Abstract
Purpose
To identify the prognostic factors related to tumor recurrence and progression in Korean patients with non-muscle-invasive bladder cancer (NMIBC).
Materials and Methods
Data were collected and analyzed for 2412 NMIBC patients from 15 centers who were initially diagnosed after transurethral resection of bladder tumor (TURBT) from January 2006 to December 2010. Using univariable and multivariable Cox proportional hazards models, the prognostic value of each variable was evaluated for the time to first recurrence and progression.
Results
With a median follow-up duration of 37 months, 866 patients (35.9%) experienced recurrence, and 137 (5.7%) experienced progression. Patients with recurrence had a median time to the first recurrence of 10 months. Multivariable analysis conducted in all patients revealed that preoperative positive urine cytology (PUC) was independently associated with worse recurrence-free survival [RFS; hazard ratio (HR) 1.56; p<0.001], and progression-free survival (PFS; HR 1.56; p=0.037). In particular, on multivariable analysis conducted for the high-risk group (T1 tumor/high-grade Ta tumor/carcinoma in situ), preoperative PUC was an independent predictor of worse RFS (HR 1.73; p<0.001) and PFS (HR 1.96; p=0.006). On multivariable analysis in patients with T1 high-grade (T1HG) cancer (n=684), better RFS (HR 0.75; p=0.033) and PFS (HR 0.33; p<0.001) were observed in association with the administration of intravesical Bacillus Calmette-Guérin (BCG) induction therapy.
Conclusion
A preoperative PUC result may adversely affect RFS and PFS, particularly in high-risk NMIBC patients. Of particular note, intravesical BCG induction therapy should be administered as an adjunct to TURBT in order to improve RFS and PFS in patients with T1HG cancer.
Keywords: Urinary bladder neoplasm, recurrence, disease progression, prognosis
INTRODUCTION
Bladder cancer is the second most common malignancy of the genitourinary tract and shows a male predominance.1,2 In the United States, bladder cancer was ranked fourth, considering the incidence among all newly diagnosed cancers in men in 2014.1 It is also estimated that in Korea, a total of 4173 cases were newly diagnosed in 2014, giving bladder cancer a rank of seventh, considering the incidence in men.2 Urothelial carcinoma of the bladder (UCB) is the most common histologic type, accounting for 80–90% of all bladder cancer. Approximately 75% of patients with UCB are initially diagnosed with non-muscle-invasive bladder cancer (NMIBC), which is confined to either the mucosa [pTa, carcinoma in situ (CIS)] or the submucosa (pT1).3 Transurethral resection of bladder tumor (TURBT) is primarily used for confirmative diagnostic and therapeutic purposes in NMIBC. However, a substantial number of patients (50–70%) experience disease recurrence within 5 years after TURBT, and 10–20% of tumors that have recurred progress to higher-stage (muscle invasion) or higher-grade disease.3,4 Therefore, the prevention of disease recurrence and progression is an important issue in the management of NMIBC, resulting in the need for adjuvant therapy in nearly all NMIBC patients.
The recognition of prognostic factors associated with the recurrence and progression of NMIBC is crucial for patient counseling and clinical decision making related to adjuvant therapy, such as intravesical chemotherapy (IVC) and Bacillus Calmette-Guérin (BCG) immunotherapy. Both pathologic tumor stage and grade are essentially determinant prognosticators for NMIBC. In particular, T1 high-grade (T1HG) UCB has a higher risk of disease recurrence and progression than other forms of NMIBC.5,6 In addition, many studies have investigated the potential prognostic value of multiple factors, including sex, age, smoking, preoperative urine cytology result, tumor size, tumor morphology, tumor multiplicity, CIS, included muscle layer, lymphovascular invasion (LVI), restaging transurethral resection (TUR), and intravesical BCG therapy, in association with the recurrence and progression of NMIBC.7,8,9,10,11,12,13 The prognostic value of several of these factors has been established, while such value remains controversial for others.
In this study, we aimed to confirm the prognostic factors significantly associated with recurrence and progression after TURBT in a large multicenter cohort of Korean patients with NMIBC.
MATERIALS AND METHODS
Study population
Before the initiation of this multicenter and retrospective study, Institutional Review Board approval was obtained for the use of individual patient data from each center. A total of 3462 patients who underwent initial TURBT for bladder cancer at their respective institutions between January 2006 and December 2010 were initially recruited from 15 centers. Inclusion criteria were as follows: 1) histologically confirmed urothelial carcinoma; 2) NMIBC including Tis or Ta or T1 tumors; and 3) follow-up for at least 1 year after TURBT. Exclusion criteria included patients with a previous or concomitant history of upper tract urothelial carcinoma, patients with malignancy of another site of origin, patients with non-urothelial carcinoma, and patients who had a history of chemotherapy, immunotherapy, or immunosuppressive agent administration within 6 months. Ultimately, a total of 2412 patients consisting of between 21 and 431 patients per center were eligible for this study.
Acquisition of data and definition of variables
Potential clinicopathological data were extracted from our multi-institutional database. Clinical (preoperative) variables included age (<65 years vs. ≥65 years), sex, urine cytology result, tumor morphology (papillary vs. non-papillary), tumor multiplicity (single vs. multiple), and tumor size (<3 cm vs. ≥3 cm). Preoperative urine cytology was examined using voided urine at the first visit prior to initial TURBT. Urine cytology result was described as one of the following three categories: negative, atypical cells, or positive for malignant cells. Positive urine cytology (PUC) was defined only as positive for malignant cells in a voided urine sample. The tumor stage (Ta vs. T1/Tis) and grade (low vs. high) of all initial TURBT specimens were assessed by genitourinary pathologists with expertise and determined according to the 2010 Tumor-Node-Metastasis classification of the American Joint Committee on Cancer (7th edition) and the 2004 World Health Organization system, respectively. The presence of concomitant CIS and muscle layer in the specimen were also identified from pathologic reports. Restaging TURBT was not routinely performed in our database and was determined at the discretion of the surgeon. Variables related to postoperative adjuvant therapy, such as immediate (within 24 h after TURBT) IVC, additional IVC and chemotherapeutic agents (e.g., mitomycin, epirubicin, and adriamycin), and intravesical BCG immunotherapy including induction and maintenance, were recorded. BCG for induction was usually initiated within 2–6 weeks following TURBT and repeated once weekly for 6 weeks (6 cycles). Any instillations beyond 6 cycles were viewed as maintenance BCG. The end-points of interest were time to disease recurrence and progression. Recurrence was defined as the first tumor relapse in the bladder or prostatic urethra irrespective of tumor stage. Progression was defined as the presence of muscle invasive disease (≥T2) or metastatic disease at the time of tumor recurrence.
Risk group stratification
All patients were stratified into each risk group according to several factors, which included tumor stage (Ta vs. T1/Tis), tumor grade (low vs. high), tumor multiplicity (single vs. multiple) and tumor size (<3 cm vs. ≥3 cm).3 Eventually, NMIBC patients were categorized into low-, intermediate-, and high-risk groups (Supplementary Table 1, only online). The high-risk group contained patients with any findings of following: T1 tumors, high-grade Ta tumors, and CIS.
Follow-up protocol
Patients were usually followed up at least every 3–4 months for the first 2 years, semiannually for the next 3 years, and annually thereafter with urine cytology, cystoscopy, and biopsy of suspicious lesions. Radiographic assessment of the upper urinary tract was generally carried out at the initial diagnosis and thereafter only in cases of disease recurrence or suspicion, such as PUC. Recurrence-free survival (RFS) was defined as the interval between the day of the TURBT and the time of the first tumor recurrence. Progression-free survival (PFS) was defined as the interval from the date of the TURBT to the date of disease progression.
Statistical analyses
For the entire study cohort, continuous and categorical variables are respectively expressed as median and interquartile range (IQR) and absolute numbers and relative percentages (%) in accordance with descriptive and frequency analyses. The Kaplan-Meier method with the log-rank test was used to estimate and compare the RFS and PFS according to each potential prognostic factor. The significant factors related to recurrence and progression identified using univariable Cox proportional hazard regression models were incorporated into a step-down multivariable Cox regression analysis to confirm the definitive prognostic factors. All statistical analyses were conducted using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA), and two-sided p values of <0.05 were considered to be statistically significant.
RESULTS
Characteristics of the study cohort
Baseline characteristics of all patients (n=2412) are summarized in Table 1. Preoperative variables showed that the median age of patients was 64.5 years, the male-to-female ratio was approximately 5:1, 39.3% were smokers at the time of diagnosis, 24.5% presented PUC results, 85.4% had papillary tumor morphology, 42.7% had multiple tumors, and 58.5% had tumors of less than 3 cm in size. On pathologic review, 56.7% of specimens showed Ta tumors, 47.4% demonstrated high-grade tumors, 8.3% had a primary or concomitant CIS, and definite muscle layer was observed in 38.5%, showing a significant difference between Ta and T1 disease (31% vs. 50.3%; p<0.001). As a result of risk stratification, a total of 550 (22.8%), 429 (17.8%), and 1433 patients (59.4%) were respectively incorporated into the low-, intermediate-, and high-risk groups. Among the entire group of patients, 684 showed T1HG disease. After TURBT, intravesical instillation within 24 h was performed in 453 (18.8%) patients, while additional IVC and induction BCG therapy were administered in 417 (17.3%) and 1299 (53.9%) patients, respectively. BCG maintenance was carried out in 648 patients (26.8%).
Table 1. Baseline Characteristics of the Entire Study Cohort (n=2412).
| Variables | Total (n=2412) |
|---|---|
| Clinical parameters | |
| Age, yr, median (IQR) | 64.5 (57.3-72.7) |
| <65, n (%) | 1099 (45.6) |
| ≥65, n (%) | 1313 (54.4) |
| Gender, n (%) | |
| Male | 1847 (76.6) |
| Female | 368 (15.3) |
| Missing/unknown | 197 (8.2) |
| Preoperative urine cytology, n (%) | |
| Negative | 1132 (46.9) |
| Atypical cells | 448 (18.6) |
| Positive for malignant cells | 593 (24.5) |
| Missing/unknown | 239 (10) |
| Tumor morphology, n (%) | |
| Papillary | 2060 (85.4) |
| Non-papillary (sessile/flat/mixed) | 346 (14.3) |
| Missing/unknown | 6 (0.3) |
| Tumor multiplicity, n (%) | |
| Single | 1357 (56.3) |
| Multiple (≥2) | 1031 (42.7) |
| Missing/unknown | 24 (1.0) |
| Tumor size, n (%) | |
| <3 cm | 1411 (58.5) |
| ≥3 cm | 664 (27.5) |
| Missing/unknown | 337 (14.0) |
| Pathological parameters | |
| Tumor stage, n (%) | |
| pTa | 1368 (56.7) |
| pT1/Tis | 1042 (43.2) |
| Missing/unknown | 2 (0.1) |
| Tumor grade, n (%) | |
| Low-grade | 1140 (47.3) |
| High-grade | 1143 (47.4) |
| Missing/unknown | 129 (5.3) |
| Carcinoma in situ, n (%) | |
| Absent | 2131 (88.3) |
| Present (primary or concomitant) | 200 (8.3) |
| Missing/unknown | 81 (3.4) |
| Muscle layer included, n (%) | |
| Absent | 1480 (61.4) |
| Present | 928 (38.5) |
| Missing/unknown | 4 (0.2) |
| Postoperative parameters | |
| Immediate intravesical instillation, n (%) | |
| No | 1951 (80.9) |
| Yes | 453 (18.8) |
| Missing/unknown | 8 (0.3) |
| Additional intravesical chemotherapy, n (%) | |
| No | 1983 (82.2) |
| Yes | 417 (17.3) |
| Missing/unknown | 12 (0.5) |
| BCG induction, n (%) | |
| No | 1113 (46.1) |
| Yes | 1299 (53.9) |
| BCG maintenance, n (%) | |
| No | 1573 (65.2) |
| Yes | 648 (26.8) |
| Missing/unknown | 191 (7.9) |
| Overall follow-up duration (months), median (IQR) | 37 (25-52) |
| Median first time to recurrence (months) | 10 (5-19) |
| Recurrence, n (%) | |
| No | 1543 (64.0) |
| Yes | 866 (35.9) |
| Missing/unknown | 3 (0.1) |
| Progression, n (%) | |
| No | 2263 (93.8) |
| Yes | 137 (5.7) |
| Muscle invasion | 79 |
| Distant metastasis | 58 |
| Missing/unknown | 12 (0.5) |
IQR, interquartile range; BCG, Bacillus Calmette-Guérin.
Clinical outcomes in the entire study cohort (n=2412)
The median follow-up duration for all patients was 37 months, with a minimum follow-up duration of 12 months and a maximum of 81 months. A total of 866 patients (35.9%) experienced a first recurrence, with a median time to recurrence of 10 months (IQR 5–19 months). Disease progression was observed in 137 patients (5.7%), divided into muscle invasion (79 patients) and distant metastasis (58 patients), with a mean follow-up duration of 37 months (IQR 25–52 months) (Table 1).
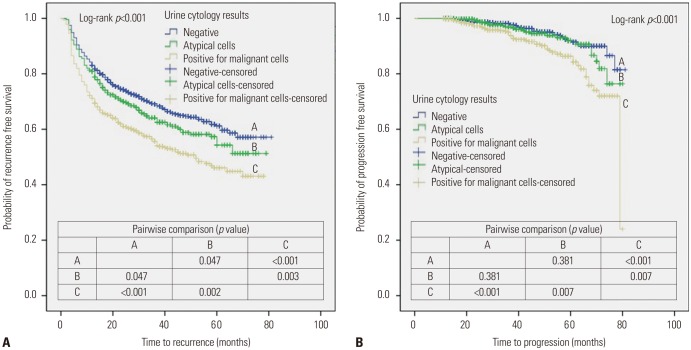
Applying the Kaplan-Meier method with the log-rank test, patients with preoperative PUC showed a worse 5-year RFS rate than those with a negative result (44.8% vs. 61.2%; p<0.001) (Fig. 1A). There was also a significant difference in the 5-year PFS rate between patients with positive and negative urine cytology results (84.3% vs. 91.4%; p<0.001) (Fig. 1B).
Fig. 1. Kaplan-Meier plots for recurrence-free survival (A) and progression-free survival (B) stratified by urine cytology result for the entire study cohort.
The results of regression analyses using univariable and multivariable Cox proportional hazards models for RFS and PFS are listed in Tables 2 and 3. Multivariable analyses revealed that a PUC result was an independent predictor of worse RFS [hazard ratio (HR) 1.56; 95% confidence interval (CI) 1.29–1.89; p<0.001] and PFS (HR 1.56; 95% CI 1.03–2.38; p<0.037). Advanced age (≥65 years) was also identified as an independent predictor of worse RFS (HR 1.34; 95% CI 1.13–1.59; p=0.001) and PFS (HR 2.13; 95% CI 1.44–3.16; p<0.001). A high tumor grade was significantly related to poor PFS (HR 2.13; 95% CI 1.39–3.25; p<0.001).
Table 2. Univariable and Multivariable Cox Regression Analyses Predicting Recurrence-Free Survival in the Entire Study Cohort (n=2412).
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Clinical parameters | ||||
| Age, yrs (≥65 vs. <65) | 1.37 (1.19-1.57) | <0.001 | 1.34 (1.13-1.59) | 0.001 |
| Gender (female vs. male) | 1.02 (0.85-1.22) | 0.852 | ||
| Preoperative urine cytology (ref. negative) | ||||
| Atypical cells | 1.20 (1.00-1.45) | 0.049 | 1.10 (0.88-1.38) | 0.411 |
| Positive for malignant cells | 1.62 (1.38-1.90) | <0.001 | 1.56 (1.29-1.89) | <0.001 |
| Tumor morphology (non-papillary vs. papillary) | 1.20 (0.99-1.43) | 0.053 | ||
| Tumor multiplicity (multiple vs. single) | 1.29 (1.13-1.48) | <0.001 | 1.07 (0.90-1.28) | 0.445 |
| Tumor size, cm (≥3 vs. <3) | 1.28 (1.10-1.49) | 0.001 | 1.07 (0.89-1.28) | 0.479 |
| Pathological parameters | ||||
| Tumor stage (pT1/Tis vs. ≤pTa) | 1.11 (0.97-1.27) | 0.114 | ||
| Tumor grade (high vs. low) | 1.54 (1.34-1.77) | <0.001 | 1.13 (0.93-1.37) | 0.206 |
| Carcinoma in situ (present vs. absent) | 1.26 (1.01-1.58) | 0.039 | 1.09 (0.81-1.46) | 0.556 |
| Muscle layer included (present vs. absent) | 0.95 (0.83-1.09) | 0.508 | ||
| Postoperative parameters | ||||
| Immediate intravesical chemotherapy (yes vs. no) | 0.89 (0.75-1.06) | 0.203 | ||
| Induction BCG (yes vs. no) | 1.29 (1.13-1.48) | <0.001 | 0.99 (0.81-1.21) | 0.914 |
| Maintenance BCG (yes vs. no) | 1.31 (1.13-1.52) | <0.001 | 1.20 (0.99-1.46) | 0.061 |
HR, hazard ratio; CI, confidence interval; BCG, Bacillus Calmette-Guérin.
Table 3. Univariable and Multivariable Cox Regression Analyses for Predicting Progression-Free Survival in the Entire Study Cohort (n=2412).
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Clinical parameters | ||||
| Age, yrs (≥65 vs. <65) | 2.02 (1.41-2.88) | <0.001 | 2.13 (1.44-3.16) | <0.001 |
| Gender (female vs. male) | 1.35 (0.86-2.12) | 0.185 | ||
| Preoperative urine cytology (ref. negative) | ||||
| Atypical cells | 1.23 (0.75-2.01) | 0.420 | 0.89 (0.53-1.51) | 0.665 |
| Positive for malignant cells | 2.32 (1.57-3.45) | <0.001 | 1.56 (1.03-2.38) | 0.037 |
| Tumor morphology (non-papillary vs. papillary) | 1.47 (0.96-2.25) | 0.078 | ||
| Tumor multiplicity (multiple vs. single) | 1.35 (0.97-1.90) | 0.078 | ||
| Tumor size, cm (≥3 vs. <3) | 1.11 (0.75-1.63) | 0.611 | ||
| Pathological parameters | ||||
| Tumor stage (pT1/Tis vs. ≤pTa) | 2.37 (1.67-3.37) | <0.001 | 1.39 (0.93-2.09) | 0.111 |
| Tumor grade (high vs. low) | 2.67 (1.82-3.93) | <0.001 | 2.13 (1.39-3.25) | <0.001 |
| Carcinoma in situ (present vs. absent) | 1.33 (0.77-2.27) | 0.303 | ||
| Muscle layer included (present vs. absent) | 1.03 (0.73-1.46) | 0.851 | ||
| Postoperative parameters | ||||
| Immediate intravesical chemotherapy (yes vs. no) | 1.10 (0.71-1.70) | 0.674 | ||
| Induction BCG (yes vs. no) | 1.16 (0.83-1.63) | 0.388 | ||
| Maintenance BCG (yes vs. no) | 0.74 (0.49-1.12) | 0.151 | ||
HR, hazard ratio; CI, confidence interval; BCG, Bacillus Calmette-Guérin.
Clinical outcomes according to each risk group in the entire cohort
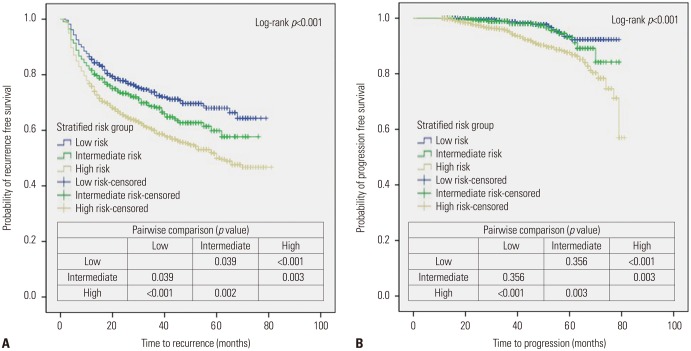
When stratifying the patients into each risk group according to the definition (Supplementary Table 1, only online), there were significant differences among risk groups in terms of age, preoperative urine cytology results, tumor morphology, included muscle layer, administration of adjuvant intravesical therapy (immediate IVC, additional IVC, induction BCG, and maintenance BCG) (Supplementary Table 2, only online). The results of the survival analysis using the Kaplan-Meier method with the log-rank test indicated that the RFS and PFS rates were well discriminated between each risk group (Fig. 2). Interestingly, these survival curves for RFS and PFS showed similar trends to those in cases of preoperative urine cytology results in the entire study cohort (Fig. 1).
Fig. 2. Kaplan-Meier plots for recurrence-free survival (A) and progression-free survival (B) according to risk stratification.
On univariable analysis of the intermediate-risk group, there were no significant prognostic factors related to recurrence or progression. For the low-risk group, multivariable analysis showed that induction BCG instillation (HR 1.76; 95% CI 1.25–2.48; p=0.001) was significantly associated with worse RFS (Supplementary Table 3, only online), while there were no relevant factors with a risk of progression on univariable analysis, except for advanced age (HR 5.81; 95% CI 1.55–20.02; p=0.008) (Supplementary Table 4, only online). Notably, only for the high-risk group, multivariable analyses revealed that preoperative PUC was confirmed as an independent predictive factor of worse RFS (HR 1.73; 95% CI 1.38–2.18; p<0.001) and PFS (HR 1.96; 95% CI 1.22–3.16; p=0.006) (Table 4).
Table 4. Multivariable Cox Regression Analyses for Predicting Recurrence-Free Survival (RFS) and Progression-Free Survival (RFS) in High-Risk Patients (n=1433).
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Clinical parameters | ||||
| Age, yrs (≥65 vs. <65) | 1.20 (0.98-1.47) | 0.074 | 1.80 (1.16-2.79) | 0.008 |
| Preoperative urine cytology (ref. negative) | ||||
| Atypical cells | 1.23 (0.93-1.62) | 0.149 | 1.13 (0.62-2.07) | 0.682 |
| Positive for malignant cells | 1.73 (1.38-2.18) | <0.001 | 1.96 (1.22-3.16) | 0.006 |
| Tumor size, cm (≥3 vs. <3) | 1.15 (0.94-1.41) | 0.181 | ||
| Postoperative parameters | ||||
| Induction BCG (yes vs. no) | 0.78 (0.48-1.25) | 0.296 | ||
| Maintenance BCG (yes vs. no) | 1.06 (0.85-1.32) | 0.632 | 0.76 (0.46-1.23) | 0.265 |
HR, hazard ratio; CI, confidence interval; BCG, Bacillus Calmette-Guérin.
Clinical outcomes in patients with T1HG cancer (n=684)
The first disease recurrence was found in 258 (37.7%) patients, with a median time to recurrence of 8 months (IQR 4.0–17.5 months). Among these patients, disease progression was noted in 71 (10.4%), with a median follow-up duration of 36 months (IQR 24–50 months). Progression to muscle invasion and distant metastasis was documented in 39 (5.7%) and 32 patients (4.8%), respectively.
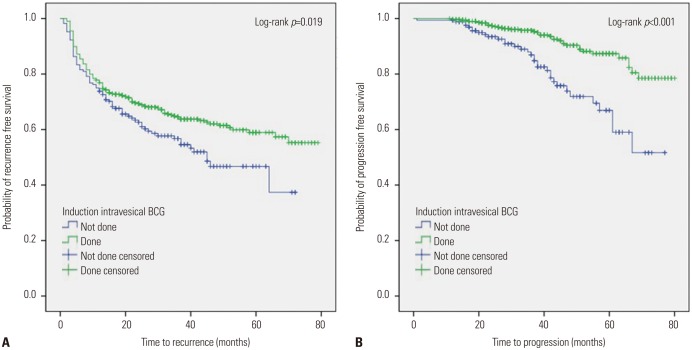
On Kaplan-Meier analysis with the log-rank test, patients who received induction BCG therapy after TURBT showed better 5-year RFS (57.3% vs. 37.4%; p=0.019) (Fig. 3A) and PFS (85.8% vs. 59.0%; p<0.001) (Fig. 3B) than those who received no induction BCG. After conducting the multivariable Cox regression analyses with adjustment for other significant variables identified on univariable analyses, induction BCG therapy remained an independent predictor of improved RFS (HR 0.75; 95% CI 0.57–0.98; p=0.033) and PFS (HR 0.33; 95% CI 0.20–0.53; p<0.001) (Table 5).
Fig. 3. Kaplan-Meier plots for recurrence-free survival (A) and progression-free survival (B) stratified by induction intravesical BCG for patients with T1 high-grade urothelial carcinoma. BCG, Bacillus Calmette-Guérin.
Table 5. Multivariable Cox Regression Analyses for Predicting Recurrence-Free Survival (RFS) and Progression-Free Survival (PFS) in Patients with T1 High-Grade Urothelial Carcinoma (n=684).
| Variables | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |
| Clinical parameters | ||||
| Age, yrs (≥65 vs. <65) | 1.39 (1.07-1.79) | 0.012 | 1.60 (0.97-2.62) | 0.065 |
| Postoperative parameters | ||||
| Induction BCG (yes vs. no) | 0.75 (0.57-0.98) | 0.033 | 0.33 (0.20-0.53) | <0.001 |
| Maintenance BCG (yes vs. no) | 0.75 (0.39-1.44) | 0.389 | ||
HR, hazard ratio; CI, confidence interval; BCG, Bacillus Calmette-Guérin.
DISCUSSION
Although NMIBC may be treated by TURBT alone, high recurrence (50–70%) and progression (10–20%) rates can be problematic in the management of most NMIBC cases.3,4 Therefore, it is necessary to consider adjuvant therapy in most patients to prevent disease recurrence and progression. To make an appropriate decision regarding adjuvant therapy, it is also important to recognize the factors predicting prognosis after TURBT.
To predict the risks of both recurrence and progression in individual patients with Ta and T1 tumors, a scoring system and risk tables, which were based on the six most significant clinical and pathological factors, including tumor multiplicity, tumor size, prior recurrence rate, T category, presence of concurrent CIS, and tumor grade, were developed by the European Organization for Research and Treatment of Cancer (EORTC) and validated by several investigators.14,15,16 The Spanish Urological Club for Oncological Treatment (CUETO) scoring model was also developed to stratify the risk of recurrence and progression in NMIBC treated with BCG.17 Incorporated variables for the model were sex, age, tumor grade, tumor status (primary, recurrent), multiplicity, and associated Tis for recurrence, and age, grade, tumor status, T category, multiplicity, and associated Tis for progression. The prognostic values of each variable involved in the EORTC risk tables or CUETO scoring model have also been described in selected patient populations. One multicenter study has reported that advanced age (70 years or older), large tumor size (3 cm or greater), and concomitant CIS showed a close association with a high risk of progression in patients with T1G3 tumors who received BCG.13 In high-risk NMIBC (Ta, T1) patients treated with BCG, tumor size (<3 cm, ≥3 cm) and T stage (Ta, T1) correlated with recurrence and progression, respectively.12 Female sex was related to worse RFS, PFS, and cancer-specific survival (CSS) in patients with T1HG disease.8,9 Other prognostic factors were also evaluated in several studies. Smoking status and immediate intravesical instillation were reported as significant predictors of recurrence in all NMIBC cases.7,18 In T1HG tumors, papillary tumor architecture (vs. sessile), no LVI, non-trigonal tumor location, intravesical BCG therapy, and presence of muscle layer in TURBT specimen were suggested as factors associated with improved PFS.5,11
In the present study, the prognostic factors mentioned above showed various clinical implications in association with prognosis after TURBT. Similarly to the existing studies,14,15,16,17 advanced age and high tumor grade were identified as significant factors related to worse RFS and/or PFS. In contrast, the definitive prognostic impact of tumor multiplicity, tumor size, and CIS on recurrence was not confirmed on multivariable analysis. Unlike in previous studies,7,8,9,11,18 sex, the presence of muscle layer, and immediate intravesical instillation had no effect on the risk of recurrence and progression in all NMIBC patients.
Although it was not described in detail in the present study, the administration of additional IVC showed no consistent effect in terms of the prevention of recurrence and progression. While IVC showed a worse result for recurrence in all NMIBC and T1HG patients, it demonstrated a better outcome for progression in all NMIBC patients. Moreover, in all risk groups, the administration of additional IVC was not associated with favorable RFS or PFS. These findings are different from the results of a previous meta-analysis, which demonstrated a significant reduction of recurrence and no effect on tumor progression with regard to additional IVC.19 Owing to these conflicting findings in the present study, we excluded the additional IVC as a covariate when performing the survival analysis. The potential causes for these conflicting findings are as followings. First of all, due to the multi-institutional and retrospective nature, there was no unified central pathologic review. Eventually, the interpretation of pathological variables, including tumor stage, grade, and urine cytology results, may be different depending on each center, such that the indications for IVC were differently applied among institutions. Additionally, as there is currently no established standard concerning the duration and frequency of IVC use in NMIBC,20 the type of agent, duration, and interval for IVC may be diversely adopted at the discretion of physicians at each hospital, allowing the generation of these conflicting findings.
Above all, the notable finding of the current study was the confirmation of the prognostic value of preoperative urine cytology results in association with recurrence and progression after TURBT. Along with cystoscopy, urine cytology is currently the standard method for the diagnosis and surveillance of bladder cancer.21,22 PUC is reported with high frequency in cases of high-grade tumor or CIS, which shows reduced intercellular adhesion and produces a greater number of cells shed into the urine. In the present study, PUC was a significant factor related to a high risk of recurrence and progression among all NMIBC patients. Particularly, on multivariable analysis conducted only in high-risk patients including high-grade and CIS disease, preoperative PUC was an independent predictor of both RFS and PFS. Moreover, survival curves for RFS and PFS showed similar tendencies between preoperative urine cytology results and risk stratification. Therefore, PUC may play a role as a surrogate marker for predicting disease recurrence and progression after TURBT. This adverse association between PUC and the prognosis of urothelial carcinoma has been found in several previous studies.23,24,25
T1HG UCBs are at a higher risk of disease recurrence and progression than other NMIBCs. Therefore, adjuvant intravesical BCG immunotherapy as an adjunct to TURBT is currently mandatory in the management of T1HG tumors to improve prognosis.3,4 In our study, approximately 75% and 35% of patients with T1HG tumors received induction and maintenance BCG therapy, respectively. T1HG patients who received induction BCG therapy showed improved RFS and PFS, and maintenance BCG was also significantly associated with better PFS on univariable analysis. The results of multivariable analysis in T1HG patients showed that improved RFS and PFS was observed only in association with induction BCG therapy. These findings are in agreement with those of a previous study, which suggests that adjuvant BCG therapy was significantly associated with prolonged RFS and worsening-free survival (PFS and CSS).5
Our study had several limitations. Owing to its multicenter and retrospective design, the accuracy in reporting prognostic factors such as sex, urine cytology results, tumor size, tumor grade, and maintenance BCG suffered from missing data. We also could not standardize the quality of TURBT or indications for adjuvant intravesical therapy and restaging TURBT. In particular, due to the various treatment patterns across institutions, it was difficult to assess which procedure, including restaging TURBT or adjuvant intravesical therapy, significantly reduced residual tumors and subsequently improved the management of NMIBC patients. In addition, we could not adjust for the number and experience of surgeons and pathologists at each center; therefore, there was no central pathology review. However, these factors can also be interpreted as additional strengths of this study, as they reflect real clinical practice and thus extend the generalizability of the results. Finally, we did not take into account comorbidities of patients, treatment-related complications, and several pathologic variables, such as LVI and variant histology of UCB, which might have affected the decision-making regarding further therapy, resulting in a possible selection bias. Consequently, the results drawn from this study should be further verified through well-designed prospective and randomized clinical trials.
In conclusion, preoperative PUC may adversely affect the prognosis after TURBT, particularly in high-risk NMIBC patients. In particular, patients with T1HG disease treated with at least an induction course of intravesical BCG after TURBT showed better RFS and PFS outcomes. Therefore, BCG immunotherapy should be considered in NMIBC patients for the prevention of disease recurrence and progression. Further prospective research will be required to verify these findings.
ACKNOWLEDGEMENTS
We thank all of the researchers who gathered the data from the fifteen Korean academic institutions, including those from the Ajou University Hospital, Yonsei University Severance Hospital, The Catholic University of Korea Hospital, Konkuk University Chungju Hospital, Kyungpook National University Hospital, Hallym University Hospital, Samsung Medical Center, Kangbuk Samsung Hospital, Asan Medical Center, Korea University Medical Center, Inha University Hospital, Chonnam National University Hospital, Chonbuk National University Hospital, Chungnam National University Hospital, and Kyung Hee University Hospital.
This research was supported in part by the Korean Urological Oncology Society Grant.
Footnotes
AUTHOR CONTRIBUTIONS: Conception and design: Hyung Suk Kim, Ja Hyeon Ku, Se Joong Kim, Sung Joon Hong, Sung Hoo Hong, Hong Sup Kim, Tae Gyun Kwon, Jin Seon Cho, Seong Soo Jeon, Kwan Joong Joo, Han Jong Ahn, Hong Seok Park, Do Hwan Seong, Dong Deuk Kwon, Hyung Jin Kim, Jae Sung Lim, Hyung-Lae Lee
Acquisition of data: Se Joong Kim, Sung Joon Hong, Sung Hoo Hong, Hong Sup Kim, Tae Gyun Kwon, Jin Seon Cho, Seong Soo Jeon, Kwan Joong Joo, Han Jong Ahn, Hong Seok Park, Do Hwan Seong, Dong Deuk Kwon, Hyung Jin Kim, Jae Sung Lim, Hyung-Lae Lee
Analysis and interpretation of data: Hyung Suk Kim, Ja Hyeon Ku
Writing or revision of the manuscript: Hyung Suk Kim, Ja Hyeon Ku, Hyung-Lae Lee
Administrative, technical, or material supports: Ja Hyeon Ku, Hyung-Lae Lee
Supervision: Ja Hyeon Ku, Hyung-Lae Lee
The authors have no financial conflicts of interest.
Supplementary Materials
Risk Group Stratification
Comparative Analysis Results of Variables among Risk Groups
Univariable and Multivariable Cox Regression Analyses Predicting Recurrence Free Survival in Low Risk Patients (n=550)
Univariable Cox Regression Analyses Predicting Progression Free Survival in Low Risk Patients (n=550)
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Prediction of cancer incidence and mortality in Korea, 2014. Cancer Res Treat. 2014;46:124–130. doi: 10.4143/crt.2014.46.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Burger M, Oosterlinck W, Konety B, Chang S, Gudjonsson S, Pruthi R, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:36–44. doi: 10.1016/j.eururo.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 5.Segal R, Yafi FA, Brimo F, Tanguay S, Aprikian A, Kassouf W. Prognostic factors and outcome in patients with T1 high-grade bladder cancer: can we identify patients for early cystectomy? BJU Int. 2012;109:1026–1030. doi: 10.1111/j.1464-410X.2011.10462.x. [DOI] [PubMed] [Google Scholar]
- 6.Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119:3219–3227. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lammers RJ, Witjes WP, Hendricksen K, Caris CT, Janzing-Pastors MH, Witjes JA. Smoking status is a risk factor for recurrence after transurethral resection of non-muscle-invasive bladder cancer. Eur Urol. 2011;60:713–720. doi: 10.1016/j.eururo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Palou J, Sylvester RJ, Faba OR, Parada R, Peña JA, Algaba F, et al. Female gender and carcinoma in situ in the prostatic urethra are prognostic factors for recurrence, progression, and disease-specific mortality in T1G3 bladder cancer patients treated with bacillus Calmette-Guérin. Eur Urol. 2012;62:118–125. doi: 10.1016/j.eururo.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Kluth LA, Fajkovic H, Xylinas E, Crivelli JJ, Passoni N, Rouprêt M, et al. Female gender is associated with higher risk of disease recurrence in patients with primary T1 high-grade urothelial carcinoma of the bladder. World J Urol. 2013;31:1029–1036. doi: 10.1007/s00345-012-0996-9. [DOI] [PubMed] [Google Scholar]
- 10.Sfakianos JP, Kim PH, Hakimi AA, Herr HW. The effect of restaging transurethral resection on recurrence and progression rates in patients with nonmuscle invasive bladder cancer treated with intravesical Bacillus Calmette-Guérin. J Urol. 2014;191:341–345. doi: 10.1016/j.juro.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shindo T, Masumori N, Kitamura H, Tanaka T, Fukuta F, Hasegawa T, et al. Clinical significance of definite muscle layer in TUR specimen for evaluating progression rate in T1G3 bladder cancer: multi-center retrospective study by the Sapporo Medical University Urologic Oncology Consortium (SUOC) World J Urol. 2014;32:1281–1285. doi: 10.1007/s00345-013-1205-1. [DOI] [PubMed] [Google Scholar]
- 12.Zachos I, Tzortzis V, Mitrakas L, Samarinas M, Karatzas A, Gravas S, et al. Tumor size and T stage correlate independently with recurrence and progression in high-risk non-muscle-invasive bladder cancer patients treated with adjuvant BCG. Tumour Biol. 2014;35:4185–4189. doi: 10.1007/s13277-013-1547-8. [DOI] [PubMed] [Google Scholar]
- 13.Gontero P, Sylvester R, Pisano F, Joniau S, Vander Eeckt K, Serretta V, et al. Prognostic factors and risk groups in T1G3 non-muscle-invasivebladder cancer patients initially treated with Bacillus Calmette-Guérin: results of a retrospective multicenter study of 2451 patients. Eur Urol. 2015;67:74–82. doi: 10.1016/j.eururo.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Seo KW, Kim BH, Park CH, Kim CI, Chang HS. The efficacy of the EORTC scoring system and risk tables for the prediction of recurrence and progression of non-muscle-invasive bladder cancer after intravesical Bacillus Calmette-Guerin instillation. Korean J Urol. 2010;51:165–170. doi: 10.4111/kju.2010.51.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Ojea A, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin: external validation of the EORTC risk tables. Eur Urol. 2011;60:423–430. doi: 10.1016/j.eururo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Hernández V, De La Peña E, Martin MD, Blázquez C, Diaz FJ, Llorente C. External validation and applicability of the EORTC risk tables for non-muscle-invasive bladder cancer. World J Urol. 2011;29:409–414. doi: 10.1007/s00345-010-0635-2. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with Bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182:2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: a meta-analysis of published results of randomized clinical trials. J Urol. 2004;171(6 Pt 1):2186–2190. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 19.Huncharek M, McGarry R, Kupelnick B. Impact of intravesical chemotherapy on recurrence rate of recurrent superficial transitional cell carcinoma of the bladder: results of a meta-analysis. Anticancer Res. 2001;21:765–769. [PubMed] [Google Scholar]
- 20.Sylvester RJ, Oosterlinck W, Witjes JA. The schedule and duration of intravesical chemotherapy in patients with non-muscle-invasive bladder cancer: a systematic review of the published results of randomized clinical trials. Eur Urol. 2008;53:709–719. doi: 10.1016/j.eururo.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavallée LT, Fergusson D, Dahm P, Scales CD, Jr, Witiuk K, Breau RH. Diagnostic tests in urology: urine cytology. BJU Int. 2012;110(11 Pt C):E789–E791. doi: 10.1111/j.1464-410X.2012.11448.x. [DOI] [PubMed] [Google Scholar]
- 22.Yafi FA, Brimo F, Auger M, Aprikian A, Tanguay S, Kassouf W. Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urol Oncol. 2014;32:27.e1–27.e6. doi: 10.1016/j.urolonc.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Y, Saika T, Miyaji Y, Saegusa M, Arata R, Akebi N, et al. Preoperative positive urine cytology is a risk factor for subsequent development of bladder cancer after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. World J Urol. 2012;30:271–275. doi: 10.1007/s00345-011-0731-y. [DOI] [PubMed] [Google Scholar]
- 24.Lodde M, Mayr R, Martini T, Comploj E, Palermo S, Trenti E, et al. Positive urine cytology and carcinoma in situ prior to second transurethral resection of the bladder correlate with positive second resection histology and the need for subsequent cystectomy. World J Urol. 2012;30:841–846. doi: 10.1007/s00345-012-0975-1. [DOI] [PubMed] [Google Scholar]
- 25.Koga F, Kobayashi S, Fujii Y, Ishioka J, Yokoyama M, Nakanishi Y, et al. Significance of positive urine cytology on progression and cancer-specific mortality of non--muscle-invasive bladder cancer. Clin Genitourin Cancer. 2014;12:e87–e93. doi: 10.1016/j.clgc.2013.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk Group Stratification
Comparative Analysis Results of Variables among Risk Groups
Univariable and Multivariable Cox Regression Analyses Predicting Recurrence Free Survival in Low Risk Patients (n=550)
Univariable Cox Regression Analyses Predicting Progression Free Survival in Low Risk Patients (n=550)