Abstract
The objectives of this study were to determine efficacy of a membrane filtration in soy hull pectin purification and evaluate combined effects of soy hull pectin and pre-emulsified fiber/oil (PE) on chemical composition and technological properties of low fat and low salt meat emulsions. Soy hull pectin was purified through two different methods (alcohol-washed (ASP) and membrane-filtered (MSP)). Insoluble soy hull residues after pectin extraction were incorporated with sunflower oil and water for the PE preparation. Meat emulsion was formulated with 58 % pork, 20 % ice, 20 % pork backfat, and 2 % NaCl as control. A total of six low fat and low salt meat emulsions (1 % NaCl and 10 % backfat) was manufactured with 1 % pectin (with/without ASP or MSP) and 10 % PE (with/without). The pectin content of ASP and MSP was 0.84 and 0.64 g L-galacturonic acid/g dry sample, respectively. The inclusion of soy hull pectin caused similar results on chemical composition, color, cooking loss, and texture of the meat emulsions, regardless of the purification method. In addition, positive impacts of the combined treatments with soy hull pectin and PE compared to single treatments on cooking loss and texture of the meat emulsions were observed. Results suggest that membrane filtration could be an effective alternative method to purify pectin, instead of alcohol-washing, and both soluble pectin and insoluble fiber from soy hulls could be used as a functional non-meat ingredient to manufacture various low fat and low salt meat products.
Keywords: Dietary fiber, Meat emulsion, Membrane filtration, Pectin, Low fat and low salt, Soy hulls
Introduction
High contents of sodium and saturated animal fat in processed meat products have been often considered one of the major health-related concerns that affect consumers’ purchasing decisions (Grasso et al. 2014). This results in a practical challenge for the meat/food industry, since salt and fat are highly associated with technological properties in emulsified meat products, such as protein solubility, water-holding capacity (WHC), or emulsifying ability. A reduction of salt and fat contents below a certain essential level causes negative impacts on cooking loss, texture, and sensory attributes of final products (Desmond 2006; Jiménez-Colmenero et al. 2001). Hence, the manufacture of low fat and/or low salt meat products formulated with various functional non-meat ingredients has received considerable interests from the meat/food industry as an alternative way to respond negative consumer perception, while not compromising functional/quality attributes of the processed meat products (Jiménez-Colmenero et al. 2001).
Dietary fiber has been extensively utilized as a natural and functional non-meat ingredient for improving WHC and modifying texture of low fat and/or salt meat emulsions, as well as providing physiological benefits to human health itself (Rodríguez et al. 2006). Moreover, the incorporation of dietary fiber and vegetable oils has been recognized as one of potential alternative formulations to manufacture functional meat products with providing additional nutritional/health benefits by replacing saturated animal fat with high amounts of unsaturated fatty acid (Choi et al. 2010; Vural et al. 2004).
Pectin, which is one of food hydrocolloids, has been practically used for several processed foods, as a cryo-protectant, emulsifier, filler, gelling agent, stabilizer, and/or thickener (Thakur et al. 1997). In addition, pectin and pectin gel have been utilized to develop low fat and/or low salt meat products, as a fat replacer and texture modifier (Candogan and Kolsarici 2003; Pappa et al. 2000; Troy et al. 1999). Recently, soy hulls, which are major agro-byproducts from soybean oil industry, have received considerable attention as a novel pectin source, as well as insoluble fiber (Kalapathy and Proctor 2001; Thakur et al. 1997). Kim et al. (2015) suggested that both isolated pectin and insoluble fiber from soy hulls could be useful non-meat ingredients for improving technological properties of meat emulsion.
In previous studies, soy hull pectin has been commonly extracted by hot acid solution, precipitated, and purified with alcohols (mainly 2-isopropanol) to remove impurities (Gnanasambandam and Proctor 1999; Kalapathy and Proctor 2001; Kim et al. 2015). However, the use of alcohols can cause a rise in a production cost and environmental pollution due to waste water generation during the alcohol washing-process (Garna et al. 2007). Alternatively, membrane filtration could be a highly viable method to purify pectin considering not only a physicochemical suitability of pectin for its molecular size (50 to 150 kDa) for the membrane filtration (Thakur et al. 1997), but also other potential benefits, such as reduced energy consumption, relatively simple processing procedure, and separation efficacy (Lau et al. 2012).
Despite of its high potential advantages, there has been no report on the substitution of alcohol washing phase with membrane filtration as an environment-friendly method and the practical use of membrane-filtered pectin as a functional food ingredient. Based on the known information, it was postulated that the development of low fat and low salt meat products formulated with membrane-filtered soy hull pectin, insoluble fiber, and vegetable oil can likely meet the consumers’ need and preference for the usage of natural ingredients and healthy meat products.
Therefore, the objectives of this study were 1) to determine the efficacy of a membrane filtration in soy hull pectin purification as a functional food ingredient and 2) evaluate combined effects of soy hull pectin and pre-emulsified fiber/oil (PE) on chemical composition and technological properties of low fat and low salt meat emulsions.
Materials and methods
Separation of pectinaceous substances from soy hulls
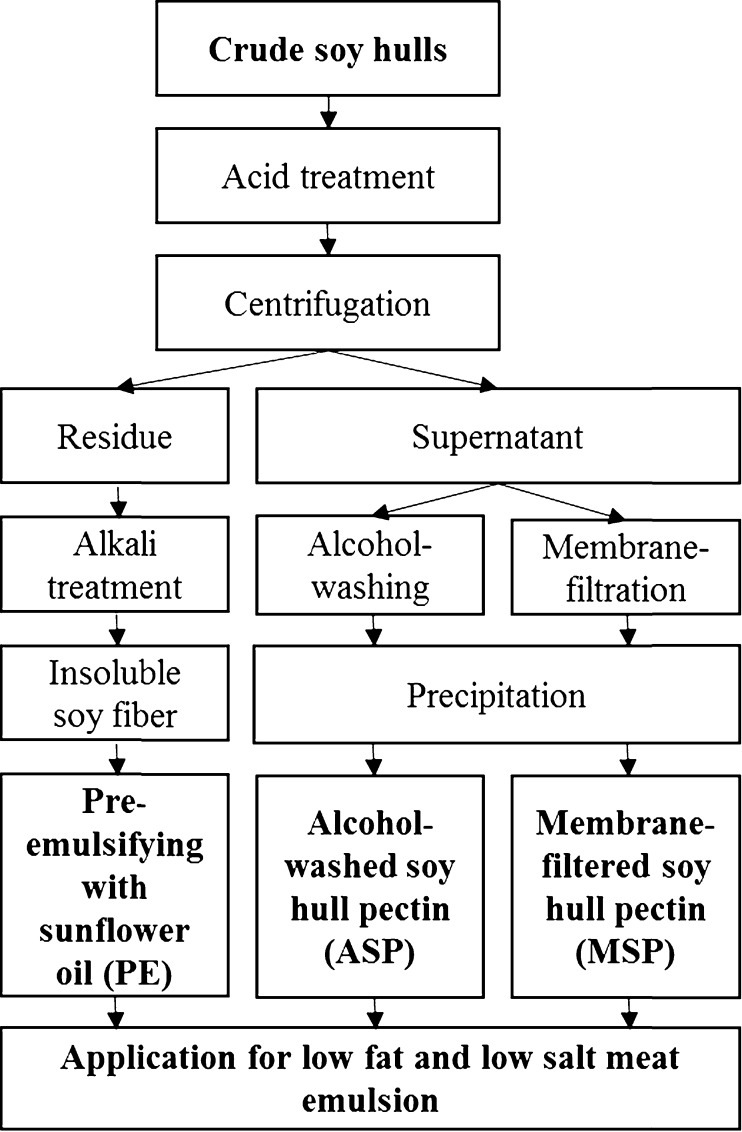
Soy hull pectin was produced with the modification of the method of Kim et al. (2015), and the schematic procedure for pectin and insoluble fiber from soy hulls is shown in Fig. 1. Crude soy hull flour was treated with 0.1 N HCl solutions (1:10 ratio) at 90 °C for 1 h, and the acid treated slurries were centrifuged at 4500 rpm for 20 min (Sorvall RC-5C Plus Centrifuge and SLA-1500 rotor, Kendro Laboratory Products, Asheville, NC, USA). The supernatants and wet cake (residue) were used for further pectin and insoluble fiber preparation, respectively.
Fig. 1.
Schematic procedure for pectin and insoluble fiber isolation from soy hulls and application for meat emulsion manufacture
Preparation of alcohol-washed soy hull pectin (ASP)
Alcohol-washed soy hull pectin (ASP) was prepared using 2-propanol (Monsoor 2005). The supernatant from acid slurries was precipitated by adjusting the pH 3.5 with 0.1 N HCl and allowed to stand for 6 h. The precipitate was collected, centrifuged (4500 rpm for 20 min), dispersed in 2-propanol, stirred for 1 h, and centrifuged for 20 min. The precipitate was washed with 70 % 2-propanol, centrifuged, and the precipitate was collected finally.
Preparation of membrane-filtered soy hull pectin (MSP)
Membrane-filtered soy hull pectin (MSP) was prepared with filtration of the supernatant from the acid slurries with stainless steel tubular composite cross flow microfiltration system (0.2 μm, SCEPTER 4-750 A-5P6, Graver Stainless Steel Membrane). Permeated liquid was re-filtered using a cross flow ultrafiltration (PCI tubular membrane, PU608), which was 8 kDa MWCO made by polysulfone. Diafiltration was conducted by adding distilled water in a feed solution tank at the rate of permeate removal, such as simple sugar, while pectin was concentrated in the retentate. Membrane technique led to approximately 65 % more pectin solutes production than the alcohol washing method.
Preparation of insoluble soy fiber
Insoluble soy fiber was prepared with the method of Kim et al. (2015). After centrifugation of the acid slurries, the insoluble residue was mixed with alkali solution (1:10 ratio, 0.2 g calcium hydroxide/g) at 90 °C for 1 h and centrifuged at 4500 rpm for 20 min. The residue was washed with deionized water until the pH reached at 6.2–6.8. All soy hull fibers including pectin were dehydrated in a 55 °C air dryer for 48 h.
Low fat and salt meat emulsion manufacture
Pre-emulsified fiber/oil was manufactured with the insoluble soy fiber, sunflower oil, and water (3:3.5:3.5 ratio) using a food blender. Meat emulsions were prepared with the manufacture procedure described by Kim et al. (2015). The formulation of meat emulsions is shown in Table 1. Fresh pork bottom round muscle (M. biceps femoris) and pork backfat were obtained at the Purdue University Meat Laboratory at 72 h postmortem, vacuum packaged, and stored at −80 °C for maximum 1 month. Frozen pork round muscle and backfat were thawed in a 2 °C cooling room for 24 h. After thawing, all subcutaneous and intramuscular fat and visible connective tissue were removed. Lean materials were initially ground through a 3/8 in. plate and re-ground 1/4 in. plate using a meat grinder (M-12-FS, Torrey, Monterrey, NL, Mexico). The pork backfat was also ground through 3/8 in. and 1/4 in. plates. Positive control ((+) Con) was formulated with 58 % ground pork, 20 % back fat, 20 % ice, and 2 % NaCl. For manufacturing low salt and low fat meat emulsions containing 10 % backfat and 1 % NaCl, a total of six treatments using a 3 × 2 factorial design (without pectin, ASP, and MSP) × 2 (with/without PE) was created. The added amounts of pectin and PE were 1 % and 10 %, respectively, and partial deficit of treatment formulations (based on 100 %) was compensated with additional ice. Sodium tri-polyphosphate (0.3 g/100 g), sodium nitrite (0.012 g/100 g), and L-ascorbic acid (0.05 g/100 g) were added in equal amounts. All treatments were individually emulsified using a food blender in a cooling room, and the final temperature of meat emulsions was maintained below 10 °C during manufacturing. A total of three independent batches per treatment was prepared.
Table 1.
Formulations of low fat and low salt meat emulsions (g/100 g)
| Ingredients | (+) Con | Low fat and low salt treatments | |||||
|---|---|---|---|---|---|---|---|
| (−) Con | ASP | MSP | PE | ASP + PE | MSP+ PE | ||
| Pork ham | 58.0 | 58.0 | 58.0 | 58.0 | 58.0 | 58.0 | 58.0 |
| Pork backfat | 20.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Ice | 20.0 | 31.0 | 30.0 | 30.0 | 20.0 | 20.0 | 20.0 |
| NaCl | 2.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| ASP1) | - | - | 1.0 | - | - | 1.0 | - |
| MSP2) | - | - | - | 1.0 | - | - | 1.0 |
| PE3) | - | - | - | - | 10.0 | 10.0 | 10.0 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
All meat emulsions were equally prepared with sodium tri-polyphosphate (0.3 g/100 g), sodium nitrite (0.012 g/100 g), and L-ascorbic acid (0.05 g/100 g)
1)ASP alcohol-washed soy hull pectin
2)MSP membrane-filtered soy hull pectin
3)PE pre-emulsified fiber/oil containing insoluble soy fiber, sunflower oil, and water (3:3.5:3.5)
Pectin content
Pectin content of ASP and MSP was determined in triplicate by the Carbazole colorimetry method (Zhang et al. 2010) and expressed as g L-galacturonic acid/g dried sample.
pH measurements
The pH values of cooked meat emulsions were determined in triplicate using an electronic pH meter (Sartorius Basic Meter PB-11, Sartorius AG, Germany). The pH values of samples were measured by blending a 3 g sample with 27 ml distilled water for 60 s in a homogenizer at 6000 rpm (Ultra-Turrax T25, Janke & Kunkel IKA-Labortechnik, Staufen, Germany).
Proximate composition and caloric content
Moisture (oven air-drying method), lipid (Soxhlet extraction), ash (muffle furnace), and total fiber content of cooked meat emulsions were determined in triplicate by the AOAC method (AOAC 2000). Protein content was analyzed by the high temperature combustion process. From the obtained data of proximate composition, caloric content was calculated according to Atwater values (Mansour and Khalil 1997).
Mineral content
Mineral content of cooked meat emulsions was analyzed in duplicate by inductively coupled plasma (ICP) emission spectroscopy after a nitric acid digestion (Havlin and Soltanpour 1980).
Instrumental color evaluation
Color of cooked meat emulsions was determined using a Hunter MiniScan EZ colorimeter (Hunter, Reston, VA, USA) equipped with a 25 mm (diameter) measuring. The setting for the illuminant was D65 source and the observer was standard 10°. Calibration of the instrument was conducted with black and white calibration tiles, according to the manual. Random five locations on cross-section of each cooked meat emulsion were taken. CIE L*, a*, and b* values were recorded. Hue angle was calculated using the following expression; hue angle = tan−1(b*/a*) (AMSA 2012).
Cooking loss
To determine cooking loss, meat emulsions (approximately 40 g) were stuffed into a 50 ml conical tube and centrifuged at 2000×g for 15 min (4 °C), to eliminate air bubbles. The meat emulsions were cooked in a 75 °C water bath until the targeted core temperature reached 71 °C monitored by using a digital thermometer equipped with a data logger, and then, cooled at room temperature for 1 h. The cooking loss of meat emulsions was determined in duplicate by calculating the difference in weight after and before cooking as follows; cooking loss (%) = [(weight of raw sample (g) - weight of cooked sample (g))/weight of raw sample (g)] × 100 (Kim et al. 2015).
Texture profile analysis (TPA)
Texture profile analysis was performed at room temperature using a TA-XT Plus Texture Analyzer (Stable Micro Systems Ltd., Surrey, UK). The samples cooked under conditions stated above were equilibrated to room temperature at 25 °C for 3 h. Two samples (cylinder shape of 2.5 cm height and 2.5 cm diameter) were taken from the central portion of each cooked sample. A twice compression cycle test (70 % compression of the original sample height) was performed with an aluminum cylinder probe (TA-25). Sample deformation curves were obtained with a 50 kg load cell and the analysis condition were as follows: pre-test speed 1.0 mm/s, post-test speed 5.0 mm/s, test speed 5.0 mm/s (n = 4/treatment/batch). Value for hardness (kg), springiness (ratio), cohesiveness, gumminess (kg), and chewiness (kg) were determined as described by Bourne (1978).
Statistical analysis
Experimental design was a completely randomized design. Data were analyzed using SPSS (SPSS Inc., Chicago, IL, USA) for one-way ANOVA to determine the significance of main effect (treatment). Duncan’s multiple range test (P < 0.05) was used to determine differences between treatment means.
Results and discussion
Pectin content of soy hull pectin
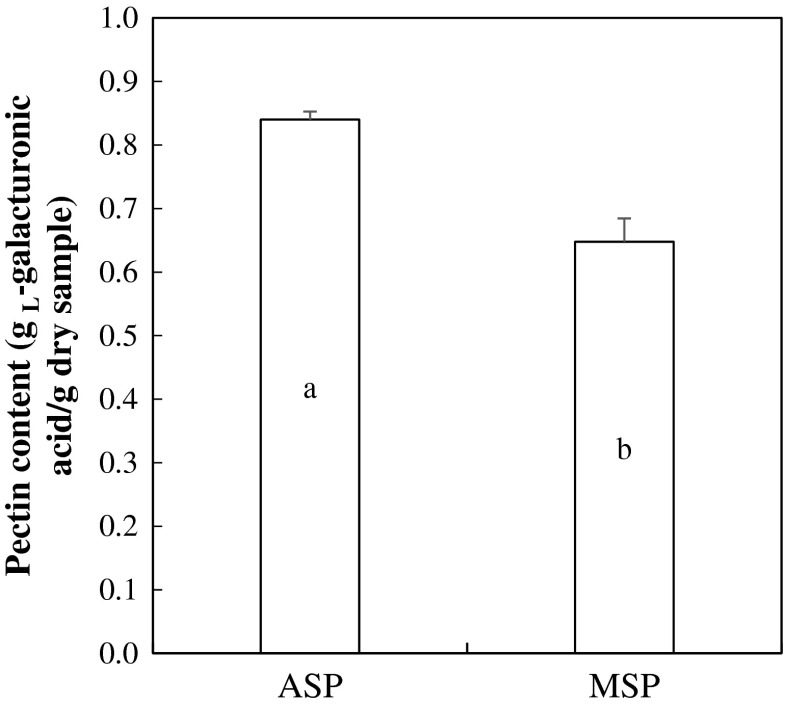
Pectin content of alcohol-washed soy hull pectin (ASP) and membrane-filtered soy hull pectin (MSP) was measured to evaluate the degree of purity by two different purification methods (Fig. 2). Galacturonic acid is a major structural compound of pectin, and its concentration has been generally used to determine pectin purity (Garna et al. 2007). The pectin content of ASP and MSP was 0.84 and 0.64 g L-galacturonic acid/g dry sample, respectively (P < 0.05). A broad scale of soy hull pectin content (54–88.3 %) was reported previously depending upon extracting conditions (Kalapathy and Proctor 2001; Gnanasambandam and Proctor 1999) and drying methods (Monsoor 2005). According to Garna et al. (2007), neutral sugars, which are formed by the degradation of carbohydrate polymers including pectin during extraction and purification process, could affect the galacturonic content of pectin. It has been suggested that the residual amount of the neutral sugars in pectin could decrease through alcohol precipitation (Garna et al. 2007). Thus, in current study, lower pectin content of MSP compared to that of ASP might be related to the inclusion of neutral sugars, which was not processed through the micro-membrane filtration.
Fig. 2.
Pectin content of alcohol-washed soy hull pectin (ASP) and membrane-filtered soy hull pectin (MSP). a, b Significance of t-test between ASP and MSP (P < 0.05)
pH, proximate composition, and caloric content of meat emulsions
The effects of membrane-filtered soy hull pectin (MSP) and pre-emulsified fiber/oil (PE) on pH, proximate composition, and caloric content of low fat and low salt meat emulsion are shown in Table 2. Positive and negative controls exhibited a similar pH value (P > 0.05), which could imply that the reduction in fat and salt had little impact on pH value of meat emulsions in the current study. The addition of soy hull pectin and PE slightly decreased the pH value (0.03–0.12 unit) of meat emulsions. In particular, the meat emulsions containing PE (PE, ASP+ PE, and MSP+ PE treatments) exhibited lower pH value than other treatments (P < 0.05). Similarly, Kim et al. (2015) reported that acid and alkali hydrolyzed soy hull flour decreased pH value of meat emulsion.
Table 2.
pH, proximate composition (g/100 g), and caloric content (kcal/100 g) of low fat and low salt meat emulsions
| Traits | (+) Con | Low fat and low salt treatmentsA | SEMC | |||||
|---|---|---|---|---|---|---|---|---|
| (−) Con | ASPB | MSP | PE | ASP + PE | MSP+ PE | |||
| pH value | 6.13ab | 6.14a | 6.10ab | 6.09b | 6.02c | 6.01c | 6.01c | 0.014 |
| Proximate composition | ||||||||
| Moisture | 56.56e | 61.81bc | 66.60a | 63.48b | 60.00 cd | 58.93cde | 57.58de | 0.814 |
| Protein | 17.51 | 16.89 | 17.61 | 16.53 | 17.86 | 16.98 | 17.10 | 0.196 |
| Fat | 23.24a | 19.82b | 15.90c | 16.45c | 19.98b | 17.04bc | 17.16bc | 0.557 |
| Ash | 1.05 | 1.10 | 1.13 | 0.89 | 0.82 | 0.68 | 0.69 | 0.073 |
| Total fiber | -D | - | - | - | 4.06 | 4.38 | 4.36 | 0.062 |
| Caloric content | 279.17a | 245.94b | 213.56d | 214.16d | 251.29b | 221.28c | 220.85c | 4.976 |
A(+) Con was formulated with 2 % NaCl and 20 % pork backfat, and low fat and salt treatments were equally prepared with 1 % NaCl and 10 % pork backfat
BASP, 1 % alcohol-washed soy hull pectin; MSP, 1 % membrane-filtered soy hull pectin; PE, 10 % pre-emulsified fiber/oil (insoluble soy fiber:sunflower oil:water = 3:3.5:3.5)
CSEM standard error of the mean
DNot measured
a-e Means within a row with different letters are significantly different (P < 0.05)
As expected, (−) Con had a higher moisture and lower fat content (P < 0.05) than (+) Con, which was likely due to an increase in ice and a decrease in pork backfat contents in the initial formulation. The addition of soy hull pectin resulted in a higher moisture content, but relatively lower fat content than (−) Con (P < 0.05). Conversely, the meat emulsions containing both soy hull pectin and PE showed lower moisture and fat contents than (−) Con (P < 0.05), which might be related to the inclusion of insoluble soy fiber. As support for this postulation, total dietary fiber of 4.36–4.38 % was observed for ASP + PE and MSP + PE treatments. In terms of protein and ash contents, there were no significant differences between all treatments, which were 16.89–17.86 % and 1.13–0.68 %, respectively. As a consequence, differently purified soy hull pectin had little impact on proximate composition of meat emulsions. The combination of soy hull pectin and PE resulted in similar moisture content (P > 0.05), but reduced fat content of 6.08–6.20 % (P < 0.05) compared to (+) Con.
The highest caloric content was found at (+) Con (279.17 kcal/100 g) (P < 0.05). (−) Con (245.94 kcal/100 g) had a higher caloric content than any other low fat and low salt treatments. The lowest caloric content was observed for ASP (213.56 kcal/100 g) and MSP (214.16 kcal/100 g), which was less 23.3–23.5 % than regular formulation of (+) Con. In general, proximate composition and caloric content of final meat products is greatly affected by initially added proportion of raw ingredients in formulation and relative changes due to water and fat releases during processing (Mittal and Zhang 2000). The lower fat content of low fat and low salt treatments compared to (−) Con was likely associated with the improvement of water-holding capacity (WHC) due to the addition of soluble (pectin) or insoluble fiber, which will be discussed further in the cooking loss section.
Mineral content of meat emulsions
In terms of mineral content (Table 3), low fat and low salt treatments (538.26–561.75 mg/100 g) had a substantially lower sodium content (≥57 %) than (+) Con (976 mg/100 g) (P < 0.05) due to reduced NaCl addition. All meat emulsions exhibited a similar potassium content (P > 0.05), which ranged from 229.48 to 266.03 mg/100 g. As a result, low fat and low salt meat emulsions had a lower Na/K ratio than (+) Con (P < 0.05). Na/K ratio is a potentially effective index, which is associated with a role to control blood pressure and hypertension in human body (Perez and Chang 2014). Moreover, it has been known that potassium ion can help to increase sodium excretion rate (Drewnowski et al. 2015). For this reason, a decrease in Na/K ratio on processed foods is as important as the absolute reduction in sodium. In our current formulation, the initially added amount of NaCl could affect the change in Na/K ratio, rather than inclusion of soy hull pectin or PE.
Table 3.
Mineral content (mg/100 g) of low fat and low salt meat emulsions
| Traits | (+) Con | Low fat and low salt treatmentsA | SEMC | |||||
|---|---|---|---|---|---|---|---|---|
| (−) Con | ASPB | MSP | PE | ASP + PE | MSP+ PE | |||
| Na | 976.00a | 561.75b | 555.54bc | 539.31d | 538.26d | 547.88 cd | 543.61 cd | 35.212 |
| K | 266.03 | 250.12 | 263.32 | 269.84 | 229.48 | 257.34 | 258.16 | 2.916 |
| Na/K ratio | 3.67a | 2.25b | 2.11bc | 2.00c | 2.35b | 2.13bc | 2.11bc | 0.127 |
| P | 214.68 | 219.47 | 205.90 | 207.68 | 220.30 | 216.63 | 218.59 | 1.292 |
| S | 136.80 | 145.90 | 141.35 | 146.37 | 147.16 | 144.28 | 147.33 | 1.836 |
| Mg | 20.03 | 21.64 | 22.97 | 27.76 | 20.92 | 25.28 | 23.96 | 0.580 |
| Ca | 7.50f | 5.95 g | 11.33e | 18.57d | 80.61c | 95.35b | 102.71a | 9.457 |
| Zn | 1.32 | 1.58 | 4.70 | 1.49 | 1.56 | 1.53 | 1.49 | 0.022 |
| Fe | 0.18 | 0.29 | 0.96 | 0.92 | 0.85 | 1.13 | 1.10 | 0.079 |
| Cu | 0.19 | 0.12 | 0.09 | 0.12 | 0.25 | 0.14 | 0.11 | 0.013 |
| Mn | 0.004 | 0.002 | 0.012 | 0.029 | 0.009 | 0.042 | 0.003 | 0.003 |
| B | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | - |
A(+) Con was formulated with 2 % NaCl and 20 % pork backfat, and low fat and salt treatments were equally prepared with 1 % NaCl and 10 % pork backfat
BASP, 1 % alcohol-washed soy hull pectin; MSP, 1 % membrane-filtered soy hull pectin; PE, 10 % pre-emulsified fiber/oil (insoluble soy fiber:sunflower oil:water = 3:3.5:3.5)
CSEM standard error of the mean
a-g Means within a row with different letters are significantly different (P < 0.05)
The calcium content of meat emulsions was greatly affected by the addition of soy hull pectin and PE (P < 0.05). Kim et al. (2015) reported that acid and alkali hydrolyzed soy hull flours contained 4065 mg/100 g of calcium due to the residue of calcium ion from calcium hydroxide used for alkali hydrolysis. Thus, the addition of PE prepared with acid and alkali hydrolyzed insoluble soy fiber could lead to the increase in calcium content of meat emulsions. However, there have been no previous reports related to an increase in calcium content due to pectin addition. Hence, this observation might be associated with a theory of low methoxyl pectin gelation: low methoxyl pectin including soy hull pectin forms pectin gel through the interaction between calcium ion and pectin molecules, which is known as “egg box model” (Gnanasambandam and Proctor 1999; Thakur et al. 1997). Gerber et al. (2009) reported that pork muscle contains 4–8 mg/100 g of calcium, which is released and decreased after cooking. Although there was a dearth of evidence concerning the interaction between pectin and calcium ion existed in animal muscle, the findings of the current study suggest that soy hull pectin might contribute to the slight increase in calcium content due to the binding of calcium ion in pectin gel.
Regarding other minerals, similar levels of phosphorus (205.90–220.30 mg/100 g), sulfur (136.80–147.33 mg/100 g), magnesium (20.03–27.76 mg/100 g), zinc (1.32–4.70 mg/100 g), iron (0.18–1.13 mg/100 g), copper (0.09–0.25 mg/100 g), manganese (0.002–0.029 mg/100 g), and boron (<0.001 mg/100 g) were detected at all meat emulsions (P > 0.05).
Color characteristics of meat emulsions
The effects of soy hull pectin and PE on color characteristics of low fat and low salt meat emulsions are shown in Table 4. Among all meat emulsions, no significant differences in CIE L* (lightness) and a* (redness) were found. However, the addition of soy hull pectin or PE slightly increased CIE b* (yellowness), when compared to positive and negative controls (P < 0.05). As a result, treatments combined with soy hull pectin and PE had a significantly higher hue angle than both (+) Con and (−) Con (P < 0.05), which indicates that the combined treatments exhibited more yellow and less red color than the other cooked meat emulsions. However, there were no significant differences in color characteristics due to the type of soy hull pectin, regardless of the presence/absence of PE. Previously, Kumar et al. (2013) reported that the addition of 3–5 % crude soy hull flour increased lightness, but decreased redness, and no significant difference in yellowness of chicken nuggets. Recently, Kim et al. (2015) reported that alcohol-washed soy hull pectin and insoluble soy fiber (acid and alkali hydrolyzed) slightly decreased redness and increased yellowness of meat emulsions. Our result was partially in agreement with the previous finding that soy hull pectin and insoluble fiber increased yellowness of meat emulsions. However, regarding no difference in redness in this study, it was probably due to an equal portion of ground pork unlike the previous study, where it was manufactured with replacement of 3 % pork lean portion with the dietary fiber suspension.
Table 4.
Color characteristics of low fat and low salt meat emulsions
| Traits | (+) Con | Low fat and low salt treatmentsA | SEMC | |||||
|---|---|---|---|---|---|---|---|---|
| (−) Con | ASPB | MSP | PE | ASP + PE | MSP+ PE | |||
| CIE L* | 75.49 | 73.99 | 73.11 | 71.59 | 71.06 | 73.29 | 72.44 | 0.594 |
| CIE a* | 6.68 | 7.59 | 7.04 | 8.20 | 7.34 | 6.56 | 7.34 | 0.176 |
| CIE b* | 10.18e | 10.74de | 11.82 cd | 13.04bc | 12.91bc | 13.82ab | 14.92a | 0.422 |
| Hue angle (°) | 56.71 cd | 54.76d | 59.23c | 57.86 cd | 60.34bc | 64.70a | 63.81ab | 0.861 |
A(+) Con was formulated with 2 % NaCl and 20 % pork backfat, and low fat and salt treatments were equally prepared with 1 % NaCl and 10 % pork backfat
BASP, 1 % alcohol-washed soy hull pectin; MSP, 1 % membrane-filtered soy hull pectin; PE, 10 % pre-emulsified fiber/oil (insoluble soy fiber:sunflower oil:water = 3:3.5:3.5)
CSEM standard error of the mean
a-e Means within a row with different letters are significantly different (P < 0.05)
Cooking loss of meat emulsions
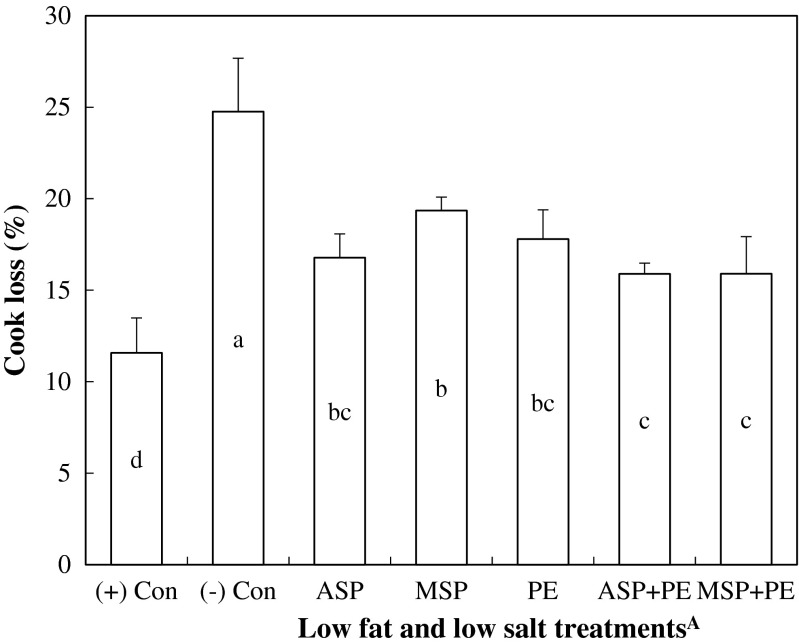
Highest cooking loss (24.76 %) was observed for (−) Con, whereas (+) Con exhibited lowest cooking loss (11.57 %) among all treatments (P < 0.05, Fig. 3). The addition of both soy hull pectin and PE significantly decreased cooking loss, when compared to (−) Con (P < 0.05). This might explain lower moisture and relatively higher fat contents of (−) Con than only soy hull pectin treatments. In addition, the greater effect to reduce cooking loss was observed for ASP+ PE (15.89 %) and MSP+ PE (15.90 %) than single addition of soy hull pectin or PE treatments (P < 0.05). In emulsified meat products, it has been generally known that a reduction in salt below 2.0 % is insufficient to solubilize myofibrillar proteins, causing the decline of WHC, emulsion stability, and protein solubility of myofibrils (Sofos 1983). In addition, a decrease in fat level from regular content (20–30 %) causes the decline of emulsifying properties and viscosity of emulsified meat products (Colmenero 1996). For these reasons, the considerable amount of water loss, especially during thermal processing, generally occurs in low fat and low salt meat products. Thus, dietary fiber has been extensively used as a binder and emulsifier to minimize the water loss of low salt and low fat meat products (Choi et al. 2010; Jiménez-Colmenero et al. 2001). Numerous studies have shown the beneficial effect of pectin and insoluble fiber (mainly cellulose) on cooking loss of processed meat products, which results from its water/oil binding and emulsifying abilities (Candogan and Kolsarici 2003; Choi et al. 2010; Troy et al. 1999; Vural et al. 2004). The results of our current study suggest that soy hull pectin, regardless of purification method, equally reduced the cooking loss of meat emulsion, and the incorporation of soy hull pectin and PE had a noticeable improvement on reducing cooking loss of low fat and low salt meat emulsions.
Fig. 3.
Cooking loss of low salt and low fat meat emulsions. A(+) Con was formulated with 2 % NaCl and 20 % pork backfat, whereas low fat and low salt treatments were equally prepared with 1 % NaCl and 10 % pork backfat. ASP, 1 % alcohol-washed soy hull pectin; MSP, 1 % membrane-filtered soy hull pectin; PE, 10 % pre-emulsified fiber/oil (insoluble soy fiber:sunflower oil:water = 3:3.5:3.5). a-d the different letters between each treatment are significantly different (P < 0.05)
Textural properties of meat emulsions
The effects of soy hull pectin and PE on textural properties of low fat and low salt meat emulsions are shown in Table 5. All low fat and low salt meat emulsions showed lower values in all textural parameters than (+) Con (P < 0.05). When compared to (−) Con, the addition of soy hull pectin increased springiness, whereas the addition of PE led to increases in hardness and springiness (P < 0.05). Inconsistent results on the effect of pectin on texture of emulsified meat products have been reported previously. Candogan and Kolsarici (2003) indicated that 20 % pectin gel prepared with 4.46 % low methoxyl pectin contributed to the formation of harder texture of low fat frankfurter. Ordóñez et al. (2001) noted that the addition of 10 % pectin gel prepared with 4 % low methoxyl pectin decreased hardness, but increased elasticity of low fat frankfurter compared to commercial low fat sausages. Sarıçoban et al. (2010) suggested that 5 % sunflower head pith, as a low methoxyl pectin source, could increase viscosity, which was directly related to the hardness of meat emulsions. Such different effect of pectin on texture of meat products might be associated with the degree of methoxylation, adding forms (dried powder or hydrated gel), or with/without the incorporation with other hydrocolloids. Previous studies found that insoluble fiber-vegetable oil emulsion could increase hardness, but had little impact on springiness for the low fat and/or low salt meat emulsions, which agreed with our finding (Choi et al. 2010; Vural et al. 2004). Our result indicates that the incorporation of soluble and insoluble fiber isolated from soy hulls rather than their single addition had a positive effect on modifying and improving textural properties of low fat and low salt meat emulsions. Further, it can be suggested that both soy hull pectin purified through alcohol washing and membrane filtration methods could result in an equivalent impact on the textural properties of meat emulsions.
Table 5.
Textural properties of low fat and low salt meat emulsions
| Traits | (+) Con | Low fat and low salt treatmentsA | SEMC | |||||
|---|---|---|---|---|---|---|---|---|
| (−) Con | ASPB | MSP | PE | ASP + PE | MSP+ PE | |||
| Hardness (N) | 79.16a | 33.89c | 36.09c | 36.96c | 44.79bc | 47.26bc | 54.09b | 3.638 |
| Springiness | 0.82a | 0.63c | 0.70b | 0.68bc | 0.68bc | 0.68bc | 0.68bc | 0.013 |
| Cohesiveness | 0.32a | 0.26b | 0.26b | 0.26b | 0.26b | 0.26 | 0.27 | 0.006 |
| Gumminess (N) | 25.36a | 8.87b | 9.35b | 9.69b | 11.83b | 12.82b | 14.47b | 1.364 |
| Chewiness (N) | 20.69a | 5.56b | 6.52b | 6.58b | 8.11b | 8.34b | 9.82b | 1.210 |
A(+) Con was formulated with 2 % NaCl and 20 % pork backfat, and low fat and salt treatments were equally prepared with 1 % NaCl and 10 % pork backfat
BASP, 1 % alcohol-washed soy hull pectin; MSP, 1 % membrane-filtered soy hull pectin; PE, 10 % pre-emulsified fiber/oil (insoluble soy fiber:sunflower oil:water = 3:3.5:3.5)
CSEM standard error of the mean
a-c Means within a row with different letters are significantly different (P < 0.05)
Conclusion
While pectin content in soy hulls was affected by two different purification methods, the two types of soy hull pectin at the adding level of 1 % similarly affected chemical composition and technological properties of low fat and low salt meat emulsions. In addition, incorporation of soy hull pectin and pre-emulsified insoluble soy fiber/sunflower oil had greater impacts on reducing cooking loss and modifying textural properties than their single addition. Consequently, the formulation used in current study, which contained 1 % soy hull pectin and 10 % pre-emulsified fiber/oil, could provide the reductions in 43.9–44.3 % of sodium content and 20.7–20.9 % of caloric content, while minimizing the changes in technological properties compared to a typical formulation. Therefore, results suggested that membrane filtration could be a highly applicable way to purify pectin, instead of alcohol-washing, as an environment friendly method. Both pectin and insoluble fiber from soy hulls could be used as a functional non-meat ingredient to develop low fat and low salt meat products.
Acknowledgments
Thanks are given to Danika K. Miller for her help in the preparation and analysis of samples, and Traci Cramer for editorial inputs.
References
- AMSA . Meat color measurement guidelines. Champaign, IL, USA: American Meat Science Association; 2012. [Google Scholar]
- AOAC . Official methods of analysis of AOAC. Vol. 41. 17. Washington DC: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Bourne MC. Texture profile analysis. Food Technol. 1978;32:62–66. [Google Scholar]
- Candogan K, Kolsarici N. The effects of carrageenan and pectin on some quality characteristics of low-fat beef frankfurters. Meat Sci. 2003;64:199–206. doi: 10.1016/S0309-1740(02)00181-X. [DOI] [PubMed] [Google Scholar]
- Choi YS, Choi JH, Han DJ, Kim HY, Lee MA, Jeong JY, Chung HJ, Kim CJ. Effects of replacing pork back fat with vegetable oils and rice bran fiber on the quality of reduced-fat frankfurters. Meat Sci. 2010;84:557–563. doi: 10.1016/j.meatsci.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Colmenero FJ. Technologies for developing low-fat meat products. Trends Food Sci Technol. 1996;7:41–48. doi: 10.1016/0924-2244(96)81327-6. [DOI] [Google Scholar]
- Desmond E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006;74:188–196. doi: 10.1016/j.meatsci.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Rehm CD, Maillot M, Monsivais P. The relation of potassium and sodium intakes to diet cost among US adults. J Hum Hypertens. 2015;29:14–21. doi: 10.1038/jhh.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garna H, Mabon N, Robert C, Cornet C, Nott K, Legros H, Wathelet B, Paquot M. Effect of extraction conditions on the yield and purity of apple pomace pectin precipitated but no washed by alcohol. J Food Sci. 2007;72:C001–C009. doi: 10.1111/j.1750-3841.2006.00227.x. [DOI] [PubMed] [Google Scholar]
- Gerber N, Scheeder MRL, Wenk C. The influence of cooking and fat trimming on the actual nutrient intake from meat. Meat Sci. 2009;81:148–154. doi: 10.1016/j.meatsci.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Gnanasambandam R, Proctor A. Preparation of soy Hull pectin. Food Chem. 1999;65:461–467. doi: 10.1016/S0308-8146(98)00197-6. [DOI] [Google Scholar]
- Grasso S, Brunton NP, Lyng JG, Lalor F, Monahan FJ. Healthy processed meat products – regulatory, reformulation and consumer challenges. Trends Food Sci Technol. 2014;39:4–17. doi: 10.1016/j.tifs.2014.06.006. [DOI] [Google Scholar]
- Havlin JL, Soltanpour PN. A nitric acid plant tissue digest method for use with inductively couple plasma spectrometry. Commun Soil Sci Plant Anal. 1980;11:969–980. doi: 10.1080/00103628009367096. [DOI] [Google Scholar]
- Jiménez-Colmenero F, Carballo J, Cofrades S. Healthier meat and meat products: their role as functional foods. Meat Sci. 2001;59:5–13. doi: 10.1016/S0309-1740(01)00053-5. [DOI] [PubMed] [Google Scholar]
- Kalapathy U, Proctor A. Effect of acid extraction and alcohol precipitation conditions on the yield and purity of soy Hull pectin. Food Chem. 2001;73:393–396. doi: 10.1016/S0308-8146(00)00307-1. [DOI] [Google Scholar]
- Kim HW, Lee YJ, Kim YHB. Efficacy of pectin and insoluble fiber extracted from soy hulls as a functional non-meat ingredient. LWT Food Sci Technol. 2015;64:1071–1077. doi: 10.1016/j.lwt.2015.07.030. [DOI] [Google Scholar]
- Kumar V, Biswas AK, Sahoo J, Chatil MK, Sivakumar S. Quality and storability of chicken nuggets formulated with green banana and soybean hulls flours. J Food Sci Technol. 2013;50:1058–1068. doi: 10.1007/s13197-011-0442-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau WJ, Ismail AF, Misdan N, Kassim MA. A recent progress in thin film composite membrane: A review. Desalination. 2012;287:190–199. doi: 10.1016/j.desal.2011.04.004. [DOI] [Google Scholar]
- Mansour EH, Khalil AH. Characteristics of low-fat beefburger as influenced by various types of wheat fibers. Food Res Int. 1997;30:199–205. doi: 10.1016/S0963-9969(97)00043-4. [DOI] [Google Scholar]
- Mittal GS, Zhang J. Prediction of temperature and moisture content of frankfurters during thermal processing using neural network. Meat Sci. 2000;55:13–24. doi: 10.1016/S0309-1740(99)00120-5. [DOI] [PubMed] [Google Scholar]
- Monsoor MA. Effect of drying methods on the functional properties of soy Hull pectin. Carbohydr Polym. 2005;61:362–367. doi: 10.1016/j.carbpol.2005.06.009. [DOI] [Google Scholar]
- Ordóñez M, Rovira J, Jaime I. The relationship between the composition and texture of conventional and low-fat frankfurters. Int J Food Sci Technol. 2001;36:749–758. doi: 10.1046/j.1365-2621.2001.00525.x. [DOI] [Google Scholar]
- Pappa IC, Bloukas JG, Arvanitoyannis IS. Optimization of salt, olive oil and pectin level for low-fat frankfurters produced by replacing pork backfat with olive oil. Meat Sci. 2000;56:81–88. doi: 10.1016/S0309-1740(00)00024-3. [DOI] [PubMed] [Google Scholar]
- Perez V, Chang ET. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv Nutr. 2014;5:712–741. doi: 10.3945/an.114.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez R, Jiménez A, Fernández-Bolaños J, Guillén R, Heredia A. Dietary fibre from vegetable products as source of functional ingredients. Trends Food Sci Technol. 2006;17:3–15. doi: 10.1016/j.tifs.2005.10.002. [DOI] [Google Scholar]
- Sarıçoban C, Yılmaz MT, Karakaya M, Tiske SS. The effect of different levels of sunflower head pith addition on the properties of model system emulsions prepared from fresh and frozen beef. Meat Sci. 2010;84:186–195. doi: 10.1016/j.meatsci.2009.08.046. [DOI] [PubMed] [Google Scholar]
- Sofos JN. Effect of reduced salt (NaCl) levels on the stability of frankfurters. J Food Sci. 1983;48:1684–1691. doi: 10.1111/j.1365-2621.1983.tb05061.x. [DOI] [Google Scholar]
- Thakur BR, Singh RK, Handa AK, Rao MA. Chemistry and uses of pectin – a review. Crit Rev Food Sci Nutr. 1997;37:47–73. doi: 10.1080/10408399709527767. [DOI] [PubMed] [Google Scholar]
- Troy DJ, Desmond EM, Buckley DJ. Eating quality of low-fat beef burgers containing fat-replacing functional blends. J Sci Food Agric. 1999;79:507–516. doi: 10.1002/(SICI)1097-0010(19990315)79:4<507::AID-JSFA209>3.0.CO;2-6. [DOI] [Google Scholar]
- Vural H, Javidipour I, Ozbas OO. Effects of interesterified vegetable oils and sugarbeet fiber on the quality of frankfurters. Meat Sci. 2004;67:65–72. doi: 10.1016/j.meatsci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen F, Yang H, Sun X, Liu H, Gong X, Jiang C, Ding C. Changes in firmness, pectin content and nanostructure of two crisp peach cultivars after storage. LWT Food Sci Technol. 2010;43:26–32. doi: 10.1016/j.lwt.2009.06.015. [DOI] [Google Scholar]