Abstract
Insoluble dietary fiber from soybean residue (SIDF) was treated with dynamic high-pressure microfluidization (DHPM) and used as adsorbent for Pb(II) ion. The effects of pressure on the Pb(II) adsorption capacity, primary cilia structure and surface topography of SIDF were determined using a gastrointestinal simulated model in vitro. SIDF (at pH 7.0) showed maximum binding capacity (261.42 ± 2.77 μmol/g), which was about 1.13 times higher than that of untreated sample (233.47 ± 1.84 μmol/g), when pressure reached 80 MPa. However, the net adsorption value of SIDF in a simulated small intestine (~ 9 μmol/g) was significantly lower than that in the stomach (~ 48 μmol/g), because of the competitive adsorption of Pb2+ by pancreatin, cholate and several enzymes in the small intestine. In addition, the adsorption capacity of SIDF exhibited good linear relationship with the physicochemical properties of total negative charges, and the adsorption behavior presumably occurred on the surface area of granules fiber.
Keywords: Dynamic high pressure microfluidization, Pb, Insoluble dietary fiber, Soybean residue
Introduction
Heavy metal pollution has caused greatest concerns because heavy metals tend to concentrate through the food chain and accumulate in living bodies, which cause serious problems for the environment, and human health. Among the heavy metals, Pb (II) is a ubiquitous and toxic agent (Goel et al. 2005). Pb2+ poisoning in humans can cause severe damage to kidneys, blood, blood-forming organs, central nervous system, and gastrointestinal tract; especially the child’s physical and mental health (Lo et al. 1999; Goel et al. 2005). Therefore, considerable researches have been conducted to reduce the concentration of Pb2+ in waste waters (Özer 2007).
Dietary fiber is considered as a health-promoting food component that has shown great potential for binding heavy metal ions (Ou et al. 1999; Özer and Pirincci 2006; Daou and Zhang 2014; Mehta et al. 2015). Consumption of dietary fiber could increase the faeces metal content, preventing the body from heavy metal’s toxicity (Serguschenko et al. 2007). Insoluble dietary fiber as a by-product is abundant, inexpensive, and has high dietary fiber content (O’Toole 1999; Ou et al. 1999; Daou and Zhang 2014). Modified dietary fiber using chemical, physical and enzymatic methods have been recently reported to enhance their heavy metal adsorption capacity (Feng et al. 2009; Park et al. 2013). However, few studies report on the modifying SIDF to enhance its heavy metal adsorption capacity.
Dynamic high pressure microfluidization (DHPM) is an emerging homogenization technology, which uses the combination-forces of high-velocity impact, high-frequency vibration, cavitation and ultra-high pressure shear to process macromolecular materials such as proteins, lipids, and carbohydrates (Iordache and Jelen 2003; Huang et al. 2013). Few reports focus on the physical properties of soybean dietary fiber influenced by DHPM. Liu et al. (2006) had reported the effect of instantaneous high-pressure treatment (IHP) on rheological properties of soybean dietary fiber. In our previous research, the composition and physicochemical properties of dietary fiber from soybean residue changed after DHPM treatment (Tu et al. 2014). However, the effect of DHPM on SIDF, in particular the adsorption capacity of Pb (II), has never been reported previously.
This study aimed to investigate the effect of DHPM-treated SIDF on the adsorption behavior of Pb2+ and its digestibility in vitro, and to analyze the relationship between the physicochemical properties of modified SIDF and its Pb(II) adsorption capacity. Meanwhile, the adsorption mechanism of Pb2+ by SIDF was also discussed by measuring structural characteristics. The modified insoluble dietary fiber is anticipated to develop products in food industries to meet consumers’ demands.
Materials and methods
SIDF preparation and DHPM treatment
Soybean residue was obtained from a local fast food restaurant (The Dongbei family, Nanchang, China). The fresh residue was dried at 55 oC for 24 h in a forced oven, ground and sieved to obtain uniform particles sizes (~0.15 mm). Starch and protein were initially removed from the samples to prepare the SIDF. After centrifugation at 3000 g for 20 min, the supernatant was discarded and the residue was washed twice with distilled water (70 οC) and then by 95 % ethanol. The washed precipitate was sequentially freeze-dried.
The freeze-dried SIDF samples were dissolved in distilled water and stirred at room temperature for 10 min. The mixture was homogenized twice in a homogenizer (GYB1003, Shanghai Donghua manufacturer, China) at 30,000 psi. The mixture was then treated with DHPM (M-110EH Microfluidics, Newton, USA) for 2 passes under 40, 80, 120 and 160 MPa, which were referred as SIDF-40, SIDF-80, SIDF-120 and SIDF-160, respectively. Samples without DHPM treatment was used as control and named SIDF-0. All SIDF were sequentially freeze-dried and stored in a desiccator.
Adsorption process of SIDF for Pb2+ under stimulated gastrointestinal tract
The preparation of digestive fluids was as follows. The simulated saliva solution comparised 2.38 g of Na2HPO4, 0.19 g of KH2PO4, 8.0 g of NaCl and 0.0054 g of α-amylase, which were dissolved in 1 L of distilled water. The solution was adjusted to pH 6.75 with 2 M NaOH or HCl. The simulated gastric juice: contained 0.32 % of pepsin prepared in 0.03 M NaCl. The solution was adjusted to pH 1.2 with 2 M HCl. Simulated intestinal juice included 0.05 g of pancreatin and 0.3 g of bile extract, which were dissolved in 35 mL of 0.1 M NaHCO3 (Gawlik-Dziki et al. 2009).
Each sample was passed through a simulated in vitro digestion model that consisted of gastric and intestinal phases (Espinal-Ruiz et al. 2014). The procedure consists of two main steps, namely an adsorption process under the simulated mouth and gastric conditions for 30 min at 37 οC and an adsorption process under the simulated colonic conditions for 60 min at 37 οC (Zacherl et al. 2011).The model of a simulated mouth included 10 mL of simulated saliva solution, which was added to 0.1 g of SIDF in a 50 mL centrifuge tube with a cap. After incubating at 37 οC for 10 min at 120 rpm in a shaker, the pH of the solution was adjusted to 2.0 with 5 M HNO3. Then 0.1 mL of Pb(NO3)2 (100 mg Pb2+/mL) and 10 mL of simulated gastric juices were added, and then incubated at 37 οC for about 20 min at 120 rpm. After incubation, counting was initiated, and 0.1 mL of supernatant (sample was performed 2 times) was collected to determine Pb(II) ions content at 0, 5, 10, 20 and 30 min. The model of gastric condition was prepared as follows: the pH of the solution was initially adjusted to 7.0 with 2 M NaOH. Afterwards, 10 mL of the simulated intestinal juice was placed into the tube and incubated at 37 οC in a shaker at 120 rpm. About 0.1 mL of supernatant (×2) was collected and used to determine the Pb(II) ions content at 0, 5, 10, 20, 30 and 60 min. The blank group was used without SIDF.
For each sample, supernatant was separated from adsorbents by centrifugation at 4000 g for 10 min and diluted with double distilled water to suitable concentrations. An atomic absorption spectrophotometer was used to determine the Pb(II) ion concentration in supernatant. The total adsorption capacity of Pb(II) ion adsorbed (mg/g) and the net adsorption capacity (mg/g) were calculated using the following equations:
where C0 is the initial concentration of Pb(II) ions in the solution (mg/L), Ct is the concentration of Pb(II) ions in solution at t minute of the experimental groups (mg/L), Ctb is the concentration of Pb(II) ions in solution at t minute of the blank group (mg/L), V is the volume of the solution (L), and W is the weight of the adsorbent (g).
The BCmax and BCmin
The BCmax and the BCmin of SIDF for Pb(II) ions were determined according to the methods of Zhang et al. (2011). Briefly, SIDF (0.2 g) was suspended in 20 mL of solution containing 10 mM of Pb(NO3)2. The pH was adjusted to 2.0 and 7.0 to simulate the conditions in the stomach and small intestine, respectively. The mixture was shaken at 37 οC for 3 h at 120 rpm in a water-bath incubator. Then, the sample (2 mL) was collected and centrifuged at 4000 g for 20 min. The concentrations of Pb2+ in the supernatant were determined by an atomic absorption spectrophotometer. SIDF (0.5 g) and Pb(NO3)2 (500 μM) were used to measure BCmin. BCmax was measured using the same processes as that of BCmin.
Water-holding capacity
The water-holding capacity (WHC) of SIDF was determined following the method of Chau and Huang (2003). SIDF was mixed with distilled water (1:10, w/v) for 24 h. After centrifugation at 3000 g for 15 min, the supernatant was discarded, the residue was weighed, and the WHC was calculated as grams of water held by 1 g of SIDF.
Oil-holding capacity
The oil-holding capacity (OHC) was measured under the same conditions as WHC measurement, but commercial peanut oil was used instead of water. OHC was expressed as grams of peanut oil held by 1 g of SIDF.
Cation exchange capacity (CEC)
The cation exchange capacity (CEC) was determined using the method of Jiménez et al. (2000). Samples (0.1 g of SIDF (×4)) were suspended in 10 mL of 2 M HCl, stirred continuously for 24 h, and centrifuged for 15 min at 2500 g. The residue was washed with distilled water until the pH of the supernatant was above 4.0. The acidic residue was suspended in 10 mL of 0.3 M NaCl (experiment was performed atleast 3 times) together with a blank containing distilled water. The supernatant was then titrated with 0.01 M NaOH. The CEC was expressed as mM of Na+ per dry sample.
Total negative charge (TNC)
The total negative charge (TNC) was determined following the method of Marshall et al. (1999). Samples (0.5 g of SIDF at pH below 3.0) were suspended in 25 mL of 0.1 M NaOH in a conical flask with stopper, shaken at 25 οC for 24 h and centrifuged for 10 min at 2650 g. Then, 10 mL of the supernatant was added to 15 mL of 0.1 M HCL. Finally, the mixture was titrated with 0.1 M NaOH. The TNC was expressed as mM of H+1 per gram dry sample.
Fourier transform infrared (FTIR) spectroscopy analysis
Spectra of the freeze-dried samples were obtained at a phase resolution of 4 cm−1 and averaged at 64 scans/min. Spectra were recorded in the transmittance mode from 4000 cm−1 to 400 cm−1 using FT-IR (Nicolet 5700;Thermonicolet, USA).
Scanning electron microscopy analysis
The microstructure of the samples were determined using an environmental scanning electron microscope (Quanta200F, FEI Deutschland GmbH, Kassel, Germany) at 10 kV voltage and 1000 × magnification.
Statistical analysis
Data was analyzed using the analysis of variance (ANOVA) and was expressed as mean values from three replicates with standard deviations. The differences between mean values were established using Duncan’s multiple range tests at the level of P ≤ 0.05. The statistical analysis was performed by SPSS (version 19.0).
Results and discussion
Adsorption process of SIDF for Pb2+ under stimulated gastrointestinal tract
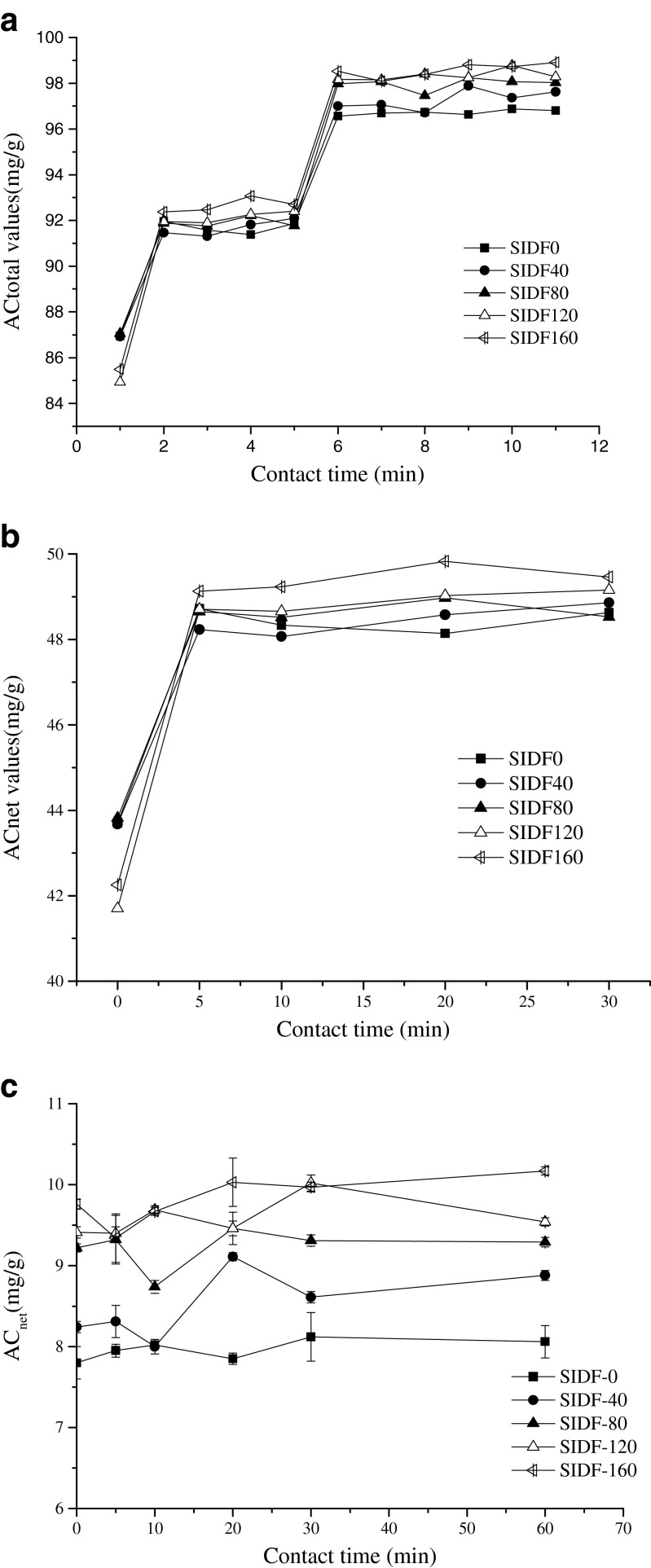
The Pb2+ adsorption process of SIDF under simulated gastrointestinal tract was shown in Fig. 1. Figure 1a shows that the total adsorption capacity of Pb(II) ions for SIDF increased rapidly during the first 5 min in the stomach. The ACtotal values of neutral small intestine were higher than those of the acidic stomach conditions, which was agreed with the results of numerous studies (Hu et al. 2010; Ou et al. 1999; Dubey and Shiwani 2012). However, the net adsorption (the adsorption capacity of samples deducting the blank value) exhibited an adverse trend. Comparing Fig.1b with Fig.1c, the net adsorption of modified SIDF in the acidic stomach (~ 48 μmol/g) was markedly higher than that in the simulated small intestine (~ 9 μmol/g). The adsorption value of unmodified SIDF in the small intestine (~ 87 μmol/g) was much higher than that in the stomach (~ 43 μmol/g). The higher value may mainly be attributed to the in vitro model that consist of complex enzymatic system, chalet and inorganic ions. The results indicated that the simulated digestive juices exhibited stronger Pb2+ binding ability than SIDF at pH 7.0. The generated suspended particles may comprise complex compounds of Pb2+ and simulated digestive juices. The results also showed that both the total and net adsorption capacities of Pb2+were the highest than others when DHPM treatment pressure reached to 160Mpa, indicating that DHPM could increase the adsorption of SIDF to some extent.
Fig. 1.
Total adsorption capacity of Pb2+ by SIDF under the simulated gastrointestinal model (contact time 1 to 5 represent 0, 5, 10, 20, 30 min in simulated stomach; contact time 6 to 11 represent 0, 5, 10, 20, 30, 60 min in simulated small intestine)(a); Net adsorption process of Pb2+ by different SIDF samples in the simulated stomach (b) and simulated small intestine (c). SIDF-0, SIDF-40, SIDF-80, SIDF-120 and SIDF-160 indicates the SIDF was treated by DHPM under 0, 40, 80, 120 and 160 MPa, respectively
The BCmax and BCmin
The values of BCmax and BCmin of SIDF for Pb (at pH = 2.0 and pH = 7.0) are shown in Table 1. The BCmax values were much higher at pH 7.0 than those at pH 2.0. This result may be due to the stronger electrostatic repulsion forces between the positively charged H3O+ and Pb2+ at acidic pH. Conversely, more speciation of the surface function groups including –COOH, −OH were exposed in the solution at pH 7.0, thereby, adsorbing more Pb2+. Consistent with the observation by Ou et al. (1999), all SIDF exhibited low BCmin values, indicating high affinity in the tested small intestine. DHPM treatment could enhance the BCmax values and treated fibers possess higher affinity for Pb(II), probably due to the changed structure and composition of SIDF. The values of ACtotal and BC were clearly in contrast, which may be due to the in vitro model that consisted of complex enzymatic system, chalet and inorganic ions. In our previous study, DHPM increased the content of soluble dietary fiber, reducing the hemicelluloses and damaging the structure of dietary fiber to form a rugged surface (Tu et al. 2014). However, no significant difference (P < 0.05) was observed between BCmax and BCmin at pH 2.0 as pressure increased. Based on the chemisorption mechanism, the adsorption effect of the exposed surface function groups may be concealed by H3O+ in the acidic solution while emerging from the neutral solution.
Table 1.
Maximum binding capacity (BCmax) and the minimum binding concentration (BCmin) of soyabean insoluble dietary fiber (SIDF) for Pb2+ in aqueous solution
| Samples | BCmax(μmol/g) | BCmin(μmol/L) | ||
|---|---|---|---|---|
| pH = 2.0 | pH = 7.0 | pH = 2.0 | pH = 7.0 | |
| SIDF-0 | 38.74a ± 1.54 | 233.47a ± 1.84 | 182.26a ± 3.40 | 5.40e ± 0.02 |
| SIDF-40 | 61.67b ± 0.80 | 257.70c ± 1.80 | 185.26a ± 2.40 | 3.83b ± 0.04 |
| SIDF-80 | 63.66b ± 0.82 | 261.4 2c ± 2.77 | 191.82a ± 3.55 | 3.68a ± 0.04 |
| SIDF-120 | 64.98b ± 2.10 | 243.69b ± 0.48 | 202.47a ± 2.15 | 4.59c ± 0.04 |
| SIDF-160 | 61.04b ± 0.56 | 240.35b ± 1.44 | 202.47a ± 3.55 | 4.71d ± 0.03 |
Values are presented as mean ± SD (n = 3). Values in the same column with different letters are significantly different according to Duncan’s multiple range tests (p < 0.05). SIDF-0, SIDF-40, SIDF-80, SIDF-120 and SIDF-160 indicates the SIDF was treated by DHPM under 0, 40, 80, 120 and 160 MPa, respectively
Physicochemical properties and their relationship with BCmax and BCmin
The effect of DHPM on the physicochemical properties including WHC, OHC, CEC and TNC of SIDF is shown in Table 2. Compared with control, DHPM could decrease the values of WHC and OHC, indicating that increasing pressure may change the structure of SIDF. SIDF-80 and SIDF-40 exhibited the highest values of WHC and OHC, respectively, implying that DHPM could expose the hydrophilic and hydrophobic groups of SIDF. SIDF exhibited the lowest values of WHC and OHC when pressure reached to 160 MPa, indicating that the SIDF structure was damaged. In addition, CEC increased as the pressure increased. This result was due to the high-frequency vibration, powerful shear and cavitation force effect of DHPM, which enhanced the content of –OH, −COOH, and -NH2 as pressure increased, thereby improving the CEC capacity. However, TNC initially increased when DHPM pressure increased from 0 MPa to 80 MPa, and then decreased as pressure increased to 120 MPa and 160 MPa. In addition, Table 3 shows that the BCmax and TNC gained a good linear relation. The BCmin values exhibited a negative correlation with TNC values, indicating that the maximum amount of Pb2+ adsorption and affinity of SIDF depended on the total negative charges of SIDF. However, the relationship among BCmax, BCmin, water holding ability and oil holding ability was very weak.
Table 2.
Physicochemical properties of insoluble dietary fiber from soybean residue
| Samples | WHC(g/g) | OHC(g/g) | CEC(mmol/g) | TNC(mmol/g) |
|---|---|---|---|---|
| SIDF-0 | 7.26c ± 0.38 | 3.60d ± 0.16 | 0.80a ± 0.23 | 2.64a ± 0.03 |
| SIDF-40 | 6.40b ± 0.19 | 2.09c ± 0.07 | 0.87a,b ± 0.05 | 3.55d ± 0.04 |
| SIDF-80 | 6.63b ± 0.02 | 1.52b ± 0.04 | 0.93a,b,c ± 0.06 | 3.65e ± 0.02 |
| SIDF-120 | 6.22a,b ± 0.30 | 1.10a ± 0.10 | 1.12b,c ± 0.09 | 3.40b ± 0.02 |
| SIDF-160 | 5.79a ± 0.17 | 1.03a ± 0.24 | 1.2c ± 0.10 | 3.04c ± 0.02 |
Values represent the means of 3 replicates (n = 3) ± standard deviation. Values in the same column with different letters are significantly different (p < 0.05). WHC water-holding capacity, OHC Oil-holding capacity, CEC cation exchange capacity, TNC total negative charge. SIDF-0, SIDF-40, SIDF-80, SIDF-120 and SIDF-160 indicates the SIDF was treated by DHPM under 0, 40, 80, 120 and 160 MPa, respectively
Table 3.
Person’s coefficient correlation (r) between the physicochemical properties and BCmax, BCmin of insoluble dietary fiber from soybean residue
| WHC | OHC | CEC | TNC | |
|---|---|---|---|---|
| BCmax | 0.639 | −0.359 | −0.174 | 0.923* |
| BCmin | −0.579 | 0.490 | 0.027 | −0.951* |
BC max maximum binding capacity, BC min minimum binding concentration, WHC water-holding capacity, OHC Oil-holding capacity, CEC cation exchange capacity, TNC total negative charge
* means significant correlation
FT-IR analyses
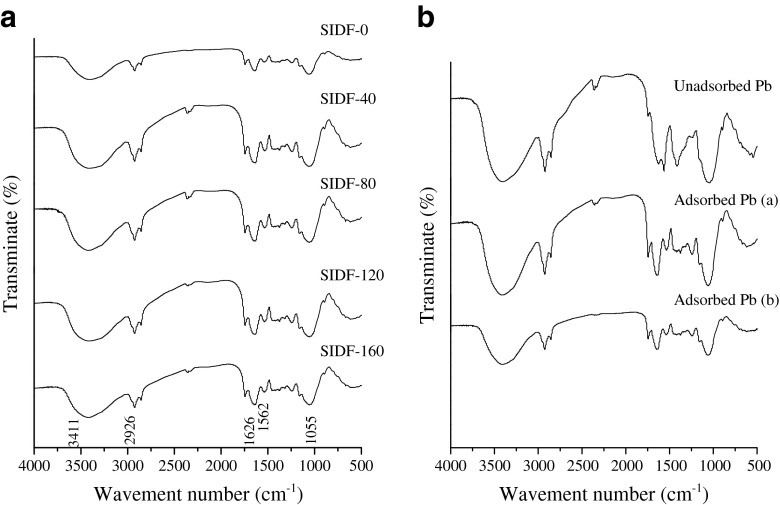
FTIR spectra of SIDF unmodified/modified by DHPM under different pressure levels are shown in Fig. 2a. The spectra were rather similar for all the samples. No significant difference in characteristic absorption bands was observed at 3411 cm−1 (v(−OH)), 2926 cm−1 (v(−CH)), 1750–1500 cm−1 (ν(C = O)), and 1055 cm−1 (ν(C-O-C)). This result showed that DHPM treatment had no effect on the primary structure of SIDF, which was agreed with findings of Chen et al. (2012) on the primary structure of pectin. The chemical structure of SIDF-0 and the ones after Pb2+ adsorption in aqueous solution and simulated digestive juices, are illustrated in Fig.2b. Compared with the un-adsorbed Pb(II), the wavenumber around 3411 cm−1 of SIDF with adsorbed Pb(II) (a and b) slightly shifted towards the left, indicating that the oxygen negative charge mainly adsorbed Pb2+. Compared with the adsorbed Pb2+ in aqueous solution (a), the wave-number of the adsorbed Pb2+/Pb(II) in simulated digestive juices (b) shifted left, demonstrating that SIDF exhibited higher Pb2+/Pb(II) adsorption capacity in simulated digestive juices.
Fig. 2.
Effect of DHPM pressure on FT-IR spectra of SIDF (A); the FTIR spectrum of untreated sample before and after adsorbing Pb2+ in aqueous solution and simulated digestive juices (B) (Adsorbed Pb2+ a SIDF-0 after adsorbing Pb2+ in aqueous solution; Adsorbed Pb2+ b SIDF-0 after adsorbing Pb2+ in simulated digestive juices). SIDF-0, SIDF-40, SIDF-80, SIDF-120 and SIDF-160 indicates the SIDF was treated by DHPM under 0, 40, 80, 120 and 160 MPa, respectively
Scanning microscopy analysis
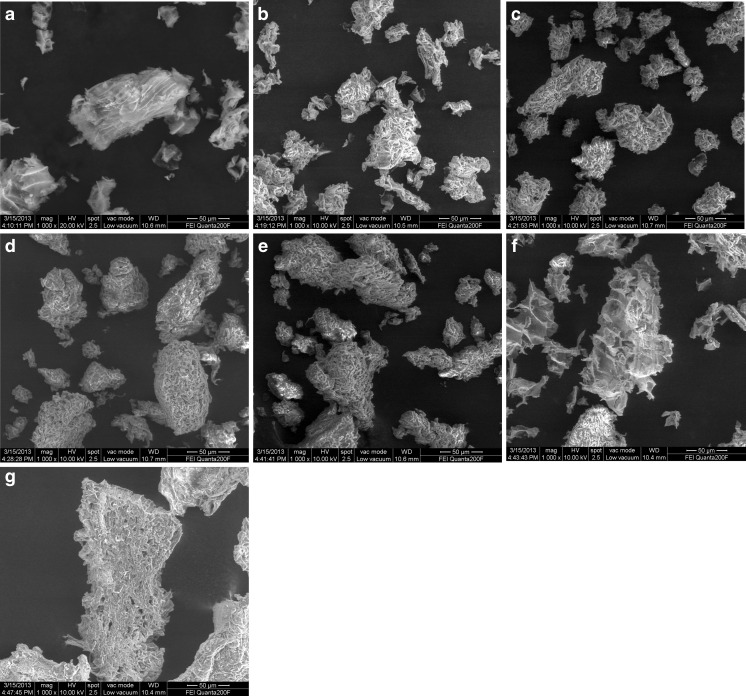
The surface structure images of unmodified/modified SIDF, native SIDF after adsorption in Pb(NO3)2 solution (pH 7.0), and SIDF in simulated small intestine are presented in Fig. 3. As shown in Fig. 3a, the unmodified SIDF exhibited a relatively tidy structure with massy fibrous. However, the thick pieces were sheared into many small and thin alveolate fragments upon treatment with DHPM at pressure 40 and 80 MPa (Fig. 3b and Fig. 3c). However, many amorphous, loose, and agglomerate pieces appeared (Fig. 3d and Fig. 3e), as treatment pressure increased to 120 MPa and 160 MPa. The appearance of agglomerates indicates that DHPM could induce degradation of dietary fiber from soybean residue because of its powerful shear force, high-velocity impact and high-frequency vibration. These results also indicated that the lacerate small pieces could be reunited by increasing the DHPM treatment intensity to a certain extent.
Fig. 3.
Environmental scanning electron micrograph of SIDF a SIDF-0: b SIDF-40; c SIDF-80; d SIDF-120; e SIDF-160; f SIDF-0 after adsorbing Pb2+ in aqueous solution; g SIDF-0 after adsorbing Pb2+ in simulated digestive juices. SIDF-0, SIDF-40, SIDF-80, SIDF-120 and SIDF-160 indicates the SIDF was treated by DHPM under 0, 40, 80, 120 and 160 MPa, respectively
The structure of fiber matrix affected its adsorption and physicochemical properties (Sangnark and Noomhorm 2003). The adsorption of Pb2+ by SIDF was dependent on the structure of fiber matrix. When pressure reached 40 to 80 MPa, the surface structure of SIDF changed from massy pieces to small and thin alveolate fragments that exposed more adsorption surface area, binding sites, and groups, resulting in increased BCmax values. However, when the fragments reunited into large pieces at 120 and 160 MPa, the surface area decreased relatively and the adsorption sites and groups were hidden, resulting in the decrease of adsorption capacity.
Figure 3f shows the image of SIDF-0 binding Pb2+ in the normal aqueous-solution-adsorption pattern. Compared with Fig. 3a, Fig.3f shows abundantly unfolded fiber bundles on the outer surface of SIDF. However, the surface seems to be completely unfolded for other small and thin particles. Assuming that Pb2+ adsorption mainly occurred on the exposed part of particles including the pores, SIDF-40 and SIDF-80 are concluded to possess the highest adsorption capacity (Table 1). Figure 3g shows that the image of the generated complex chunk was completely different from that of the dietary fiber. The mixture may contain the complexes of small fiber fragments, cholate, pancreatin and some enzymes that entrapped large amounts of Pb(II) ions and other inorganic ions. Meanwhile, the abnormal phenomenon of the net adsorption capacity of Pb2+ by SIDF in the simulated small intestine can result from the Pb(II) ions adsorption capacity by pancreatin, cholate and other enzymes superior to that of SIDF.
Conclusions
DHPM could increase the Pb2+ adsorption by SIDF to some extent. DHPM treatment changed the surface structure of SIDF, but did not affect the primary structure. Adsorption may occur on the surface area of fiber granules, and adsorption ability depended on its structure and physicochemical property. The adsorption capacity (BCmax) and affinity exhibited a good linear relationship with the physicochemical property of total negative charges. Moreover, we found that the net absorption of SIDF in the simulated small intestine (~9 μmol/g) was much lower than that in acidic stomach (~48 μmol/g) in the in vitro simulated gastrointestinal model. This result may be due to the competitive adsorption of Pb2+ by pancreatin, cholate, and other enzymes in the small intestine.
Acknowledgments
This study was supported by the Freedom Explore Program of State Key Laboratory of Food Science and Technology, Nanchang University (SKLF-ZZB-201310), The Key Project for Science and Technology Innovation of Jiangxi Province (20124ACB00600), and Earmarked fund for Jiangxi Agriculture Research System (JXARS-04).
Abbreviations
- DHPM
dynamic high pressure microfluidization
- SIDF
soybean insoluble dietary fiber
- BCmax
maximum binding capacity
- BCmin
the minimum binding concentration
- WHC
water-holding capacity
- TNC
total negative charge
References
- Chau C-F, Huang Y-L. Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L. Cv Liucheng J Agr Food Chem. 2003;51(9):2615–2618. doi: 10.1021/jf025919b. [DOI] [PubMed] [Google Scholar]
- Chen J, Liang R-H, Liu W, Liu C-M, Li T, Tu Z-C, Wan J. Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocoll. 2012;28(1):121–129. doi: 10.1016/j.foodhyd.2011.12.018. [DOI] [Google Scholar]
- Daou C, Zhang H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Technol. 2014;51(12):3878–3885. doi: 10.1007/s13197-013-0925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey A, Shiwani S. Adsorption of lead using a new green material obtained from portulaca plant. Int J Environ Sci Te. 2012;9(1):15–20. doi: 10.1007/s13762-011-0012-8. [DOI] [Google Scholar]
- Espinal-Ruiz M, Parada-Alfonso F, Restrepo-Sánchez L-P, Narváez-Cuenca C-E, McClements DJ. Impact of dietary fibers [methyl cellulose, chitosan, and pectin] on digestion of lipids under simulated gastrointestinal conditions. Food Funct. 2014;5(12):3083–3095. doi: 10.1039/C4FO00615A. [DOI] [PubMed] [Google Scholar]
- Feng N, Guo X, Liang S. Adsorption study of copper (II) by chemically modified Orange peel. J Hazard Mater. 2009;164(2):1286–1292. doi: 10.1016/j.jhazmat.2008.09.096. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki U, Dziki D, Baraniak B, Lin R. The effect of simulated digestion in vitro on bioactivity of wheat bread with Tartary buckwheat flavones addition. LWT Food Sci Technol. 2009;42(1):137–143. doi: 10.1016/j.lwt.2008.06.009. [DOI] [Google Scholar]
- Goel J, Kadirvelu K, Rajagopal C, Garg VK. Removal of lead (II) by adsorption using treated granular activated carbon: batch and column studies. J Hazard Mater. 2005;125(1):211–220. doi: 10.1016/j.jhazmat.2005.05.032. [DOI] [PubMed] [Google Scholar]
- Hu G, Huang S, Chen H, Wang F. Binding of four heavy metals to hemicelluloses from rice bran. Food Res Int. 2010;43(1):203–306. doi: 10.1016/j.foodres.2009.09.029. [DOI] [Google Scholar]
- Huang X, Tu Z, Wang H, Zhang Q, Hu Y, Zhang L, Niu P, Shi Y, Xiao H. Glycation promoted by dynamic high pressure microfluidisation pretreatment revealed by high resolution mass spectrometry. Food Chem. 2013;141(3):3250–3259. doi: 10.1016/j.foodchem.2013.05.159. [DOI] [PubMed] [Google Scholar]
- Iordache M, Jelen P. High pressure microfluidization treatment of heat denatured whey proteins for improved functionality. Innov Food Sci Emerg. 2003;4(4):367–376. doi: 10.1016/S1466-8564(03)00061-4. [DOI] [Google Scholar]
- Jiménez A, Rodríguez R, Fernández-Caro I, Guillén R, Fernández-Bolaños J, Heredia A. Dietary fibre content of table olives processed under different European styles: study of physico-chemical characteristics. J Sci Food Agric. 2000;80(13):1903–1908. doi: 10.1002/1097-0010(200010)80:13<1903::AID-JSFA720>3.0.CO;2-N. [DOI] [Google Scholar]
- Liu C, Liu W, Xiong H, Liang H, Tu Z, Ruan R. Effect of instantaneous high pressure (IHP) treatment on solubility and rheologic properties of soybean dietary fiber. Abstr Pap Am Chem Soc. 2006;232:165–165. [Google Scholar]
- Lo W, Chua H, Lam K-H, Bi S-P. A comparative investigation on the biosorption of lead by filamentous fungal biomass. Chemosphere. 1999;39(15):2723–2736. doi: 10.1016/S0045-6535(99)00206-4. [DOI] [PubMed] [Google Scholar]
- Marshall WE, Wartelle LH, Boler DE, Johns MM, Toles CA. Enhanced metal adsorption by soybean hulls modified with citric acid. Bioresour Technol. 1999;69(3):263–268. doi: 10.1016/S0960-8524(98)00185-0. [DOI] [Google Scholar]
- Mehta N, Ahlawat S, Sharma D, Dabur R. Novel trends in development of dietary fiber rich meat products—a critical review. J Food Sci Technol. 2015;52(2):633–647. doi: 10.1007/s13197-013-1010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole DK. Characteristics and use of okara, the soybean residue from soy milk production a review. J Agric Food Chem. 1999;47(2):363–371. doi: 10.1021/jf980754l. [DOI] [PubMed] [Google Scholar]
- Ou S, Gao K, Li Y. An in vitro study of wheat bran binding capacity for Hg, Cd, and Pb. J Agric Food Chem. 1999;47(11):4714–4717. doi: 10.1021/jf9811267. [DOI] [PubMed] [Google Scholar]
- Özer A. Removal of Pb (II) ions from aqueous solutions by sulphuric acid-treated wheat bran. J Hazard Mater. 2007;141(3):753–761. doi: 10.1016/j.jhazmat.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Özer A, Pirincci H. The adsorption of Cd (II) ions on sulphuric acid-treated wheat bran. J Hazard Mater. 2006;137(2):849–855. doi: 10.1016/j.jhazmat.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Park KH, Lee KY, Lee HG. Chemical composition and physicochemical properties of barley dietary fiber by chemical modification. Int J Biol Macromol. 2013;60:360–365. doi: 10.1016/j.ijbiomac.2013.06.024. [DOI] [PubMed] [Google Scholar]
- Sangnark A, Noomhorm A. Effect of particle sizes on in-vitro calcium and magnesium binding capacity of prepared dietary fibers. Food Res Int. 2003;36(1):91–96. doi: 10.1016/S0963-9969(02)00112-6. [DOI] [Google Scholar]
- Serguschenko I, Kolenchenko E, Khotimchenko M. Low esterified pectin accelerates removal of lead ions in rats. Nutr Res. 2007;27(10):633–639. doi: 10.1016/j.nutres.2007.06.005. [DOI] [Google Scholar]
- Tu Z, Chen L, Wang H, Ruan C, Zhang L, Kou Y. Effect of fermentation and dynamic high pressure microfluidization on dietary fibre of soybean residue. J Food Sci Technol. 2014;51(11):3285–3292. doi: 10.1007/s13197-012-0838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacherl C, Eisner P, Engel K-H. In vitro model to correlate viscosity and bile acid-binding capacity of digested water-soluble and insoluble dietary fibres. Food Chem. 2011;126(2):423–428. doi: 10.1016/j.foodchem.2010.10.113. [DOI] [Google Scholar]
- Zhang N, Huang C, Ou S. In vitro binding capacities of three dietary fibers and their mixture for four toxic elements, cholesterol, and bile acid. J Hazard Mater. 2011;186(1):236–239. doi: 10.1016/j.jhazmat.2010.10.120. [DOI] [PubMed] [Google Scholar]