Abstract
Objective
To assess the correlation between the anorectal function and bladder detrusor function in patients with complete spinal cord injury (SCI) according to the type of lesion.
Methods
Medical records of twenty-eight patients with SCI were included in this study. We compared the anorectal manometric and urodynamic (UD) parameters in total subjects. We analyzed the anorectal manometric and UD parameters between the two groups: upper motor neuron (UMN) lesion and lower motor neuron (LMN) lesion. In addition, we reclassified the total subjects into two groups according to the bladder detrusor function: overactive and non-overactive.
Results
In the group with LMN lesion, the mean value of maximal anal squeeze pressure (MSP) was slightly higher than that in the group with UMN lesion, and the ratio of MSP to maximal anal resting pressure (MRP) was statistically significant different between the two groups. In addition, although the mean value of MSP was slightly higher in the group with non-overactive detrusor function, there was no statistical correlation of anorectal manometric parameters between the groups with overactive and non-overactive detrusor function.
Conclusion
The MSP and the ratio of MSP to MRP were higher in the group with LMN lesion. In this study, we could not identify the correlation between bladder and bowel function in total subjects. We conclude that the results of UD study alone cannot predict the outcome of anorectal manometry in patients with SCI. Therefore, it is recommended to perform assessment of anorectal function with anorectal manometry in patients with SCI.
Keywords: Neurogenic bowel, Neurogenic urinary bladder, Spinal cord injuries, Manometry, Urodynamics
INTRODUCTION
Neurogenic bladder that develops after spinal cord injury (SCI) has become an active area of research; on the other hand, there is a lack of systematic research on neurogenic bowel because it is not a threat to life [1]. After SCI, 27%–62% of patients complain of chronic gastrointestinal dysfunction such as constipation, abdominal discomfort, etc. [2,3]. Changes in bladder and bowel function have a considerable impact on the quality of life of patients with SCI [4].
The genitourinary tract and the gastrointestinal tract share the same embryology called the cloaca, location, and sacral innervations [5]. There are analogies between colorectal and urinary dysfunction following SCI. Both are reservoir organs that require coordinated smooth and striated muscle interaction for functioning [6]. Defecation and voiding are the result of simultaneous relaxation of striated muscle and contraction of smooth muscle. This may be disturbed following SCI and result in contraction of both types of muscles leading to incomplete evacuation or obstruction of urinary outflow [7]. This has served as a basis for imparting an understanding of the interconnected nature of lower urinary tract and anorectal dysfunction.
Urodynamic (UD) study is commonly performed to observe the detrusor function. Although anorectal manometry can evaluate the anal sphincter function, this test has rarely been used clinically in SCI patients. There has been little study to investigate correlations between functional parameters of neurogenic bladder and bowel. The aim of this study was to assess whether the UD study can predict the anorectal manometry outcome through a correlation between the two studies in patients with SCI.
MATERIALS AND METHODS
In this retrospective study, medical records of twenty-eight patients with complete SCI aged from 20 to 77 years (23 males, 5 females; mean age, 43.5±16.5 years; mean time since injury, 67.2±69.5 months) were included. Patients with peripheral neuropathy, an evidence of urinary tract infection, concurrent brain injury, and those who were taking drugs that may affect the intravesical pressure, such as anticholinergics or cholinergics were excluded from the study. In addition, we also excluded the patients who were taking drugs that may affect the gastrointestinal motility from the study. Maximal anal resting pressure (MRP), maximal anal squeeze pressure (MSP), and the ratio of MSP to MRP were evaluated with anorectal manometry. In the UD study, maximal detrusor pressure, maximal bladder volume, and bladder compliance were measured.
Anorectal manometry was performed with a Polygraf ID A500 eight-channel manometer (Given Imaging, Yokneam, Israel). A radial silicone catheter with a diameter of 0.5 cm was used for measurement of anal canal pressures. During the test, the examiner induced psychological stability and limited the factors that can affect the intra-abdominal pressure including coughing, laughing, sighing, etc. Normal ranges were defined as follows: resting anal pressure, 40–70 mmHg; and squeeze anal pressure, 100–180 mmHg.
Patients were placed in the left lateral decubitus position and the catheter was placed at the height of the anus for calibration. After calibration, the catheter was lubricated with gel, introduced into the anus, and advanced until the distal end was 6 cm from the anal verge. At the start of the examination, patients were instructed to relax the anal region for more than 1 minute for measurement of resting pressure. Patients were then asked to contract the anus as much as possible for MSP measurement at each position. Using the stationary pull-through method, the examiner manually withdrew the catheter 1 cm caudad every 30 seconds and measured the anal resting and squeeze pressures.
UD study was performed using Solar Gold (MMS, Enschede, The Netherlands). Detrusor pressure (Pdet) was calculated indirectly by subtracting the abdominal pressure (Pabd) from the vesical pressure (Pves): Pdet = Pves – Pabd. One urinary catheter was employed: a 6-Fr catheter for Pves recording. Pabd was measured with a 10-Fr balloon catheter inflated with 20 mL distilled water. Patients were placed in the supine position and the urethral and rectal catheters were placed after cleansing of the genital area using an aseptic technique. A saline solution (NaCl 0.9%) was warmed to 32℃ and infused into the bladder at a rate of 24 mL/min. The diagnosis of overactive bladder was confirmed according to the standardization of the 2002 International Continence Society, and it is defined as a condition in which there are spontaneous or provoked involuntary detrusor contractions during the filling phase [8].
According to the type of SCI lesion, total subjects were divided into two groups: the upper motor neuron (UMN) lesion and the lower motor neuron (LMN) lesion. On physical examination, patients with one or more of the following symptoms suggesting an UMN lesion were classified into the UMN lesion group; Babinski sign, ankle clonus, increased deep tendon reflex, bulbocavernosus or clitocavernous reflex. In addition, we reclassified the total subjects into two groups according to the detrusor function. This study was a retrospective study based on the patient's record. Institutional Review Board approval was obtained for this study. The t-test was used for between-group comparison of anorectal manometric parameters. Statistical significance was set at p<0.05.
RESULTS
The mean MRP, MSP, the ratio of MSP to MRP, maximal bladder detrusor pressure, maximal bladder volume, and bladder compliance in the 28 patients with complete SCI were 54.5±23.3 mmHg, 70.0±28.2 mmHg, 1.3±0.4, 23.5±17.1 cmH2O, 439.6±170.8 mL, and 31.1±27.9 mL/cmH2O, respectively. MRP was within the normal range; however, MSP showed a low value compared to the normal values in total subjects.
In the group with UMN lesion, the mean MRP, MSP, the ratio of MSP to MRP, maximal bladder detrusor pressure, maximal bladder volume, and bladder compliance were 55.2±21.8 mmHg, 64.1±25.8 mmHg, 1.2±0.1, 22.9±13.7 cmH2O, 409.4±191.0 mL, and 27.4±27.1 mL/cmH2O, respectively. In the group with LMN lesion, the mean MRP, MSP, the ratio of MSP to MRP, maximal bladder detrusor pressure, maximal bladder volume, and bladder compliance were 53.4±26.5 mmHg, 79.2±30.5 mmHg, 1.6±0.5, 24.4±22.1 cmH2O, 486.4±128.2 mL, and 36.8±29.5 mL/cmH2O, respectively (Table 1). In the group with LMN lesion, the MSP was slightly higher and the ratio of MSP to MRP was statistically significantly different between the two groups. In addition, there was no statistically significant difference in the urodynamic parameters (Table 2).
Table 1. Demographic and clinical variables according to the type of spinal cord injury lesion.
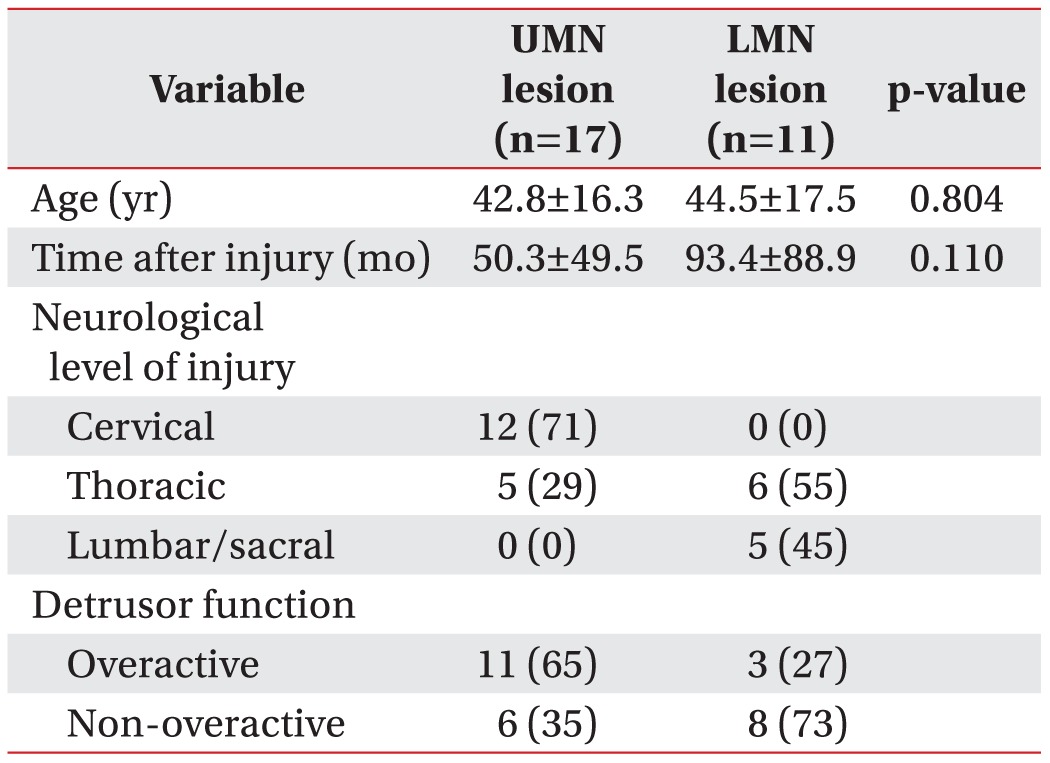
Values are presented as mean±standard deviation or number (%).
UMN, upper motor neuron; LMN, lower motor neuron.
Table 2. Differences in anorectal manometric and urodynamic parameters between the two groups: the UMN and LMN lesion.

Values are presented as mean±standard deviation.
UMN, upper motor neuron; LMN, lower motor neuron; MRP, maximal anal resting pressure; MSP, maximal squeeze pressure.
*p<0.05.
We reclassified the total subjects into two groups according to the detrusor function: overactive and nonoveractive detrusor function (Table 3). Although the MSP was slightly higher in the group with non-overactive detrusor function, there was no statistically significant difference in the anorectal manometric parameters between the two groups (Table 4).
Table 3. Demographic and clinical variables according to the detrusor function.
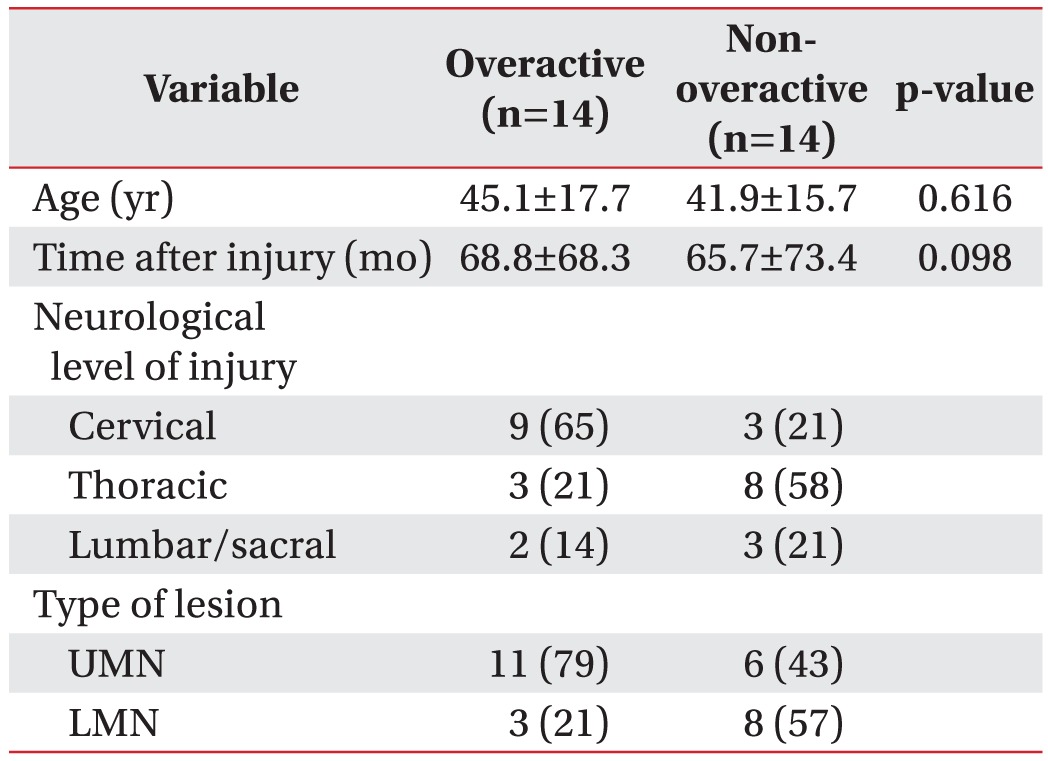
Values are presented as mean±standard deviation or number (%).
UMN, upper motor neuron; LMN, lower motor neuron.
Table 4. Differences in anorectal manometric and urodynamic parameters between the two groups: overactive and non-overactive detrusor function.
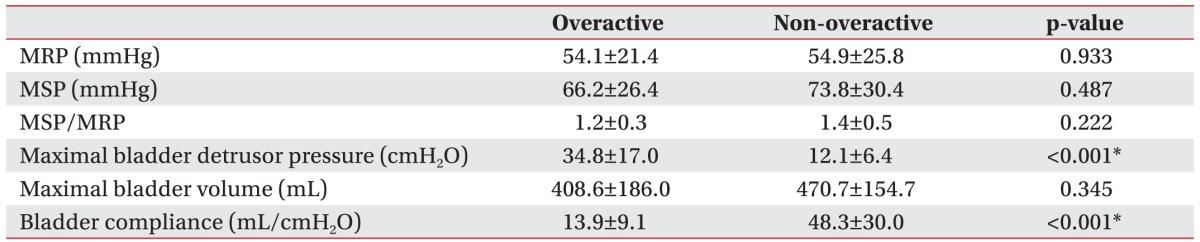
Values are presented as mean±standard deviation.
MRP, maximal anal resting pressure; MSP, maximal squeeze pressure.
*p<0.05.
DISCUSSION
The anal sphincter has both smooth muscle and striated muscle components. Both sensory and motor innervations occur through several nerves including the lumbar colonic nerves, the pudendal nerves and pelvic nerves with connections to the spinal cord levels from at least L2 to S4 [9]. Fecal continence requires the ability to maintain resting internal anal sphincter (IAS) tone and external anal sphincter (EAS) contraction in response to increased intra-abdominal pressure, rectal distention, and rectal contraction [10]. IAS tone is increased by the sympathetic nerve stimulation of the hypogastric plexus originating from the L1-2 segment and IAS tone is reduced by the parasympathetic nerve stimulation of the pelvic nerve originating from the S2-4 segment. This contributes to most of the resting anal pressure (55%). EAS is under voluntary control through the pudendal nerve originating from the S2-4 segment and it contributes to the squeeze pressure [11].
Sacral function is clinically assessed through physical examination including neurological test; therefore, the variation of test results could differ depending on the subjectivity of the examiner. In addition, it is difficult to describe the symptoms by simply evaluating the anal sphincter function because the mechanism of neurogenic bowel is complex [1]. In anorectal manometry, MRP indicates the IAS function and MSP indicates the EAS function. In patients with SCI, anorectal manometry has the advantage of being able to obtain direct and objective indicators for measuring the IAS and EAS function.
Bowel dysfunction in patients with SCI could show different patterns according to the type of SCI lesion. Spinal cortical sensory pathway deficits in cases of upper motor neurogenic bowel can lead to a decreased ability to sense the urge to defecate. The upper motor neurogenic bowel can show a hyperreflexic response resulting from sacral reflexes caused by passive filling of the rectum [12]. Patients with lower motor neurogenic bowel dysfunction can show decreased anal tone and the anocutaneous reflex may be absent or decreased because of injury to afferent or efferent sacral pathways [13].
Anorectal manometry is a test that evaluates functional anorectal disorders and pelvic floor disorders. The anorectal manometry results were classified into four patterns on the basis of rectal pressure and sphincter tone in response to rectal distention and the patterns were similar to those in cystometrograms; however, there was no significant relationship with bowel dysfunction [10]. Women with overactive bladder had increased incidence of paradoxical puborectalis contraction than those in the control group; however, there were no significant differences between groups in the following anorectal manometric parameters: rectoanal inhibitory reflex, rectal sensitivity, maximum tolerable volume, resting pressure, and hypertonia at rest [14]. In another study, the mean MSP showed lower values compared with the normal range in patients with complete SCI. In contrast, the mean MSP showed relatively normal values in patients with incomplete SCI [1]. Pudendal neuropathy and impaired rectoanal inhibitory reflex are common and may be important in the pathogenesis of bowel dysfunction in patients with spinal cord lesions [15]. Another study evaluated the clinical, neurological, and pathophysiological counterparts of neurogenic bowel in subjects with motor complete SCI classified into the following three groups: lesion above T7 with preserved spinal sacral reflexes, lesion below T7 with preserved sacral reflexes, and lesion below T7 without sacral reflexes. Resting pressure showed near normal results in all groups and MSP showed a tendency to increase by 23% and 86% in the groups with lesion above T7 and below T7 with preserved sacral reflexes. However, in the group with lesion below T7 without sacral reflexes, MSP did not show a tendency to increase [16].
We analyzed the anorectal manometric and urodynamic parameters depending on the type of SCI lesion. The mean MRP was within the normal range; however, the mean MSP showed lower values compared with the normal range in total subjects. Based on the results of this study, the IAS may have a normal function in some patients, resulting in almost normal resting anal canal pressure. This suggests that the majority of the resting anal sphincter pressure generated by the contraction of the IAS and the ability are well preserved in patients with complete SCI.
Reflex arc control is preserved in most patients with upper motor neurogenic bowel dysfunction. In this study, the group with UMN lesion included 65% of the patients with overactive detrusor function and the group with LMN lesion included 73% of the patients with non-overactive detrusor function. The mean MSP was lower in the group with UMN lesion than in the group with LMN lesion. The urodynamic parameters showed no statistically significant differences between the two groups, unlike anorectal manometric parameters. We expected that there would be accompanying weakness of abdominal wall muscle contraction in patients with UMN lesion. We assumed that MSP measured with anorectal manometry is difficult to rule out various pressures exerted on the abdominal wall or pelvic floor [17]. We also suspected that neurogenic bladder and bowel dysfunction in total subjects would be affected by a large variation in time since injury and consumption of drugs for a long time. Another study in patients with SCI who had complete upper motor neurogenic bowel lesions showed that total gastrointestinal transit time was longer during the acute rather than the chronic phase [17].
We reclassified the total subjects into two groups according to the detrusor function in order to analyze the correlation between neurogenic bladder and bowel dysfunction. The MSP and the ratio of MSP to MRP showed slightly decreased values in the group with overactive detrusor function. However, there was no statistically significance in the anorectal manometric parameters between the groups with overactive and non-overactive detrusor function. Despite similarities between colorectal and urinary function, the correlation between neurogenic bladder and bowel was found to be low in our study.
There are some limitations in this retrospective study. The sample size was small and there was a large variation in time since injury among the subjects. The sample size was small; hence, we could not analyze the difference in results according to the period after SCI. Although subjects who were not taking drugs that may affect the bladder or bowel at the time of assessment were enrolled, we could not completely rule out the effects of a remnant drug in the body on the bowel. We did not measure the maximal rectal pressure, anal sphincter relaxation rate, and the defecation index.
In conclusion, the MSP and the ratio of MSP to MRP were lower in the group with UMN lesion. However, there was no statistically significant correlation of the bladder detrusor function between the two groups with UMN and LMN lesion. As a result, there was a statistically significant difference in some anorectal manometric parameters according to the type of SCI lesion. We could not identify the correlation between the anorectal manometric and urodynamic parameters in patients with complete SCI. We conclude that the UD results alone cannot predict the outcome of anorectal manometry in complete SCI patients. Therefore, in order to obtain accurate and objective indicators of bowel dysfunction in SCI patients, it is recommended to perform anorectal manometry. Further complementary studies involving a larger number of cases are needed to confirm the relationship between anorectal manometric and urodynamic findings.
ACKNOWLEDGMENTS
This study was supported by a 2014 research grant from Pusan National University Yangsan Hospital.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim DU, Song GA, Kim GH, Heo J. Usefulness of anorectal manometry in patients with spinal cord injury. Korean J Neurogastroenterol Motil. 2009;15:52–57. [Google Scholar]
- 2.Stone JM, Nino-Murcia M, Wolfe VA, Perkash I. Chronic gastrointestinal problems in spinal cord injury patients: a prospective analysis. Am J Gastroenterol. 1990;85:1114–1119. [PubMed] [Google Scholar]
- 3.Han TR, Kim JH, Kwon BS. Chronic gastrointestinal problems and bowel dysfunction in patients with spinal cord injury. Spinal Cord. 1998;36:485–490. doi: 10.1038/sj.sc.3100616. [DOI] [PubMed] [Google Scholar]
- 4.Krogh K, Nielsen J, Djurhuus JC, Mosdal C, Sabroe S, Laurberg S. Colorectal function in patients with spinal cord lesions. Dis Colon Rectum. 1997;40:1233–1239. doi: 10.1007/BF02055170. [DOI] [PubMed] [Google Scholar]
- 5.Klijn AJ, Asselman M, Vijverberg MA, Dik P, de Jong TP. The diameter of the rectum on ultrasonography as a diagnostic tool for constipation in children with dysfunctional voiding. J Urol. 2004;172(5 Pt 1):1986–1988. doi: 10.1097/01.ju.0000142686.09532.46. [DOI] [PubMed] [Google Scholar]
- 6.Pedersen E. Regulation of bladder and colon: rectum in patients with spinal lesions. J Auton Nerv Syst. 1983;7:329–338. doi: 10.1016/0165-1838(83)90086-3. [DOI] [PubMed] [Google Scholar]
- 7.Ruutu M. Cystometrographic patterns in predicting bladder function after spinal cord injury. Paraplegia. 1985;23:243–252. doi: 10.1038/sc.1985.40. [DOI] [PubMed] [Google Scholar]
- 8.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 9.Sagar PM, Pemberton JH. Anorectal and pelvic floor function. Relevance of continence, incontinence, and constipation. Gastroenterol Clin North Am. 1996;25:163–182. doi: 10.1016/s0889-8553(05)70370-8. [DOI] [PubMed] [Google Scholar]
- 10.Lynch AC, Anthony A, Dobbs BR, Frizelle FA. Anorectal physiology following spinal cord injury. Spinal Cord. 2000;38:573–580. doi: 10.1038/sj.sc.3101076. [DOI] [PubMed] [Google Scholar]
- 11.Krogh K, Christensen P, Laurberg S. Colorectal symptoms in patients with neurological diseases. Acta Neurol Scand. 2001;103:335–343. doi: 10.1034/j.1600-0404.2001.103006335.x. [DOI] [PubMed] [Google Scholar]
- 12.Tjandra JJ, Ooi BS, Han WR. Anorectal physiologic testing for bowel dysfunction in patients with spinal cord lesions. Dis Colon Rectum. 2000;43:927–931. doi: 10.1007/BF02237352. [DOI] [PubMed] [Google Scholar]
- 13.Bartolo DC, Read NW, Jarratt JA, Read MG, Donnelly TC, Johnson AG. Differences in anal sphincter function and clinical presentation in patients with pelvic floor descent. Gastroenterology. 1983;85:68–75. [PubMed] [Google Scholar]
- 14.Goncalves ML, Fernandes SF, de Almeida RM, Diaz FA, de Oliveira PG, de Sousa JB. Anorectal manometry evaluation in adult women with clinical and urodynamic diagnostics of overactive bladder. Arq Bras Cir Dig. 2013;26:280–285. doi: 10.1590/s0102-67202013000400006. [DOI] [PubMed] [Google Scholar]
- 15.Tjandra JJ, Ooi BS, Han WR. Anorectal physiologic testing for bowel dysfunction in patients with spinal cord lesions. Dis Colon Rectum. 2000;43:927–931. doi: 10.1007/BF02237352. [DOI] [PubMed] [Google Scholar]
- 16.Valles M, Vidal J, Clave P, Mearin F. Bowel dysfunction in patients with motor complete spinal cord injury: clinical, neurological, and pathophysiological associations. Am J Gastroenterol. 2006;101:2290–2299. doi: 10.1111/j.1572-0241.2006.00729.x. [DOI] [PubMed] [Google Scholar]
- 17.Krogh K, Mosdal C, Laurberg S. Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord. 2000;38:615–621. doi: 10.1038/sj.sc.3101066. [DOI] [PubMed] [Google Scholar]


