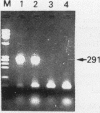
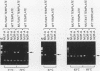
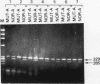
Abstract
AIM: Development of a specific polymerase chain reaction (PCR) assay for detection of the pre-core, stop codon, mutant of hepatitis B virus (HBV). METHODS: PCR primers, specific at the 3'-end for nucleotide 1896 of either the pre-core, stop codon, mutant or wild type HBV, were synthesised using published sequence data. Positive control templates for both types of virus were synthesised by the PCR, incorporating sequences specific for each virus type at the appropriate position. These templates were used to optimise the specificity of the procedure. Formalin fixed, paraffin wax embedded human tissue from acute or fulminant HBV hepatitis from Hong Kong or Oxford was then investigated for presence of mutant or wild type virus. The HBV DNA was amplified from this tissue using a two step procedure, with an initial amplification phase followed by a second diagnostic phase on optimally diluted target DNA. RESULTS: Specific detection of mutant or wild type HBV was achieved. An important factor in determining specificity was the temperature of annealing, 70 degrees C proving to be highly specific. To overcome the inherent variation of target copy number in clinical samples and to provide an intrinsic positive control, it was important to generate and standardise the amount of target HBV used for the specific PCR. Two cases of fulminant hepatitis and four cases of acute hepatitis from Hong Kong, and one case of fulminant hepatitis from Oxford, contained only wild type HBV, with no evidence of a mutant virus. CONCLUSION: This method can be applied to FFPE tissues. It is rapid, non-radioactive, and specific for the stop codon mutation at nucleotide 1896 of HBV. Preliminary investigation of a small number of cases of fulminant hepatitis from Oxford and Hong Kong showed only wild type virus. The result differs from results published from Japan and Israel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An S. F., Fleming K. A. Removal of inhibitor(s) of the polymerase chain reaction from formalin fixed, paraffin wax embedded tissues. J Clin Pathol. 1991 Nov;44(11):924–927. doi: 10.1136/jcp.44.11.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetto M. R., Giarin M. M., Oliveri F., Chiaberge E., Baldi M., Alfarano A., Serra A., Saracco G., Verme G., Will H. Wild-type and e antigen-minus hepatitis B viruses and course of chronic hepatitis. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4186–4190. doi: 10.1073/pnas.88.10.4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman W. F., Jacyna M. R., Hadziyannis S., Karayiannis P., McGarvey M. J., Makris A., Thomas H. C. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989 Sep 9;2(8663):588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Murray K. Expression of the hepatitis B virus surface, core and E antigen genes by stable rat and mouse cell lines. J Mol Biol. 1982 Nov 25;162(1):43–67. doi: 10.1016/0022-2836(82)90161-9. [DOI] [PubMed] [Google Scholar]
- Kaneko S., Feinstone S. M., Miller R. H. Rapid and sensitive method for the detection of serum hepatitis B virus DNA using the polymerase chain reaction technique. J Clin Microbiol. 1989 Sep;27(9):1930–1933. doi: 10.1128/jcm.27.9.1930-1933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka Y., Takase K., Kojima M., Shimizu M., Inoue K., Yoshiba M., Tanaka S., Akahane Y., Okamoto H., Tsuda F. Fulminant hepatitis B: induction by hepatitis B virus mutants defective in the precore region and incapable of encoding e antigen. Gastroenterology. 1991 Apr;100(4):1087–1094. doi: 10.1016/0016-5085(91)90286-t. [DOI] [PubMed] [Google Scholar]
- Lo Y. M., Mehal W. Z., Fleming K. A. In vitro amplification of hepatitis B virus sequences from liver tumour DNA and from paraffin wax embedded tissues using the polymerase chain reaction. J Clin Pathol. 1989 Aug;42(8):840–846. doi: 10.1136/jcp.42.8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata M., Ehata T., Yokosuka O., Hosoda K., Ohto M. Mutations in the precore region of hepatitis B virus DNA in patients with fulminant and severe hepatitis. N Engl J Med. 1991 Jun 13;324(24):1699–1704. doi: 10.1056/NEJM199106133242404. [DOI] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]