Abstract
Quercetagetin, the major flavonoid in marigold (Tagetes erecta L.) inflorescence residues was extracted and purified. The content of quercetagetin after the purification was 89.91 ± 0.26 %. The in vitro antioxidant activity of quercetagetin and its potential in controlling diabetes mellitus and obesity were investigated and compared to quercetin and rutin. The 50 % inhibitory concentration (IC50) values of quercetagetin on scavenging 1, 1-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azinobis-(3-ethylbenzothiazolin-6-sulfonic acid) (ABTS) and hydroxyl radicals were 27.12 ± 1.31 μmol/L, 12.16 ± 0.56 μmol/L and 1833.97 ± 6.66 μmol/L, respectively. The IC50 values of quercetagetin on α-glucosidase, α-amylase and pancreatic lipase were 180.11 ± 3.68 μmol/L, 137.71 ± 3.55 μmol/L and 2327.58 ± 12.37 μmol/L, respectively. These results indicated that quercetagetin exhibited strong in vitro antioxidant, anti-diabetic and antilipemic activities. Lineweaver-Burk plots analysis elucidated that quercetagetin inhibited α-glucosidase and α-amylase non-competitively, while its inhibition against pancreatic lipase was involved in a mixed-type pattern. Moreover, strong correlations were found between ABTS·+/DPPH· scavenging activities and lipase inhibitory activity (R2 > 0.90), as well as ·OH scavenging activity and α-amylase inhibitory activity (R2 = 0.8967).
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2228-6) contains supplementary material, which is available to authorized users.
Keywords: Marigold (Tagetes erecta L.) inflorescence residues, Quercetagetin, Diabetes mellitus, Pancreatic lipase, Enzyme inhibition
Introduction
The number of people suffering from metabolic syndrome such as diabetes mellitus and obesity is growing rapidly worldwide. According to the International Diabetes Federation, more than 415 million people worldwide suffer from diabetes in 2015 and the incidence is expected to be 642 million by 2040 (IDF 2015). Globally, it is estimated that 1.9 billion adults are overweight and 600 million are obese by 2014 (Hosseinpanah et al. 2016). Meanwhile, metabolic syndrome can cause serious damage to body systems, such as blood vessels and nerves (Matsui et al. 2007; Cohen and Goedert 2004). One of the major causes of the metabolic syndrome is excessive consumption of carbohydrates and triglycerides. Triglycerides can cause abnormal accumulation of body fat since it has the highest energy density (9 kcal/g) (Zielinska-Przyjemska et al. 2009). One therapeutic approaches is to retard carbohydrates and lipids absorption by inhibiting the activity of the key digestive enzymes in the digestive system. α-Glucosidase, α-amylase and pancreatic lipase are the key enzymes in the digestive system, catalyzing the hydrolysis of carbohydrates and triglycerides to easily digestible molecules (Shobana et al. 2009). Some synthetic inhibitors of key digestive enzymes (acarbose, miglitol, orlistat etc.) have been successfully applied for the treatment of diabetes mellitus and obesity in clinics. However, their side effects, such as flatulence, abdominal distention and liver toxicity have also been reported (Shobana et al. 2009). Hence, some studies have focused on developing natural inhibitors of these enzymes from plants (Dong et al. 2012; Marrelli et al. 2013; You et al. 2012). Recent findings indicate that inhibitory properties of the enzymes activity, in particular, correspond to the flavonoid compounds present in plants, such as quercetin and its glycoside derivatives, ellagic acid, chlorogenic acid, kaempferol, myricitrin and rutin (Marrelli et al. 2013; Wang et al. 2010; You et al. 2012). In addition, oxidative stress is well known to play an important role in the pathogenesis of diabetes and obesity (Chen and Yen 2007). Therefore, screening natural compounds possessing antioxidant activity combined with inhibitory activities against α-glucosidase, α-amylase and pancreatic lipase should be a wise option for the treatment of diabetes mellitus and obesity (Yuan et al. 2013).
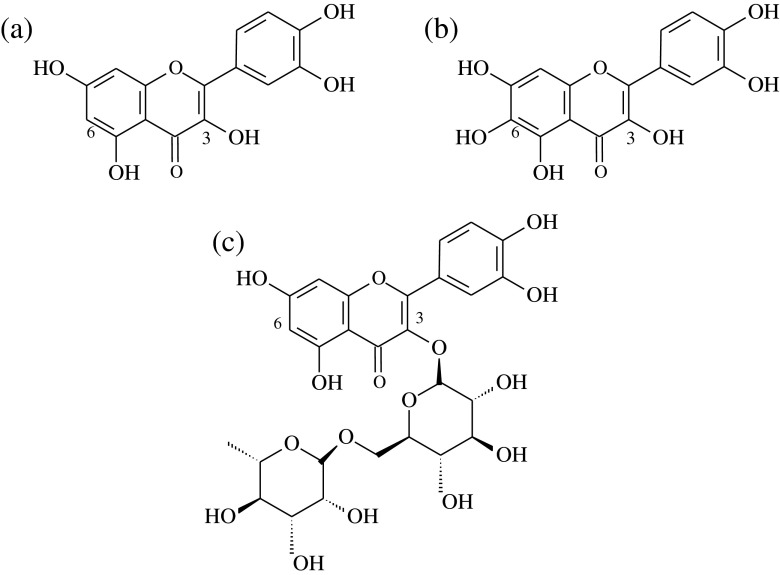
Marigold (Tagetes erecta L.) inflorescence, known as a common ornamental plant and a traditional Chinese medicine, is available in many parts of the world (Gong et al. 2012b). In China, a large amount of marigold is cultivated for the purpose of lutein extraction. However, the residues after extracting lutein with hexane are usually discarded or just used as a fertilizer. There are considerable bioactive substances such as polyphenols and flavonoids in the residues (Parejo et al. 2004). Quercetagetin, the major flavonoid component in the extract from marigold (Tagetes erecta L.) inflorescence residues, has a characteristic flavonol compound that has an additional C6-OH group based on the molecular structure of quercetin (Fig. 1). There are many reports focused on its antioxidant activity (Gong et al. 2012a, b; Xu et al. 2014), anti-inflammatory activity (Kang et al. 2013) and copigment effect (Xu et al. 2015). Some scientific researches reported that quercetin has strong inhibition of α-glucosidase, α-amylase and lipase (Dong et al. 2012; You et al. 2012). However, to the best of our knowledge, there were no investigation into α-glucosidase, α-amylase and pancreatic lipase inhibitory activities of quercetagetin by far. Therefore, the aims of this study were to (1) compare and evaluate the in vitro anti-diabetic and antilipemic activities between quercetagetin and some other natural flavonoids including quercetin and rutin; (2) explore the inhibitive mode of quercetagetin on α-glucosidase, α-amylase and pancreatic lipase; and (3) interpret the intermolecular interactions between quercetagetin and these three enzymes by fluorescence spectroscopy. These findings from present study are expected to provide the potential application of quercetagetin as an anti-diabetic and antilipemic nutraceutical.
Fig. 1.
Chemical structures of quercetin (a), quercetagetin (b) and rutin (c)
Materials and methods
Materials
The defatted marigold (Tagetes erecta L.) inflorescence residues were provided by Saite Natural Pigments Co. Ltd. (Qingdao, China). Marigold (Tagetes erecta L.) inflorescence was authenticated at Department of Analysis for Chinese Materia Medica, China Pharmaceutical University, where a voucher specimen was deposited (Voucher number 20639). 2,2-Azinobis-(3-ethylbenzothiazolin-6-sulfonic acid) (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), α-glucosidase (from Saccharomyces cerevisiae), α-amylase (from porcine pancreatin), lipase (from porcine pancreas), p-nitrophenyl-α-D-glucopyranoside (PNPG), orlistat, 4-nitrophenyl octanoate (4-NPC), and the standard of quercetagetin (98 %), quercetin (99 %) and rutin (95 %) were purchased from Sigma-Aldrich Co. LLC. (Shanghai, China). Acarbose was supplied by Bayer Korea (Seoul, South Korea). Other reagents (analytical grade) were purchased from Beijing Chemical Co. (Beijing, China). All standards including quercetagetin, quercetin, rutin, acarbose and orlistat were prepared as stock solutions in following assays.
Extraction and purification of quercetagetin
The marigold inflorescence residues (1 kg) were first defatted by Soxhlet method with n-hexane at 80 °C for 2 h. The defatted marigold inflorescence residues were extracted with ethanol aqueous solution (70 %, v/v) for 1.5 h. At the end of extraction, the crude extract was filtered with a quantifying filtration paper, and the liquid was concentrated with a vacuum rotary evaporator (Model R203B, Shanghai Senco Technology Co. Ltd., Shanghai, China) at 40 °C. The concentrated extract (219.3 g) was recovered and then dispersed in distilled water (extract/water mass ratio 1:10) and stirred for 30 min. The suspension was then centrifuged (3155×g, 20 min, ambient temperature), separated and rewashed with distilled water. The triple washed extract was freeze dried to yield approximately 43.4 g yellow powder and stored at 4 °C. The purified marigold residues extract (PMRE) was dissolved with 70 % ethanol aqueous solution before used.
HPLC analysis
The PMRE (0.75 mg/mL in 70 % ethanol aqueous solution) filtered by Millipore filter (0.45 μm) was injected into an Agilent 1100 series analytical HPLC system (20 μL), equipped with an Agilent Zorbax SB C18 column (150 × 4.6 mm i.d., 5 μm). The elution was performed at a flow rate of 0.7 mL/min using a mixture of methanol (solvent A) and methanol/water/formic acid (20:80:0.25, v/v/v; solvent B) as a mobile phase. Gradient elution program was as follows: solvent A increased in 5 min from 0 to 10 %, in 20 min from 10 to 20 %, in 40 min from 20 to 40 %, in 50 min from 40 to 80 % and returned to 0 in the next 5 min, and solvent B reached to 100 % at the same time. All programs were operated at 30 °C. The detection wavelength of the DAD detector was set at 360 nm. Quercetagetin was identified by direct comparison of its retention time with that of the authentic compound under identical condition and by spiking the samples with the standard, and further calculated from the calibration curve analyzed under the same HPLC condition.
Identification of the major compounds in PMRE
In order to identify the major compounds in PMRE, a mass detector fitted with an electrospray ionization (ESI) source was used (Agilent 6500 Series LC/MSD Trap). The HPLC separation program of the major compounds in PMRE was the same as described previously. Mass spectrometry was operated in negative scan mode. The electrospray capillary voltage was set at 4000 V. Nitrogen was used as a nebulizing gas at a pressure of 30 psi with a flow rate of 10.0 L/min and drying gas temperature of 300 °C. Scan range of the mass spectrometry was m/z 100–900.
Antioxidant capacity assays
DPPH radical-scavenging assay
The DPPH radical scavenging activity assay was performed according to the method described by Gong et al. (2012a) with minor modification. The sample or positive controls (quercetin and rutin) (2 mL) was mixed with 2 mL of freshly prepared DPPH (0.175 mM in methanol) and the mixture was vibrated for 20 s at room temperature. A control, in which the sample was replaced by methanol, was measured in the same way. The decrease in absorbance of the mixture at 517 nm was recorded by UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) after reacting in the dark for 1 h.
ABTS radical-scavenging assay
The ABTS radical scavenging activity assay was performed according to the method described by Gong et al. (2012a). The ABTS•+ was prepared by adding 7 mM ABTS stock solution into 2.45 mM potassium persulfate and keep the mixture for 16 h at room temperature in the dark. Before used, ABTS•+ solution was diluted with 70 % methanol aqueous solution to an absorbance of 0.700 ± 0.020 at 734 nm. The sample (1 mL) was mixed with 3 mL of ABTS•+ solution and shaken thoroughly. The reactive mixture was allowed to stand for 1 h in the dark at room temperature and the absorbance was recorded at 734 nm using UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
Scavenging activity of hydroxyl radicals (·OH) assay
The ·OH scavenging activity assay was performed according to the method described by Kaur et al. (2008) with some modifications. The reaction mixture contained 150 μL of 30 mM 2-deoxy-2-ribose (prepared in 45 mM sodium phosphate buffer, pH 8.0), 450 μL solution of various concentrations of the sample, 200 μL of 10 mM FeSO4-EDTA and 100 μL H2O2 (1 mM). After incubation at 37 °C for 2 h, the reaction was stopped by adding 3 mL of thiobarbituric acid (0.5 %, w/v). The absorbance was recorded at 536 nm using UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) against the blank solution.
α-Glucosidase and α-amylase inhibitory activities analysis
The α-glucosidase (from Saccharomyces cerevisiae) inhibitory activity was determined following the method of Boath et al. (2012). The quercetagetin and controls (rutin, quercetin and acarbose) were diluted in 70 % aqueous ethanol solution to a range of concentrations. Briefly, 80 μL of the sample and 50 μL of 0.1 M sodium phosphate buffer (pH 6.8) containing α-glucosidase (0.4 U/mL) were incubated for 15 min at room temperature. After incubation, 100 μL of PNPG solution (4 mM) was added and incubated at 37 °C for another 15 min. The blank was prepared by adding distilled water instead of α-glucosidase. Then the reaction was stopped by adding 600 μL of 0.1 M Na2CO3 and the absorbance was recorded at 405 nm using UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
The α-amylase (from porcine pancreatin) inhibitory activity was determined following the method described by Kim et al. (2005) with some modifications. Briefly, 80 μL of the sample was premixed with 150 μL of the amylase (0.5 U/mL) for 10 min at room temperature and then followed by the addition of 200 μL of 0.5 % potato soluble starch solution and incubated at 37 °C for 5 min to start the reaction. Finally, 1.0 mL of 3,5-dinitrosalicylic acid solution (1 %, w/v) was added and the mixture was incubated in boiling water for 5 min then cooled down to room temperature. The total volume was made up to 9.0 mL with distilled water. The blank was prepared by adding distilled water instead of the α-amylase. The absorbance was measured at 540 nm using UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
The percentage of inhibition of the sample was calculated as follows:
| 1 |
Where A = absorbance
The control was prepared without the sample by replacing the sample volume with buffer solution. The IC50 value was defined as the concentration of the inhibitor required for inhibiting 50 % of the enzymatic activity.
Pancreatic lipase inhibitory activity analysis
The lipase inhibitory activity of quercetagetin was performed using a method of Conforti et al. (2012) with minor modification. Orlistat was used as a positive control. The extract, orlistat, quercetinand rutin were all dissolved in ethanol aqueous solution (70 %, v/v). The total mixture was composed of 100 μL 4-NPC (5 mM), 4 mL Tris–HCl buffer (50 mM, pH 7.4) and 100 μL of the sample was incubated at 37 °C for 25 min. Then, 100 μL of pancreatic lipase solution (5 U/mL) was added to initiate the reaction. Solution without sample was used as the control. Solution without pancreatic lipase was used as blank. The absorbance was measured at 410 nm with UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan).
The inhibition mode of α-glucosidase, α-amylase and pancreatic lipase
For kinetic analyses, the Vmax and Km constants were estimated by using the Lineweavere-Burk plots from the Michaelis-Menten equations. Km represents the Michaelis constant. Vmax is the maximum rate of the enzymatic reaction. The substrate solutions with the concentrations of 0.10, 0.25, 0.50, 0.167 and 1.00 mM were used for the α-glucosidase and α-amylase inhibition mode assays. The substrate solutions at concentrations of 0.05, 0.08, 0.10, 0.50 and 1.00 mM were used for the pancreatic lipase inhibition mode assay.
Fluorescence spectroscopy
Fluorescence measurements were carried out using a fluorescence spectrophotometer (F-7000, Hitachi, Japan). PMRE solution with various concentrations was added into α-glucosidase (0.4 U/mL), α-amylase (0.5 U/mL) and lipase (5 U/mL) solutions, respectively. Spectra were collected when the mixture was kept for 5 min at room temperature. The interaction between quercetagetin and different enzymes were investigated using tryptophan fluorescence quenching. Fluorescence quenching spectra were recorded in the range around 300–450 nm and the excitation wavelength of 280 nm was chosen. Both excitation and emission slit widths were set at 5 nm.
The fluorescence quenching data were analyzed by fitting to the Stern − Volmer equation (Li et al. 2009a):
| 2 |
The binding constant Ka and the number of binding sites can be calculated according to a double-logarithmic equation:
| 3 |
Where F0 and F are the fluorescence intensities in the absence and presence of a quencher, kq is the biomolecular quenching constant, τ0 is the lifetime of fluorescence in the absence of a quencher, Ksv is the Stern-Volmer quenching constant, [Q] is the concentration of the quencher, Ka is the binding constant and n is the number of binding sites (Soares et al. 2007).
Statistical analysis
All experiments were performed at least in triplicate. The values were expressed as the mean ± SD. The results were statistically analyzed by ANOVA and Fisher’s least significant difference (LSD) with SPSS 18.0 software. The IC50 values were calculated from linear regression analysis. Statistical differences were accepted at a level of p < 0.05.
Results and discussion
Identification of the compounds
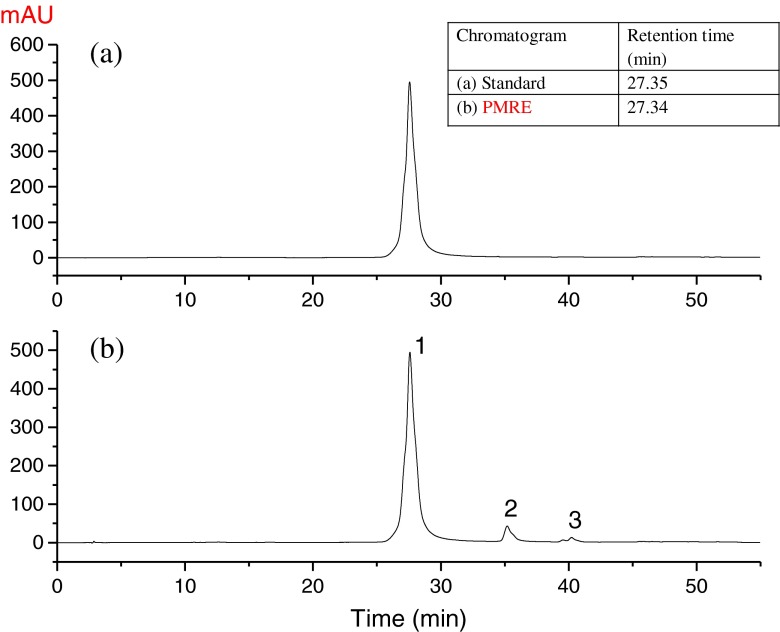
The content of quercetagetin in the crude marigold residues extract was 62.8 %, and more than 8 antioxidant compounds were detected in our preciously studies (Gong et al. 2012b; Xu et al. 2014). In present study, we focused on recovering the major bioactive compound, quercetagetin in the ethanolic extract. The ethanolic extract (219.3 g), extracted from 1 kg residuce, was first washed with distilled water three times and then freeze dried to yield 43.4 g yellow powder finally. SI. 1 (see supplementary information) shows the method of the extraction and purification, the yield and the quantification of quercetagetin throughout the process. To get high purity quercetagetin, the water-washing procedure was adopted in this study and the content of quercetagetin was significantly (p < 0.05) improved to 89.91 ± 0.26 % and the yield of quercetagetin was 4.34 ± 0.05 % (see supplementary information, SI. 1 and SI. 2). The HPLC profile of PMRE is presented in Fig. 2.
Fig. 2.
HPLC Chromatogram of (a) standard (0.75 mg/mL) and (b) PMRE (0.75 mg/mL). (Separated on a Zorbax SB C18 column with a detection wavelength of 360 nm)
HPLC-DAD-ESI-MS/MS analysis was conducted to identify the main compounds in the extract. Based on the DAD and MS/MS spectra, three major compounds were tentatively identified and spectral data are shown in Table 1. The HPLC–ESI–MS and HPLC–ESI–MS/MS of the main compounds in PMRE are shown in SI. 3 (see supplementary information).
Table 1.
Mass spectral characteristics and identification of compounds in marigold residue extracts
a Tentatively identified compound
b Tentatively identified as 6-Hydroxykaempferol-3-O-hexoside
c Tentatively identified as O-coumaroylhexoside or O-rhamnosylhexoside derivative
Peak 1 exhibited UV maxima adsorption at 260 and 360 nm together with fragment ions at m/z 317, and thus compound 1 was tentatively identified as quercetagetin by comparison with the published data (Parejo et al. 2004; Abad-García et al. 2009). The MS ions of peak 2 were observed at m/z 625 and m/z 301, the MS/MS data showed a loss of m/z 324 from the deprotonated molecular ion [M-H]− of m/z 625 to the deprotonated aglycon ion [Y0-H]− of m/z 301. Those signals suggested that the aglycon ion was probable 6-hydroxykaempferol (Parejo et al. 2004). By analyzing the MS/MS data of the ion at m/z 324, it indicated the presence of a dihexose, which most probably attached to the 3-position of the molecule. The MS ions of peak 3 was observed at m/z 639, and its MS/MS produced ion was at m/z 331, which was in accord with the literature data of patuletin (Parejo et al. 2004). The results of the MS/MS experiments in neutral loss scan mode of 308 mass units from m/z 639 to m/z 331 suggested the loss of a coumaroylhexose or rhamnosylhexose group. In a word, compound 3 was tentatively identified as patuletin-O-hexoside.
Antioxidant capacity of quercetagetin
In vitro antioxidant potentials of plant extracts were generally evaluated using DPPH•, ABTS•+ and · OH scavenging assays (Danino et al. 2009). Table 2 summarizes the IC50 (μmol/L) values of quercetagetin with antioxidant activity assays using quercetin and rutin as positive controls. DPPH radical scavenging activity of quercetagetin (IC50 = 27.12 μmol/L) was a little lower than that of quercetin (IC50 = 27.85 μmol/L), but obviously higher than that of rutin (IC50 = 17.82 μmol/L). A same sequence of IC50 values were found by ABTS radical scavenging assay. With · OH scavenging assay, rutin displayed the highest IC50 value of 870.15 ± 3.04 μmol/L, followed by quercetin, 1662.29 ± 5.09 μmol/L and finally quercetagetin, 1833.97 ± 6.66 μmol/L (Table 2).
Table 2.
IC50 values of quercetagetin and controls
| IC50 value (μmol/L) | |||||
|---|---|---|---|---|---|
| Quercetin | Rutin | Quercetagetin | Acarbose | Orlistat | |
| ABTS•+ | 12.38 ± 0.50 | 7.60 ± 0.37 | 12.16 ± 0.56 | ND | ND |
| DPPH• | 27.85 ± 1.13 | 17.82 ± 0.84 | 27.12 ± 1.31 | ND | ND |
| ·OH | 1662.29 ± 5.09 | 870.15 ± 3.04 | 1833.97 ± 6.66 | ND | ND |
| Pancreatic lipase | 2950.23 ± 7.93 | 2557.37 ± 8.81 | 2327.58 ± 12.37 | ND | 1.37 ± 0.15 |
| α-Amylase | 71.49 ± 2.88 | 43.29 ± 1.73 | 137.71 ± 3.55 | 5.80 ± 0.34 | ND |
| α-Glucosidase | 163.44 ± 3.44 | 99.13 ± 1.99 | 180.11 ± 3.68 | 810.85 ± 5.96 | ND |
| α-Glucosidasea | 50.33b | 96.10c | – | 601.76d | – |
Quercetagetin, quercetin and rutin have typical flavonoid structure (Fig. 1). However, rutin had stronger ABTS and DPPH radicals scavenging activities. This could be explained by the structural difference that quercetagetin and quercetin contain hydroxyl group at C3 of C-ring, while rutin is derived from the quercetin with the replacement of C3-OH by the rutinoside. Based on the structure-activity relationship of flavonoids, Li et al. (2009b) revealed that the glycosylation of C3-OH had no effect on antioxidant activity. However, Afanas’ev et al. (1989) reported that the antioxidant activity of rutin was much weaker than that of quercetin. This suggested that C3-OH was an important determinant of the scavenging effect.
Inhibitory activities and kinetic analysis of α-glucosidase and α-amylase
α-Glucosidase and α-amylase, two important carbohydrate-hydrolyzing enzymes, are responsible for carbohydrate digestion and glucose absorption in alimentary tract, and have been identified to be the therapeutic targets for controlling diabetes (Kim et al. 2005). Acarbose is a drug commonly applied in the management of diabetes mellitus (Dong et al. 2012). As shown in Table 2, quercetagetin exhibited good α-glucosidase and α-amylase inhibitory activities. No significant difference (p > 0.05) of the IC50 values were found between quercetagetin (180.11 ± 3.68 μmol/L) and quercetin (163.44 ± 3.44 μmol/L) for α-glucosidase inhibitory activity, but they were greatly higher than that of rutin (99.13 ± 1.99 μmol/L) and significantly (p < 0.05) lower than that of acarbose (810.85 ± 5.96 μmol/L). The IC50 value of acarbose (positive control) for α-glucosidase inhibitor was observed to be much higher, which was similar to the result from Youn et al. (2004). In this study, quercetin and rutin were found to be about 5 and 8 times more effective than acarbose in inhibiting α-glucosidase inhibitory activity, respectively. This was further substantiated when compared the effectiveness of quercetin and rutin with published data of acarbose (Table 2). This was expected that acarbose showed to be a potent inhibitor of mammalian sucrase and maltase and inactive against yeast enzyme.
On the basis of the IC50 values in Table 2, it was found that the sequence of inhibitory activities of these flavonoids against α-amylase are as follows: acarbose > rutin > quercetin > quercetagetin. These results demonstrated that rutin exhibited a stronger inhibitory activity against α-glucosidase and α-amylase compared with quercetin and quercetagetin. Quercetagetin had lower α-glucosidase and α-amylase inhibitory activities than quercetin. Their structural difference is that quercetagetin has an additional C6-OH group based on the molecule of quercetin. Edenharder et al. (1997) reported that the enzyme activity could be inhibited by the flavanone and its structure, hydroxyl replaced on C6 of flavanone had a significant influence on enzyme inhibition activities. We speculated that C6-OH group weakened the inhibitory activities of quercetagetin to α-glucosidase and α-amylase.
As the main driving force of the interaction between quercetagetin, quercetin, rutin and α-glucosidase was hydrophobic, with the replacement of C3-OH by rutinoside, quercetin turns into rutin, and its polarity become larger than that of quercetin, and the inhibitory ability of rutin was stronger than that of quercetin. This suggested that the effect of steric hindrance was far less influential than that of the substitution by glycosyl unit, and also showed that the mechanism of interaction between molecules may be very complex (Li et al. 2009b).
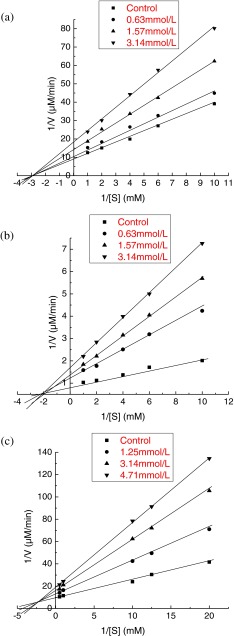
The inhibition property of quercetagetin was analyzed by using Lineweaver-Burk plots. Figure 3a shows the plot of α-glucosidase inhibitory activity versus different substrate concentrations of PNPG in the presence of quercetagetin at 0.63, 1.57 and 3.14 mmol/L and absence of the inhibitor. The linear regression equations of quercetagetin at 0.63, 1.57 and 3.14 mmol/L were y = 3.309x + 12.339 (R2 = 0.9928), y = 4.7426x + 14.644 (R2 = 0.9976) and y = 6.3027x + 18.207 (R2 = 0.9945), respectively. With the increase of the inhibitor concentration, the vertical axis intercept (1/V) was increased, whereas the horizontal axis intercept (1/[S]) remained the same value. These results suggested that the rates of α-glucosidase catalyzed reactions were slowed down with the rise of quercetagetin concentration, and the Km value was not affected. Interestingly, trilobatin extracted from Lithocarpus polystachyus Rehd also exhibited a non-competitive inhibition of α-glucosidase inhibition and the Km value of trilobatin was 0.25 mM (Dong et al. 2012).
Fig. 3.
The Lineweavere-Burk plots analysis of α-glucosidase (a), α-amylase (b) and pancreatic lipase (c) inhibitory effects by quercetagetin
A similar trend was found for α-amylase inhibitory activity. As shown in Lineweavere-Burk plots (Fig. 3b), lines y = 5.2388x + 1.5446 (R2 = 0.9892), y = 7.5429x + 1.9664 (R2 = 0.9912) and y = 9.2841x + 2.2345 (R2 = 0.9995) represent the quercetagetin with concentration of 1.25, 3.14 and 4.71 mmol/L, respectively. Obviously, all the lines crossed at the same point in the horizontal axis. Therefore, quercetagetin exhibited a non-competitive inhibition mechanism of α-amylase like α-glucosidase.
The kinetic inhibition parameters on α-glucosidase, α-amylase and pancreatic lipase were calculated according to Lineweaver-Burk plots, and the results were listed in Table 3. In the presence of quercetagetin, Vmax values for α-glucosidase and α-amylase were decreased with the increase of quercetagetin concentration, while the values of Km remained unchanged. The results of the Km and IC50 values for the α-glucosidase and α-amylase inhibitory activities indicated that quercetagetin was comparable to other reported natural strong inhibitors like quercetin, catechin and trilobatin against α-glucosidase and α-amylase (Dong et al. 2012; You et al. 2012).
Table 3.
Kinetic inhibition parameters on α-glucosidase, α-amylase and pancreatic lipase
| α-Glucosidase | α-Amylase | Pancreatic lipase | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quercetagetin (mmol/L) | 0 | 0.63 | 1.57 | 3.14 | 0 | 0.63 | 1.57 | 3.14 | 0 | 1.25 | 3.14 | 4.71 |
| K m (mM) | 0.34 | 0.33 | 0.33 | 0.33 | 2.62 | 2.61 | 2.61 | 2.62 | 0.17 | 0.22 | 0.28 | 0.31 |
| V max (μM/min) | 0.111 | 0.081 | 0.068 | 0.054 | 1.136 | 0.647 | 0.509 | 0.447 | 0.105 | 0.076 | 0.062 | 0.053 |
| Inhibition type | non-competitive | non-competitive | mixed-type | |||||||||
Inhibitory activity and kinetic analysis of pancreatic lipase
Pancreatic lipase plays a key role in triglycerides metabolism (Shobana et al. 2009). Inhibition of pancreatic lipase is a valuable method for the treatment of diet-induced hyperglycemia (Kim et al. 2011). Many flavonoids extracted from plants were previously reported for outstanding pancreatic lipase inhibitory activity (Conforti et al. 2012; You et al. 2012).
The inhibitory abilities of quercetagetin, quercetin, rutin and orlistat against pancreatic lipase were investigated and the results were shown in Table 2. Orlistat exhibited the highest inhibitory activity against the enzyme (IC50 = 1.37 ± 0.15 μmol/L), followed by quercetagetin (IC50 = 2327.58 ± 12.37 μmol/L), rutin (IC50 = 2557.37 ± 8.81 μmol/L) and finally quercetin (IC50 = 2950.23 ± 7.93 μmol/L). The inhibitory activities of quercetagetin, rutin and quercetin against pancreatic lipase were significantly (p < 0.05) lower than that of orlistat.
According to Fig. 3c, the lines intersected with each other in the beta quadrant, which indicated that the pancreatic lipase inhibition by quercetagetin was mixed-type. The Km value was increased from 0.17 mM in the absence of inhibitor to 0.22, 0.28, and 0.31 mM in the presence of 1.25, 3.14 and 4.71 mmol/L of quercetagetin, respectively (Table 3). However, the Vmax without the inhibitor was 0.105 μM and decreased when quercetagetin was added. Thus, kinetic analysis indicated that quercetagetin was a mixed-type inhibitor of pancreatic lipase. This result showed that quercetagetin might bind, not only with free enzyme, but also with the enzyme-substrate complex.
Quenching of α-glucosidase, α-amylase and pancreatic lipase fluorescence spectra
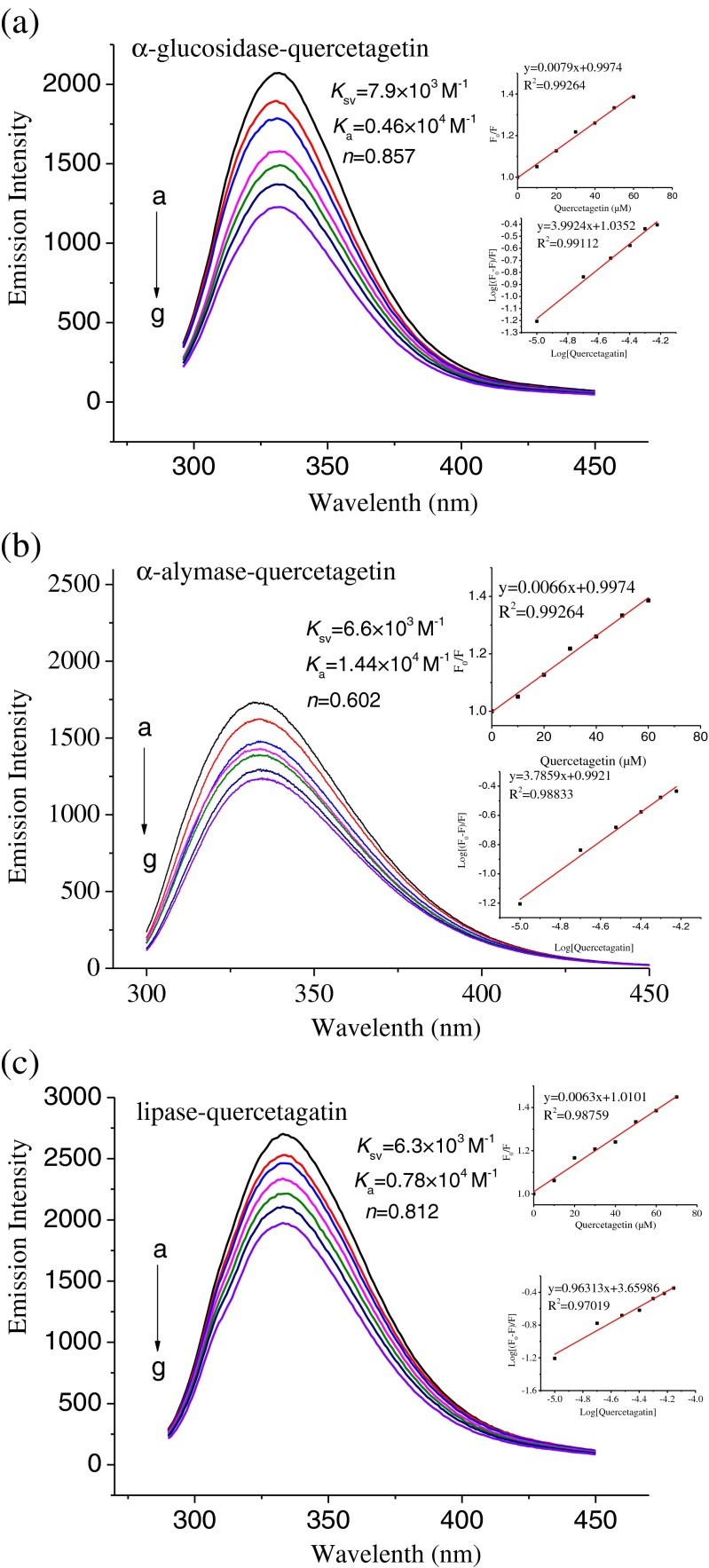
Fluorescence spectroscopy, as a highly sensitive method for protein/enzyme tertiary structural characterization, is the most commonly used technique to investigate the structural change in protein/enzyme upon the association with flavonoids (Soares et al. 2007). Moreover, it is a useful approach to investigate intermolecular interactions. The tryptophan (Trp) residues are often found fully or partially buried in the hydrophobic core of protein/enzyme interiors. There are a number of Trp residues in the α-glucosidase, α-amylase and pancreatic lipase molecules (Fei et al. 2014; Gonçalves et al. 2010). By monitoring the emission peak change, some information might be obtained concerning the structural change and the microenvironment surrounding the fluorophore in these enzymes. The fluorescence intensities of α-glucosidase, α-amylase and pancreatic lipase were regularly decreased with the increase of quercetagetin concentration, and this implied that there existed a binding behavior between the three enzymes and quercetagetin (Fig. 4). The fluorescence quenching parameters are shown in Fig. 4 (inset) according to Eq. (3). Most of the Ka values were in the range of 104 M−1. In the linear range of the Stern-Volmer regression curve, the average quenching constants (Ksv) of α-glucosidase, α-amylase and pancreatic lipase were estimated as 7.9 × 103 M−1, 6.6 × 103 M−1, and 6.3 × 103 M−1. It is well known that τ0 of Trp depends on pH and buffer composition. In buffer solution at pH < 7.0 (pH 6.5, 20 mM phosphate buffer was used in this assay), τ0 of Trp was 2.16 ± 0.11 ns (Gudgin et al. 1981). The number of binding sites of quercetagetin with three enzymes was ranked as α-glucosidase (0.857) > lipase (0.812) > α-amylase (0.602). Based on the aforementioned result, we concluded that the affinity of quercetagetin toward the enzymes was dependent on the types of enzymes.
Fig. 4.
Fluorescence emission spectra of α-glucosidase-quercetagetin complexes (a), α-amylase-quercetagetin complexes (b) and lipase-quercetagetin complexes (c) at different concentrations of quercetagetin. Insets: Plot of F 0/F versus [quercetagetin] as per Eq. (2) (top); log[(F 0−F)/F] versus log [quercetagetin] as per Eq. (3) (bottom). (a-g) the quercetagetin concentration from 0 to 60 μM.
The lowest Ksv value estimated in present study was 6.3 × 103 M−1, yielding kq above 1.99 × 1012 M−1 s−1, at least 2 orders of magnitude higher than 2.0 × 1010 M−1 s−1, the maximum dynamic collisional quenching constant for various quenchers interacting with biopolymers (Gonçalves et al. 2010). These results thus implied that the quenching process was static quenching involving a formation of quercetagetin-enzyme complex.
Correlation between the antioxidant activities and enzyme inhibitory activities
To gain a better understanding of the relationship between the antioxidant activities and the enzyme inhibitory activities of quercetin, quercetagetin and rutin, the correlation analysis was performed (Table 4). The ABTS·+ and DPPH· values had a moderate correlation with α-glucosidase inhibitory activity (R2 > 0.60), a poor correlation with α-amylase inhibitory activity (R2 < 0.060) and a strong correlation with lipase inhibitory activity (R2 > 0.90). A strong correlation was also found between the levels of ABTS·+/DPPH· values and lipase inhibitory activities of natural compounds (Chigorimbo-Murefu et al. 2009).
Table 4.
Relationship between the antioxidant activity and inhibitory activity against α-glucosidase, α-amylase and pancreatic lipase
| ABTS | DPPH | ·OH | α-Glucosidase | α-Amylase | Pancreatic lipase | |
|---|---|---|---|---|---|---|
| ABTS•+ | 1 | 0.9813 | 0.1042 | 0.6529 | 0.0324 | 0.9837 |
| DPPH• | 1 | 0.1580 | 0.6012 | 0.0540 | 0.9674 | |
| ·OH | 1 | 0.4705 | 0.8967 | 0.1140 |
With regards to the relationship between the · OH scavenging activity and the enzyme inhibitory activities, different results were obtained. The · OH scavenging activity had a moderate correlation with α-glucosidase inhibitory activity (R2 = 0.4705), a strong correlation with α-amylase inhibitory activity (R2 = 0.8967), and a poor correlation with pancreatic lipase inhibitory activity (R2 = 0.1140). These findings therefore revealed that the flavonoid with higher · OH scavenging activity exhibited higher α-glucosidase and α-amylase inhibitory activities, which was in agreement with the result reported by Farsi et al. (2011).
Conclusions
The present study investigated the potential anti-diabetic and antilipemic activities of quercetagetin extracted from marigold (Tagetes erecta L.) inflorescence residues, focusing on the inhibitory effects of α-glucosidase, α-amylase and pancreatic lipase. Quercetagetin exhibited strong inhibitory activity against α-glucosidase and pancreatic lipase, as well as a moderate inhibitory effect against α-amylase. In addition, the results demonstrated that quercetagetin possessed the great antioxidant capacity. In conclusion, the results from this study offered essential scientific support to the application of quercetagetin as nutraceutical for the treatment of diabetes and obesity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 1028 kb)
Acknowledgments
The research was funded by the National Natural Science Foundation of China under Grant No.31371835.
References
- Abad-García B, Berrueta LA, Garmón-Lobato S, Gallo B, Vicente F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J Chromatogr A. 2009;1216:5398–5415. doi: 10.1016/j.chroma.2009.05.039. [DOI] [PubMed] [Google Scholar]
- Afanas’ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- Arumugam B, Manaharan T, Heng CK, Kuppusamy UR, Palanisamy UD. Antioxidant and antiglycemic potentials of a standardized extract of Syzygium malaccense. LWT-Food Sci Technol. 2014;59:707–712. doi: 10.1016/j.lwt.2014.06.041. [DOI] [Google Scholar]
- Boath AS, Stewart D, McDougall GJ. Berry components inhibit α-glucosidase in vitro: synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012;135:929–936. doi: 10.1016/j.foodchem.2012.06.065. [DOI] [PubMed] [Google Scholar]
- Chen HY, Yen GC. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chem. 2007;101:686–694. doi: 10.1016/j.foodchem.2006.02.047. [DOI] [Google Scholar]
- Chigorimbo-Murefu NT, Riva S, Burton SG. Lipase-catalysed synthesis of esters of ferulic acid with natural compounds and evaluation of their antioxidant properties. J Mol Catal B Enzym. 2009;56:277–282. doi: 10.1016/j.molcatb.2008.05.017. [DOI] [Google Scholar]
- Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Conforti F, Perri V, Menichini F, Marrelli M, Uzunov D, Statti GA. Wild Mediterranean dietary plants as inhibitors of pancreatic lipase. Phytother Res. 2012;26:600–604. doi: 10.1002/ptr.3603. [DOI] [PubMed] [Google Scholar]
- Danino O, Gottlieb HE, Grossman S, Bergman M. Antioxidant activity of 1,3-dicaffeoylquinic acid isolated from Inula viscosa. Food Res Int. 2009;42:1273–1280. doi: 10.1016/j.foodres.2009.03.023. [DOI] [Google Scholar]
- Dong H, Li M, Zhu F, Liu F, Huang J. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012;130:261–266. doi: 10.1016/j.foodchem.2011.07.030. [DOI] [Google Scholar]
- Edenharder R, Rauscher R, Platt KL (1997) The inhibition by flavonoids of 2-amino-3-methylimidazo [4, 5-f] quinoline metabolic activation to a mutagen: a structure–activity relationship study. Mutat Res-Fund Mol M 379:21–32 [DOI] [PubMed]
- Farsi E, Shafaei A, Hor SY, Ahamed MBK, Yam MF, Idress HA. Correlation between enzymes inhibitory effects and antioxidant activities of standardized fractions of methanolic extract obtained from Ficus deltoidea leaves. Afr J Biotechnol. 2011;10:15184–15194. doi: 10.5897/AJB11.1365. [DOI] [Google Scholar]
- Fei Q, Gao Y, Zhang X, Sun Y, Hu B, Zhou L. Effects of oolong tea polyphenols, EGCG, and EGCG3 ″ Me on pancreatic α-amylase activity in vitro. J Agric Food Chem. 2014;62:9507–9514. doi: 10.1021/jf5032907. [DOI] [PubMed] [Google Scholar]
- Gonçalves R, Mateus N, De Freitas V. Study of the interaction of pancreatic lipase with procyanidins by optical and enzymatic methods. J Agric Food Chem. 2010;58:11901–11906. doi: 10.1021/jf103026x. [DOI] [PubMed] [Google Scholar]
- Gong Y, Hou Z, Gao Y, Xue Y, Liu X, Liu G. Optimization of extraction parameters of bioactive components from defatted marigold (Tagetes erecta L.) residue using response surface methodology. Food Bioprod Process. 2012;90:9–16. doi: 10.1016/j.fbp.2010.12.004. [DOI] [Google Scholar]
- Gong Y, Liu X, He W, Xu H, Yuan F, Gao Y. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia. 2012;83:481–489. doi: 10.1016/j.fitote.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Gudgin E, Lopez-Delgado R, Ware WR. The tryptophan fluorescence lifetime puzzle: a study of decay times in aqueous solution as a function of pH and buffer composition. Can J Chem. 1981;59:1037–1044. doi: 10.1139/v81-154. [DOI] [Google Scholar]
- Hosseinpanah F, Mirbolouk M, Mossadeghkhah A, Barzin M, Serahati S, Delshad H, Azizi F. Incidence and potential risk factors of obesity among Tehranian adults. Prev Med. 2016;82:99–104. doi: 10.1016/j.ypmed.2015.11.015. [DOI] [PubMed] [Google Scholar]
- IDF (2015) International Diabetes Federation, Diabetes Atlas 2015. Available from http://www.idf.org/diabetesatlas. Accessed 30 Dec 2015
- Kang GJ, Han SC, Ock JW, Kang HK, Yoo ES. Anti-inflammatory effect of quercetagetin, an active component of immature Citrus unshiu, in HaCaT human keratinocytes. Biomol Ther. 2013;21:138–145. doi: 10.4062/biomolther.2013.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R, Arora S, Singh B. Antioxidant activity of the phenol rich fractions of leaves of Chukrasia tabularis A. Juss. Bioresour Technol. 2008;99:7692–7698. doi: 10.1016/j.biortech.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Kim Y, Jeong Y, Wang M, Lee W, Rhee H. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005;21:756–761. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kim TH, Kim JK, Ito H, Jo C. Enhancement of pancreatic lipase inhibitory activity of curcumin by radiolytic transformation. Bioorg Med Chem Lett. 2011;21:1512–1514. doi: 10.1016/j.bmcl.2010.12.122. [DOI] [PubMed] [Google Scholar]
- Li Y, Gao F, Shan F, Bian J, Zhao C. Study on the interaction between 3 flavonoid compounds and α-amylase by fluorescence spectroscopy and enzymatic kinetics. J Food Sci. 2009;74:C199–C203. doi: 10.1111/j.1750-3841.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou F, Gao F, Bian J, Shan F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J Agric Food Chem. 2009;57:11463–11468. doi: 10.1021/jf903083h. [DOI] [PubMed] [Google Scholar]
- Marrelli M, Loizzo MR, Nicoletti M, Menichini F, Confort F. Inhibition of key enzymes linked to obesity by preparations from Mediterranean dietary plants: effects on α-amylase and pancreatic lipase activities. Plant Food Hum Nutr. 2013;68:340–346. doi: 10.1007/s11130-013-0390-9. [DOI] [PubMed] [Google Scholar]
- Matsui T, Tanaka T, Tamura S, Toshima A, Tamaya K, Miyata Y. α-Glucosidase inhibitory profile of catechins and the aflavins. J Agric Food Chem. 2007;55:99–105. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- Parejo I, Jauregui O, Viladomat F, Bastida J, Codina C. Characterization of acylated flavonoid-O-glycosides and methoxylated flavonoids from Tagetes maxima by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:2801–2810. doi: 10.1002/rcm.1697. [DOI] [PubMed] [Google Scholar]
- Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- Soares S, Mateus N, De Freitas V. Interaction of different polyphenols with bovine serum albumin (BSA) and human salivary α-amylase (HSA) by fluorescence quenching. J Agric Food Chem. 2007;55:6726–6735. doi: 10.1021/jf070905x. [DOI] [PubMed] [Google Scholar]
- Wang H, Du YJ, Song HC. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010;123:6–13. doi: 10.1016/j.foodchem.2010.03.088. [DOI] [Google Scholar]
- Xu H, Wang W, Jiang J, Yuan F, Gao Y. Subcritical water extraction and antioxidant activity evaluation with on-line HPLC-ABTS·+assay of phenolic compounds from marigold (Tagetes erecta L.) flower residues. J Food Sci Technol. 2014;52:3803–3811. doi: 10.1007/s13197-014-1449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Liu X, Yan Q, Yuan F, Gao Y. A novel copigment of quercetagetin for stabilization of grap skin anthocyanins. Food Chem. 2015;166:50–55. doi: 10.1016/j.foodchem.2014.05.125. [DOI] [PubMed] [Google Scholar]
- You Q, Chen F, Wang X, Jiang Y, Lin S. Anti-diabetic activities of phenolic compounds in muscadine against α-glucosidase and pancreatic lipase. LWT Food Sci Technol. 2012;46:164–168. doi: 10.1016/j.lwt.2011.10.011. [DOI] [Google Scholar]
- Youn JY, Park HY, Cho KH. Anti-hyperglycemic activity of Commelina communis L. inhibition of α-glucosidase. Diabetes Res Clin Pract. 2004;66:149–155. doi: 10.1016/j.diabres.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Yuan T, Wan CP, Ma H, Seeram NP. New phenolics from the flowers of Punica granatum and their in vitro α-glucosidase inhibitory activities. Planta Med. 2013;79:1674–1679. doi: 10.1055/s-0033-1350925. [DOI] [PubMed] [Google Scholar]
- Zielinska-Przyjemska M, Olejnik A, Dobrowolska-Zachwieja A, Grajek W. In vitro effects of beetroot juice and chips on oxidative metabolism and apoptosis in neutrophils from obese individuals. Phytother Res. 2009;23:49–55. doi: 10.1002/ptr.2535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 1028 kb)