, Patten C, McGuirk TE, Lu X, Triggs WJ. Does inhibitory repetitive transcranial magnetic stimulation augment functional task practice to improve arm recovery in chronic stroke. Stroke Res Treat. 2014;2014:305236. doi: 10.1155/2014/305236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65:466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- 6.Jin X, Wu X, Wang J, Huang B, Wang Q, Zhang T, et al. Effect of transcranial magnetic stimulation on rehabilitation of motor function in patients with cerebral infarction. Zhonghua Yi Xue Za Zhi. 2002;82:534–537. [PubMed] [Google Scholar]
- 7.Lefaucheur JP. Stroke recovery can be enhanced by using repetitive transcranial magnetic stimulation (rTMS) Neurophysiol Clin. 2006;36:105–115. doi: 10.1016/j.neucli.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki N, Mizutani S, Kakuda W, Abo M. Comparison of the effects of high- and low-frequency repetitive transcranial magnetic stimulation on upper limb hemiparesis in the early phase of stroke. J Stroke Cerebrovasc Dis. 2013;22:413–418. doi: 10.1016/j.jstrokecerebrovasdis.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Kim BR, Chun MH, Kim DY, Lee SJ. Effect of high- and low-frequency repetitive transcranial magnetic stimulation on visuospatial neglect in patients with acute stroke: a double-blind, sham-controlled trial. Arch Phys Med Rehabil. 2013;94:803–807. doi: 10.1016/j.apmr.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, Choi HE, Jung H, Lee BJ, Lee KH, Lim YJ. Comparison of the effects of 1 Hz and 20 Hz rTMS on motor recovery in subacute stroke patients. Ann Rehabil Med. 2014;38:585–591. doi: 10.5535/arm.2014.38.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JH, Kim SB, Lee KW, Kim MA, Lee SJ, Choi SJ. Factors associated with upper extremity motor recovery after repetitive transcranial magnetic stimulation in stroke patients. Ann Rehabil Med. 2015;39:268–276. doi: 10.5535/arm.2015.39.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emara T, El Nahas N, Elkader HA, Ashour S, El Etrebi A. MRI can predict the response to therapeutic repetitive Transcranial Magnetic Stimulation (rTMS) in stroke patients. J Vasc Interv Neurol. 2009;2:163–168. [PMC free article] [PubMed] [Google Scholar]
- 13.Tecchio F, Zappasodi F, Pasqualetti P, De Gennaro L, Pellicciari MC, Ercolani M, et al. Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol. 2008;119:675–682. doi: 10.1016/j.clinph.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Jung HY, Park BK, Shin HS, Kang YK, Pyun SB, Paik NJ, et al. Development of the Korean version of Modified Barthel Index (K-MBI): multi-center study for subjects with stroke. J Korean Acad Rehabil Med. 2007;31:283–297. [Google Scholar]
- 15.Bembenek JP, Kurczych K, Karli Nski M, Czlonkowska A. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke :a systematic review of the literature. Funct Neurol. 2012;27:79–84. [PMC free article] [PubMed] [Google Scholar]
- 16.Kunitz SC, Gross CR, Heyman A, Kase CS, Mohr JP, Price TR, et al. The pilot Stroke Data Bank: definition, design, and data. Stroke. 1984;15:740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 17.Safaz I, Yilmaz B, Yasar E, Alaca R. Brunnstrom recovery stage and motricity index for the evaluation of upper extremity in stroke: analysis for correlation and responsiveness. Int J Rehabil Res. 2009;32:228–231. doi: 10.1097/MRR.0b013e32832a62ad. [DOI] [PubMed] [Google Scholar]
- 18.Beebe JA, Lang CE. Active range of motion predicts upper extremity function 3 months after stroke. Stroke. 2009;40:1772–1779. doi: 10.1161/STROKEAHA.108.536763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smania N, Paolucci S, Tinazzi M, Borghero A, Manganotti P, Fiaschi A, et al. Active finger extension: a simple movement predicting recovery of arm function in patients with acute stroke. Stroke. 2007;38:1088–1090. doi: 10.1161/01.STR.0000258077.88064.a3. [DOI] [PubMed] [Google Scholar]
- 20.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G EPOS Investigators. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke. The EPOS cohort study. Stroke. 2010;41:745–750. doi: 10.1161/STROKEAHA.109.572065. [DOI] [PubMed] [Google Scholar]
- 21.Kang Y, Na DL, Hahn S. A validity study on the Korean Mini-Mental State Examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997;15:300–308. [Google Scholar]
- 22.Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res. 2008;186:59–66. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujiyama H, Hinder MR, Schmidt MW, Garry MI, Summers JJ. Age-related differences in corticospinal excitability and inhibition during coordination of upper and lower limbs. Neurobiol Aging. 2012;33:1484.e1–1484.e14. doi: 10.1016/j.neurobiolaging.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Levin O, Fujiyama H, Boisgontier MP, Swinnen SP, Summers JJ. Aging and motor inhibition: a converging perspective provided by brain stimulation and imaging approaches. Neurosci Biobehav Rev. 2014;43:100–117. doi: 10.1016/j.neubiorev.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66:298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- 26.Corti M, Patten C, Triggs W. Repetitive transcranial magnetic stimulation of motor cortex after stroke: a focused review. Am J Phys Med Rehabil. 2012;91:254–270. doi: 10.1097/PHM.0b013e318228bf0c. [DOI] [PubMed] [Google Scholar]
- 27.Wang RY, Tseng HY, Liao KK, Wang CJ, Lai KL, Yang YR. rTMS combined with task-oriented training to improve symmetry of interhemispheric corticomotor excitability and gait performance after stroke: a randomized trial. Neurorehabil Neural Repair. 2012;26:222–230. doi: 10.1177/1545968311423265. [DOI] [PubMed] [Google Scholar]
- 28.Kakuda W, Abo M, Watanabe S, Momosaki R, Hashimoto G, Nakayama Y, et al. High-frequency rTMS applied over bilateral leg motor areas combined with mobility training for gait disturbance after stroke: a preliminary study. Brain Inj. 2013;27:1080–1086. doi: 10.3109/02699052.2013.794973. [DOI] [PubMed] [Google Scholar]
- 29.Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- 30.Kalra L, Rossini PM. Influencing poststroke plasticity with electromagnetic brain stimulation: myth or reality. Neurology. 2010;75:2146–2147. doi: 10.1212/WNL.0b013e31820204d9. [DOI] [PubMed] [Google Scholar]
- 31.Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke: a double-blind placebocontrolled crossover trial. Restor Neurol Neurosci. 2007;25:461–465. [PubMed] [Google Scholar]
- 32.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–1232. doi: 10.1016/S1474-4422(10)70247-7. [DOI] [PubMed] [Google Scholar]