Abstract
Properties of fish skin gelatin film incorporated with palm oil at 50 and 75 % (w/w) as affected by glycerol at 0–30 % (w/w) were investigated. Increases in water vapour permeability and elongation at break along with decrease in tensile strength were noticed when levels of glycerol were increased (p < 0.05). Decrease in L*- and a*-values with coincidental increase in b*- and ΔE*-values were observed in emulsified films when amount of palm oil incorporated increased (p < 0.05). Light transmittance of all films increased as glycerol levels were increased (p < 0.05). FTIR results suggested that the protein–protein interaction in film matrix decreased when palm oil was incorporated. Films added with palm oil had lower glass transition and degradation temperatures than control films. The addition of 75 % palm oil and 10 % glycerol improved water vapour barrier property of fish skin gelatin films without drastic alteration of mechanical properties.
Keywords: Emulsified film, Gelatin film, Palm oil, Glycerol, Thermal properties
Introduction
Biopolymer films have gained interest in their use as edible food packaging. Edible films can be defined as thin continuous layer of biopolymer materials which can be applied as a coating on food, used as a wrap or made into pouch to hold the food or to protect it against external factors (Fakhouri et al. 2015; Falguera et al. 2011). Proteins from various sources have been used as materials for biodegradable film because of their relative abundance and good film-forming ability (Hamaguchi et al. 2007). Gelatin possesses excellent film-forming property and can be used for edible coatings and film making (Fakhouri et al. 2015; Jongjareonrak et al. 2006). Nevertheless, gelatin films have a poor water vapour barrier ability (Sobral et al. 2001). Hydrophobic substances such as lipid have been incorporated to improve water barrier property of gelatin film (Limpisophon et al. 2010). Those substances do not dissolve in aqueous phase, in which film-forming dispersion or emulsion must be prepared before making the films. The homogeneity of oil droplets and stability of emulsion in film-forming dispersion (FFD) during film drying give rise to a homogeneous distribution of lipid droplets in the film, which in turn contributes to efficient control of water vapour migration (Debeaufort et al. 2000). To disperse oil in film-forming aqueous phase, appropriate surfactant and homogenisation condition are required. Microfluidisation is a potential technique to bring about fine and more uniform oil droplets as well as more emulsion stability, compared to typical homogenisation.
Palm oil is natural hydrophobic substance obtained from the mesocarp of palm seed. Throughout the world, 90 % of palm oil is used for human consumption (Edem 2002). Due to its abundance and cheap price, palm oil could be used as a promising hydrophobic substance to improve water vapour barrier property of gelatin film. Recently, the incorporation of palm oil in combination with 30 % glycerol using lecithin as surfactant in film-forming dispersion could reduce the water vapour transfer through the film to some degree (Tongnuanchan et al. 2015). The use of reduced amount of hydrophilic glycerol might help lower hydrophilicity of resulting film. However, no information regarding the impact of glycerol in combination with palm oil on properties of gelatin films exists. Thus, this study aimed to investigate the influence of glycerol amounts on mechanical, physical and thermal properties as well as molecular interaction of fish skin gelatin films incorporated with palm oil at different levels.
Materials and methods
Chemicals and gelatin
Glycerol and soy lecithin were purchased from Sigma–Aldrich (St. Louis, MO, USA). All chemicals were of analytical grade. Tilapia skin gelatin (~240 bloom) was procured from Lapi Gelatine S.p.A (Empoli, Italy). Palm oil was obtained from OLEEN Company Limited (Bangkok, Thailand).
Preparation of film
Gelatin powder was mixed with distilled water and heated at 70 °C for 30 min to obtain the protein concentration of 3.5 % (w/v). Glycerol at 0, 10, 20 and 30 % (w/w, based on protein) was used as a plasticiser. Palm oil previously mixed with lecithin at 50 % (w/w, based on palm oil) was added into gelatin solution at levels of 50 and 75 % (w/w, based on protein). The mixtures were emulsified using a rotor–stator homogeniser (IKA Labortechnik homogeniser, Selangor, Malaysia) at 22,000 rpm for 3 min and a Microfluidics homogeniser (Model HC-5000, Microfluidiser, Newton, MA, USA) at 2,000 psi for two passes. Film-forming dispersion (FFD) was subjected to the removal of dissolved air by a vacuum pump (Diaphragm vacuum pump, Wertheim Germany) for 30 min at room temperature (28–30 °C). FFD (4 mL) was cast onto a rimmed silicone resin plate (50 × 50 mm2) and air-blown for 12 h at room temperature prior to further drying at 25 °C and 50 ± 5 % RH for 24 h in an environmental chamber (WTB Binder, Tuttlingen, Germany). Control films (without palm oil) were prepared from film-forming solution in the absence and presence of glycerol (30 %). All films were manually peeled off and subjected to analysis.
Analysis of films
Film thickness
The thickness of film was measured using a digital micrometer (Mitutoyo, Model ID-C112PM, Serial No. 00320, Mitutoyo Corp., Kawasaki-shi, Japan). Five random locations around each film of ten film samples were used for average thickness determination.
Mechanical properties
Prior to testing, films were conditioned for 48 h at 25 °C and 50 ± 5 % RH. Tensile strength (TS) and elongation at break (EAB) were determined as described by Iwata et al. (2000) with a slight modification using the Universal Testing Machine (Lloyd Instrument, Hampshire, UK). Ten samples (2 × 5 cm2) with initial grip length of 3 cm were used for testing. Cross-head speed was set at 30 mm/min.
Water vapour permeability (WVP)
WVP was measured using a modified ASTM method (ASTM 1989) as described by Shiku et al. (2004). The films were sealed on an aluminium permeation cup containing dried silica gel (0 % RH) with silicone vacuum grease and a rubber gasket to hold the films in place. The cups were placed in a desiccator containing the distilled water at 30 °C. The cups were weighed at 1-h intervals over a 10-h period. WVP of the film was calculated as follows:
where w is the weight gain of the cup (g); l is the film thickness (m); A is the exposed area of film (m2); t is the time of gain (s); P2-P1 is the vapour pressure difference across the film (4242 Pa at 30 °C).
Colour
Film samples were subjected to colour measurement using a CIE colorimeter (Hunter associates laboratory, Inc., Reston, VA, USA). D65 (day light) and a measure cell with opening of 30 mm was used. The colour of the films was expressed as L*-value (lightness), a*-value (redness/greenness) and b*-value (yellowness/blueness). Total colour difference (ΔE*) was calculated as follows (Gennadios et al. 1996):
where , and are the differences between the colour parameters of the samples and those of the white standard ( = 93.63, a* = −0.95 and b* = 0.46).
Light transmittance and transparency value
The light transmittance of films was measured in the range of 200–800 nm using a UV–Vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) as described by Shiku et al. (2004) The transparency value of film was calculated using the following equation (Han and Floros 1997):
where T600 is the fractional transmittance at 600 nm and x is the film thickness (mm). The greater transparency value represents the lower transparency of film.
Differential scanning calorimetry
Thermal transitions of films were scanned using a differential scanning calorimeter (DSC) (Perkin Elmer, Model DSC-7, Norwalk, CT, USA) as described by Tongnuanchan et al. (2015). Temperature calibration was performed using the indium thermogram. Film samples (2–5 mg) were accurately weighed into aluminium pans, hermetically sealed, and scanned over the temperature range of −20 to 150 °C, with a heating rate of 5 °C/min. Liquid nitrogen was used as cooling medium and the system was equilibrated at −20 °C for 5 min prior to the scan. An empty aluminium pan was used as the reference. A second scan was also performed in the same manner, followed by quench-cooling of the sample after completing the first scan. Glass transition temperature (Tg), melting transition temperature (Tmax) and the melting enthalpy (ΔH) were determined.
Thermo-gravimetric analysis (TGA)
Films were scanned using a thermo-gravimetric analyser (TGA7, PerkinElmer, Norwalk, CT, USA) from 25 to 800 °C at a rate of 10 °C/min (Nuthong et al. 2009). Thermal degradation temperature (Td) and weight loss of film samples were determined.
Attenuated total reflectance-fourier transform infrared (ATR-FTIR) spectroscopy
Films were scanned with a Bruker Model Equinox 55 FTIR spectrometer (Bruker Co., Ettlingen, Germany) equipped with a horisontal ATR Trough plate crystal cell (45° ZnSe; 80 mm long, 10 mm wide and 4 mm thick) (PIKE Technology Inc., Madison, WI, USA) at 25 °C as described by Nuthong et al. (2009) Films were placed onto the crystal cell and the cell were clamped into the mount of FTIR spectrometer. The spectra in range of 650–4000 cm−1 were determined.
Statistical analysis
Completely randomised design (CRD) was used throughout the study. Three different lots of films were prepared for all experiments. The determinations were performed in triplicate. Data were subjected to analysis of variance (ANOVA), and mean comparisons were carried out by the Duncan’s multiple range test (Steel et al. 1980). Analysis was performed using the SPSS package (SPSS for windows, SPSS Inc., Chicago, IL, USA).
Results and discussion
Thickness
Thickness of fish skin gelatin films containing glycerol at various levels (0–30 % w/w, based on protein) in the presence of palm oil at levels of 50 and 75 % (w/w, based on protein) is shown in Table 1. For the control films, that containing 30 % glycerol had higher thickness than that without glycerol addition (p < 0.05). Emulsified films generally had higher film thickness than the control films, regardless of glycerol levels (p < 0.05). The thickness of film incorporated with 75 % palm oil was higher than that of film containing 50 % palm oil, when the same glycerol level was used (p < 0.05). When the same level of palm oil was incorporated, continuous increases in film thickness were found when glycerol levels were increased from 0 to 30 % (p < 0.05). The highest film thickness was obtained in film containing 75 % palm oil in the presence of 20 or 30 % glycerol (p < 0.05). Addition of glycerol, which is a small hydrophilic molecule, into the film as a plasticiser reduced the protein–protein interaction (Gontard et al. 1993). As a result, the less compactness was found as evidenced by the increased film thickness in the film containing a larger amount of glycerol. The result was in agreement with that reported by Limpisophon et al. (2010). Moreover, oil droplets also lowered the interaction between peptide chains. Oil droplets could distribute and insert between the protein chains, thereby lowering the compactness of film network (Tongnuanchan et al. 2013). Thus, palm oil and glycerol levels directly affected free volume of film, which in turn determined thickness of gelatin films.
Table 1.
Thickness, mechanical properties and water vapour permeability of fish skin gelatin films incorporated without and with palm oil and glycerol at various levels
| Palm oils (%) | Glycerols (%) | Thickness (mm) | TS (MPa) | EAB (%) | WVP (×10−12 gm−1 s−1 Pa−1) |
|---|---|---|---|---|---|
| Without palm oil | 0 | 0.046 ± 0.002*h§ | 77.96 ± 1.56a | 7.47 ± 0.90j | 21.21 ± 0.57b |
| 30 | 0.054 ± 0.004g | 26.04 ± 1.59ef | 38.17 ± 2.37g | 22.9 ± 0.69a | |
| 50 | 0 | 0.077 ± 0.002f | 41.95 ± 3.89b | 23.45 ± 4.01i | 7.96 ± 0.19e |
| 10 | 0.079 ± 0.002ef | 35.55 ± 3.74c | 47.46 ± 5.57f | 8.51 ± 0.04d | |
| 20 | 0.082 ± 0.002de | 26.63 ± 1.85e | 70.20 ± 6.10d | 8.89 ± 0.12d | |
| 30 | 0.085 ± 0.003d | 19.50 ± 2.32g | 108.64 ± 7.19b | 10.56 ± 0.22c | |
| 75 | 0 | 0.088 ± 0.001c | 35.80 ± 1.38c | 31.63 ± 1.63h | 5.87 ± 0.28g |
| 10 | 0.090 ± 0.002bc | 30.77 ± 1.42d | 57.51 ± 4.07e | 6.65 ± 0.15f | |
| 20 | 0.092 ± 0.001ab | 23.35 ± 1.29f | 80.35 ± 5.55c | 7.06 ± 0.08f | |
| 30 | 0.095 ± 0.002a | 14.12 ± 2.38h | 116.25 ± 8.10a | 7.92 ± 0.11e |
§ Different lowercase superscripts in the same column indicate significant differences (p < 0.05)
TS tensile strength, EAB elongation at break, WVP water vapour permeability
* Mean ± SD (n = 3)
Mechanical properties
Tensile strength (TS) and elongation at break (EAB) of gelatin films containing glycerol (0–30 % w/w, based on protein) and palm oil (50 and 75 % w/w, based on protein) are shown in Table 1. For the control films (without palm oil), the lower TS and higher EAB were found for the control film containing 30 % glycerol (p < 0.05). At either 0 or 30 % glycerol, the emulsified films showed lower TS and higher EAB than those without palm oil (p < 0.05). At the same palm oil level used, the decreases in TS with coincidental increases in EAB were observed when the level of glycerol increased (p < 0.05). The film incorporated with 75 % palm oil showed the lower TS, but higher EAB than those containing 50 % palm oil (p < 0.05), regardless of glycerol levels. As a consequence, the decrease in rigidity with concomitant increase in extensibility/elasticity of film was gained. With the addition of 75 % palm oil, TS of film decreased from 35.80 to 14.12 MPa (p < 0.05) with increasing glycerol levels from 0 to 30 %. In contrast, EAB of film increased from 31.63 to 116.25 %. Limpisophon et al. (2009) reported that increasing glycerol content enhanced the flexibility of gelatin film from blue shark (Prionace glauca) skin. Glycerol with small size could distribute in the aqueous phase uniformly, in which the interaction between protein chains could be suppressed. Palm oil particularly at higher levels in conjunction with glycerol was able to lower the interaction between protein chains in film matrix. This could increase the elasticity and flexibility of films as evidenced by the higher EAB along with lower TS. Furthermore, the addition of iodine at higher concentrations (2–8 %) was found to decrease TS of zein film to a higher extent (Singh et al. 2009). Therefore, the mechanical properties of resulting films were influenced by both palm oil and glycerol.
Water vapour permeability (WVP)
WVP of fish skin gelatin films containing palm oil and glycerol at various levels is shown in Table 1. Generally, high WVP was observed in the control films (without palm oil). The control film with 30 % glycerol showed higher WVP than that without glycerol (p < 0.05). For emulsified films, WVP increased with increasing glycerol contents (p < 0.05). The film incorporated with 75 % palm oil showed lower WVP than those containing 50 % palm oil (p < 0.05), regardless of glycerol levels. Addition of hydrophobic substances such as palm oil could increase the hydrophobicity of films, thereby reducing the water vapour adsorption and also migration through the film. This result was in agreement with Tongnuanchan et al. (2013) who reported that root essential oils (ginger, turmeric and plai) incorporated into fish skin gelatin film decreased WVP of resulting films, especially with increasing essential oil amount. Limpisophon et al. (2009) also reported that the increasing stearic acid from 0 to 100 % in the film-forming solution decreased WVP of gelatin-fatty acid emulsified films. It was noted that the addition of palm oil into gelatin film in combination with reduced amount of glycerol could decrease WVP of gelatin film effectively. The incorporation of high palm oil level (75 %) with low glycerol level (0 and 10 %) yielded the film with high water vapour barrier property.
Colour, light transmittance and film transparency
The colour of gelatin films containing glycerol (0-30 %) and palm oil (50 and 75 %) is shown in Table 2. In general, the addition of palm oil resulted in the changes in colour of gelatin films. Film incorporated with palm oil showed lower L*- and a*-values along with higher b* and ΔE*-values than the control films (without palm oil) (p < 0.05). Those changes were more pronounced with increasing amount of palm oil added. In general, films added with palm oil became more yellowish as indicated by the increased b*-value. This result suggested that the colouring components in palm oil most likely contributed to the colour of gelatin film (Hopkins et al. 2015; Tongnuanchan et al. 2013). No differences in colour between films containing different levels of glycerol were found (p > 0.05), when the palm oil at the same level was used. Therefore, the level of palm oil noticeably affected the colour of gelatin film.
Table 2.
Colour parameters of fish skin gelatin films incorporated without and with palm oil and glycerol at various levels
| Palm oils (%) | Glycerols (%) | L * | a * | b * | ΔE * |
|---|---|---|---|---|---|
| Without palm oil | 0 | 90.42 ± 0.33*a§ | −0.96 ± 0.04a | 0.62 ± 0.07a | 3.20 ± 0.32a |
| 30 | 90.41 ± 0.22a | −0.95 ± 0.04a | 0.64 ± 0.02a | 3.21 ± 0.21a | |
| 50 | 0 | 88.35 ± 0.10b | −1.54 ± 0.02b | 7.52 ± 0.36b | 8.84 ± 0.29b |
| 10 | 88.35 ± 0.22b | −1.54 ± 0.03b | 7.52 ± 0.39b | 8.83 ± 0.41b | |
| 20 | 88.34 ± 0.17b | −1.55 ± 0.01b | 7.53 ± 0.17b | 8.85 ± 0.15b | |
| 30 | 88.33 ± 0.25b | −1.53 ± 0.03b | 7.53 ± 0.37b | 8.85 ± 0.19b | |
| 75 | 0 | 87.17 ± 0.06c | −1.81 ± 0.02c | 9.68 ± 0.19c | 11.29 ± 0.18c |
| 10 | 87.16 ± 0.24c | −1.81 ± 0.02c | 9.68 ± 0.19c | 11.30 ± 0.21c | |
| 20 | 87.16 ± 0.36c | −1.80 ± 0.02c | 9.68 ± 0.13c | 11.30 ± 0.18c | |
| 30 | 87.15 ± 0.24c | −1.82 ± 0.02c | 9.68 ± 0.19c | 11.30 ± 0.20c |
§ Different lowercase superscripts in the same column indicate significant differences (p < 0.05)
* Mean ± SD (n = 3)
Light transmission at selected wavelengths from 200 to 800 nm in UV and visible ranges and transparency value of films from fish skin gelatin containing palm oil at levels of 50 and 75 % in the presence of glycerol at various levels is shown in Table 3. All gelatin films had excellent barrier property against UV light at 200 and 280, especially when palm oil was incorporated. Protein-based films had the excellent UV light barrier capacity owing to their high amount of aromatic amino acids that absorb UV light parameters (Hamaguchi et al. 2007). Similar result was found for gelatin films from skins of tilapia (Tongnuanchan et al. 2013). All emulsified films showed the lower transmission of visible light in the range of 350-800 nm, compared with control film (without palm oil). The higher barrier property toward light transmission was obtained for films incorporated with 75 % palm oil in comparison with the films containing 50 % palm oil, regardless of glycerol level. This was more likely associated with the increase in opaqueness of films containing palm oils. Oil droplets distributed throughout the films might hinder light transmission through the films (Ma et al. 2012). It was noted that transmission increased when glycerol content increased. Glycerol more likely reduced the compactness of film matrix, thereby facilitating the light transmission of films. For transparency values, the lower transparency value indicated that the film was more transparent. Films added with palm oil had higher transparency value than the control films (p < 0.05) (Table 3). This result suggested that the incorporation of palm oil yielded less transparent films. Oil droplets localised in the film matrix had light scattering effect, thus lowering the transparency of gelatin film (Tongnuanchan et al. 2012). With increasing glycerol levels (0–30 %), transparency value decreased, indicating increased transparency of films. Thus, levels of both palm oil and glycerol played an essential role in colour, light transmittance and transparency of emulsified gelatin films.
Table 3.
Light transmittance and transparency value of fish skin gelatin films incorporated without and with palm oil and glycerol at various levels
| Palm oils (%) | Glycerols (%) | Light transmittance (%) at different wavelength (nm) | Transparency value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 200 | 280 | 350 | 400 | 500 | 600 | 700 | 800 | |||
| Without palm oil | 0 | 0.02 | 37.14 | 73.39 | 77.74 | 81.74 | 83.96 | 85.48 | 86.64 | 1.40 ± 0.001*h§ |
| 30 | 0.03 | 42.07 | 83.13 | 85.14 | 87.39 | 88.56 | 89.23 | 89.70 | 1.15 ± 0.002g | |
| 50 | 0 | 0.00 | 0.82 | 21.69 | 35.54 | 53.68 | 63.05 | 71.49 | 76.17 | 2.53 ± 0.029a |
| 10 | 0.00 | 1.03 | 22.83 | 36.75 | 55.05 | 64.96 | 72.89 | 76.28 | 2.36 ± 0.027b | |
| 20 | 0.00 | 1.23 | 23.58 | 37.39 | 55.58 | 65.25 | 73.17 | 77.67 | 2.29 ± 0.017c | |
| 30 | 0.00 | 1.32 | 25.92 | 40.37 | 58.30 | 66.53 | 74.92 | 79.13 | 2.08 ± 0.004e | |
| 75 | 0 | 0.00 | 0.31 | 16.08 | 29.65 | 48.52 | 62.29 | 68.07 | 73.41 | 2.36 ± 0.011b |
| 10 | 0.00 | 0.45 | 17.20 | 30.86 | 49.51 | 62.99 | 68.50 | 73.66 | 2.26 ± 0.017d | |
| 20 | 0.00 | 0.78 | 20.69 | 35.41 | 53.88 | 64.42 | 71.40 | 75.89 | 2.10 ± 0.014e | |
| 30 | 0.00 | 0.87 | 21.09 | 35.70 | 53.88 | 64.95 | 71.08 | 75.51 | 1.88 ± 0.003f | |
§ Different lowercase superscripts in the same column indicate significant differences (p < 0.05)
* Mean ± SD (n = 3)
Differential scanning calorimetry (DSC)
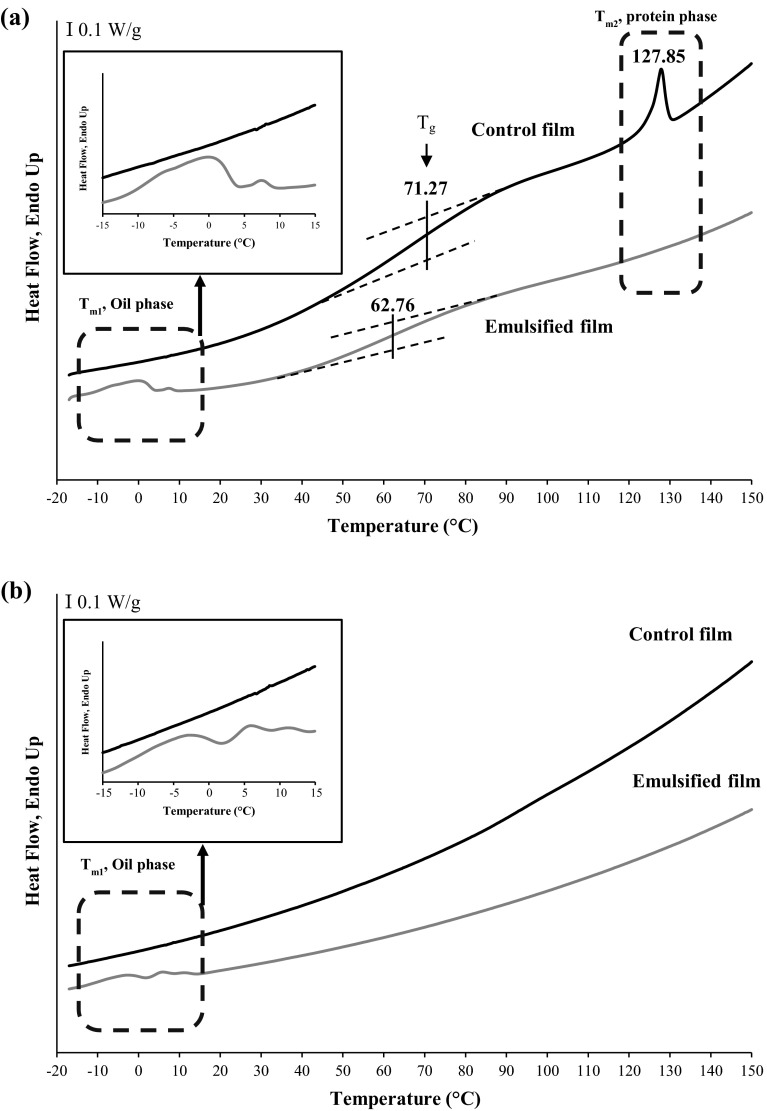
DSC thermograms of the 1st and 2nd-heating scans of selected emulsified film (containing 10 % glycerol incorporated with 75 % palm oil) and the control film (containing 30 % glycerol without palm oil), showing highest WVP, are illustrated in Fig. 1a and b, respectively. From thermogram of the 1st heating scan (from −20 to 150 °C), the control film showed a step-like transitions, indicating the glass transition temperature (Tg), and an endothermic melting transition (Tmax). The glass transition is associated with molecular segmental motion of disordered (amorphous phase) structure which undergoes from a brittle glassy solid state to a rubbery or highly viscous state, whereas the melting transition of the protein film indicated the temperature causing a disruption of ordered or aggregated structure (Tang et al. 2009). Tg of the control gelatin film was found at temperature of 71.27 °C, which was more likely associated with Tg of plasticised gelatin-rich phase. Gelatin-based films had various Tg, depending upon gelatin sources, compositions of film and process used (Tongnuanchan et al. 2015). Tg of gelatin film decreased from 71.27 °C (control film) to 62.76 °C, when palm oil at the levels of 75 % was incorporated. It was suggested that the addition of palm oil more likely impeded protein–protein interaction in film matrix, thereby increasing the mobility of gelatin chain. The losses of cohesive structure integrity were found in film network when oil droplets were present (Tongnuanchan et al. 2014). This result was in agreement with the decreased strength and increased EAB (Table 1.) of emulsified gelatin film.
Fig. 1.
DSC thermograms of 1st-heating scan (a) and 2nd-heating scan (b) of the control gelatin film and film incorporated with 75 % palm oil and 10 % glycerol
For endothermic/melting transition, the control film showed endothermic peak with Tmax of 127.85 °C. This endothermic transition obtained after the glass transition was possibly associated with the helix-coil transition of gelatin (Vanin et al. 2005) as well as the disruption of other kinds of ordered or aggregated molecular structure. Gelatin chains could undergo partial renaturation during film formation process (Hoque et al. 2011b). However, melting/ordered-phase transition peak disappeared when palm oil was incorporated. It was noted that the addition of palm oil might disrupt the protein–protein chain interaction in the resulting film. When palm oil droplets were dispersed in the gelatin film matrix, the weaker film structure was developed, particularly with large amount of oil added. The weaker film structure had lower thermal stability, which required a lower enthalpy for destroying the inter-molecular interaction. Moreover, thermograms of films incorporated with 75 % palm oil exhibited another endothermic transition peak (Tmax1), observed at temperature of −0.82 °C. As expected, no endothermic transition peak at this range of temperature was found in the control film. This endothermic transition was most likely attributed to the melting transition of palm oil droplets dispersed in the film network. This result was in agreement with Tongnuanchan et al. (2015) who reported that fish gelatin film incorporated with palm oil at levels of 25–100 % exhibited endothermic peak in the range of −2.32 to 0.52 °C which was due to the melting peak of palm oil.
From the thermograms of the 2nd-heating scan (Fig. 1b), the endothermic peak, which was related to gelatin-rich phase transition (Tmax2), was not observed for both film samples. It was suggested that gelatin was not able to rearrange themselves into ordered structure upon quench cooling during DSC scan. These results suggested that gelatin molecules were completely disrupted along with the loss of adsorbed water after the first-heating scan. Furthermore, the endothermic melting peak was observed for films incorporated with palm oil, but it was not found for the control film. This endothermic transition was correlated with the melting transition of palm oil as previously described. Thus, the incorporation of palm oil directly affected the thermal behavior of gelatin film.
Thermo-gravimetric analysis (TGA)
The degradation temperatures (Td), weight loss (Δw) and residue of selected emulsified films (containing 10 % glycerol and 75 % palm oil) and control film (containing 30 % glycerol without palm oil) are presented in Table 4. The control film exhibited three stages of weight loss, whilst the emulsified film had four stages of weight loss. For both films, the first stage weight loss (Δw1 = 5.76–6.1 %) was observed over the temperature (Td1) ranging from 54.50 to 56.36 °C. The weight loss at this temperature range was more likely associated with the loss of free and bound water absorbed in the film. Emulsified film had lower weight loss than the control film. This might be due to the lower amount of water in film matrix, associated with higher hydrophobicity of film containing palm oil (Tongnuanchan et al. 2014). The second stage of weight loss of films appeared approximately at the onset temperature of 211.19–213.70 °C (Td2) with Δw2 of 4.11–19.5 %. Weight loss at this stage was mostly owing to the loss of low molecular weight protein fraction and glycerol as well as structural bound water (Hoque et al. 2011a). It was noted that Td2 and Δw2 of films incorporated with palm oil was lower than those of control film. This might be due to the lower proportion of glycerol in film matrix. However, this range of temperature was higher than the boiling point of glycerol (182 °C) (Guerrero et al. 2011). Some kinds of interaction such as hydrogen bond were plausibly formed between protein fractions and glycerol (Guerrero et al. 2011). For the third stage of weight loss, Δw3 of 37.35–55.83 % and Td3 of 286.80–295.62 °C were observed for both films. This stage of weight loss was most likely associated with the degradation or decomposition of larger size or highly interacted protein fractions. In general, the Td3 for films incorporated with palm oil was lower than the control film. The results suggested that film incorporated with palm oil showed lower heat resistance than the control film. Incorporation of palm oil yielded a weaker film network due to the lower inter/intra-molecular protein interaction in film matrix. This led to the lower heat resistance of resulting films. Moreover, Td3 was higher than the smoke point of palm oil (~235 °C) (Guzman et al. 2010). The loss of volatile compounds or low molecular weight free fatty acids in palm oil could occur at this temperature. For the fourth stage of weight loss, Δw4 of 36.98 % with Td4 of 361.84 °C was obtained for emulsified films, suggesting the loss of high thermal stable components possibly gelatin molecules closely associated with soy lecithin. However, the fourth stage of weight loss (Δw4) was undetectable for the control film. In general, gelatin films incorporated with palm oil had lower residue mass from thermal degradation, compared with the control film. This confirmed that films containing palm oil had weaker interaction between protein molecules in film network than the control film as evidenced by the lowered TS (Table 1). As a consequence, the residue or char in films added with palm oil was found at a lower extent. Thus, the incorporation of palm oil could loosen film network, leading to higher degree of thermal degradation.
Table 4.
Thermal degradation temperatures (Td, °C) and weight loss (Δw, %) of the control fish skin gelatin film and film incorporated with 75 % palm oil and 10 % glycerol
| Palm oils (%) | Glycerols (%) | Δ1 | Δ2 | Δ3 | Δ4 | Residual (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Td1,onset | Δw 1 | Td2,onset | Δw 2 | Td3,onset | Δw 3 | Td4,onset | Δw 4 | |||
| 0 | 30 | 54.50 | 6.10 | 211.19 | 19.50 | 295.62 | 55.83 | – | – | 18.57 |
| 75 | 10 | 56.36 | 5.76 | 213.70 | 4.11 | 286.80 | 37.35 | 361.84 | 36.98 | 15.80 |
Δ1, Δ2, Δ3 and Δ4 denote the first, second, third and fourth stage weight loss, respectively, of film during heating scan
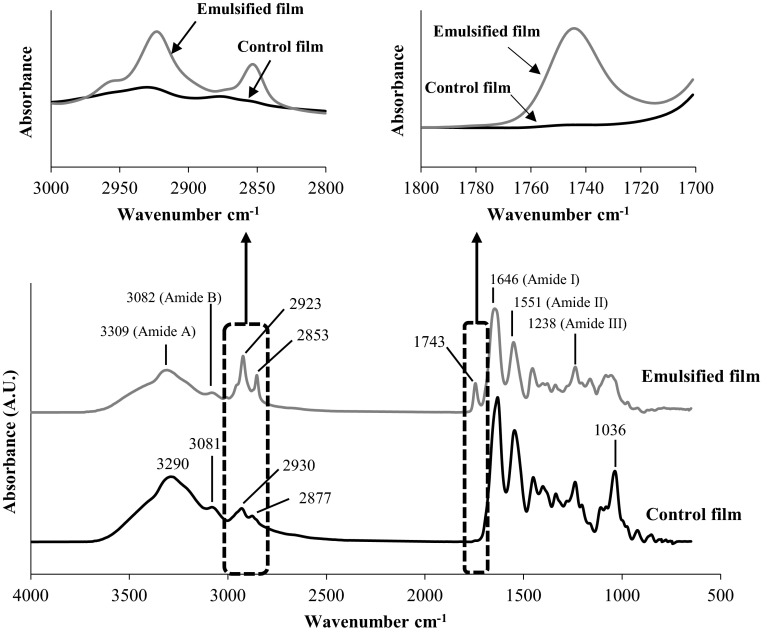
Fourier-transform infrared (FTIR) spectroscopy
FTIR spectra of control film and the selected emulsified film are illustrated in Fig. 2. Generally, control film and emulsified film showed similar major peaks but the amplitudes of peaks varied as influenced by both glycerol and palm oil incorporated. The band situated at the wavenumber of 1035–1038 cm−1 was found in both film samples, corresponding to the OH group, mainly from glycerol added as a plasticiser (Bergo and Sobral 2007). It was noted that the amplitude of the OH group of control film was higher than that found in film incorporated with palm oil. This was because glycerol proportion in the emulsified film was lower than that of control film. Both films had similar spectra in the range of 1700–700 cm−1, covering amide-I, II and III bands. Both films had major bands at 1646 cm−1 (amide-I, illustrating C=O stretching/hydrogen bonding coupled with COO), 1551 cm−1 (amide-II, presenting the bending vibrations of N–H groups and stretching vibrations of C–N groups) and 1238 cm−1 (amide-III, illustrating the vibrations in-plane of C–N and N–H groups of bound amide or vibrations of CH2 groups of glycine) (Aewsiri et al. 2009; Muyonga et al. 2004). It was noted that the amplitudes of amide-I, II and III of control film were slightly higher than film incorporated with palm oil. This was mainly due to the dilution effect caused by palm oil incorporated into gelatin film. Amide-I and Amide-II bands of zein film were found at the wavenumbers of 1650 and 1545 cm−1, respectively. The amplitude of amide-II band of zein film plasticized with 30 % glycerol increased as the concentration of iodine added increased from 2 to 8 % (Singh et al. 2009). Amide-A peak of control film found at wavenumber of 3290 cm−1, which was lower than that of emulsified film (3309 cm−1). The amide-A band represents the NH-stretching coupled with hydrogen bonding. Glycerol at higher proportion in the control films might undergo interaction via hydrogen bonding with–NH in the gelatin chains to a higher extent as indicated by the shift to the lower wavenumbers. Emulsified film also showed the lower amplitude of amide-A band. The result suggested that the incorporation of palm oil into gelatin film showed the dilution effect on proteins in the film network. Amide-B band was found at 3081–3082 cm−1 for both film samples. Amide-B band illustrated NH-stretching vibration and asymmetric CH-stretching vibration (Kong and Yu 2007). Additionally, peaks at wavenumbers of 2930 and 2877 cm−1 for the control film were shifted to lower wavenumbers of 2923 and 2853 cm−1 when palm oil was incorporated. The amplitude of both peaks was much higher in films added with palm oil, in comparison with the control film. Peaks at wavenumbers around 2853 cm−1 and 2924 cm−1 represented the methylene asymmetrical and symmetrical stretching vibration of the aliphatic C–H in CH2 and CH3 groups, respectively (Muik et al. 2007). Those vibration stretching bands are obviously found in most lipids and hydrophobic substances (Guillén and Cabo 2004). The result confirmed the presence of hydrophobic substance, palm oil, in the film. Furthermore, the peak at wavenumber of 1743 cm−1 was observed only in films containing palm oil. The carbonyl absorption of triglyceride ester linkage was observed at 1746 cm−1 (Setiowaty et al. 2000). Thus, the stretching vibration peak assignable to the C=O group of triglycerides was noticeable when the palm oil was incorporated.
Fig. 2.
ATR-FTIR spectra of the control gelatin film and film incorporated with 75 % palm oil and 10 % glycerol
Conclusion
Incorporation of palm oil and glycerol affected mechanical, physical and thermal properties of gelatin films differently, depending upon the levels used. The lowest WVP of gelatin film was observed when 75 % palm oil was incorporated and glycerol was excluded. The incorporation of palm oil directly affected colours, transparency and thermal properties of films. Thus, the use of 75 % palm oil and 10 % glycerol could improve water vapour barrier properties of gelatin-based film, whereas the satisfactory mechanical properties were still obtained.
Acknowledgements
The authors would like to thank the Graduate School of Prince of Songkla University, Thailand for the financial support. The TRF Distinguished Research Professor Grant was also acknowledged.
References
- Aewsiri T, Benjakul S, Visessanguan W. Functional properties of gelatin from cuttlefish (Sepia pharaonis) skin as affected by bleaching using hydrogen peroxide. Food Chem. 2009;115:243–249. doi: 10.1016/j.foodchem.2008.12.012. [DOI] [Google Scholar]
- ASTM . Annual book of ASTM standards. Philadelphia: American Society for Testing and Materials; 1989. [Google Scholar]
- Bergo P, Sobral PJA. Effects of plasticizer on physical properties of pig skin gelatin films. Food Hydrocoll. 2007;21:1285–1289. doi: 10.1016/j.foodhyd.2006.09.014. [DOI] [Google Scholar]
- Debeaufort F, Quezada-Gallo J-A, Delporte B, Voilley A. Lipid hydrophobicity and physical state effects on the properties of bilayer edible films. J Membr Sci. 2000;180:47–55. doi: 10.1016/S0376-7388(00)00532-9. [DOI] [Google Scholar]
- Edem DO. Palm oil: biochemical, physiological, nutritional, hematological and toxicological aspects: a review. Plant Foods Hum Nutr. 2002;57:319–341. doi: 10.1023/A:1021828132707. [DOI] [PubMed] [Google Scholar]
- Fakhouri FM, Martelli SM, Caon T, Velasco JI, Mei LHI. Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol Technol. 2015;109:57–64. doi: 10.1016/j.postharvbio.2015.05.015. [DOI] [Google Scholar]
- Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22:292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- Gennadios A, Weller CL, Hanna MA, Froning GW. Mechanical and barrier properties of egg albumen films. J Food Sci. 1996;61:585–589. doi: 10.1111/j.1365-2621.1996.tb13164.x. [DOI] [Google Scholar]
- Gontard N, Guilbert S, Cuq JL. Water and glycerol as plasticizers affect mechanical and water vapor barrier properties of an edible wheat gluten film. J Food Sci. 1993;58:206–211. doi: 10.1111/j.1365-2621.1993.tb03246.x. [DOI] [Google Scholar]
- Guerrero P, Nur Hanani ZA, Kerry JP, de la Caba K. Characterization of soy protein-based films prepared with acids and oils by compression. J Food Eng. 2011;107:41–49. doi: 10.1016/j.jfoodeng.2011.06.003. [DOI] [Google Scholar]
- Guillén MD, Cabo N. Study of the effects of smoke flavourings on the oxidative stability of the lipids of pork adipose tissue by means of Fourier transform infrared spectroscopy. Meat Sci. 2004;66:647–657. doi: 10.1016/S0309-1740(03)00185-2. [DOI] [PubMed] [Google Scholar]
- Guzman A, Torres JE, Prada LP, Nuñez ML. Hydroprocessing of crude palm oil at pilot plant scale. Catal Today. 2010;156:38–43. doi: 10.1016/j.cattod.2009.11.015. [DOI] [Google Scholar]
- Hamaguchi PY, Weng W, Kobayashi T, Runglertkreingkrai J, Tanaka M. Effect of fish meat quality on the properties of biodegradable protein films. Food Sci Technol Res. 2007;13:200–204. doi: 10.3136/fstr.13.200. [DOI] [Google Scholar]
- Han JH, Floros JD. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J Plast Film Sheeting. 1997;13:287–298. [Google Scholar]
- Hopkins EJ, Chang C, Lam RSH, Nickerson MT. Effects of flaxseed oil concentration on the performance of a soy protein isolate-based emulsion-type film. Food Res Int. 2015;67:418–425. doi: 10.1016/j.foodres.2014.11.040. [DOI] [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T. Effects of partial hydrolysis and plasticizer content on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. Food Hydrocoll. 2011;25:82–90. doi: 10.1016/j.foodhyd.2010.05.008. [DOI] [Google Scholar]
- Hoque MS, Benjakul S, Prodpran T, Songtipya P. Properties of blend film based on cuttlefish (Sepia pharaonis) skin gelatin and mungbean protein isolate. Int J Biol Macromol. 2011;49:663–673. doi: 10.1016/j.ijbiomac.2011.06.028. [DOI] [PubMed] [Google Scholar]
- Iwata KI, Ishizaki SH, Handa AK, Tanaka MU. Preparation and characterization of edible films from fish water-soluble proteins. Fish Sci. 2000;66:372–378. doi: 10.1046/j.1444-2906.2000.00057.x. [DOI] [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006;20:492–501. doi: 10.1016/j.foodhyd.2005.04.007. [DOI] [Google Scholar]
- Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Limpisophon K, Tanaka M, Weng W, Abe S, Osako K. Characterization of gelatin films prepared from under-utilized blue shark (Prionace glauca) skin. Food Hydrocoll. 2009;23:1993–2000. doi: 10.1016/j.foodhyd.2009.03.014. [DOI] [Google Scholar]
- Limpisophon K, Tanaka M, Osako K. Characterisation of gelatin–fatty acid emulsion films based on blue shark (Prionace glauca) skin gelatin. Food Chem. 2010;122:1095–1101. doi: 10.1016/j.foodchem.2010.03.090. [DOI] [Google Scholar]
- Ma W, Tang CH, Yin SW, Yang XQ, Qi JR, Xia N. Effect of homogenization conditions on properties of gelatin-olive oil composite films. J Food Eng. 2012;113:136–142. doi: 10.1016/j.jfoodeng.2012.05.007. [DOI] [Google Scholar]
- Muik B, Lendl B, Molina-Diaz A, Valcarcel M, Ayora-Cañada MJ. Two-dimensional correlation spectroscopy and multivariate curve resolution for the study of lipid oxidation in edible oils monitored by FTIR and FT-Raman spectroscopy. Anal Chim Acta. 2007;593:54–67. doi: 10.1016/j.aca.2007.04.050. [DOI] [PubMed] [Google Scholar]
- Muyonga JH, Cole CGB, Duodu KG. Characterisation of acid soluble collagen from skins of young and adult Nile perch (Lates niloticus) Food Chem. 2004;85:81–89. doi: 10.1016/j.foodchem.2003.06.006. [DOI] [Google Scholar]
- Nuthong P, Benjakul S, Prodpran T. Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int J Biol Macromol. 2009;44:143–148. doi: 10.1016/j.ijbiomac.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Setiowaty G, Che Man YB, Jinap S, Moh MH. Quantitative determination of peroxide value in thermally oxidized palm olein by Fourier transform infrared spectroscopy. Phytochem Anal. 2000;11:74–78. doi: 10.1002/(SICI)1099-1565(200003/04)11:2<74::AID-PCA498>3.0.CO;2-E. [DOI] [Google Scholar]
- Shiku Y, Hamaguchi PY, Benjakul S, Visessanguan W, Tanaka M. Effect of surimi quality on properties of edible films based on Alaska pollack. Food Chem. 2004;86:493–499. doi: 10.1016/j.foodchem.2003.09.022. [DOI] [Google Scholar]
- Singh N, Georget DMR, Belton PS, Barker SA. Zein−iodine complex studied by FTIR spectroscopy and dielectric and dynamic rheometry in films and precipitates. J Agric Food Chem. 2009;57:4334–4341. doi: 10.1021/jf900436q. [DOI] [PubMed] [Google Scholar]
- Sobral PJA, Menegalli FC, Hubinger MD, Roques MA. Mechanical, water vapor barrier and thermal properties of gelatin based edible films. Food Hydrocoll. 2001;15:423–432. doi: 10.1016/S0268-005X(01)00061-3. [DOI] [Google Scholar]
- Steel RGD, Torrie JH, Dicky DA. Principles and procedures of statistics: a biometrical aproach. New York: McGraw-Hill; 1980. [Google Scholar]
- Tang C-H, Xiao M-L, Chen Z, Yang X-Q, Yin S-W. Properties of cast films of vicilin-rich protein isolates from phaseolus legumes: influence of heat curing. LWT Food Sci Technol. 2009;42:1659–1666. doi: 10.1016/j.lwt.2009.05.020. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012;134:1571–1579. doi: 10.1016/j.foodchem.2012.03.094. [DOI] [PubMed] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T. Physico-chemical properties, morphology and antioxidant activity of film from fish skin gelatin incorporated with root essential oils. J Food Eng. 2013;117:350–360. doi: 10.1016/j.jfoodeng.2013.03.005. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T. Structural, morphological and thermal behaviour characterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014;41:33–43. doi: 10.1016/j.foodhyd.2014.03.015. [DOI] [Google Scholar]
- Tongnuanchan P, Benjakul S, Prodpran T, Nilsuwan K. Emulsion film based on fish skin gelatin and palm oil: physical, structural and thermal properties. Food Hydrocoll. 2015;48:248–259. doi: 10.1016/j.foodhyd.2015.02.025. [DOI] [Google Scholar]
- Vanin FM, Sobral PJA, Menegalli FC, Carvalho RA, Habitante AMQB. Effects of plasticizers and their concentrations on thermal and functional properties of gelatin-based films. Food Hydrocoll. 2005;19:899–907. doi: 10.1016/j.foodhyd.2004.12.003. [DOI] [Google Scholar]