Abstract
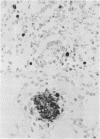
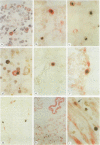
AIMS: To compare the use of biotinylated and digoxigenin labelled probes for diagnosis of human fetal parvovirus B19 infection in formalin fixed, paraffin wax embedded tissues; and to assess the cellular distribution of the virus in positive cases. METHODS: Sections of lung tissue from 23 cases of anatomically normal non-immune fetal hydrops presenting between 1984 and 1989, and from 13 control cases of hydrops due to chromosomal abnormality were probed for B19 DNA by in situ hybridisation using both biotinylated and digoxigenin labelled probes. The distribution of the virus was then investigated in all cases of fetal B19 infection confirmed in this laboratory to date (n = 11) by combining in situ hybridisation for viral DNA (using the digoxigenin system) with immunohistological labelling for a range of cellular antigens. RESULTS: Five unequivocal cases of B19 infection were identified among the 23 fetuses with unexplained hydrops using both probe labels. When combined with data from previous studies of the period 1974-1983, the results indicate that B19 infection was responsible for 27% of cases of anatomically normal non-immune hydrops and 8% of all cases, of non-immune hydrops presenting to this hospital over 15 years. False positive signal was seen in an additional three cases, using biotinylated probes. Digoxigenin labelled probes gave greater specificity and permitted detailed investigation of tissues high in endogenous biotin. Though most cells containing B19 DNA colabelled as erythroid precursors, viral DNA was frequently detected within mononuclear-phagocytic cells. In three cases viral signal was also found within occasional myocardial cells labelled by antibody to desmin. CONCLUSIONS: A relatively high proportion of cases of anatomically normal, non-immune hydrops are caused by B19 infection. Digoxigenin is a more reliable probe label than biotin for in situ hybridisation in archival fetal tissues. Double labelling for cellular antigens and viral nucleic acid is a powerful technique for investigating virus-host cell interactions, and provides evidence that cell types other than those of erythroid lineage may have a role in human fetal parvovirus infection.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Lewis E., Kidd I. M., Hall S. M., Cohen B. J. An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984 Aug;93(1):85–93. doi: 10.1017/s0022172400060964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Anand A., Ritchie L. D., Clewley J. P., Reid T. M. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984 Nov 3;2(8410):1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- Burns J., Graham A. K., Frank C., Fleming K. A., Evans M. F., McGee J. O. Detection of low copy human papilloma virus DNA and mRNA in routine paraffin sections of cervix by non-isotopic in situ hybridisation. J Clin Pathol. 1987 Aug;40(8):858–864. doi: 10.1136/jcp.40.8.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P. A., Caul E. O. Fetal cell tropism of human parvovirus B19. Lancet. 1988 Apr 2;1(8588):767–767. doi: 10.1016/s0140-6736(88)91572-3. [DOI] [PubMed] [Google Scholar]
- Burton P. A. Intranuclear inclusions in marrow of hydropic fetus due to parvovirus infection. Lancet. 1986 Nov 15;2(8516):1155–1155. doi: 10.1016/s0140-6736(86)90555-6. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Cohen B. J., Field A. M. Detection of parvovirus B19 DNA, antigen, and particles in the human fetus. J Med Virol. 1987 Dec;23(4):367–376. doi: 10.1002/jmv.1890230409. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Schmidtke J., Gosden J. R. Characterisation of a human Y chromosome repeated sequence and related sequences in higher primates. Chromosoma. 1982;87(5):491–502. doi: 10.1007/BF00333470. [DOI] [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., McKie V. C., Anderson L. J., Astell C. R., Tattersall P. Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol. 1986 Nov;60(2):548–557. doi: 10.1128/jvi.60.2.548-557.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming K. A., Evans M., Ryley K. C., Franklin D., Lovell-Badge R. H., Morey A. L. Optimization of non-isotopic in situ hybridization on formalin-fixed, paraffin-embedded material using digoxigenin-labelled probes and transgenic tissues. J Pathol. 1992 May;167(1):9–17. doi: 10.1002/path.1711670104. [DOI] [PubMed] [Google Scholar]
- Franciosi R. A., Tattersall P. Fetal infection with human parvovirus B19. Hum Pathol. 1988 Apr;19(4):489–491. doi: 10.1016/s0046-8177(88)80505-7. [DOI] [PubMed] [Google Scholar]
- Greywoode G. I., McCarthy S. P., McGee J. O. Labelling of cells of the mononuclear phagocyte system in routinely processed archival biopsy specimens with monoclonal antibody EBM/11. J Clin Pathol. 1990 Dec;43(12):992–996. doi: 10.1136/jcp.43.12.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassam S., Briner J., Tratschin J. D., Siegl G., Heitz P. U. In situ hybridization for the detection of human parvovirus B19 nucleic acid sequences in paraffin-embedded specimens. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(5):257–261. doi: 10.1007/BF02899412. [DOI] [PubMed] [Google Scholar]
- Katz V. L., Chescheir N. C., Bethea M. Hydrops fetalis from B19 parvovirus infection. J Perinatol. 1990 Dec;10(4):366–368. [PubMed] [Google Scholar]
- Kurtzman G. J., Ozawa K., Cohen B., Hanson G., Oseas R., Young N. S. Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med. 1987 Jul 30;317(5):287–294. doi: 10.1056/NEJM198707303170506. [DOI] [PubMed] [Google Scholar]
- Lenghaus C., Studdert M. J. Acute and chronic viral myocarditis. Acute diffuse nonsuppurative myocarditis and residual myocardial scarring following infection with canine parvovirus. Am J Pathol. 1984 May;115(2):316–319. [PMC free article] [PubMed] [Google Scholar]
- Morey A. L., O'Neill H. J., Coyle P. V., Fleming K. A. Immunohistological detection of human parvovirus B19 in formalin-fixed, paraffin-embedded tissues. J Pathol. 1992 Feb;166(2):105–108. doi: 10.1002/path.1711660204. [DOI] [PubMed] [Google Scholar]
- Naides S. J., Weiner C. P. Antenatal diagnosis and palliative treatment of non-immune hydrops fetalis secondary to fetal parvovirus B19 infection. Prenat Diagn. 1989 Feb;9(2):105–114. doi: 10.1002/pd.1970090205. [DOI] [PubMed] [Google Scholar]
- Nascimento J. P., Hallam N. F., Mori J., Field A. M., Clewley J. P., Brown K. E., Cohen B. J. Detection of B19 parvovirus in human fetal tissues by in situ hybridisation. J Med Virol. 1991 Feb;33(2):77–82. doi: 10.1002/jmv.1890330203. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Porter H. J., Heryet A., Quantrill A. M., Fleming K. A. Combined non-isotopic in situ hybridisation and immunohistochemistry on routine paraffin wax embedded tissue: identification of cell type infected by human parvovirus and demonstration of cytomegalovirus DNA and antigen in renal infection. J Clin Pathol. 1990 Feb;43(2):129–132. doi: 10.1136/jcp.43.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter H. J., Khong T. Y., Evans M. F., Chan V. T., Fleming K. A. Parvovirus as a cause of hydrops fetalis: detection by in situ DNA hybridisation. J Clin Pathol. 1988 Apr;41(4):381–383. doi: 10.1136/jcp.41.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter H. J., Quantrill A. M., Fleming K. A. B19 parvovirus infection of myocardial cells. Lancet. 1988 Mar 5;1(8584):535–536. doi: 10.1016/s0140-6736(88)91332-3. [DOI] [PubMed] [Google Scholar]
- Saint-Martin J., Choulot J. J., Bonnaud E., Morinet F. Myocarditis caused by parvovirus. J Pediatr. 1990 Jun;116(6):1007–1008. doi: 10.1016/s0022-3476(05)80677-8. [DOI] [PubMed] [Google Scholar]
- Salimans M. M., van de Rijke F. M., Raap A. K., van Elsacker-Niele A. M. Detection of parvovirus B19 DNA in fetal tissues by in situ hybridisation and polymerase chain reaction. J Clin Pathol. 1989 May;42(5):525–530. doi: 10.1136/jcp.42.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. F., Nerlich A., Hottenträger B., Jäger G., Wiest I., Kantimm S., Roggendorf H., Schultz M., Gloning K. P., Schramm T. Parvovirus B19 infection of the fetus. Histology and in situ hybridization. Am J Clin Pathol. 1991 Jul;96(1):121–126. doi: 10.1093/ajcp/96.1.121. [DOI] [PubMed] [Google Scholar]
- Stross W. P., Warnke R. A., Flavell D. J., Flavell S. U., Simmons D., Gatter K. C., Mason D. Y. Molecule detected in formalin fixed tissue by antibodies MT1, DF-T1, and L60 (Leu-22) corresponds to CD43 antigen. J Clin Pathol. 1989 Sep;42(9):953–961. doi: 10.1136/jcp.42.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Muijen G. N., Ruiter D. J., Warnaar S. O. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987 Oct;57(4):359–369. [PubMed] [Google Scholar]
- Weiland H. T., Vermey-Keers C., Salimans M. M., Fleuren G. J., Verwey R. A., Anderson M. J. Parvovirus B19 associated with fetal abnormality. Lancet. 1987 Mar 21;1(8534):682–683. doi: 10.1016/s0140-6736(87)90442-9. [DOI] [PubMed] [Google Scholar]