Abstract
The aim of this study was to investigate antioxidant, antimicrobial and anticancerous activity of Melanelia subaurifera and Melanelia fuliginosa. The phytochemical analysis was determined by HPLC–UV method. Antioxidant activity was evaluated by DPPH and reducing power assay while antimicrobial activity was determined by minimal inhibitory concentration. The cytotoxic activity was tested using MTT method. The method for quantification of 2′-O-methyl anziaic acid and lecanoric acid in these lichens using RF-HPLC was also developed and validated. The depsides (lecanoric acid, gyrophoric acid, atranorin, anziaic acid and 2′-O-methyl anziaic acid), and dibenzofurane (usnic acid) were identified in these lichens. The antioxidant activity (IC50) of lichens extracts ranged from 121.52 to 424.51 μg/ml. 2′-O-Methyl anziaic acid showed the highest antimicrobial activity with MIC ranging from 0.0625 to 1 mg/ml. M. subaurifera extract showed the highest cytotoxic activity against the tested cell lines (IC50 = 9.88 to 31.64 μg/ml).
Keywords: HPLC analysis, Lichens secondary metabolites, Biological activities
Introduction
The global problem of today are uncontrolled and more frequent use of antibiotics and other synthetic or semisynthetic drugs, which leads to resistance, an increasing number of pathogens. These pathogens challenger’s different diseases in humans, animals and plants. In order to prevent disease occurrence and spread of resistance, recorded an increase of interest for bioactive natural products, organic foods and natural cosmetics. The reason for popularizing the “natural” product is insecurity and toxicity of newly synthesized compounds to human health and the environment. The study of chemical, biological and pharmacological properties of the used natural products in traditional medicine around the world were obtained by many therapeutic agents that are still used today in modern medicine (Berdy 2012). Looking for new bioactive preparations of natural origin, lichens are the subject of numerous studies.
Lichens are presented as an association of two symbionts: fungal partner, the mycobiont and algae partner, the photobiont. The appearance of lichen thallus is primarily determined by the mycobiont. Lichens have worldwide distribution and produce a wide array of both primary and secondary metabolites (Karagoz et al. 2009). These secondary metabolites are bioactive compounds, which are, often, unique to the organisms and produced primarily by the mycobiont. Secondary metabolites of lichens, so-called lichen substances, are product of metabolic pathways, and many of them originate from acetyl-polymalonyl pathway. Secondary metabolites from lichens can be extracted into organic solvent: benzene, chloroform, acetone, ethanol or methanol. To today, there are 1050 lichens substances identified with different methods (Shukla et al. 2010). They have various biological activities such as antibiotic, antimycobacterial, antioxidant, antitumor, antiviral, antiherbivore, analgesic and antipyretic properties, and have been used for treatment of various conditions in traditional medicine (Boustie et al. 2011). New substances are still being discovered every year and their structures determined.
The genus Melanelia is cosmopolitan and comprises about 40 species (Blanco et al. 2004). However, the bioactivity with respect to antioxidant capacities, antimicrobial potential and cytotoxic activity of this genus have not been reported till date. For this rationale, the present investigation was designed to estimate the in vitro different biological activities of this lichen.
Continuing our investigation of lichen extracts and bioactive phenolic substances isolated from lichens, we relate here the results of the phytochemical analysis of Melanelia subaurifera and M. fuliginosa acetone extracts and their antioxidant, antimicrobial and anticancer activities as well as isolated lecanoric acid and 2′-O-methyl anziaic acid. We also validated RF-HPLC methods for quantification of two biomarker compounds in tested lichens.
Materials and methods
Collection of lichen sample
Lichen material of M. subaurifera (Nyl.) Essl. and M. fuliginosa (Fr. ex Duby) Laundon., were collected from Kopaonik, Serbia, with the following geographical coordinates: 43°16′09″N 20°49′21″E, during May 2013. Identification was done using relevant key and monographs (Dobson 2011; Wirth 1995). The demonstration samples are preserved in facilities of the Department of Biology and Ecology of Kragujevac, Faculty of Science. Specimen of each species has been retained in our laboratory for future reference.
Preparation of the lichen extracts
The lichen material was air dried at room temperature (25 ± 1 °C) for 2 week, after which it was grinded. Dry ground thalli of the investigated lichens (50 g) were extracted using acetone in a Soxhlet extractor (IKA, Werke, Staufen, Germany). The extracts were filtered and then concentrated under reduced pressure in a rotary evaporator (IKA, RV 10, Werke, Staufen, Germany). The dry extracts were stored at −18 °C until they were used in the tests. The extracts were dissolved in 5 % dimethyl sulphoxide (DMSO) for the experiments.
High performance liquid chromatography (HPLC) analysis
The lichen extracts were redissolved in 500 µm of acetone and analzed on an HPLC (agilant Technologies, 1200 Series) instrument with C18 column (C18; 25 cm × 4.6 mm, 10 m) using UV spectrophotometric detector with methanol–water-phosphoroc acid (85:15:0,9, v/v/v) solvent. Deionized water used throughout the experiments was generated by a Milli-Q academic water purification system (Milford, MA, USA). Phosphoric acid was analytical-grade reagent. Methanol was of HPLC grade and was purchased from Merck (Darmstadt, Germany). The sample injection volume was 10 µL. The flow rate was 1.0 ml min−1. The standards used were obtained from the following sources: atranorin (tR = 16.21 ± 0.20 min), was isolated from lichen P. furfuraceae, usnic acid (tR = 14.32 ± 0.20 min) from Usnea barbata, lecanoric acid (tR = 2.47 ± 0.10 min) from Parmotrema tinctorum, gyrophoric acid (tR = 4.25 ± 0.20 min) from Umbilicaria crustulosa, and anziaic acid (tR = 6.15 ± 0.10 min) was synthesized from olivetol by the procedure described by Lin et al. (2013). Nuclear magnetic resonance (NMR) spectra were recorded employing a Bruker AM-400 spectrometer. Chemical shifts (δ) were expressed in ppm, and J values were expressed in Hz. Mass (MS) spectra were recorded on a Varian 500-MS IT mass spectrometer.
Isolation of lecanoric acid from Melanelia subaurifera
The dried acetone extract of the lichen M. subaurifera (100 mg) was dissolved in toluene. After the precipitate was removed, the solution was concentrated using an evaporator under reduced pressure. The residue was precrystallized from methanol yielding lecanoric acid which was identified by spectroscopic data (Huneck and Yoshimura 1996) and used for determination of antioxidant, antimicrobial and cytotoxic activities. Lecanoric acid: HPLC purity: 99.9 % (254 nm), tR: 2.47 min.
Isolation of 2′-O-methyl anziaic acid from Melanelia fuliginosa
The dried acetone extract of the lichen M. fuliginosa (200 mg) was dissolved in toluene. After the precipitate was removed, the solution was concentrated using an evaporator under reduced pressure and the residue was fractioned on a silica gel column (0.149–0.074 mm; 100–200 mesh). The column was eluted with methanol-chloroform gradient solvent (20:1, 10:1 and 5:1) yielding eight fractions. The third eluted fraction of the lichen extract contains 2′-O-methyl anziaic acid (antimicrobial activity was checked by using different microorganisms v.i.z. 80 mg), which was further purified by crystallization from toluene-cyclohexane and used for structure identification and determination of antioxidant, antimicrobial and cytotoxic activities. The structure of 2′-O-methyl anziaic acid was confirmed by spectral data (Huneck and Yoshimura, 1996). 2′-O-Methyl anziaic acid: 1H NMR (400 MHz, CD3OD, ppm) δ 0.95 (t, 3H, CH3), 0.99 (t, 3H, CH3), 1.41 (sext., 4H, 2× CH2), 1.68 (m, 4H, 2× CH2), 2.98 (m, 4H, 2× CH2), 3.87 (s, 3H, OCH3), 6.41 (2H, Abq, H-3, H-5), 6.66 (1H, d, J = 7.5 Hz, H-3′), 6.77 (1H, d, J = 7.5 Hz, H-5′). MS, m/z 239 (16), 238 (100), 224 (40), 221 (21), 207 (39), 206 (50), 195 (12), 194 (24), 182 (94), 181 (20), 178 (18), 177 (48), 168 (16), 164 (9), 150 (21), 138 (62), 137 (16), 124 (12). Calcd for C25H32O7: 444.050, Found: 444.0511; HPLC purity: 99.8 % (254 nm), tR: 7.44 min.
Antioxidant activity
Scavenging DPPH radicals
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method is an antioxidant assay for measured the free radical scavenging activity of lichen extracts. This method is similar to the method previously used by some authors (Dorman et al. 2004) but was modified in details. Two milliliters of methanol solution of DPPH radical in the concentration of 0.05 mg/ml and 1 ml of lichen extract (1 mg/ml) were placed in cuvettes. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. Then the absorbance was measured at 517 nm in spectrophotometer (Bibby Scientific Limited, Stone, UK). Ascorbic acid was used as positive control. The DPPH radical concentration was calculated using the following equation:
where A0 is the absorbance of the negative control and A1 is the absorbance of reaction mixture or standard. All the measurements were repeated three times, and the results are presented as the mean ± standard error.
The inhibition concentration at 50 % inhibition (IC50) was the parameter used to compare the radical scavenging activity. A lower IC50 meant better radical scavenging activity.
Reducing power
The reducing power of extracts was determined according to the method of Oyaizu (1986). One milliliter of extracts (1 mg/ml) were mixed with 2.5 ml of phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1 %). The mixtures were incubated at 50 °C for 20 min. Then, trichloroacetic acid (10 %, 2.5 ml) was added to the mixture and centrifuged. Finally, the upper layer was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml; 0.1 % ml). The absorbance of the solution was measured at 700 nm in spectrophotometer (Bibby Scientific Limited, Stone, UK). Ascorbic acid was used as positive control. Higher absorbance of the reaction mixture indicated that the reducing power is increased.
Determination of total phenolic compounds
Total soluble phenolic compounds in the lichen extracts were determined with Folin-Ciocalteu reagent according to the method of Slinkard and Singleton (1997) using pyrocatechol as a standard phenolic compound. Briefly, 1 ml of the lichen extract (1 mg/ml) in a volumetric flask diluted with distilled water (46 ml). One milliliter of Folin Ciocalteu reagent was added and the content of the flask was mixed thoroughly. After 3 min 3 ml of sodium carbonate (2 %) was added and then was allowed to stand d for 2 h with intermittent shaking. The absorbance was measured at 760 nm in in spectrophotometer (Bibby Scientific Limited, Stone, UK). The total concentration of phenolic compounds in the extract determined as microgram of pyrocatechol equivalent (PE) per milligram of dry extract by using an equation that was obtained from a standard pyrocatechol graph as follows:
Antimicrobial activity
Microorganisms
Bacillus cereus (ATCC 11778), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Proteus mirabilis (ATCC 12453). All the bacteria used were obtained from the American Type Culture Collection (ATCC). Bacterial cultures were maintained on Müller-Hinton agar substrates (Torlak, Belgrade, Serbia).
The fungi used as test organisms were: Aspergillus flavus (ATCC 9170), Aspergillus niger (ATCC 16888), Candida albicans (ATCC 10231), Mucor mucedo (ATCC 20094), Trichoderma viride (ATCC 13233), Cladosporium cladosporioides (ATCC 11275), Fusarium oxysporum (ATCC 62506), Alternaria alternata (ATCC 11680), Penicillium expansum (ATCC 20466), Penicillium chrysogenum (ATCC 10106). All fungi were procured from the mycological collection maintained at the Mycological Laboratory within the Department of Biology of Kragujevac University’s Faculty of Science (DBFS). Fungal cultures were maintained on potato dextrose (PD) agar except Candida albicans that was maintained on Sabourad dextrose (SD) agar (Torlak, Belgrade). All cultures were stored at 4 °C and subcultured every 15 days.
Suspension preparation
A loopful of isolated bacterial colonies were inoculated into of their respective medium and incubated at 37 °C for 24 h. The turbidity of actively growing bacterial and yeast suspension was adjusted to match the turbidity standard of 0.5 McFarland (bio- Mérieux, Marcy l0Etoile, France) to approximately 108 CFU/ml. Suspensions of fungal spores were prepared from fresh mature (3- to 7-day-old) cultures that grew at 30 °C on potato dextrose (PD) agar substrate. Spores were rinsed with sterile distilled water, used to determine turbidity spectrophotometrically (Jenway, Bibby Scientific Limited, Stone, UK) at 530 nm and then further diluted to approximately 106 CFU/ml according to the procedure recommended by NCCLS (1998).
Minimal inhibitory concentration (MIC)
The minimal inhibitory concentration (MIC) was determined by broth micro dilution method using 96-well micro-titer plates (Spektar, Čačak, Serbia) (Sarker et al. 2007). A series of dilutions with concentrations ranging from 40 to 1.25 mg/ml for extracts and 2 to 0.0625 mg/ml for compounds was used in the experiment against every microorganism tested. Two-fold dilutions of test samples were prepared in Müller-Hinton broth for bacterial cultures and SD broth for fungal cultures. Each plate had a set of controls: a column with a broad-spectrum antibiotic as positive control inhibition, streptomycin was used in the case of bacteria, ketoconazole in the case of fungi. DMSO solution was used as a negative control. The minimal inhibitory concentration was determined with resazurin. To each well 10 μl of resazurin indicator solution was added. Resazurin is an oxidation reduction indicator which is a blue non- fluorescent dye that becomes pink and fluorescent when reduced to resorufin by oxidoreductases within viable cells. The boundary dilution without any changing color of resazurin was defined as the minimal inhibitory concentration (MIC) for the tested microorganism at the given concentration. All experiments were performed in triplicate.
Cytotoxic activity
Cell lines
Human epithelial carcinoma Hela cells, human lung carcinoma A549 cells and human colon carcinoma LS174 cells were obtained from American Type Culture Collection (Manassas, VA, USA). All cancer cell lines were cultured as a monolayer in the RPMI 1640 nutrient medium, with 10 % heat-inactivated (56 °C) fetal bovine serum, (Sigma Chemical Co. St Louis, MO, USA) supplemented with 3 mmol L−1l-glutamine, 100 mg/ml streptomycin, 100 IU/ml penicillin. Cells were grown in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C.
Treatment of cell lines
In vitro assay for cytotoxic activity of investigated samples was performed when the cells reached 70–80 % of confluence. Stock solution of extracts and compounds was dissolved in corresponding medium to the required working concentrations. Neoplastic Hela cells (5000 cells per well), A549 cells (5000 cells per well) and LS174 cells (5000 cells per well) were seeded into 96-well microtiter plates, and 24 h later, after cell adherence, 5 different, double diluted concentrations of investigated extracts were added to the wells. Final concentrations of the extracts were 200, 100, 50, 25, and 12.5 μg/ml except for the control wells, where only nutrient medium was added. The cultures were incubated for the next 72 h.
Determination of cell survival (MTT test)
The effect on cancer cell survival was determined 72 h after the addition of extract, by the MTT test (Mosmann 1983). Briefly, 20 μl of MTT solution (5 mg/ml PBS) was added to each well and incubated for a further 4 h at 37 °C in 5 % CO2 and humidified air. Subsequently, 100 μl of 10 g/l SDS was added to solubilise the formazan crystals formed from MTT after the conversion by mitochondrial dehydrogenases of viable cells. Absorbance was measured using a microplate reader (Multiskan EX, Thermo Scientific, Finland) at 570 nm. Cytotoxic activity of tested samples was compared against normal human fetal lung fibroblast MRC5 cells. Each experiment was performed in triplicate and independently repeated at least four times.
Method validation
The method was validated according to the ICH guidelines for linearity, precision, accuracy, selectivity, limit of detection and limit of quantification (Saran et al. 2013). The sensitivity of method was evaluated by determining the limit of detection (LOD) and limit of quantitation (LOQ) by measuring the magnitude of analytical background by injecting the blank. The LOD of an individual analytical procedure is defined as the lowest amount of an analyte in a sample that can be detected but not necessarily quantified. The LOQ of an individual analytical procedure is the lowest amount of analyte in a sample that can be determined with suitable precision and accuracy. The LOD and LOQ were determined by injecting a series of diluted standard solutions until the signal-to-noise ratio (S/N) for the standards reached a 3:1 ratio for LOD and 10:1 for LOQ, respectively. Calibration curves for 2′-O-methyl anziaic acid and lecanoric acid were prepared by injecting different concentrations of standard samples, recording their peak areas and plotting peak areas vs concentration of the standard. At least six concentrations of the analyte solutions were analyzed in triplicate. Calibration curves were linear over a large concentration range of 15–1000 µg/ml for 2′-O-methyl anziaic acid and 1.5–600 µg/ml for lecanoric acid. The calibration curves and the results of linearity ranges are shown in Fig. 1. The precision was represented by the intra- and inter-day relative standard deviation (%RSD). The inter-day precision (RSD) was determined by analyzing standard solution of 2′-O-methyl anziaic acid and lecanoric acid over the entire calibration range for six different days. The intra-day precision (RSD) was determined by analyzing standard solution of 2′-O-methyl anziaic acid lecanoric acid over the entire calibration range for six times on the same day. By introducing small changes in the mobile phase and flow rate change, the effects on the results were examined. Robustness of the method was done six times and the retention times recorded for our parameters was well within the limit of 1 min. System suitability was evaluated by six replicate analysis of the formulation at a concentration of 10 ppm. The retention time of their areas were recorded subsequently. Mean area and SD was calculated to determine relative SD and the criteria is ≤2 %, respectively. The solution stability of working standard solution of anziaic acid and lecanoric acid was tested at day 0, 24 h and 20 days respectively. The solution stability was determined comparing per cent area and peak purity of these compounds from chromatograms.
Fig. 1.
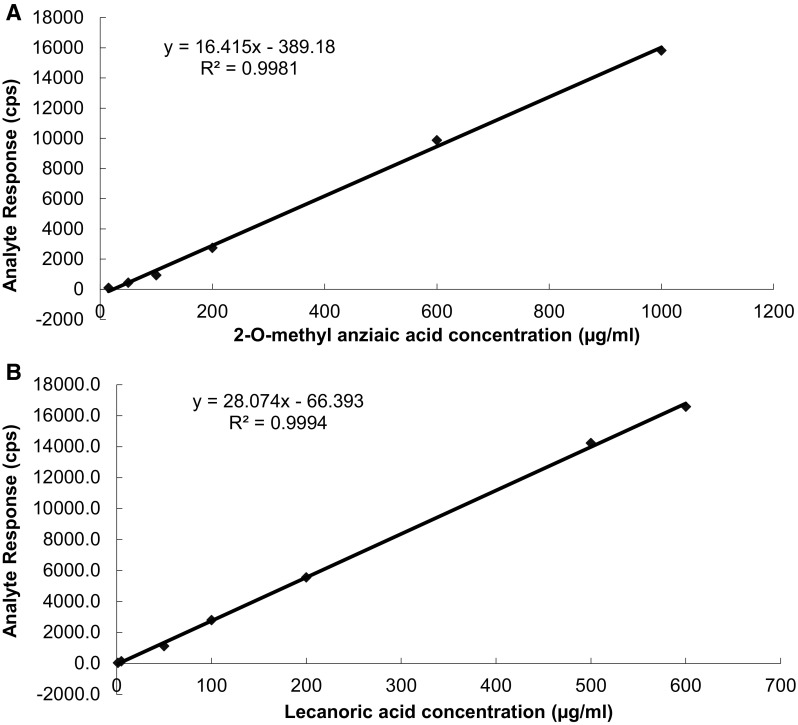
Linearity plot of 2′-O-methyl anziaic acid (a) and lecanoric acid (b)
Statistical analyses
All data were presented as means ± standard deviations (mean ± SD) of three parallel measurements. Statistical analyses were performed using Microsoft Excel and SPSS software software packages. To determine the statistical significance of antioxidant activity, student’s t test was used.
Results
This research indicates significant results of antioxidant, antimicrobial and antitumor activities of acetone extract of lichens species viz. M. subaurifera and M. fuliginosa, and compounds which are isolated from mentioned species, lecanoric acid and 2′-O-methyl anziaic acid.
Chromatograms for standards and M. subaurifera and M. fuliginosa acetone extracts eluted by HPLC are represented in Fig. 2. Depsides (two aromatic rings bonded with ester group), tridepside (three aromatic rings bonded with ester group) and dibenzofuran were observed to be the as the most abundant substances in the extracts.
Fig. 2.
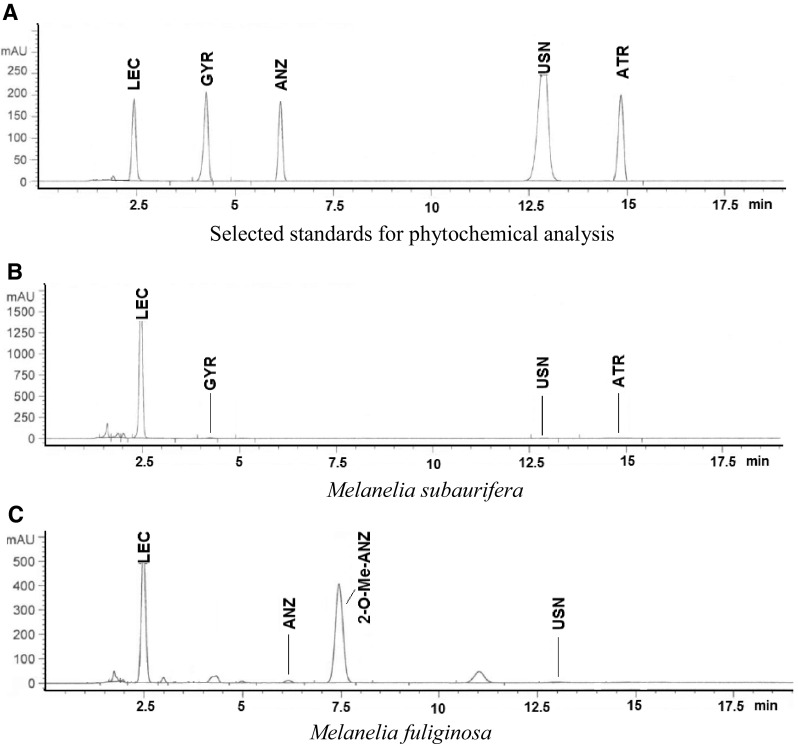
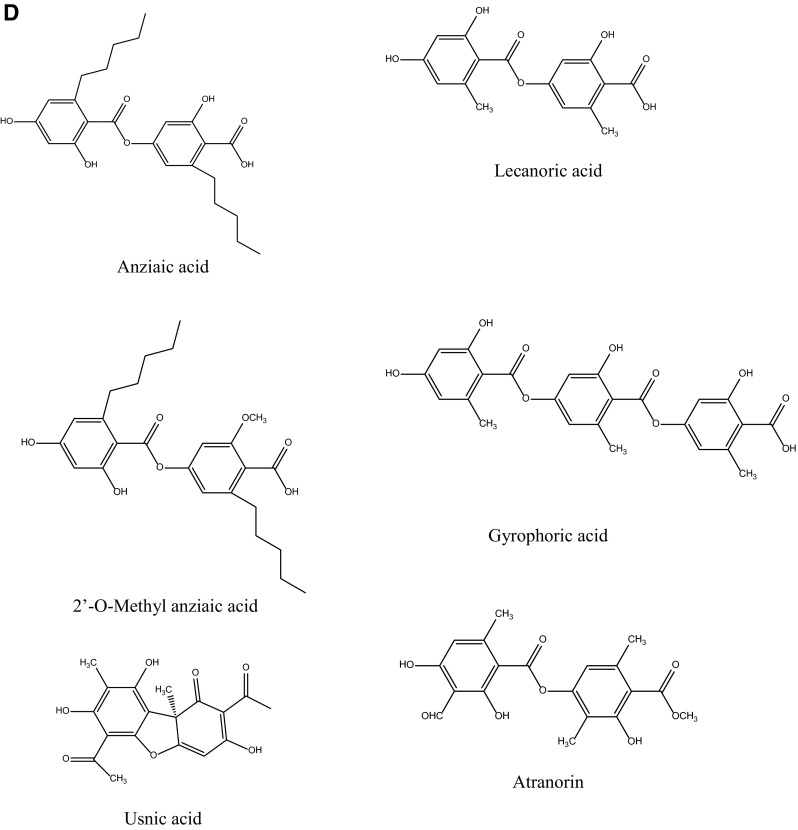
Chromatograms for selected standards (a), for the acetone extracts of Melanelia subaurifera (b) and Melanelia fuliginosa (c) at 254 nm and chemical structures of the isolated compound (d)
Comparing the retention times (tR) and UV spectra (200–400 nm) from HPLC–UV with those of authentic substances, it is readily confirmed that four secondary metabolites, lecanoric acid (tR = 2.47 ± 0.10 min), gyrophoric acid (tR = 4.25 ± 0.20 min), usnic acid (tR = 12.87 ± 0.10 min) and atranorin (tR = 14.87 ± 0.20 min), were identified in M. subaurifera lichen extract.
Lecanoric acid has the most intense peaks in the HPLC chromatogram. This compound belongs to the depsides and has three characteristic absorption maxima at 212, 270, 304 nm. From the HPLC chromatograms of M. fuliginosa acetone extract, we detected anziaic acid (tR = 6.15 ± 0.10 min) and usnic acid. In addition to these minor components, the compounds with the most intense peaks in the chromatogram lecanoric acid and 2′-O-methyl anziaic acid (tR = 7.44 ± 0.10 min and UV spectrum with 213, 270 and 306 nm) were identified. Table 1. show the retention time of the detected lichen substances and their absorbance maxima (nm). After identification of the present metabolites in lichens, two major lichen compounds in these species, lecanoric acid and 2′-O-methyl anziaic acid, were isolated by chromatographic column using different solvent systems and used for investigations of antioxidant, antimicrobial and anticancer activities. The chemical structures of the isolated compounds are shown in Fig. 2.
Table 1.
Retention time of the examined lichen substances and their absorbance maxima (nm)
| Compound | Substance class | Retention time (t R ± SD)a (min) | Absorbance maxima (nm) UV spectrum |
|---|---|---|---|
| Lecanoric acid | Depside | 2.47 ± 0.10 | 212, 240, 320m |
| Gyrophoric acid | Tridepside | 4.25 ± 0.20 | 213, 270, 304 |
| Anziaic acid | Depside | 6.15 ± 0.10 | 214, 270, 309 |
| 2-O-Methyl anziaic acid | Depside | 7.44 ± 0.10 | 213, 270, 306 |
| Usnic acid | Dibenzofurane | 12.87 ± 0.10 | 234, 282 |
| Atranorin | Depside | 14.87 ± 0.20 | 212, 278, 312m |
aValues are the means of three determinations ± SD, m—minor absorbance maximum
Antioxidant activities as assessed by DPPH radical scavenging capacities were determined and shown in Table 2. The tested extracts as well as isolated components showed a dose dependent activity. The results of this assay present that IC50 values of lichen extracts and tested compounds range from 121.52 to 424.51 µg/ml. Acetone extract of M. subaurifera exhibit largest DPPH radical scavenging activity (IC50 = 165.13 µg/ml), than extract of M. fuliginosa (IC50 = 266.32 µg/ml). The IC50 value of compound lecanoric acid is higher (IC50 = 424.51) compared to other compound 2′-O-methyl anziaic acid (IC50 = 121.52). There was a statistically significant difference between samples and control (p < 0.05).
Table 2.
DPPH radical scavenging activity, reducing power and phenolics content of acetone extracts of lichens Melanelia subaurifera and Melanelia fuliginosa, and their major metabolites
| Tested lichen species and compounds | DPPH radical scavenging IC50 (μg/ml) |
|---|---|
| Melanelia subaurifera | 165.13 ± 1.78 |
| Melanelia fuliginosa | 266.32 ± 2.08 |
| Lecanoric acid | 424.51 ± 1.42 |
| 2′-O-methyl anziaic acid | 121.52 ± 1.12 |
| Ascorbic acida | 6.42 ± 0.18 |
| Tested lichen species and compounds | Reducing power Absorbance (700 nm) |
|||
|---|---|---|---|---|
| 1000 µg/ml | 500 µg/ml | 250 µg/ml | 125 µg/ml | |
| Melanelia subaurifera | 0.1189 ± .031 | 0.0744 ± .004 | 0.0327 ± .001 | 0.0316 ± .003 |
| Melanelia fuliginosa | 0.0905 ± .006 | 0.0623 ± .003 | 0.0468 ± .002 | 0.0252 ± .002 |
| Lecanoric acid | 0.0336 ± .001 | 0.0178 ± .002 | 0.0170 ± .001 | 0.0165 ± .001 |
| 2′-O-methyl anziaic acid | 0.2048 ± .042 | 0.0335 ± .002 | 0.0128 ± .002 | 0.0056 ± .000 |
| Ascorbic acida | 2.113 ± .032 | 1.654 ± .021 | 0.0957 ± .008 | 0.0478 ± .004 |
| Lichen species | Phenolics content (μg PE/mg of extract) |
|||
|---|---|---|---|---|
| Melanelia subaurifera | 41.33 ± 1.065 | |||
| Melanelia fuliginosa | 39.68 ± 1.013 | |||
Values are expressed as mean ± SD of three parallel measurements
PE pyrocatechol equivalents
aAscorbic acid was used as standard
The results of the reducing power assay of lichen extracts and isolated compounds are summarized in Table 2. The tested extracts as well as isolated components showed a dose dependent activity. Tested extract showed strong reducing power activity. There was a statistically significant difference between samples and control (p < 0.05). Measured values of absorbance varied from 0.0336 to 0.2048. High absorbance of extracts means high reducing power. Among tested extracts, acetone extract of lichen M. subaurifera gave highest reducing power than M. fuliginosa. The isolated compounds 2′-O-methyl anziaic acid showed higher reducing power than lecanoric acid.
Total phenolic constituents of tested extracts are given in Table 2. The total phenolic compounds was determined as the pyrocatechol equivalent using an equation obtained from a standard pyrocatechol graph (y = 0.0057x − 0.1646, R2 = 0.9934). Result show that total phenolics contents of the acetone extracts of M. subaurifera and M. fuliginosa were 39.68 and 41.33 µg PE/mg.
Various antioxidant activities were compared to ascorbic acid. The results showed that standard antioxidant had stronger activity than tested samples.
In our experiments, it was recorded relatively strong antimicrobial activity of tested lichen extracts and their metabolites. Both tested compounds inhibited all tested bacteria and fungi, while the antimicrobial activity of the tested extracts was selective. The maximum antimicrobial activity was found in the 2′-O-methyl anziaic acid, compound which is isolated from lichen M. fuliginosa and which in relatively low concentrations inhibited the tested microorganisms. The MIC for isolated compound ranged from 0.0625 to 1 mg/ml. both tested compounds inhibiting all tested bacteria and fungi species.
The antimicrobial activity was compared with the standard antibiotics, streptomycin (for bacteria) and ketoconazole (for fungi). The results showed that standard antibiotics had stronger activity than tested samples (Table 3). In a negative control, DMSO had no inhibitory effect on the tested organisms.
Table 3.
Minimum inhibitory concentration (MIC) of acetone extracts of Melanelia subaurifera, Melanelia fuliginosa, lecanoric acid and 2-O-methyl-anziaic acid and their growth inhibitory effects on Hela, A549 and LS174l malignant cells and MRC5 normal cell
| Minimum inhibitory concentration [MIC (mg/ml)] | |||||
|---|---|---|---|---|---|
| Lichen species Test microbes |
Melanelia subaurifera | Melanelia fuliginosa | Lecanoric acid | 2′-O-methyl anziaic acid | Antibiotics |
| S. aureus | 20* | 10 | 1 | 1 | 0.031 |
| B. subtilis | 5 | 10 | 1 | 0.25 | 0.016 |
| B. cereus | 1.25 | 2.5 | 0.5 | 0.0625 | 0.016 |
| E. coli | / | 20 | 1 | 1 | 0.062 |
| P. mirabilis | 10 | 5 | 1 | 0.5 | 0.062 |
| M. mucedo | 10 | 10 | 1 | 0.5 | 0.156 |
| T. viride | 5 | 10 | 1 | 0.25 | 0.078 |
| C. cladosporioides | 10 | 5 | 1 | 0.25 | 0.039 |
| F. oxysporum | 10 | 2.5 | 1 | 0.25 | 0.078 |
| A. alternata | 10 | 5 | 1 | 0.25 | 0.078 |
| A. flavus | / | / | 1 | 1 | 0.312 |
| A. niger | 20 | 20 | 1 | 0.5 | 0.078 |
| C. albicans | 5 | 2.5 | 0.5 | 0.25 | 0.039 |
| P. expansum | 20 | 10 | 1 | 0.5 | 0.156 |
| P. chrysogenum | 10 | 20 | 1 | 0.5 | 0.078 |
| Growth inhibitory effects [IC50 (μg/ml)] | |||||
|---|---|---|---|---|---|
| Lichen species Cell lines |
Melanelia subaurifera | Melanelia fuliginosa | Lecanoric acid | 2′-O-methyl anziaic acid | Cis-DDP |
| Hela | 9.88 ± 0.56 | 45.24 ± 1.23 | 123.97 ± 2.06 | 151.79 ± 2.72 | 0.86 ± 0.33 |
| A549 | 31.25 ± 1.02 | 125.76 ± 2.11 | >200 | 154.11 ± 3.58 | 4.91 ± 0.42 |
| LS174 | 31.64 ± 1.55 | 142.87 ± 2.07 | >200 | >200 | 3.18 ± 0.29 |
| MRC5 | >200 | >200 | >200 | >200 | 13.21 ± 0.37 |
Antibiotics: Streptomycin (for bacteria), Ketoconazole (for fungi)
The result of cytotoxic activity of the studied lichens extracts and their mentioned metabolites, related to tested cell lines was shown in the Table 3. The tested extracts as well as isolated components showed a dose dependent activity. M. subaurifera extract shows the higher cytotoxic activity. The IC50 against Hela cells, A549 cells and LS174 cells was 9.88, 31.25 and 31.64 μg/ml, respectively. M. fuliginosa manifested a slightly lower cytotoxic activity. The IC50 value was 45.24 μg/ml related to Hela cell, 125.76 related to A549 cells and 142.87 μg/ml related to LS174 cell line. Both isolated metabolites lecanoric acid and 2′-O-methyl anziaic acid exhibited weaker activity compared to the extracts. Cytotoxic activity of tested samples on normal MRC5 cells was not observed. Furthermore, all samples showed less activity compared to cis-DDP as a positive control.
Results of analytical RP-HPLC method validation offers reliable evaluation of the marker compounds 2′-O-methyl anziaic acid and lecanoric acid such as chemical and active markers that possess biological activity of the different lichen species. This method offered adequate method validation parameters such as calibration curve, linearity, limit of detection (LOD), limit of quantification (LOQ), precission, accuracy, solution stability, ruggednes/robustness method application and system suitability. In the case of 2′-O-methyl anziaic acid, a very good linearity was successfully achieved in the concentration range of 15–1000 µg/ml. For lecanoric acid in the concentration range of 1.5–600 µg/ml good linearity is successful. The correlation coefficient (R2) and regression equation was found to be 0.9981 and y = 16.415x − 389.18 for 2′-O-methyl anziaic acid. For lecanoric acid, these parameters were R2 = 0.9994 and y = 28.074x − 66,393. The relative peak area (RPA) and relative retention time (RRT) for both lichen samples were used for quantifying 2′-O-methyl anziaic acid and lecanoric acid from investigated lichen species. The concentration of above-mentioned compounds (mg/gm) and %CV are shown in Table 4.
Table 4.
Application of method to quantification and method validation parameters for quantification of 2-O-methyl-anziaic acid and lecanoric acid from the lichen Melanelia subaurifera and Melanelia fuliginosa
| Application of method to quantification of 2-O-methyl-anziaic acid and lecanoric acid | ||
|---|---|---|
| Sample | Concentration (mg/gm)a | % CV |
| 2-O-methyl-anziaic acid | ||
| Melanelia fuliginosa | 4.24 ± 0.04 | 4.39 |
| Lecanoric acid | ||
| Melanelia subaurifera | 8.71 ± 0.01 | 3.24 |
| Method validation parameters | ANZ | LEC |
|---|---|---|
| LOD (µg/ml) | 0.71 | 0.26 |
| LOQ (µg/ml) | 2.37 | 0.87 |
| Linear range (µg/ml) | 15–1000 | 1.5–600 |
| Mean correlation coefficient (r2) | 1 | 1 |
| System suitability (% CV, n = 6) | 0.89 | 0.92 |
| Retention time (min) | 7.44 | 2.47 |
| Accuracy and precision (% CV, n = 3) | ||
| Intraday | 0.64–1.11 | 0.89–1.11 |
| Interday | 0.96–1.42 | 1.00–1.56 |
| Stability | 24 h | |
| Melanelia subaurifera | 97.18 % | |
| Melanelia fuliginosa | 94.53 % | |
| Stability | 20 days | |
| Melanelia subaurifera | 95.52 % | |
| Melanelia fuliginosa | 92.87 % | |
| Mobile phase MeOH: d/w, v/v | Retention time (min) | |
|---|---|---|
| ANZ | LEC | |
| Ruggedness | ||
| 81:19 | 7.32 | 2.38 |
| 80:20 | 7.44 | 2.47 |
| 79:21 | 7.69 | 2.54 |
| Flow rate change (ml/min) | ||
| 0.9 | 7.31 | 2.35 |
| 1.0 | 7.44 | 2.47 |
| 1.1 | 6.80 | 2.10 |
aMean ± SD, n = 3 (batches)
As shown in Table 4, the limit of determination (LOD) for 2′-O-methyl anziaic acid and lecanoric acid were found to be 0.71 and 0.26 µg/ml, respectively. The limits of quantification (LOQ) were 2.37 µg/ml for 2′-O-methyl anziaic acid and 0.87 µg/ml for lecanoric acid. The Table 4 also presents the accuracy and intraday and interday precision data for both marker compounds. These data suggested that this method is validated, satisfactory and highly reproducible. Stability of 2′-O-methyl anziaic acid and lecanoric acid for 24 h and 20 days are shown in Table 4. The different retention times (within the limit 1 min) were recorded by using different composition of mobile phase and changing flow rate and are given in Table 4. System suitability for six replicate analyses (%CV, n = 6) were found to be 0.89 for 2′-O-methyl anziaic acid and 0.92 for lecanoric acid. The relative retention time (RRT) and relative peak area (RPA) of each characteristic from samples related to the reference peak was calculated for quantifying compounds 2′-O-methyl anziaic acid and lecanoric from the lichens M. subaurifera and M. fuliginosa.
Discussion
Identification of compounds was achieved by comparison of their tR values with the standard substances previously isolated from lichens. The results of UV absorbance spectral analysis were in agreement with (200–400 nm) Yoshimura et al. (1994). 2′-O-Methyl anziaic acid is less widespread in lichens than anziaic acid and has previously been report in Lecidea diducens and Lecidea speirodes (Culberson and Hertel 1972).
Recent studies have shown that anziaic acid exhibits antibacterial activity and have been also found to act as an inhibitor of human topoisomerase II but had little effect on human topoisomerase I. This investigation showed that anziaic acid can act as a topoisomerase poison, suggesting that depsides could be exploited to provide novel leads for antimicrobial and anticancer agents (Cheng et al. 2013). The preliminary structure–activity relationship (SAR) has been shown that lipophilic n-pentil groups play an important role in enhance topoisomerase I and gyrase inhibition as well as antibacterial activity (Lin et al. 2013).
Antioxidant activities of various lichen species investigated many researchers. Lichen extracts have been reported for antioxidant properties due to their phenolic content by few authors (Behera et al. 2009). In most lichens, phenols, including depsides, depsidones, and dibenzofurans, are important antioxidants because of their ability to scavenge free radicals such as singlet oxygen, superoxide, and hydroxyl radicals. The antioxidant activities of the individual phenolic compounds may depend on structural factors, such as the number of phenolic hydroxyl or methoxyl groups and free carboxylic groups and other structural features (Jayabalan et al. 2008). Researches proved antioxidant activity of some lichen species such as Bryoria fuscescens, Cetraria islandica, Lecanora muralis, Dermatocarpon intestiniformis, Parmelia saxatilis, Parmeliopsis ambigua, Peltigera rufescens, Platismatia glauca, Ramalina pollinaria, R. polymorph, Umbilicaria nylanderiana,Umbilicaria crustulosa, Usnea ghattenis, and U. longissima (Behera et al. 2009; Kosanić et al. 2012). Lopes et al. (2008) indicated on free radical scavenging activity of lecanoric acid and its derivatives. Jayaprakasha and Rao (2000) reported that phenolic constituents from the lichen Parmotrema stuppeum, including methyl orsenillate, orsenillic acid, atranorin and lecanoric acid showed moderate antioxidant activity.
It is necessary to understand that extracts are mixtures of natural compounds, and their antioxidant activity is not only a result of the different activities of individual components but may be the result of their interactions, which can have different effects on the overall activity of extracts. In our case, the antioxidant activity of the Melanelia subaurifera extract may be the result of synergistic effect of several compounds present in the extract, which in associated action contribute a relatively high total antioxidant effect of extract.
The antimicrobial activity of the acetone extracts of the lichens M. subaurifera and M. fuliginosa and their metabolites, was tested against the above mentioned Gram-positive and Gram-negative bacteria and several species of fungus by minimal inhibitory concentration (MIC). Antibacterial activity was stronger than antifungal activity for tested metabolites, but it was relatively identical for lichens extracts. Reason for greater sensitivity of bacteria than fungi is differences in the composition and permeability of the cell wall. The cell wall of Gram-positive bacteria is made of peptidoglucanes and teichoic acids, the cell wall of Gram-negative bacteria is made of peptidoglucanes, lipopolysacharides and lipoproteins, while the cell wall of fungi is poorly permeable and it consists of polysaccharides such as hitchin and glucan (Kosanić et al. 2012). In terms on antimicrobial activity we found we found interesting result that P. mirabilis (Gram-negative bacteria) was sensitive than S. aureus (Gram-positive bacteria). This observation is comparable with some other studies (Bnyan et al. 2013; Hisham 2014) that have also found that P. mirabilis is sensitive than S. aureus.
Of total number of lichens, about 50 % have been reported to possess antibiotic activities (Sharnoff 1997). Accordingly, lichen extract and their antibiotic properties are of special interest to scientists, and because of that they are the subject of many studies (Swathi et al. 2010; Kosanić et al. 2012). Most of the antibacterial activities were tested on Staphylococcus aureus, Bacillus, Pseudomonas, Escherichia coli, Kleibsiella, Candida, Salmonella, Yersinia and Proteus sp., (Swathi et al. 2010). The intensity of the manifestation of inhibition depends on the solvent used in the extraction. Because of that, various solvents are used in research. Methanol is the most commonly used solvent for extraction of bioactive compound from lichens. However, different organisms respond the differently to same concentration of a given solvent, as apparent in the results of some researches. Knowing this, we used in this research acetone for extraction of lichens materials.
In this study the most sensitive organisms was Bacillus cereus, and the most resistant was Gram-negative bacteria E. coli and fungi species Aspergillus flavus. Although some literature data reported that among the Bacillus species, B. sublitis was the most sensitive microorganism to lichen substances such as atranorin, usnic acid, norstictic acid, protoacetraric acid, fumaroprotoacetraric acid, atranol, lecanoric acid, stictic acid, divericatic acids and zeorin (Vasudeo and Lew 2011). It was also observed that the tested extract was inactive against E.coli, whereas the isolated compound exhibited a antimicrobial effect. However, in several previous experiments (Schinor et al. 2007; Dos Santos et al. 2012) also it has been found that the extracts do not inhibit the grow of microorganisms, but their isolated compounds demonstrate an antimicrobial effect. This is because components are present in the extract act antagonistically and thus reduce the activity of the extract.
In cytotoxicity, tested extracts exhibited better activity than the isolated components. Our results may indicate the possible participation of active substances other than lecanoric acid and 2 O-methyl anziaic, which in the combined effect exhibit higher cytotoxic activity than single tested substances, which is reflected on the overall activity of the tested extracts.
Until now, only few researchers proved that lichen have anticancer activity. Bezivin et al. (2003) reported significant anticancer effect for Parmelia caperata, Cladonia convoluta, C. rangiformis, Platisma glauca and Ramalina cuspidata. Manojlović et al. (2010) explored anticancer properties of Thamnolia vermicularis. Lecanoric acid and its derivatives had been found to have a lot of bioactivities in previous studies. Bogo et al. (2010) reported that lecanoric acid, a secondary metabolite from Parmotrema tinctorum, exerted anticancer activities against HEp-2 larynx carcinoma, MCF7 breast carcinoma, 786-0 kidney carcinoma and B16- F10 murine melanoma cell lines. Also, cytotoxic activities of some other lichen constituents have been reviewed by some authors (Vasudeo and Lew 2011).
Is very known that constituents present in extracts may interact to produce synergistic or antagonistic antioxidant, antimicrobial and cytotoxic effects with each other, and these interactions may influence the total biological activity of extracts. We believe that our results on antioxidant, antimicrobial and cytotoxic activity of tested lichens come from these various interactions. Tested extracts showed a stronger activity than tested isolated components because they are contained and other highly active components such as usnic acid, gyrophoric acid, atranorin, for which earlier in numerous works is proved to be extremely active. Therefore, extracts in the common synergistic effect of these components showed stronger activity than the single isolated components.
In this study, we have explored chemical composition of acetone extracts of the foliose lichens M. subaurifera and M. fuliginosa and the antioxidant, antimicrobial and antitumor activities of their extracts as well as some their major metabolites. Lichen acids which we tested were depsides: lecanoric acid and 2′-O-methyl anziaic acid. As we expected, lichen extracts and isolated compounds show strong antioxidant, antimicrobial and cytotoxic activities. RP-HPLC methods was developed and validated for quantitative estimation of lecanoric acid and 2′-O-methyl anziaic acid from lichens M. subaurifera and M. fuliginosa successfully. The concentrations (mg/gm) and % CV of 2-O-methyl-anziaic acid in the Melanelia fuliginosa were 4.24 ± 0.04 mg/gm and 4.39, respectively. These values for lecanoric acid in Melanelia subaurifera were 8.71 ± 0.01 mg/gm and 3.24, respectively. This method can be successfully applied for quality control of the lichen and other botanical drugs containing lecanoric acid and 2′-O-methyl anziaic acid and for their identification and standardization.
These results show that lichens and their secondary metabolites are good sources of natural antibiotics, antioxidants and anticarcinogens. Further investigations should be done to search new bioactive compounds from lichens, with stronger biological activity.
Acknowledgments
This work was financed in part by the Ministry of Science, Technology, and Development of the Republic of Serbia and was carried out within the framework of Project Nos. 173032, 175011 and 172015.
References
- Behera BC, Verma N, Sonone A, Makhija U. Optimization of culture conditions for lichen Usnea ghattensis G. awasthi to increase biomass and antioxidant metabolite production. Food Technol Biotechnol. 2009;47:7–12. [Google Scholar]
- Berdy J. Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot. 2012;65:385–395. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- Bezivin C, Tomasi S, Lohezic-Le Devehat F, Boustie C. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine. 2003;10:499–503. doi: 10.1078/094471103322331458. [DOI] [PubMed] [Google Scholar]
- Blanco O, Crespo A, Divakar PK, Esslinger TL, Hawksworth DL, Lumbsch HT. Melanelixia and Melanohalea, two new genera segregated from Melanelia (Parmeliaceae) based on molecular and morphological data. Mycol Res. 2004;108:873–884. doi: 10.1017/S0953756204000723. [DOI] [PubMed] [Google Scholar]
- Bnyan I, Hasan H, Ewadh M. Antimicrobial activity of Citrullus colocynthis against different types of bacteria. Adv Life Sci Technol. 2013;7:48–51. [Google Scholar]
- Bogo D, de Matos MF, Honda NK, Pontes EC, Oguma PM, da Santos EC, et al. In vitro antitumour activity of orsellinates. Z Naturforsch C. 2010;65:43–48. doi: 10.1515/znc-2010-1-208. [DOI] [PubMed] [Google Scholar]
- Boustie J, Tomasi S, Grube M. Bioactive lichen metabolites: alpine habitats as an untapped source. Phytochem Rev. 2011;10:287–307. doi: 10.1007/s11101-010-9201-1. [DOI] [Google Scholar]
- Cheng B, Cao S, Vasquez V, Annamalai T, Tamayo-Castillo G, Clardy J, et al. Identification of anziaic acid, a lichen depside from Hypotrachyna sp., as a new topoisomerase poison inhibitor. PLoS ONE. 2013;8:e60770. doi: 10.1371/journal.pone.0060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culberson CF, Hertel H. 2′-O-methylanziaic acid, a new depside in Lecidea diducens and Lecidea speirodes. The Brylogist. 1972;75:372–376. doi: 10.2307/3241483. [DOI] [Google Scholar]
- Dobson FS. Lichens. An illustrated guide to the British and Irish species. 6. London: Richmond Publishing Co; 2011. [Google Scholar]
- Dorman HJ, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem. 2004;52:762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- Dos Santos FM, de Souza MG, Crotti AE, Martins CH, Ambrósio SR, Veneziani RC, Silva EML, Cunha WR. Evaluation of antimicrobial activity of extracts of Tibouchina candolleana (melastomataceae), isolated compounds and semi-synthetic derivatives against endodontic bacteria. Braz J Microbiol. 2012;43:793–799. doi: 10.1590/S1517-83822012000200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisham AA. Comparative antibacterial and antibiofilm activities of manuka honey and Egyptian clover honey. Asian J Appl Sci. 2014;2:110–115. [Google Scholar]
- Huneck S, Yoshimura I. Identification of lichen substances. Berlin Heidelberg: Springer; 1996. [Google Scholar]
- Jayabalan R, Subathradevi P, Marimuthu S, Sathishkumar M, Swaminathan K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008;109:227–234. doi: 10.1016/j.foodchem.2007.12.037. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha GK, Rao LJ. Phenolic constituents from the lichen Parmotrema stuppeum (Nyl.). Hale and their antioxidant activities. Z Naturforsch C. 2000;55:1018–1022. doi: 10.1515/znc-2000-11-1227. [DOI] [PubMed] [Google Scholar]
- Karagoz A, Dogruoz N, Zeybek Z, Aslan A. Antibacterial activity of some lichen extracts. J Med Plants Res. 2009;3:1034–1039. [Google Scholar]
- Kosanić M, Ranković B, Stanojković T. Antioxidant, antimicrobial, and anticancer activities of three Parmelia species. J Sci Food Agric. 2012;92:1909–1916. doi: 10.1002/jsfa.5559. [DOI] [PubMed] [Google Scholar]
- Lin H, Annamalai T, Bansod P, Tse-Dinhb Y, Sun D. Synthesis and antibacterial evaluation of anziaic acid and analogues as topoisomerase I inhibitors. Med Chem Commun. 2013;4:1613–1618. doi: 10.1039/c3md00238a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes TI, Coelho RG, Yoshida NC, Honda NK. Radicalscavenging activity of orsellinates. Chem Pharm Bull. 2008;56:1151–1554. doi: 10.1248/cpb.56.1551. [DOI] [PubMed] [Google Scholar]
- Manojlović N, Vasiljević P, Jusković M, Najman S, Janković S, Milenković-Andjelković A. HPLC analysis and cytotoxic potential of extracts from the lichen, Thamnolia vermicularis var. Subuliformis. J Med Plants Res. 2010;4:817–823. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards) Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: Proposed standard M38-p. Wayne: NCCLS; 1998. [Google Scholar]
- Oyaizu M. Studies on products of browning reaction prepared from glucoseamine. Jpn J Nutr. 1986;44:307–314. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Saran S, Menon S, Shailajan S, Pokharna P. Validated RP-HPLC method to estimate eugenon from commercial formulations like Caturjata Churna, Lavangadi Vati, Jatiphaladi Churna, Sitopaladi Churna and clove oil. J Pharm Res. 2013;6:53–60. [Google Scholar]
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinor EC, Salvador MJ, Ito IY. Evaluation of the antimicrobial activity of crude extracts and isolated constituents from Chresta scapigera. Braz J Microbiol. 2007;38:145–149. doi: 10.1590/S1517-83822007000100030. [DOI] [Google Scholar]
- Sharnoff SD (1997) Lichens and people. Online, http://www.lichen.com/people.html
- Shukla V, Joshi G, Rawat M. Lichens as a potential natural source of bioactive compounds: a review. Phytochem Rev. 2010;9:303–314. doi: 10.1007/s11101-010-9189-6. [DOI] [Google Scholar]
- Slinkard K, Singleton VL. Total phenolic analyses: automation and comparison with manual method. Am J Enol Vitic. 1997;28:49–55. [Google Scholar]
- Swathi D, Suchitha Y, Prashith Kekuda TR, Venugopal TM, Vinayaka KS, Mallikarjun N, et al. Antimicrobial, antihlmintic and insecticidal activity of a macrolichen Everniastrumcirrhatum (FR.) Hale. IJDDR. 2010;2:780–789. [Google Scholar]
- Vasudeo PZ, Lew C. Biopharmaceutical potential of lichens. Pharm Biol. 2011;50:778–798. doi: 10.3109/13880209.2011.633089. [DOI] [PubMed] [Google Scholar]
- Wirth V. Die Flechten Baden-Würtembergs, Verbreitungsatlas, 1and2. Stuttgart: Eugen Ulmer GmbHandCo; 1995. [Google Scholar]
- Yoshimura I, Kinoshita Y, Yamamoto Y, Huneck S, Yamada Y. Analysis of secondary metabolites from lichen by high performance liquid chromatography with a photodiode array detector. Phytochem Anal. 1994;5:197–205. doi: 10.1002/pca.2800050405. [DOI] [Google Scholar]


