Abstract
Sweet Sherry wines from Pedro Ximénez and Muscat sun and chamber-dried grapes during vintages 2009 and 2010 were aged in American oak wood for 12 months. Their volatile content was periodically analyzed using SBSE-GC-MS. Cluster analysis and principal component analysis demonstrated that the volatile compounds considered can be used to detect grape variety and vintage. Principal component analysis for each grape variety, clearly differentiated aging time and vintage. Drying type was the least significant factor. Sweet wines produced using chamber driers were from Pedro Ximénez and Muscat grapes provides similar in volatile constituents as those produced by traditional process.
Keywords: Sweet Sherry wines, Volatile compounds, Oak cask, Chamber drying, Muscat, Pedro Ximénez
Introduction
Volatile compounds play an important role in the organoleptic characteristics of wines. Several hundred compounds from different families, such as alcohols, esters, aldehydes, ketones, volatile acids, terpenes, etc., contribute to wine flavour. The combination of all these compounds constitutes the character of wine and differentiates one wine from another (García-Jares and García-Martín 1995).
The presence or absence of all of these volatile compounds in a wine depends on several factors: climate and soil, ripeness and grape variety, winemaking conditions, and aging. Some of them are already present in the grapes and constitute the varietal aroma. Many of these volatile compounds are terpenes and terpenols, although there are some other compounds that are present in certain grape varieties such as norisoprenoids, benzene compounds, C6 alcohols, etc. (Schreier 1979; Rapp and Mandery 1986). Volatile compounds also arose from grape metabolism and may vary as a function of grape variety and different cultural and climate-related factors; on the other hand, the extent to which these compounds come from the grape to the final wine also influenced by the conditions of its vinification and aging processes (Rapp 1998).
Some volatile compounds are formed during the processes of fermentation and aging (Schreier 1979; Rapp and Mandery 1986; García-Jares and García-Martín 1995) and may play an important role in the overall aroma of wine. The alcoholic fermentation process generates the greater part of the aromatic compounds present in the wine (Lee et al. 2004; Hernández-Orte et al. 2005).
Normally, all varietal and vinification volatile compounds are subjected to the later process of aging in wood. During this stage, the wine acquires aromatic complexity as a result of important and diverse reactions (Ortega-Heras et al. 2004).
Esterification, hydrolysis and redox reactions together with the slow but continuous diffusion of oxygen through wood pores and the cession of different compounds from the wood to the aged wine take place during the period of aging in oak wood. Additionally, most varietal and fermentative volatile compounds decrease during the aging in wood (Ortega-Heras et al. 2004; Cámara et al. 2006; Ruiz-Bejarano et al. 2013).
In Andalusia, special sweet wines are produced from two grape varieties, Muscat and Pedro Ximénez. These wines are produced in Malaga, Montilla-Moriles and Jerez Denominations of Origin (D.O.) following a traditional method. Grapes of these varieties, in bunches, are spread out on esparto grass mats under sun before pressing. During this period, bunches are turned several times and kept covered at night. This traditional process is affected by the attack of insects and possible rainfall. This leads to growth of fungi which resulted in loss of high amount of grapes and give rise to the formation of toxins in the wine (Valero et al. 2008; Ruíz Bejarano et al. 2010).
After this, the grapes attain sugar levels above 300 g/L and are crushed and pressed employing vertical presses. After pressing and partial fermentation, musts are fortified with ethanol to ensure that the wine will contain at least 15–18 % alcohol. These young sweet wines are, generally, subjected to aging in American oak wood following a system of dynamic oxidative aging (Casas 1985).
Alternative to this traditional sun-drying methodology, that avoids the problems of fungi and insects, has been developed. It is being used for the drying of horticultural products, in general, and grapes, in particular, and consists in forcing the loss of water by means of forced convection with hot air in drying chambers (Vega-Mercado et al. 2001; Ruíz Bejarano et al. 2010; Serratosa et al. 2014).
Ruiz et al. (2009) found that the musts from chamber-dried grapes from Pedro Ximenez exhibited similar volatile profiles than those obtained from sun-dried grapes.
The objective of this work was to study the use of climatic chambers as feasible alternative to the traditional sun drying. For this purpose, the volatile profiles of sweet Sherry wines aged in wood for one year and produced from chamber and sun dried grapes have been studied.
Two grape varieties (Muscat and Pedro Ximenez) and two vintages (2009 and 2010) were studied. Wine aging was periodically monitored for one year.
Material and methods
Samples
Each grape variety and vintage, about 2000 kg of ripe grape bunches were collected from a local winery in the Jerez-Xérès-Sherry D.O. All of them were dried using a climatic chamber (Ibercex A. S. L., S. A., Spain) for about five days at 40 °C and relative humidity of 10 %. Bunches were spread uniformly in the chamber forming a single layer and water loss was monitored everyday. At the end of the chamber drying process, moisture content was about 35 % with 20–21 °Baume (Table 1).
Table 1.
Mean values found for musts before alcoholic fermentation and wines before aging in wood
| Sample | Vintage | Drying type | Grape variety | Baume | pH | Total acidity* | Alcoholic degree (% v/v) | Free SO2 |
|---|---|---|---|---|---|---|---|---|
| Must | 2009 | Sun | Muscat | 21.4 | 3.6 | 5.7 | - | - |
| Pedro Ximénez | 20.9 | 3.5 | 6.7 | - | - | |||
| Chamber | Muscat | 20.2 | 3.4 | 6.9 | - | - | ||
| Pedro Ximénez | 21.1 | 3.7 | 7.5 | - | - | |||
| 2010 | Sun | Muscat | 21.3 | 3.5 | 5.8 | - | - | |
| Pedro Ximénez | 20.4 | 3.8 | 5.8 | - | - | |||
| Chamber | Muscat | 20.8 | 3.7 | 5.9 | - | - | ||
| Pedro Ximénez | 20.7 | 3.8 | 6.3 | - | - | |||
| Wine | 2009 | Sun | Muscat | - | 3.3 | 5.9 | 17.3 | 15.7 |
| Pedro Ximénez | - | 3.4 | 8.2 | 17.9 | 13.8 | |||
| Chamber | Muscat | - | 3.5 | 9.1 | 17.1 | 19.0 | ||
| Pedro Ximénez | - | 3.3 | 8.5 | 17.5 | 15.4 | |||
| 2010 | Sun | Muscat | - | 3.5 | 7.6 | 19.1 | 13.8 | |
| Pedro Ximénez | - | 3.4 | 8.1 | 16.5 | 20.5 | |||
| Chamber | Muscat | - | 3.5 | 9.2 | 18.3 | 20.0 | ||
| Pedro Ximénez | - | 3.5 | 7.7 | 16.3 | 16.4 |
*g/L tartaric acid
In the case of sun drying process, grapes were dried for about 10–15 days (10 and 12 days for PX 2009 and 2010, respectively; and 13 and 15 days for Muscat 2009 and 2010, respectively) using esparto grass mats and being turned over and covered at night. Bunches were also spread uniformly forming a single layer and the water loss was monitored everyday. At the end of the sun drying process, the moisture content was about 35 % with 20–21 ºBaume (Table 1).
After both drying processes, and for each grape variety and vintage, grapes were separately destemmed, crushed and pressed using a vertical press. The highest pressure of 300 bars was applied in three cycles. Must pH was adjusted to 3.5 with tartaric acid (Agrovin, Spain). The concentration of total sulphur dioxide was also set at 120 mg/L by adding potassium metabisulfite (Agrovin, Spain).
For each vintage, musts dried by sun and chamber from each grape variety, were separately fermented in duplicate at temperature of 10 °C with S. Bayanus (40 g/hL, Uvaferm 43, Lallemand, Australia).
In all cases, the fermentation was stopped by adding alcohol up to 17°–18° (process known as fortification). The final sugar content of wines was around 90–100 g/L.
Wines from the same grape variety, drying system and vintage were aged, in duplicate, in 30 L medium toasted American oak casks. In the case of wines obtained from Pedro Ximénez grapes corresponding to vintage 2009, they were aged in triplicate. During this period, all the wines were situated in the same room at about 20 °C.
The sampling was carried out just after fermentation (S0, 0 days), and periodically for one year of aging (S1, 30 days; S3, three months; S5, five months; S9, nine months; and S12, twelve months). During this period, the intervening months did not evaluated. Samples were stored at 4 °C until their analysis.
Analysis of volatile compounds
Chemicals and reagents
All the aroma standards employed in this work were supplied by Merck (Darmstadt, Germany) and Sigma (Steinheim, Germany). 4-methyl-2-pentanol was used as internal standard.
Sample preparation
Volatile compounds were analysed by SBSE-GC-MS according to the method proposed by Alves et al. (2005). In brief, five milliliters of ultra-purewater, 5 ml of wine sample, 30 μl of a solution of 4-methyl-2-pentanol (2.52 g/L in Milli-Q water containing 15 % v/v of ethanol) and a PDMS stir bar (20 mm × 0.5 mm (length x film thickness)) supplied by Gerstel (GmbH, Mülheim a/d Ruhr, Germany) were used for the extractions. For each extraction process, the stir bar was stirred for 1 h (800 rpm) at room temperature (20 °C). Then, it was transferred into a glass thermal desorption tube and then thermal desorption was carried out. All samples were analysed in duplicate.
Instrumentation
A commercial TDS-2 thermal desorption unit (Gerstel) connected to a programmed-temperature vaporisation (PTV) injector CIS-4 (Gerstel) by a heated transfer line were used for the thermal desorption of the coated stir bars. The PTV was installed in an Agilent 6890 GC-5973 MS system (Agilent Technologies, Palo Alto, CA, USA). An empty baffled liner was used in the PTV. The thermodesorption unit was equipped with a MPS 2 L autosampler (Gerstel). The desorption temperature was programmed from 40 °C to 300 °C (held for 10 min) at 60 °C/min under a helium flow (75 ml/min) and the desorbed analytes were cryofocused in the PTV system with liquid nitrogen at −140 °C. Finally, the PTV system was programmed from −140 °C to 300 °C (held for 5 min) at 10 °C/s for analysis by GC-MS. A DB-Wax capillary column (J&W Scientific, Folsom, CA, USA), 60 m × 0.25 mm I.D., with a 0.25 μm coating was used in order to perform the capillary GC-MS analysis in the electron impact mode. Helium was used as carrier gas (1.0 ml/min). The GC oven was fixed as follows: held at 35 °C for 10 min, then ramped at 5 °C/min to 100 °C. Then it was raised to 210 °C at 3 °C/min and held for 40 min. The mass detector operated in EI+ mode at 70 eV from 30 to 400 amu.
Peak identification was carried out using the Wiley 7 N Edition Library (Wiley Registry of Mass Spectral Data, 7th Edition, 2000) library by analogy of mass spectra (with a minimum of 90 % of correspondence) and conformed by retention times of standards when they were available. Additionally, in order to guarantee the identifications, the retention indices were experimentally determined on a polar column (DB-Wax) and compared with those found in the bibliography. The objective of this study was to evaluate the differences between different treatments, therefore, it was considered that quantification using calibration lines was not necessary. Semi-quantitative data were obtained by measuring the relative quantifying ion peak area in relation to that of the area of 4-methyl-2-pentanol, the internal standard.
Statistical analysis
Statistical analyzes were carried out by using Statgraphics Centurion, version 15.0 (Statpoint Inc., USA) for Windows XP. Analysis of variance (ANOVA) was applied to establish the possible significant differences among samples for each volatile compound. Furthermore, principal component analysis (PCA), and cluster analysis (CA) were carried out with the aim of highlighting the similarity of the samples and the main contributors to the variance found among them.
Results and discussion
Table 1 shows some oenological parameters obtained for musts before alcoholic fermentation and wines before aging in oak casks.
As can be seen, after drying stage, a mean value of 20.9 °Be was obtained. All wines were aged in wood with contents in free SO2 about 15–20 mg/L.
Analysis of variance ANOVA
In this study, 51 individual volatile compounds, belong to different chemical families, have been identified in the different wines (Table 2).
Table 2.
Identification and quantification parameters for volatile compounds found in the sweet wines studied. LRI: linear retention indices. S: Standard; MS: Mass spectrum
| Number | LRI | Compound | m/z | Identification |
|---|---|---|---|---|
| 1 | 1038 | ethyl butanoate | 71 | S, MS |
| 2 | 1067 | ethyl 3-methylbutanoate | 88 | S, MS |
| 3 | 1080 | n-butyl acetate | 43 | MS |
| 4 | 1088 | hexanal | 56 | S, MS |
| 5 | 1120 | isoamyl acetate | 43 | MS |
| 6 | 1147 | ß-myrcene | 93 | MS |
| 7 | 1197 | 4-methyl-2-pentanol (IS) | 45 | S, MS |
| 8 | 1218 | 2-hexenal | 41 | S, MS |
| 9 | 1229 | 3-methyl-1-butanol | 55 | S, MS |
| 10 | 1231 | ethyl hexanoate | 88 | S, MS |
| 11 | 1276 | octanal | 43 | MS |
| 12 | 1336 | 6-methyl-5-hepten-2-one | 43 | MS |
| 13 | 1344 | 1-hexanol | 56 | S, MS |
| 14 | 1378 | nonanal | 57 | MS |
| 15 | 1414 | ethyl octanoate | 88 | S, MS |
| 16 | 1423 | 2-furaldehyde | 95 | S, MS |
| 17 | 1441 | nerol oxide | 68 | MS |
| 18 | 1451 | linalool oxide | 59 | MS |
| 19 | 1489 | benzaldehyde | 106 | S, MS |
| 20 | 1547 | linalool | 93 | S, MS |
| 21 | 1567 | 5-methyl-2-furaldehyde | 110 | S, MS |
| 22 | 1613 | 4-terpineol | 111 | S, MS |
| 23 | 1646 | ethyl decanaote | 88 | MS |
| 24 | 1711 | diethyl succinate | 101 | S, MS |
| 25 | 1737 | α-terpineol | 93 | S, MS |
| 26 | 1769 | β-citronellol | 69 | MS |
| 27 | 1783 | naphthalene,1,2-dihydro-1,1,6-trimethyl (TDN) | 157 | MS |
| 28 | 1810 | ethyl phenyl acetate | 91 | S, MS |
| 29 | 1836 | nerol | 69 | MS |
| 30 | 1849 | phenylethyl acetate | 104 | MS |
| 31 | 1854 | diethyl pentanedioate | 143 | MS |
| 32 | 1879 | geraniol | 69 | S, MS |
| 33 | 1890 | ethyl dodecanoate | 101 | MS |
| 34 | 1944 | 2-phenylethanol | 91 | S, MS |
| 35 | 1956 | 2,6 di-tert-butyl-p cresol (BHT) | 205 | MS |
| 36 | 1979 | 2-ethyl hexanoic acid | 73 | MS |
| 37 | 1989 | phenol | 94 | MS |
| 38 | 2001 | nerolidol | 69 | MS |
| 39 | 2101 | octanoic acid | 60 | S, MS |
| 40 | 2145 | ethyl myristate | 88 | MS |
| 41 | 2195 | eugenol | 164 | S, MS |
| 42 | 2200 | nonanoic acid | 73 | MS |
| 43 | 2202 | m-thymol | 135 | MS |
| 44 | 2204 | 4-ethylphenol | 107 | S, MS |
| 45 | 2232 | carvacrol | 135 | MS |
| 46 | 2317 | decanoic acid | 60 | S, MS |
| 47 | 2325 | ethyl hexadecanoate | 88 | MS |
| 48 | 2371 | farnesol | 222 | MS |
| 49 | 2389 | ethyl octadecanoate | 88 | MS |
| 50 | 2494 | dodecanoic acid | 73 | MS |
| 51 | 2556 | tetradecanoic acid | 73 | MS |
| 52 | 2850 | hexadecanoic acid | 73 | MS |
For both varieties, volatile compounds found in this study had been previously identified (Franco et al. 2004; Márquez et al. 2008; López de Lerma et al. 2012; Ruiz et al. 2014).
Table 3 shows mean values found for the different chemical families considered (ethyl esters; acids; acetates; terpenes and terpenols; aldehydes, ketones and alcohols; and miscellaneous) according to each factor (aging time, grape variety, vintage and drying type) considering the different values of the remaining factors. This fact could explain the high standard deviation values obtained. Most of these factors seem to be significant for some of the chemical families. As could be expected, wines from Muscat grape variety exhibited a higher content in terpenic compounds (Sánchez-Palomo et al. 2005) whereas aged wines showed higher contents in ethyl esters and aldehydes, ketones and alcohols (Table 3).
Table 3.
Mean values (relative chromatographic area) and standard deviations for each chemical family
| Volatile Compounds | Aging time (months) | Grape variety | Vintage | Drying type | ||||
|---|---|---|---|---|---|---|---|---|
| 0 | 12 | Muscat | PX | 2009 | 2010 | Chamber | Sun | |
| Ethyl esters | 0.409 ± 0.946 | 0.555 ± 1.258 | 0.578 ± 1.091 | 0.473 ± 1.018 | 0.371 ± 0.904 | 0.628 ± 1.140 | 0.447 ± 0.961 | 0.582 ± 1.137 |
| Acids | 0.070 ± 0.081 | 0.083 ± 0.099 | 0.075 ± 0.077 | 0.068 ± 0.085 | 0.054 ± 0.065 | 0.086 ± 0.095 | 0.065 ± 0.072 | 0.078 ± 0.096 |
| Acetates | 0.264 ± 0.389 | 0.314 ± 0.373 | 0.265 ± 0.300 | 0.282 ± 0.334 | 0.212 ± 0.193 | 0.338 ± 0.403 | 0.299 ± 0.305 | 0.279 ± 0.350 |
| Terpenes and terpenols | 0.017 ± 0.034 | 0.018 ± 0.059 | 0.046 ± 0.077 | 0.003 ± 0.007 | 0.013 ± 0.048 | 0.018 ± 0.044 | 0.020 ± 0.056 | 0.009 ± 0.026 |
| Aldehydes, ketones and alcohols | 0.236 ± 0.376 | 0.330 ± 0.485 | 0.304 ± 0.453 | 0.289 ± 0.412 | 0.278 ± 0.403 | 0.307 ± 0.443 | 0.313 ± 0.451 | 0.265 ± 0.382 |
| Miscellaneous | 0.006 ± 0.034 | 0.032 ± 0.057 | 0.025 ± 0.044 | 0.026 ± 0.048 | 0.026 ± 0.046 | 0.026 ± 0.048 | 0.024 ± 0.044 | 0.028 ± 0.051 |
In order to study statistically the differences in the volatile content of all the wines studied, data were submitted to analysis of variance (ANOVA). For this one, four independent factors were considered: aging time, grape variety, vintage, and type of drying. Table 4 shows the results obtained from ANOVA and the mean values obtained for each volatile compound and grape variety, at the initial (S0) and after one year of aging in oak cask (S12), taking into account, for each case, both ways of drying (sun and chamber drying) and vintages (2009 and 2010).
Table 4.
Mean values (relative chromatographic area) and standard deviations for volatile compounds studied
| Volatile Compounds | Relative areas | p value aging time | p value grape variety | p value vintage | p value drying type | ||
|---|---|---|---|---|---|---|---|
| Muscat | Pedro Ximénez | ||||||
| Ethyl Esters | |||||||
| ethyl butanoate | S0 | 0.109 ± 0.047 | 0.110 ± 0.061 | 0.7330 | 0.0152 | 0.0000* | 0.3537 |
| S12 | 0.134 ± 0.034 | 0.109 ± 0.056 | |||||
| ethyl 3-methylbutanoate | S0 | 0.019 ± 0.004 | 0.017 ± 0.006 | 0.0000* | 0.0338* | 0.0237 | 0.4503 |
| S12 | 0.111 ± 0.012 | 0.093 ± 0.029 | |||||
| ethyl hexanoate | S0 | 0.999 ± 0.531 | 1.01 ± 1.02 | 0.6628 | 0.1201 | 0.0000* | 0.8030 |
| S12 | 1.129 ± 0.799 | 0.740 ± 0.531 | |||||
| diethyl pentanedioate | S0 | 0.001 ± 0.000 | 0.004 ± 0.004 | 0.0000* | 0.3594 | 0.0156 | 0.1507 |
| S12 | 0.029 ± 0.008 | 0.026 ± 0.008 | |||||
| ethyl octanoate | S0 | 1.52 ± 1.18 | 2.45 ± 2.31 | 0.0528 | 0.4729 | 0.0000* | 0.3658 |
| S12 | 1.68 ± 1.25 | 0.982 ± 0.827 | |||||
| ethyl decanoate | S0 | 0.369 ± 0.293 | 0.495 ± 0.400 | 0.0000* | 0.0043* | 0.0000* | 0.0000* |
| S12 | 0.255 ± 0.170 | 0.096 ± 0.086 | |||||
| diethyl succinate | S0 | 0.312 ± 0.139 | 0.686 ± 1.126 | 0.0000* | 0.0071* | 0.0028* | 0.0129 |
| S12 | 5.30 ± 0.665 | 4.42 ± 1.12 | |||||
| ethyl dodecanoate | S0 | 0.063 ± 0.052 | 0.024 ± 0.001 | 0.0035* | 0.0000* | 0.0001* | 0.0052* |
| S12 | 0.032 ± 0.023 | 0.008 ± 0.010 | |||||
| ethyl tetradecanoate | S0 | 0.025 ± 0.003 | 0.015 ± 0.006 | 0.1533 | 0.0003* | 0.4078 | 0.0079* |
| S12 | 0.018 ± 0.009 | 0.010 ± 0.008 | |||||
| ethyl hexadecanoate | S0 | 0.099 ± 0.060 | 0.065 ± 0.052 | 0.1362 | 0.0030* | 0.4672 | 0.0000* |
| S12 | 0.051 ± 0.017 | 0.043 ± 0.044 | |||||
| ethyl octadecanoate | S0 | 0.014 ± 0.008 | 0.009 ± 0.009 | 0.1072 | 0.0085* | 0.9202 | 0.0000* |
| S12 | 0.005 ± 0.001 | 0.006 ± 0.006 | |||||
| Acids | |||||||
| 2-ethylhexanoic acid | S0 | 0.006 ± 0.003 | 0.012 ± 0.015 | 0.9726 | 0.0000* | 0.0000* | 0.0000* |
| S12 | 0.006 ± 0.002 | 0.012 ± 0.010 | |||||
| octanoic acid | S0 | 0.098 ± 0.064 | 0.157 ± 0.134 | 0.1887 | 0.2243 | 0.0000* | 0.0429 |
| S12 | 0.113 ± 0.052 | 0.099 ± 0.068 | |||||
| nonanoic acid | S0 | 0.006 ± 0.000 | 0.007 ± 0.003 | 0.0000* | 0.1006 | 0.4415 | 0.4323 |
| S12 | 0.021 ± 0.005 | 0.016 ± 0.005 | |||||
| decanoic acid | S0 | 0.093 ± 0.056 | 0.141 ± 0.105 | 0.0116 | 0.4005 | 0.0000* | 0.6451 |
| S12 | 0.109 ± 0.053 | 0.086 ± 0.068 | |||||
| dodecanoic acid | S0 | 0.050 ± 0.030 | 0.034 ± 0.015 | 0.3468 | 0.0001* | 0.0039* | 0.0372 |
| S12 | 0.049 ± 0.018 | 0.037 ± 0.034 | |||||
| tetradecanoic acid | S0 | 0.036 ± 0.003 | 0.051 ± 0.029 | 0.0054* | 0.4658 | 0.8695 | 0.5192 |
| S12 | 0.093 ± 0.015 | 0.104 ± 0.089 | |||||
| hexadecanoic acid | S0 | 0.127 ± 0.031 | 0.122 ± 0.054 | 0.2249 | 0.4442 | 0.9436 | 0.5373 |
| S12 | 0.174 ± 0.043 | 0.211 ± 0.189 | |||||
| Acetates | |||||||
| n-butyl acetate | S0 | 0.004 ± 0.001 | 0.004 ± 0.001 | 0.0000* | 0.0003* | 0.0436 | 0.0000* |
| S12 | 0.030 ± 0.004 | 0.040 ± 0.013 | |||||
| isoamyl acetate | S0 | 0.785 ± 0.464 | 0.749 ± 0.455 | 0.0060* | 0.0006* | 0.0000* | 0.0001* |
| S12 | 0.846 ± 0.291 | 0.854 ± 0.327 | |||||
| phenylethyl acetate | S0 | 0.084 ± 0.076 | 0.046 ± 0.025 | 0.0000* | 0.0000* | 0.0000* | 0.0049* |
| S12 | 0.296 ± 0.062 | 0.209 ± 0.053 | |||||
| ethyl 2-phenyl acetate | S0 | 0.131 ± 0.090 | 0.208 ± 0.147 | 0.3938 | 0.0000* | 0.0000* | 0.0000* |
| S12 | 0.171 ± 0.077 | 0.220 ± 0.084 | |||||
| Terpenes and terpenols | |||||||
| nerol oxide | S0 | 0.011 ± 0.000 | 0.000 ± 0.000 | 0.0009* | 0.0000* | 0.0429 | 0.0021* |
| S12 | 0.048 ± 0.014 | 0.001 ± 0.000 | |||||
| linalool oxide | S0 | 0.013 ± 0.006 | 0.001 ± 0.000 | 0.0163 | 0.0000* | 0.8873 | 0.0084* |
| S12 | 0.015 ± 0.003 | 0.001 ± 0.000 | |||||
| linalool | S0 | 0.181 ± 0.045 | 0.004 ± 0.002 | 0.0011* | 0.0000* | 0.9931 | 0.0069* |
| S12 | 0.337 ± 0.070 | 0.004 ± 0.002 | |||||
| 4-terpineol | S0 | 0.001 ± 0.000 | 0.000 ± 0.000 | 0.0176 | 0.0000* | 0.8557 | 0.0068* |
| S12 | 0.002 ± 0.000 | 0.001 ± 0.000 | |||||
| α-terpineol | S0 | 0.063 ± 0.004 | 0.01 ± 0.009 | 0.0549 | 0.0000* | 0.7817 | 0.0177 |
| S12 | 0.093 ± 0.027 | 0.002 ± 0.001 | |||||
| β-citronellol | S0 | 0.123 ± 0.103 | 0.010 ± 0.005 | 0.0105 | 0.0000* | 0.0001* | 0.0002* |
| S12 | 0.033 ± 0.026 | 0.002 ± 0.002 | |||||
| nerol | S0 | 0.037 ± 0.027 | 0.002 ± 0.001 | 0.6397 | 0.0000* | 0.0022* | 0.0002* |
| S12 | 0.029 ± 0.008 | 0.001 ± 0.000 | |||||
| nerolidol | S0 | 0.007 ± 0.004 | 0.008 ± 0.004 | 0.8796 | 0.0000* | 0.0235 | 0.0003* |
| S12 | 0.021 ± 0.020 | 0.023 ± 0.015 | |||||
| geraniol | S0 | 0.065 ± 0.003 | 0.003 ± 0.001 | 0.4596 | 0.0000* | 0.0919 | 0.0102 |
| S12 | 0.089 ± 0.025 | 0.001 ± 0.000 | |||||
| thymol | S0 | 0.000 ± 0.000 | 0.001 ± 0.000 | 0.0001* | 0.5748 | 0.0046* | 0.0090* |
| S12 | 0.001 ± 0.000 | 0.001 ± 0.001 | |||||
| carvacrol | S0 | 0.001 ± 0.000 | 0.001 ± 0.001 | 0.0001* | 0.0153 | 0.0000* | 0.0000* |
| S12 | 0.003 ± 0.002 | 0.002 ± 0.001 | |||||
| β-myrcene | S0 | 0.002 ± 0.000 | 0.000 ± 0.000 | 0.0085* | 0.0000* | 0.4759 | 0.2776 |
| S12 | 0.002 ± 0.001 | 0.001 ± 0.000 | |||||
| Farnesol | S0 | 0.063 ± 0.059 | 0.036 ± 0.024 | 0.0000* | 0.0436 | 0.0000* | 0.0027* |
| S12 | 0.012 ± 0.001 | 0.005 ± 0.008 | |||||
| Aldehydes, ketones and alcohols | |||||||
| hexanal | S0 | 0.008 ± 0.001 | 0.006 ± 0.002 | 0.0000* | 0.0346 | 0.2857 | 0.6599 |
| S12 | 0.012 ± 0.002 | 0.009 ± 0.002 | |||||
| 2-hexenal | S0 | 0.628 ± 0.325 | 0.601 ± 0.165 | 0.0000* | 0.7338 | 0.0000* | 0.0739 |
| S12 | 1.01 ± 0.362 | 0.909 ± 0.210 | |||||
| octanal | S0 | 0.004 ± 0.000 | 0.006 ± 0.002 | 0.00027* | 0.2992 | 0.2530 | 0.0612 |
| S12 | 0.007 ± 0.001 | 0.006 ± 0.002 | |||||
| 6-methyl-5-hepten-2-one | S0 | 0.014 ± 0.002 | 0.013 ± 0.009 | 0.3028 | 0.0000* | 0.0068* | 0.6166 |
| S12 | 0.013 ± 0.001 | 0.011 ± 0.005 | |||||
| 1-hexanol | S0 | 0.016 ± 0.001 | 0.022 ± 0.006 | 0.0000* | 0.0008* | 0.1913 | 0.0001* |
| S12 | 0.046 ± 0.021 | 0.036 ± 0.006 | |||||
| nonanal | S0 | 0.027 ± 0.003 | 0.030 ± 0.013 | 0.0008* | 0.0872 | 0.0005* | 0.4327 |
| S12 | 0.045 ± 0.004 | 0.033 ± 0.009 | |||||
| benzaldehyde | S0 | 0.030 ± 0.007 | 0.028 ± 0.022 | 0.0000* | 0.5868 | 0.0689 | 0.5625 |
| S12 | 0.147 ± 0.036 | 0.143 ± 0.035 | |||||
| 3-methyl-2-butanol | S0 | 0.913 ± 0.549 | 0.779 ± 0.211 | 0.0000* | 0.0419 | 0.0000* | 0.0313 |
| S12 | 1.51 ± 0.618 | 1.27 ± 0.257 | |||||
| 2-phenylethanol | S0 | 0.624 ± 0.095 | 0.580 ± 0.490 | 0.0107 | 0.0021 | 0.0909 | 0.0000* |
| S12 | 0.816 ± 0.188 | 0.949 ± 0.427 | |||||
| Miscellaneous | |||||||
| phenol | S0 | 0.014 ± 0.003 | 0.016 ± 0.007 | 0.0193 | 0.3855 | 0.0734 | 0.9031 |
| S12 | 0.021 ± 0.004 | 0.023 ± 0.015 | |||||
| eugenol | S0 | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.0000* | 0.0000* | 0.0001* | 0.0523 |
| S12 | 0.018 ± 0.003 | 0.012 ± 0.002 | |||||
| 4-ethylphenol | S0 | 0.000 ± 0.000 | 0.001 ± 0.000 | 0.0000* | 0.0003* | 0.7184 | 0.1561 |
| S12 | 0.001 ± 0.000 | 0.001 ± 0.000 | |||||
| 2-furaldehyde | S0 | 0.018 ± 0.002 | 0.019 ± 0.005 | 0.0000* | 0.5470 | 0.6292 | 0.0450 |
| S12 | 0.180 ± 0.051 | 0.194 ± 0.063 | |||||
| 5-methylfuraldehyde | S0 | 0.002 ± 0.001 | 0.002 ± 0.001 | 0.0000* | 0.1261 | 0.8953 | 0.0019* |
| S12 | 0.053 ± 0.017 | 0.060 ± 0.018 | |||||
| TDN | S0 | 0.001 ± 0.000 | 0.002 ± 0.002 | 0.0002* | 0.2822 | 0.0340 | 0.0148 |
| S12 | 0.003 ± 0.001 | 0.004 ± 0.002 | |||||
| BHT | S0 | 0.002 ± 0.001 | 0.004 ± 0.002 | 0.6875 | 0.0371 | 0.0000* | 0.0068* |
| S12 | 0.003 ± 0.002 | 0.003 ± 0.001 | |||||
S0 sampling after fermentation, S12 sampling after 12 months of wood aging
*Analysis of variance. Values are significant at p < 0.01. TDN: 1,1,6-trimethyl-1,2-dihydronaphthalene; BHT: 2,6 di-tert-butyl-p cresol
As can be seen in Table 4, practically all the volatile compounds were significant affected (p < 0.01) by one or more factors.
Ethyl esters
Ethyl esters of C6, C8 and C10 fatty acids, together with other volatile compounds such as higher alcohols, acetates and certain volatile acids are the main responsible for the fermentation aroma in wines (Karagiannis et al. 2000).
Concerning the factor “aging time”, both grape varieties exhibited similar changes. As it was previously observed for wines from Muscat grapes aged in oak casks and stainless steel vessels (Ruiz-Bejarano et al. 2013), several ethyl esters (ethyl 3-methylbutanoate, diethyl pentanedioate, and diethyl succinate) showed significant increases whereas ethyl decanoate and ethyl dodecanoate decreased as the time of aging increased.
Cámara et al. (2006) found great decreases in fatty acids ethyl esters (C6-C16) and acetates and high increases of ethyl esters of diprotic acids such as diethyl succinate in the case of Madeira wines aged in oak wood for twenty five years. Chaves et al. (2007) observed a high content in ethyl acetate and ethyl hexanoate for Pedro Ximénez sweet wines aged in wood.
Hydrolysis and esterification reactions can affect this type of volatile compounds during the period of aging in wood (Cámara et al. 2006; Chaves et al. 2007). The high content in ethanol that these wines present (17°) could explain these increases by esterification reactions. However, it must be taken into account that both reactions depend on different factors, such as pH, temperature, alcoholic degree, and type of acid, so all of them should be considered in order to explain the variations in the ethyl ester content found for these wines.
In relation to grape variety, Muscat wines exhibited a higher content in long-chain ethyl esters (Tables 3 and 4; ethyl decanoate, ethyl dodecanoate, ethyl tetradecanoate, ethyl hexadecanoate and ethyl octadecanoate). This fact was also observed in a previous study about the volatile fraction of commercial Andalusian sweet wines from Muscat and Pedro Ximénez grapes (Márquez et al. 2008).
About the factor “vintage”, ethyl butanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, and ethyl dodecanoate were found in higher amounts for wines from vintage 2010 (Tables 3 and 4).
On one hand, climatic conditions seem to have a clear effect on amino acid content of grape must (Ortega-Heras et al. 2014), and therefore a significant influence on volatile compounds of a wine (Hernández-Orte et al. 2002). Ortega-Heras et al. (2014) observed that a year with low rainfall produced grapes with higher amino acid content.
On the other hand, in the Jerez-Xérès-Sherry D.O, the total precipitations from January to October for the vintages 2009 and 2010 were 275 and 601 L/m2, respectively (Ministerio de Agricultura, Alimentación y Medioambiente 2015). This notably difference in the precipitation value for both vintages could explain that this factor appears as significant for this type of compounds and others as it will be seen later.
Concerning the factor drying type, only long-chain ethyl esters (ethyl decanoate, ethyl dodecanoate, ethyl tetradecanoate, ethyl hexadecanoate and ethyl octadecanoate) showed significantly different values for both drying types, with higher contents for those wines obtained from sun dried grapes (Tables 3 and 4).
Acids
In relation to acids, the most influential factor was vintage, with some higher values, as it was observed for ethyl esters, for those wines from vintage 2010 (Table 4).
The factors “aging time”, “grape variety”, and “drying type” had a low influence on this type of compounds. The factor drying type was only significant for 2-ethylhexanoic acid.
Acetates
Regarding acetates, n-butyl acetate, isoamyl acetate and phenylethyl acetate showed significant increases as the aging time increased (Table 3). A different evolution was observed by Cámara et al. (2006) and Ruiz-Bejarano et al. (2013). Bordiga et al. (2014) observed that certain ethyl esters together with acetates such as isoamyl acetate, 1-hexyl acetate or 2-phenylethyl acetate gradually reduced their concentrations and, on the contrary, the levels of diethyl succinate and isobutyl acetate increased progressively in Nebbiolo-based wine during its aging in wood.
Ramey and Ough (1980) found different evolutions during the aging in wood for some acetates and ethyl esters according to the variety grape. The initial postfermentation level of each volatile compound seems to be crucial for its latter evolution during aging. Both types of volatile compounds may be hydrolyzed, be formed through chemical esterification, or remain at constant equilibrium concentrations depending on their initial concentration.
About the influence of drying type, all acetates showed a higher amount for those wines obtained from climatic chamber-dried grapes (Table 3 and Table 4). Ruiz et al. (2014) observed significant differences between the volatile contents found for musts from Pedro Ximénez grapes chamber-dried at two different temperatures, so, it seems logical to consider that the specific drying conditions used may play a decisive role in some volatile compounds of a must and latter, of the wine obtained.
In relation to factor vintage, it was significant for isoamyl acetate, phenylethyl acetate and ethyl 2-phenyl acetate, with, in general, higher contents for vintage 2010 (Tables 3 and 4). In this sense, Dennis et al. (2012) observed that the postfermentation concentration of an acetate was influenced by the prefermentation concentration of its respective alcoholic precursor, being this last one dependent on the specific climatic and cultural conditions under which grapes were cultivated and vinificated.
Terpenes and terpenols
Concerning terpenes and terpenols, as can be seen in Table 4, the most significant factors were “grape variety”, “drying type” and “aging time”. Various authors (Sánchez-Palomo et al. 2005; Fenoll et al. 2009) have established that compounds such as linalool, geraniol, citronellol and nerol are the main responsible for the typical floral aroma of Muscat grapes.
In relation to the factor aging, in a previous study about Muscat grapes (Ruiz-Bejarano et al. 2013), terpenols such as linalool, α-terpineol, β-citronellol, nerol, and geraniol showed significant decreases as the time of aging increased whereas their oxides increased. These changes were higher for wines aged in wood than those aged in stainless steel.
In the present work, only farnesol decreased significantly whereas nerol oxide, linalool, thymol, carvacrol and β-myrcene showed statistically significant increases as the aging time increased (Table 3). Several authors (Marais and van Wyk 1986; Loscos et al. 2010) have observed that some terpenic monoalcohols (linalool, nerol, and geraniol) may be transformed into α-terpineol and other terpenes during the aging and that this conversion depends on different factors.
In the case of factor “drying type”, higher amounts were found for wines from grapes dried in chamber (Table 3). Ruiz et al. (2009) observed that musts from chamber-dried grapes presented the same aroma terms as those from sun-dried grapes, although with higher odor activity values, particularly those of the floral and fruity terms, which are normally ascribable to terpenols, ethyl esters and acetates.
Aldehydes, alcohols and ketones
For this type of volatile compounds, the most significant factor was “aging time”. As can be seen in Table 3, most of alcohols and aldehydes showed significant increases as the aging in wood increased.
Some authors have found significant increases for higher alcohols during aging (Cámara et al. 2006). In this study, 3-methyl-2-butanol and 1-hexanol exhibited significant increases during aging. For aldehydes, benzaldehyde, nonanal, octanal, hexanal and 2-hexenal exhibited a clear rising tendency during aging. Chaves et al. (2007) found that certain aldehydes such as decanal together with 2,3-butanedione and linalool could be used as reliable fingerprints of the aging in wood of PX wines. These authors suggested that the increases in the aldehyde concentrations during aging may be the result of oxidation reactions of their alcoholic type precursors.
In relation to the factor “vintage”, this was significant for 2-hexenal, 6-methyl-5-hepten-2-one, nonanal and 3-methyl-2-butanol with higher values for those wines from vintage 2010 (Table 3).
Concerning the factor drying type, it was only significant for 1-hexanol and 2-phenylethanol. It revealed a low influence of this parameter on this family of volatile compounds, as it was previously observed for other compounds.
Miscellaneous
For this type of compounds, the most significant factor was aging time (Table 3 and Table 4). Aged wines showed higher contents for eugenol, 4-ethylphenol, 2-furaldehyde, 5-methylfuraldehyde and 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN, Table 4).
Some authors (Cámara et al. 2006; Márquez et al. 2008) have found clear increases for 2-furaldehyde and 5-methyl-2-furaldehyde in wines during their aging in wood. Both of them are formed by degradation of carbohydrates during the toasting of the barrel and then are transferred to the wine during the period of aging.
2-Furaldehyde and 5-methyl-2-furaldehyde have been found in musts from Pedro Ximénez grapes after their sun drying (Franco et al. 2004). 2-Furfuraldehyde is produced from the heating of xylose and 5-methyl-2-furfuraldehyde emerges from rhamnose. Therefore, both factors, aging in wood and drying stage, could explain the content found for these compounds in the wines studied.
Eugenol exhibited an obvious increase during aging. It is already present in oak wood without toasting and its concentration augments with the barrel toasting process, being transferred to the wine during the period of aging (Chatonnet 1999).
Regarding TDN, present in wines after fermentation, its content was higher for those wines aged in wood for a year. The presence of this compound in wines is principally related to the maturation process by carotenoid-degradation (Versini et al. 2002). Different authors (Silva et al. 2003; Ruiz-Bejarano et al. 2013) have found that its content increases during aging depending on factors such as temperature and time.
Taking into account the results obtained from ANOVA study, in which most of the volatile compounds were significantly affected by some of the factors studied, a multivariate study was carried out.
Cluster analysis CA
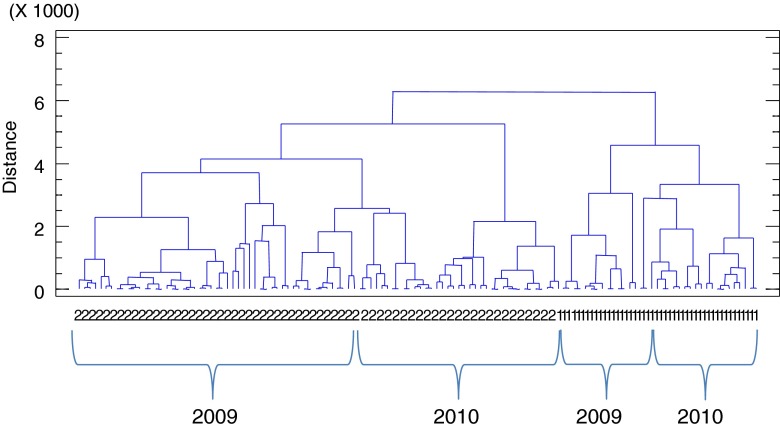
The data matrix was subjected to a hierarchical agglomerative cluster analysis of cases, taking the squared euclidean distance as metric and the Ward method as amalgamation rule. The dendrogram obtained is shown in Fig. 1.
Fig. 1.
Cluster analysis (CA). Dendrogram obtained using squared Euclidean distance and the Ward method as amalgamation rule. 1: Muscat; 2: Pedro Ximénez
Two main clusters can be appreciated: one cluster for wines from Muscat grapes and another one for wines from PX grapes. Later, a clear influence of the factor vintage can be observed inside of each one.
Therefore, considering all volatile compounds studied, they possess sufficient explanatory power to detect grape variety and vintage. It seems that these two factors, variety and vintage, were more influential in the volatile profile of wines studied than aging time and drying type. This same differentiation was observed in a previous study carried out about the characterization of the volatile fraction of Andalusian sweet wines (Márquez et al. 2008).
Ortega-Heras et al. (2004) observed that all the grape varieties have not the same extraction capacity from wood and that the aging in wood can accentuate the varietal aroma differences.
Principal component analysis PCA
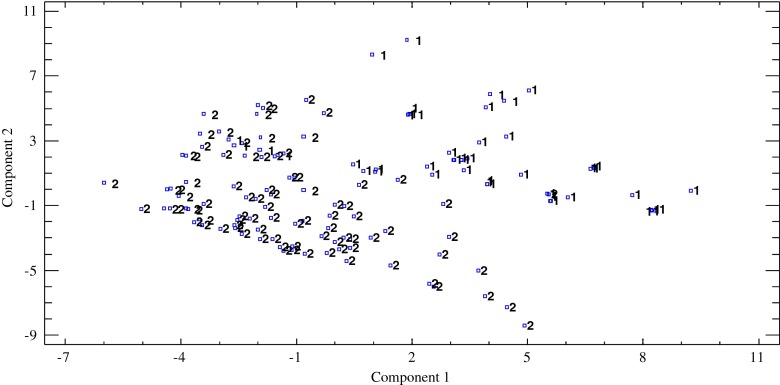
To corroborate the results obtained from cluster analysis (CA) and check the volatile compounds that allow the differentiation of wines studied, a principal component analysis (PCA) was performed.
10 PCs (principal components) which explained the 87.3 % of the total variance were extracted.
Figure 2 shows the score plot of all wines onto the plane defined by the two first principal components. These two first PCs accounted for 50.1 % of the variance (30.8 and 19.3 %, respectively). As can be seen (Fig. 2), wines were differentiated according to grape variety, with those wines from Muscat grapes presenting positive values for both PCs.
Fig. 2.
Principal component analysis (PCA) for all samples. 1: Muscat wines; 2: Pedro Ximénez wines
The main contributors to these PCs were: terpenols such as linalool, 4-terpineol, geraniol, nerol; some ethyl esters (ethyl 3-methylbutanoate, ethyl succinate and diethyl pentanedioate); and acetates (phenylethyl acetate, n-butyl acetate and ethyl octanoate). All these volatile compounds are varietal compounds and/or are formed during the alcoholic fermentation process (Schreier 1979; Rapp and Mandery 1986).
Taking into account the results obtained from PCA carried out on all samples, a new PCA was performed, but in this case, both grape varieties were separately studied.
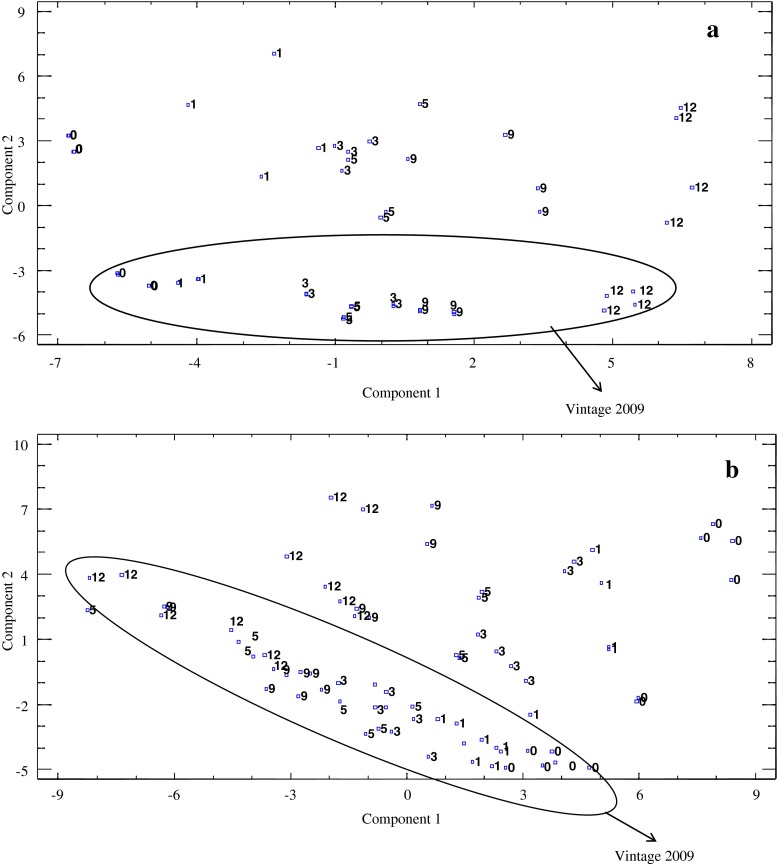
For Muscat wines, nine PCs were obtained, which explained the 92.8 % of the total variance, with 53.8 % of this one explained by the two first PCs (32.8 % by PC1 and 21 % by PC2). In the case of PX wines, the 87.4 % of the total variance was explained by ten PCs. PC1 and PC2 explained 30.1 % and 20.2 % of this one, respectively.
Figure 3 shows the distribution of both varietal wines onto the plane defined by the first PCs. As can be seen, for each grape variety, wines were separated according to their aging time. For Muscat wines, wines aged in wood for 5, 9 and 12 months presented positive values for PC1 whereas for PX wines, those wines aged a high period of time (5 months or more) exhibited negative values for this PC.
Fig. 3.
Principal component analysis (PCA) for: a: Muscat wines; b: Pedro Ximénez Wines. 0, 1, 3, 5, 9 and 12: months of aging in wood
The loadings of each volatile compound on PC1 show clearly that volatile compounds related to aging in wood (2-furaldehyde, benzaldehyde, 5-methylfuraldehyde, eugenol, 4-ethylphenol) are the main responsible for PC1, with positive values for Muscat wines and negative values for PX wines.
For each grape variety, those wines from vintage 2009 showed negative values for PC2, so this PC seems to be related to the factor “vintage”. In both cases, the main contributors, with positive values, to this PC were some ethyl esters (ethyl butanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate and ethyl dodecanoate) and acids (octanoic acid, decanoic acid and dodecanoic acid). Most of these volatile compounds are formed during alcoholic fermentation and their content in the wine depends on the must amino acid composition (Hernández-Orte et al. 2002).
In the previous ANOVA study, the factor vintage had showed a significant influence on most of these compounds. The high difference between the precipitation values of both vintages (Ministerio de Agricultura, Alimentación y Medioambiente 2015) could explain the high significance found for this factor in the volatile content of wines studied.
In summary, from the results obtained, the factor drying type was the least influential on the volatile profiles of sweet Sherry wines studied, while aging time, grape variety, and vintage showing to be significant on them.
Taking into account the global volatile profile, the factors grape variety and vintage have demonstrated the highest influence on the volatile content of the wines.
Conclusions
It can be concluded that the use of climatic chamber for drying Muscat and Pedro Ximénez grapes can produce wines with similar volatile contents to those obtained following a traditional drying process, but with lower losses in raw material due to attack of insects or possible rainfall.
In order to completely validate this alternative drying system to produce sweet Sherry wines, further studies about sensory evaluation of wines obtained from grapes dried by both drying systems would be required.
References
- Alves RF, Nascimento AMD, Nogueira JMF. Characterization of the aroma profile of Madeira wine by sorptive extraction techniques. Anal Chim Acta. 2005;546:11–21. doi: 10.1016/j.aca.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bordiga M, Piana G, Coïsson JD, Travaglia F, Arlorio M. Headspace solid-phase micro extraction coupled to comprehensive two-dimensional with time-of-flight mass spectrometry applied to the evaluation of nebbiolo-based wine volatile aroma during ageing. Int J Food Sci Technol. 2014;49:787–796. doi: 10.1111/ijfs.12366. [DOI] [Google Scholar]
- Cámara JS, Alves MA, Marques JC. Changes in volatile composition of Madeira wines during their oxidative ageing. Anal Chim Acta. 2006;563:188–197. doi: 10.1016/j.aca.2005.10.031. [DOI] [Google Scholar]
- Casas J. University Of Cadiz Press (ed) III jornadas universitarias sobre el Jerez. Cadiz: A. Jiménez-Mena; 1985. Descripción resumida de la técnica enológica de los vinos de Jerez; pp. 333–361. [Google Scholar]
- Chatonnet P. Discrimination and control of toasting intensity and quality of oak wood barrels. Am J Enol Vitic. 1999;50:479–494. [Google Scholar]
- Chaves M, Zea L, Moyano L, Medina M. Changes in color and odorant compounds during oxidative aging of Pedro Ximenez sweet wines. J Agric Food Chem. 2007;55:3592–3598. doi: 10.1021/jf063506v. [DOI] [PubMed] [Google Scholar]
- Dennis EG, Keyzers RA, Kalua CM, Maffei SM, Nicholson EL. Grape contribution to wine aroma: production of hexyl acetate, octyl acetate, and benzyl acetate during yeast fermentation is dependent upon precursors in the must. J Agric Food Chem. 2012;60:2638–2646. doi: 10.1021/jf2042517. [DOI] [PubMed] [Google Scholar]
- Fenoll J, Manso A, Hellín P, Ruiz L, Flores P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009;114:420–428. doi: 10.1016/j.foodchem.2008.09.060. [DOI] [Google Scholar]
- Franco M, Peinado RA, Medina M, Moreno J. Off-vine grape drying effect on volatile compounds and aromatic series in must from Pedro Ximénez grape variety. J Agric Food Chem. 2004;52:3905–3910. doi: 10.1021/jf0354949. [DOI] [PubMed] [Google Scholar]
- García-Jares C, García-Martín S. Analysis of some highly volatile compounds of wine by means of purge and cold trapping injector capillary gas chromatography. Application to the differentiation of Rias Baixas Spanish white wines. J Agric Food Chem. 1995;43:764–768. doi: 10.1021/jf00051a037. [DOI] [Google Scholar]
- Hernández-Orte P, Cacho JF, Ferreira V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J Agric Food Chem. 2002;50:2891–2899. doi: 10.1021/jf011395o. [DOI] [PubMed] [Google Scholar]
- Hernández-Orte P, Ibarz M, Cacho J, Ferreira V. Effect of the addition of ammonium and amino acids to musts of airen variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005;89:163–174. doi: 10.1016/j.foodchem.2004.02.021. [DOI] [Google Scholar]
- Karagiannis S, Economou A, Lanaridis P. Phenolic and volatile composition of wines made from Vitis vinifera cv. Muscat Lefko Grapes from the Island of Samos. J Agric Food Chem. 2000;48:5369–5375. doi: 10.1021/jf000459c. [DOI] [PubMed] [Google Scholar]
- Lee S, Rathbone D, Asimont S, Roland A, Ebeler SE. Dynamic changes in ester formation during chardonnay juice fermentations with different yeast inoculation and initial brix conditions. Am J Enol Vitic. 2004;55:346–354. [Google Scholar]
- López de Lerma N, García-Martínez T, Moreno J, Mauricio JC, Peinado RA. Volatile composition of partially fermented wines elaborated from sun dried Pedro Ximénez grapes. Food Chem. 2012;135:2445–2452. doi: 10.1016/j.foodchem.2012.07.058. [DOI] [PubMed] [Google Scholar]
- Loscos N, Hernández-Orte P, Cacho J, Ferreira V. Evolution of the aroma composition of wines supplemented with grape flavour precursors from different varietals during accelerated wine ageing. Food Chem. 2010;120:205–216. doi: 10.1016/j.foodchem.2009.10.008. [DOI] [Google Scholar]
- Marais J, Van WyK CJ. Effect of grape maturity and juice treatments on terpene concentrations and wine quality of Vitis vinifera L. cv. weisser Riesling and Bukettraube. S Afric J Enol Vitic. 1986;7:26–35. [Google Scholar]
- Márquez R, Castro R, Natera R, García-Barroso C. Characterisation of the volatile fraction of andalusian sweet wines. Eur Food Res Technol. 2008;226:1479–1484. doi: 10.1007/s00217-007-0679-8. [DOI] [Google Scholar]
- Ministerio de Agricultura, Alimentación y Medioambiente (2015) Agencia Estatal de Meteorología website (Spanish Goverment). http://www.aemet.es. Accessed 17/05/15
- Ortega-Heras M, González-Huerta C, Herrera P, González-Sanjosé ML. Changes in wine volatile compounds of varietal wines during ageing in wood barrels. Anal Chim Acta. 2004;513:341–350. doi: 10.1016/j.aca.2003.10.051. [DOI] [Google Scholar]
- Ortega-Heras M, Pérez-Magariño S, Del-Villar-Garrachón V, González-Huerta C, Moro LC, Guadarrama A, Villanueva S, Gallo R, Martín de la Helguera S. Study of the effect of vintage, maturity degree, and irrigation on the amino acid and biogenic amine content of a white wine from the verdejo variety. J Sci Food Agric. 2014;94:2073–2082. doi: 10.1002/jsfa.6526. [DOI] [PubMed] [Google Scholar]
- Ramey D, Ough C. Volatile ester hydrolysis or formation during storage of model solutions and wines. J Agric Food Chem. 1980;25:928–934. doi: 10.1021/jf60231a021. [DOI] [Google Scholar]
- Rapp A. Volatile flavour of wine correlation between instrumental analysis and sensory perception. Nahrung. 1998;42:351–363. doi: 10.1002/(SICI)1521-3803(199812)42:06<351::AID-FOOD351>3.3.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Rapp A, Mandery H. Wine aroma. Experientia. 1986;42:873–884. doi: 10.1007/BF01941764. [DOI] [Google Scholar]
- Ruíz Bejarano MJ, Rodríguez Dodero MC, Barroso CG. Optimizing the process of making sweet wines to minimize the content of ochratoxin A. J Agric Food Chem. 2010;58:13006–13012. doi: 10.1021/jf103245z. [DOI] [PubMed] [Google Scholar]
- Ruiz MJ, Zea L, Moyano L, Medina M. Aroma active compounds during the drying of grapes cv. Pedro Ximenez destined to the production of sweet Sherry wine. Eur Food Res Technol. 2009;230:429–435. doi: 10.1007/s00217-009-1183-0. [DOI] [Google Scholar]
- Ruiz MJ, Moyano L, Zea L. Changes in aroma profile of musts from grapes cv. Pedro Ximenez Chamber-dried at controlled conditions destined to the production of Sweet Sherry Wine. LWT - Food Sci Technol. 2014;59:560–565. doi: 10.1016/j.lwt.2014.04.056. [DOI] [Google Scholar]
- Ruiz-Bejarano MJ, Castro-Mejías R, Rodríguez-Dodero MC, García-Barroso C. Study of the content in volatile compounds during the aging of sweet Sherry wines obtained from grapes cv. Muscat and fermented under different conditions. Eur Food Res Technol. 2013;237:905–922. doi: 10.1007/s00217-013-2061-3. [DOI] [Google Scholar]
- Sánchez-Palomo E, Díaz-Maroto MC, Pérez-Coello MS. Rapid determination of volatile compounds in grapes by HS-SPME coupled with GC-MS. Talanta. 2005;66:1152–1157. doi: 10.1016/j.talanta.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Schreier P. Flavor composition of wines: a review. Crit Rev Food Sci Nutr. 1979;12:59–111. doi: 10.1080/10408397909527273. [DOI] [PubMed] [Google Scholar]
- Serratosa MP, Márquez A, Moyano L, Zea L, Mérida J. Chemical and morphological characterization of chardonnay and Gewürztraminer grapes and changes during chamber-drying under controlled conditions. Food Chem. 2014;159:128–136. doi: 10.1016/j.foodchem.2014.02.167. [DOI] [PubMed] [Google Scholar]
- Silva AC, Hogg T, Guedes de Pinho P. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J Agric Food Chem. 2003;51:1377–1381. doi: 10.1021/jf025847o. [DOI] [PubMed] [Google Scholar]
- Valero A, Marín S, Ramos AJ, Sanchis V. Survey: ochratoxin A in European special wines. Food Chem. 2008;108:593–599. doi: 10.1016/j.foodchem.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Vega-Mercado H, Marcela Góngora-Nieto M, Barbosa-Cánovas GV. Advances in dehydration of foods. J Food Eng. 2001;49:271–289. doi: 10.1016/S0260-8774(00)00224-7. [DOI] [Google Scholar]
- Versini G, Carlin S, Dalla A, Nicolini G, Rapp A. Formation of 1,1,6 trimethyl-1,2-dihydronaphthalene and other norisoprenoids in wine: considerations of the kinetics. In: Winterhalter P, Rouseff R, editors. Carotenoid-derived aroma compounds, ACS symposium series 802. Washington DC: American Chemical Society; 2002. pp. 285–299. [Google Scholar]