Abstract
Purpose
The efficacy and safety of denosumab was compared with placebo in Korean postmenopausal women with osteoporosis in this phase III study.
Materials and Methods
Women aged 60 to 90 years with a T-score of <-2.5 and ≥-4.0 at the lumbar spine or total hip were randomized to a single 60 mg subcutaneous dose of denosumab or placebo for the 6-month double-blind phase. Eligible subjects entered the 6-month open-label extension phase and received a single dose of denosumab 60 mg.
Results
Baseline demographics were similar in the 62 denosumab- and 64 placebo-treated subjects who completed the double-blind phase. Treatment favored denosumab over placebo for the primary endpoint {mean percent change from baseline in lumbar spine bone mineral density (BMD) at Month 6 [3.2% (95% confidence interval 2.1%, 4.4%; p<0.0001)]}; and secondary endpoints (mean percent change from baseline in lumbar spine BMD at Month 1, total hip, femoral neck, and trochanter BMD at Months 1 and 6, and median percent change from baseline in bone turnover markers at Months 1, 3, and 6). Endpoint improvements were sustained over 12 months in the open-label extension (n=119). There were no new or unexpected safety signals.
Conclusion
Denosumab was well tolerated and effective in increasing BMD and decreasing bone turnover markers over a 12-month period in Korean postmenopausal women. The findings of this study demonstrate that denosumab has beneficial effects on the measures of osteoporosis in Korean postmenopausal women.
Keywords: Denosumab, postmenopausal osteoporosis, Korea, bone mineral density, biochemical markers of bone turnover
INTRODUCTION
Osteoporosis, a metabolic bone disease characterized by low bone mineral density (BMD), is a global concern. It is estimated that 35.5% of women 50 years of age or older have osteoporosis based on data from the 2008–2009 Korean National Health and Nutrition Examination Survey (KNHANES).1 Furthermore, 37.7% of Korean menopausal women 50 years of age or older are at high risk of osteoporotic fracture according to the 2010 KNHANES survey.2 In addition, age increases the proportion of women with high fracture risk: 49.3% of those 55 years and older compared with 67.7% of those 65 years and older and the incidence of hip fracture was 20432 in 2008.3 Despite this major health problem, diagnosis and treatment rates are low at 29.9% and 14.4%, respectively.1
Denosumab, a fully human monoclonal antibody, reduces bone resorption by inhibiting binding of receptor activator of nuclear factor-κB ligand (RANKL), a tumor necrosis factor that regulates osteoclast activity, to its receptor.4 In the pivotal Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study, denosumab 60 mg every 6 months for 36 months reduced fracture risk in postmenopausal women with osteoporosis (n=7868) from North America, Australia, and Europe.5 In open-label extension studies (n=4550), denosumab maintained reduced bone turnover, increased BMD, and lowered fracture rates in patients for an additional 2 years and 3 years.6,7
Limited studies are available on denosumab treatment in Korean subjects. The current study (ClinicalTrials.gov identifier: NCT01457950; study number: DPH114163) compared the efficacy and safety of denosumab 60 mg versus placebo in Korean postmenopausal women with osteoporosis. The open-label extension was conducted to provide safety and efficacy data on treatment with denosumab over 12 months and confirm treatment benefit in subjects who previously received placebo.
MATERIALS AND METHODS
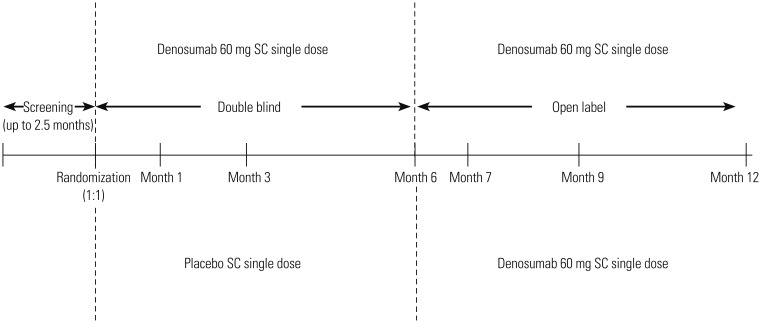
This phase III, randomized, double-blind, placebo-controlled, parallel-group 6-month study with a 6-month open-label extension (Fig. 1) was conducted at 10 centers in Korea from June 2012 to July 2013 (ClinicalTrials.gov identifier: NCT01457950; study number: DPH114163).
Fig. 1. Study design. SC, subcutaneous.
Ethics statement
The study was approved by an ethics committee and conducted according to Good Clinical Practice, and each subject gave written informed consent prior to study entry. Subjects were free to withdraw at any time throughout the study.
Study participants
Korean-born postmenopausal women aged 60 to 90 years with 4 ethnic Korean grandparents, fluency in Korean, and a T-score of <-2.5 and ≥-4.0 at either the lumbar spine or total hip were enrolled. The reference group for the T-score was Korean. Exclusion criteria included bone metabolic diseases other than osteoporosis, an increased risk of developing osteonecrosis of the jaw (ONJ) due to dental conditions, hypocalcemia or hypercalcemia, treatment with bone metabolism drugs, or vitamin D deficiency (<20 ng/mL).
Study design
Subjects with vitamin D levels <20 ng/mL at screening were repleted and retested prior to study entry. Eligible subjects were randomized to receive a single subcutaneous dose of either denosumab 60 mg (Prolia®, Amgen, Thousand Oaks, CA, USA) or placebo at the baseline visit. All subjects received oral calcium ≥1000 mg and vitamin D ≥400 international units (IU) daily.
Changes in BMD and bone turnover markers were assessed over the course of 6 months. Study sites were randomly selected based on Institutional Review Board approval, number of subjects in their databases, and their ability to conduct the study.
The choice of DXA device was left up to the investigator. Sites could measure BMD and T-scores by dual-energy X-ray absorptiometry (DXA) using Hologic and GE Lunar DXA scanners, depending upon what machine the site had available. Cross calibration of the DXA machines was unnecessary because the data are expressed as percent change from each individual patient's baseline. This is the usual method for performing DXA measurements and does not rely on the individual machine. This is standard practice in protocols that examine BMD.5,8,9 All measurements for an individual subject were to be performed on the same DXA scanner. Synarc, Inc. (Portland, OR, USA) provided the central DXA analyses for the study for both Lunar and Hologic DXA machines including the standardization of the DXA scanning procedures among the clinical centers participating in the study. A DXA quality control program was put in place for this study (Synarc, Inc., Portland, OR, USA). As part of that program, the calibration of each DXA scanner was monitored by regular measurements of the local spine phantom. The phantom measurements were collected and reviewed centrally, which confirmed DXA calibration stability throughout the duration of the study. The coefficient of variation of the phantom measurements was maintained below 0.5% for all scanners.
All statistical analyses of DXA data were performed on DXA data analysed by Synarc including machine equivalence data collection requirements. Bone turnover marker [serum C-terminal telopeptide of type I collagen (s-CTX) and serum procollagen type I N-terminal propeptide (s-PINP)] levels were measured using blood samples from fasted subjects. The intra- and inter assay CVs of these bone markers are shown in Table 1. Assays were performed at a central laboratory (Quest Diagnostics, Nichols Institute, San Juan Capistrano, CA, USA). The assays used were the Human Serum CrossLaps® ELISA Test Kit (Nordic Bioscience A/S, Herlev, Denmark), an enzyme immunological test for the quantification of degradation products of s-CTX, and the Orion Diagnostica (Espoo, Finland) UniQ™ PlNP RIA kit, a radioimmunoassay for the quantification of intact PINP. Baseline risk of a major osteoporotic fracture (i.e., hip, clinical spine, humerus, or wrist fracture) was calculated for the subjects using an algorithm tool [Fracture Risk Assessment Tool (FRAX)] that was developed by the World Health Organization and calibrated for Korea (version 3.7).10 The FRAX model is based on the following clinical risk factors with the inclusion of femoral neck BMD: body mass index (BMI), history of fracture, parental history of hip fracture, use of oral glucocorticoids, other secondary causes of osteoporosis (e.g., rheumatoid arthritis), current smoking, and alcohol intake (≥3 units daily). The FRAX calculation is designed to provide the risk of hip fracture and the risk of a major osteoporotic fracture (clinical spine, forearm, hip or shoulder fracture only). There are no provisions to calculate a clinical spine fracture alone. Thus, we provided the data that are available to us with the FRAX algorithm.
Table 1. Intra- and Inter Assay CVs of Bone Turnover Markers s-CTX and s-PINP.
| s-CTX | s-PINP | ||||
|---|---|---|---|---|---|
| Control (ng/mL) | Intra (%) | Inter (%) | Control (ng/mL) | Intra (%) | Inter (%) |
| 0.39 | 3.1 | 9.1 | 41 | 3.3 | 3.0 |
| 0.62 | 3.7 | 4.9 | 98 | 3.4 | 6.2 |
| 1.43 | 8.5 | 8.0 | 130 | 4.0 | 5.2 |
s-CTX, serum C-terminal telopeptide of type I collagen; s-PINP, serum procollagen type I N-terminal propeptide.
To qualify for continuing the open-label extension, subjects had to complete the double-blind phase and have a normal serum albumin-adjusted calcium value and no increased risk of developing ONJ. At the start of the open-label phase, all eligible subjects received a single subcutaneous denosumab 60-mg injection.
Study endpoints
The primary endpoint was the mean percent change in lumbar spine BMD from baseline to Month 6. Secondary endpoints included the mean percent change in lumbar spine BMD from baseline to Month 1, the mean percent change in total hip, femoral neck, and trochanter BMD from baseline to Months 1 and 6, and the median percent change in s-CTX and s-PINP from baseline to Months 1, 3, and 6.
Safety was assessed by monitoring adverse events (AEs), including serious AEs (SAEs), vital signs, laboratory tests, electrocardiogram and physical examination at screening, and denosumab antibody assays. The serum of subjects was screened for anti-denosumab binding antibodies using an electrochemiluminescent (ECL) bridging immunoassay. An ONJ Adjudication Committee and an Atypical Femoral Fracture (AFF) Adjudication Committee (AFFAC), both blinded to group assignments, were created to assess ONJ and AFF events. If the investigator suspected a case of ONJ, the information was forwarded to an independent ONJ Adjudication Committee. This Committee was blinded to group assignments, and gathered additional information from the site and adjudicated all events. The same ONJ adjudication Committee that adjudicated events for the FREEDOM trial was used.5
The endpoints of the open-label extension phase for subjects who received denosumab throughout both the double-blind and open-label phases consisted of the percent change in BMD (lumbar spine, total hip, femoral neck, and trochanter) and bone turnover markers (s-CTX and s-PINP) from baseline to Month 12. The same endpoints were assessed for subjects who switched from placebo to denosumab in the open-label phase, but only within the time frame of Month 6 to Month 12. Safety assessments (AEs, vital signs, laboratory tests, immunogenicity) continued throughout the 12 months.
Statistical analysis
The planned sample size of 50 evaluable subjects in the denosumab group and 50 evaluable subjects in the placebo group would provide 99% power to detect a 3.5% difference (denosumab-placebo) in the primary endpoint, assuming standard deviation of 4 and a two-sided significance level of α=0.05.
The primary efficacy analysis for BMD used an analysis of covariance (ANCOVA) model adjusting for treatment and baseline BMD (as a continuous covariate). The analysis of the secondary efficacy endpoints used the ANCOVA model for BMD parameters at Months 1 and 6 and the Hodges-Lehmann estimate of difference and 2-sided Wilcoxon rank sum test for bone turnover markers at Months 1, 3, and 6. For the open-label phase, percent changes in BMD parameters were calculated and 95% confidence intervals (CIs) were estimated for the exploratory analysis using the t-test statistic. The 95% CIs for bone turnover marker percent reductions were estimated using the Wilcoxon signed rank tests. For the BMD analyses, last observation carried forward (LOCF) was used to impute missing values for a given time point. LOCF was performed such that values from the double-blind phase were not carried forward into the open-label extension. There was no imputation for missing values in the bone turnover marker analyses. In addition to the pre-specified analyses described, a post hoc summary of changes in calcium by study visit was performed. All safety endpoints were summarized with descriptive statistics. SAS 9.2 was the statistical software (SAS Institute Inc., Cary, NC, USA) used in this analysis.
RESULTS
Subject disposition
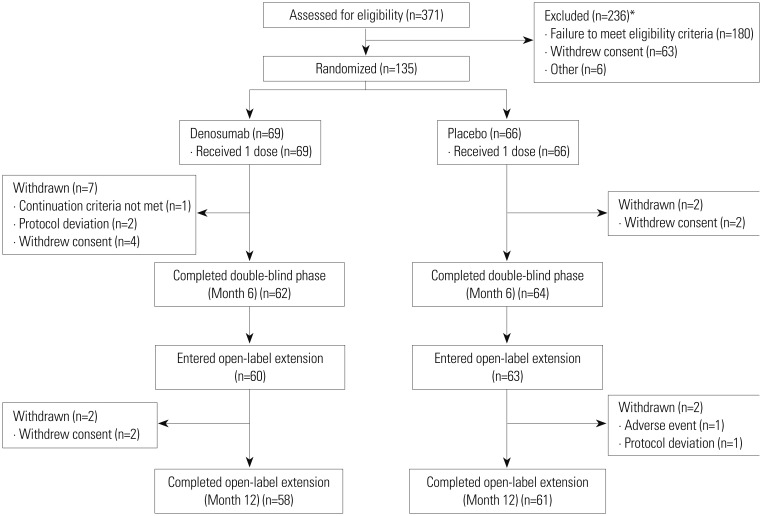
Two hundred thirty six (236) of the 371 subjects failed screening (Fig. 2). The reasons for screen failures were failure to meet the inclusion/exclusion criteria (180 subjects), subject withdrawal of consent (63 subjects), lost to follow-up (4 subjects), and investigator discretion (2 subjects). The intent-to-treat (ITT) population (n=135) included 69 and 66 subjects who were randomized to the denosumab or placebo group, respectively, and received study treatment at baseline. The ITT efficacy population also consisted of 135 subjects because the entire ITT population had at least 1 baseline and post-baseline efficacy assessment. Twenty-nine subjects in each group did not need vitamin D repletion, and 40 and 37 subjects in the denosumab and placebo groups, respectively, were successfully vitamin D-repleted during the screening phase. Sixty-two denosumab-treated and 64 placebo-treated subjects completed the double-blind phase; the most common reason for dropping out of the double-blind phase was withdrawal of consent.
Fig. 2. Subject disposition. *Some subjects had more than 1 reason for failing screening.
Baseline demographics were similar for both treatment groups, with the exception that numerically more subjects in the denosumab group had a history of fracture (30% vs. 23% for denosumab vs. placebo group, respectively) and numerically more subjects in the placebo group had a history of hip fracture in a parent (1% vs. 8% for denosumab vs. placebo group, respectively) (Table 2). Fracture risk was similar between treatment groups (Table 2).
Table 2. Subject Baseline Characteristics (ITT Population of Double-Blind Phase).
| Variable | Denosumab (n=69) | Placebo (n=66) |
|---|---|---|
| Age, mean±SD (range) | 67.0±4.86 (60-81) | 66.0±4.77 (60-78) |
| Height, cm, mean±SD | 152.2±4.94 | 154.3±5.25 |
| Weight, kg, mean±SD | 54.4±6.68 | 56.5±6.31 |
| BMI, kg/m2, mean±SD (range) | 23.5±2.83 (14.0-30.0) | 23.7±2.29 (18.9-27.9) |
| Years since menopause, mean±SD | 19.0±7.02 | 17.5±6.20 |
| Previous fracture*, n (%) | 21 (30) | 15 (23) |
| Parent history of hip fracture, n (%) | 1 (1) | 5 (8) |
| Corrected T-score, mean±SD | ||
| Femoral neck | -2.5±0.56 | -2.4±0.61 |
| Total hip | -2.0±0.64 | -1.9±0.65 |
| Total spine | -3.0±0.59 | -2.9±0.58 |
| Trochanter | -2.2±0.63 | -2.2±0.66 |
| s-CTX, µg/L, mean±SD | 0.611±0.2975 | 0.537±0.2421 |
| s-PINP, µg/L, mean±SD | 60.2±25.17 | 57.7±25.90 |
| 10-yr probability (%) of hip fracture† | ||
| Hologic | 4.9±4.40 | 4.8±3.47 |
| Lunar | 3.9±2.79 | 3.9±3.56 |
| 10-yr probability (%) of major osteoporotic fracture† | ||
| Hologic | 11.4±6.54 | 11.6±4.53 |
| Lunar | 10.2±4.46 | 10.2±6.00 |
BMD, bone mineral density; BMI, body mass index; ITT, intent to treat; SD, standard deviation; s-CTX, serum C-terminal telopeptide of type I collagen; s-PINP, serum procollagen type I N-terminal propeptide.
*Most common was prior wrist fracture (6 in denosumab group and 4 in placebo group), †Fracture probability was calculated using screening femoral neck BMD assessments in the World Health Organization's Fracture Risk Assessment Tool (FRAX) model.
Sixty denosumab-treated and 63 placebo-treated subjects were eligible to enter the open-label extension. These 123 subjects represented the ITT population of the open-label phase (ITT-OL population). Subsequently, 2 subjects from each group withdrew, thus, a total of 119 subjects completed the open-label extension.
Efficacy
Double-blind phase
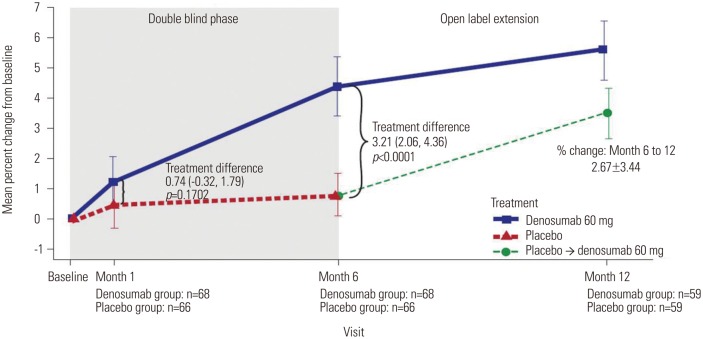
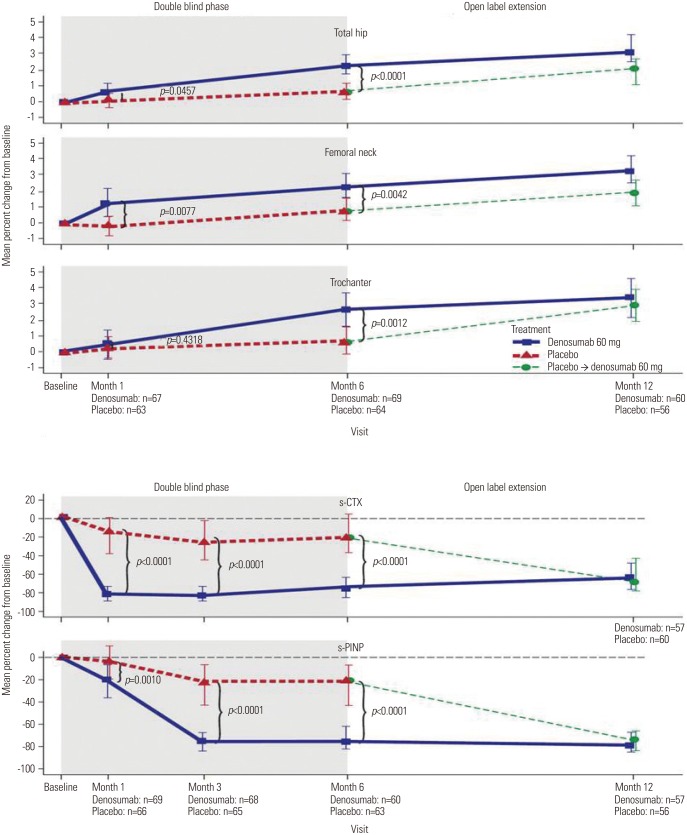
A treatment difference of 3.2% (95% CI, 2.1%, 4.4%; p<0.0001) was seen between the denosumab and placebo groups for the primary endpoint (percent change in lumbar spine BMD from baseline to Month 6) (Fig. 3). For the secondary endpoints, denosumab increased BMD levels at all sites measured at Months 1 and 6 and reduced s-CTX and s-PINP at Months 1, 3, and 6. At Month 6, denosumab demonstrated a treatment difference compared with placebo for the mean percent change in BMD for the total hip [1.7% (95% CI, 1.0%, 2.4%; p<0.0001)], femoral neck [1.4% (95% CI, 0.4%, 2.3%; p=0.0042)], and trochanter [2.0% (95% CI, 0.8%, 3.2%; p=0.0012)] and median percent change in serum bone turnover marker levels, s-CTX [-52.1% (95% CI, -60.8%, -43.2%; p<0.0001)] and s-PINP [-48.2% (95% CI, -56.8%, -39.4%; p<0.0001)] (Fig. 4).
Fig. 3. Primary endpoint - Mean percent change from baseline in BMD in lumbar spine. BMD measurements consisted of last observation carried forward (LOCF) values. Error bars are 95% confidence intervals for the mean percent change from baseline BMD at the lumbar spine (unadjusted). Baseline for the placebo → denosumab group is the end of double-blind phase. BMD, bone mineral density.
Fig. 4. Secondary endpoints - mean/median percent change from baseline in BMD in total hip, femoral neck, and trochanter and in bone turnover markers, s-CTX and s-PINP. BMD measurements consisted of last observation carried forward (LOCF) values, and bone turnover marker measurements consisted of observed values. Error bars for BMD endpoints are 95% confidence intervals for the mean percent change from baseline BMD. Error bars for bone turnover markers are (Q1, Q3). Baseline for the placebo → denosumab group is the end of double-blind phase. BMD, bone mineral density; s-CTX, serum C-terminal telopeptide of type I collagen; s-PINP, serum procollagen type I N-terminal propeptide.
Open-label extension
In subjects who received denosumab for all 12 months, the mean percent change in lumbar spine BMD from baseline to Month 12 was 5.6% (95% CI, 4.6%, 6.6%) (Fig. 3). In subjects who switched from placebo to denosumab at Month 6, the mean percent change in lumbar spine BMD from Month 6 to Month 12 was 2.7% (95% CI, 1.8%, 3.6%) (Fig. 3). Denosumab also increased total hip, femoral neck, and trochanter BMD and reduced bone turnover marker levels in both groups at Month 12 (Fig. 4).
Safety
Denosumab was well tolerated throughout the 12 months. There were no cases of binding anti-denosumab antibodies in any subject at Month 6 or Month 12, no events of AFF or ONJ, and no new or unexpected safety signals.
Double-blind phase
Thirty-eight denosumab-treated subjects (55%) and 32 placebo-treated subjects (48%) experienced AEs. The most common AEs (≥5%) were constipation, myalgia, nasopharyngitis, dyspepsia, and arthralgia (Table 3). Most AEs in both groups were either mild or moderate in intensity. Severe AEs included a motorcycle accident in 1 denosumab-treated subject, constipation in 1 denosumab-treated subject, and appendicitis in 1 placebo-treated subject; none of these were considered treatment-related. There were 3 treatment-related AEs (2 reports of nausea and 1 report of myalgia) for the denosumab group and 2 treatment-related AEs (1 report each of seborrheic keratosis and myalgia) for the placebo group. SAEs occurred in 6 subjects (9%) in the denosumab group and 2 subjects (3%) in the placebo group: 2 reports of intervertebral disc protrusion, and 1 report each of bronchitis, tendon rupture, inadequate diabetic control, and hemorrhoids in denosumab-treated subjects, and 1 report each of appendicitis and rotator cuff syndrome in placebotreated subjects. None of the SAEs were deemed treatment-related by the investigator. No subjects in either treatment group withdrew because of AEs. There was 1 fatality in the denosumab group, which was caused by trauma from a motorcycle accident and was deemed unrelated to the study drug. In addition to a skull fracture caused by the motorcycle accident, 1 subject in the denosumab group had a left metatarsus fracture, and 1 placebo-treated subject had a right toe phalange fracture. The fractures healed without complications and were not attributed to the study treatment.
Table 3. Adverse Events.
| Adverse event, n (%) | Double-blind phase | Open-label extension | ||
|---|---|---|---|---|
| Denosumab (n=69) | Placebo (n=66) | Denosumab → denosumab (n=60) | Placebo → denosumab (n=63) | |
| Any AE | 38 (55) | 32 (48) | 22 (37) | 29 (46) |
| Serious AEs (SAEs) | 2 (3) | 1 (2) | 1 (2) | 3 (5) |
| Deaths | 1 (1)* | 0 (0) | 0 (0) | 0 (0) |
| Treatment-related AEs | 2 (3) | 1 (2) | 0 (0) | 0 (0) |
| Withdrawal due to AE | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Most common adverse events† | ||||
| Constipation | 5 (7) | 2 (3) | 1 (2) | 1 (2) |
| Myalgia | 4 (6) | 1 (2) | 1 (2) | 2 (3) |
| Nasopharyngitis | 4 (6) | 8 (12) | 6 (10) | 6 (10) |
| Dyspepsia | 2 (3) | 4 (6) | 3 (5) | 1 (2) |
| Arthralgia | 1 (1) | 3 (5) | 1 (2) | 4 (6) |
| Headache | 1 (1) | 1 (2) | 1 (2) | 3 (5) |
| Ligament sprain | 3 (4) | 1 (2) | 3 (5) | 1 (2) |
| Gastritis | 3 (4) | 1 (2) | 3 (5) | 0 (0) |
AE, adverse event; SAE, serious adverse event.
*Cause of death was traumatic subdural hemorrhage due to motorcycle accident, †Adverse events are listed if reported by ≥5% in either group in either the double-blind phase or the open-label extension. Values of ≥5% are shaded.
Laboratory results revealed that 1 subject in the placebo group had hypercalcemia, however, it was not considered an AE. A post hoc analysis determined that there were 7 subjects at Month 1 whose calcium value was below the lower limit of the normal range (2.12 mmol/L). However, all of these subjects were above the lower limit of the range of potential clinical concern (1.8 mmol/L). There were no reports of symptomatic hypocalcemia in either treatment group. The maximum calcium level decreases were 0.32 mmol/L and 0.27 mmol/L in denosumab-treated and placebo-treated subjects, respectively. One denosumab-treated subject had high total bilirubin at Month 1 (30 µmol/L) and Month 6 (36 µmol/L), but the subject did not experience associated increases in transaminases or AEs and entered the open-label phase of the study.
Open-label extension
AEs occurred in 22 (37%) subjects in the denosumab → denosumab group (i.e., subjects who received 2 injections of denosumab) and in 29 (46%) subjects in the placebo → denosumab group (i.e., subjects who received 1 injection of denosumab), with the most common AEs (≥5%) being nasopharyngitis, arthralgia, headache, dyspepsia, ligament sprain, and gastritis (Table 2). No AEs were considered treatment-related. Four SAEs occurred in the open-label phase. In the denosumab → denosumab group, 1 subject had macular hole in the eye, and in the placebo → denosumab group, 1 subject had colon adenoma, 1 had spinal compression fracture due to a fall, and 1 had a perforated appendicitis. The SAE of colon adenoma led to study withdrawal. In each treatment group, 1 subject had asymptomatic hypercalcemia but neither subject withdrew from the study. There were no deaths during the open-label phase.
DISCUSSION
Denosumab was well tolerated and effective in increasing BMD at the lumbar spine, total hip, femoral neck, and trochanter and decreasing bone turnover markers (s-CTX and s-PINP) in Korean postmenopausal women. The frequency and types of AEs were consistent with prior studies in non-Korean subjects, with no significant differences seen between denosumab and placebo.5 None of the reported SAEs were attributed to the study drug by investigators.
Although laboratory reports indicated more calcium values in the hypocalcemic range in the denosumab group, there were no adverse events due to hypocalcemia reported.
The double-blind placebo-controlled phase of this study was 6 months in duration and demonstrated a significant treatment difference for denosumab compared with placebo for the primary endpoint lumbar spine BMD of 3.2% (95% CI, 2.1%, 4.4%; p<0.0001). The increase in BMD continued up to 12 months (5.6%; 95% CI, 4.6%, 6.6%) and was trending upwards when the study ended. This response is similar in magnitude to other denosumab studies.5,11,12 The small change vs. placebo in the first 6 months in the denosumab group may be accounted for by a good response in the placebo group (Fig. 3), resulting from vitamin D repletion prior to study entry. The topic of dietary calcium and vitamin D and the changes in BMD is a complex one. It is not uncommon that studies in the field of osteoporosis have shown an increase in BMD in the placebo group.9,13 Many have speculated on the reasons which might include repletion of vitamin D to levels that are no longer considered "insufficient"; better calcium intake; the effect of simply being in a clinical trial; and other reasons. One other reason would be the phenomenon of "regression to the mean".5 It would not be anticipated that the addition of calcium and vitamin D would greatly enhance BMD. However, in older individuals, there have been very small increments in BMD.14 This could be the explanation for the slight increase in the placebo group, although this is not likely because of the lesser time frame in our study.
The increases in lumbar spine BMD and improvements in other secondary endpoints at 6 and 12 months in this Korean study of postmenopausal women with osteoporosis is similar in magnitude and are consistent with 3 other trials of similar design. These observations provide additional evidence that denosumab provides an alternative treatment for osteoporosis in Korean women.5,11,12
The efficacy and safety of denosumab has also been confirmed in other Asian populations: Indian and Japanese in shorter-term studies.11,12 In a similarly designed 6-month, randomized, double-blind, placebo-controlled, parallel-group, multicenter study in 250 Indian postmenopausal women with osteoporosis, a single subcutaneous dose of denosumab 60 mg was also effective compared with placebo in improving secondary endpoints: increasing BMD at the total hip, femoral neck, and trochanter and decreasing bone turnover markers, s-PINP and s-CTX. In a dose-response study of denosumab on BMD and bone turnover markers in Japanese postmenopausal women with osteoporosis, denosumab 60 mg increased lumbar spine BMD by 6.73% (p<0.0001) compared with placebo at 12 months.12 The lumbar BMD increment of 3.1% in Indian and 6.73% in Japanese women after treatment for 6 and 12 months, respectively, were comparable to 3.21% and 5.6% at 6 and 12 months in the current study (all values were compared to placebo). These findings are further supported by the Denosumab Fracture Intervention Randomized Placebo Controlled Trial (DIRECT Trial).15 The primary endpoint in this trial, that compared denosumab 60 mg every 6 months with placebo and included Japanese women and men with osteoporosis, was the reduction in vertebral fracture risk at 24 months. Denosumab significantly reduced the risk of new or worsening vertebral fractures by 65.7%, with incidences of 3.6% in denosumab and 10.3% in placebo group at 24 months (hazard ratio 0.343; 95% CI, 0.194–0.606; p=0.0001). For secondary endpoints, mean BMD percentage change from baseline at 24 months was 9.1% and 0.1% at the lumbar spine in the denosumab and placebo groups, 4.6% and -1.1% at the total hip, 4.0% and -1.1% at the femoral neck, and 0.5% and -1.8% at distal one third radius, respectively. The difference between the two groups was significant as early as 3 months at the lumbar spine, total hip, and femoral neck (p<0.0001) and 6 months at the distal one third radius (p<0.0001). The median percentage change from baseline in serum CTX-1 and BSAP in the denosumab group was reduced by 70.9% at 1 month and 50.2% at 3 months, respectively, and maintained significant reduction levels thereafter. The difference in serum CTX-1 and BSAP between the denosumab and placebo groups was significant as early as 1 month (p<0.0001).
Denosumab has also been shown to be effective in the FREEDOM study, a large, multinational clinical trial, which did not include a Korean population.5 Compared with the FREEDOM study, the Korean study is limited by a smaller sample size and a shorter time frame. Compared with 36 months for the FREEDOM study, the double-blind phase of the Korean study was 6 months and, therefore, safety and clinical data in comparison with placebo were limited to 6 months of treatment. The Korean study had a 6-month open-label extension compared with a 7-year extension conducted in the FREEDOM study; results from the first 2 and 3 years of the FREEDOM extension have been reported.6,7 Open-label extensions in both studies were limited by the lack of comparator data. Whereas the FREEDOM study assessed the effect of denosumab on reducing fracture risk, the Korean study was not designed to evaluate fractures; it measured BMD and bone turnover marker levels. A greater placebo response was seen in the Korean study, which may be due to calcium and vitamin D supplementation in a population with low dietary calcium and vitamin D intake.16 Despite these differences, both studies demonstrated that denosumab has a favorable treatment effect compared with placebo in postmenopausal women with osteoporosis. The 6- and 12-month increase in the primary endpoint lumbar spine BMD in the current study is similar in magnitude and consistent with the data reported in the subgroup analysis of the FREEDOM study; 3.3% and 5.6% vs. 3.9% and 5.5%, respectively, vs. placebo.
The measurement of BMD as early as 1 month may raise concern because this is not done in clinical practice. It is, however, important to note that the purpose of the current study was to bridge to the Phase III FREEDOM study which measured BMD at 1 month. In the larger subset of patients in the FREEDOM trial, there was a statistically significant increase in BMD at the lumbar spine at 1 month. Although the measurement is dependent on the CV of the measurement, the standard error can be reduced and prove significance with a large number of subjects. In our trial, the purpose was to compare the magnitude of change across trials for the registration of denosumab in Korea.
Another potential concern in this study is the percent change in lumbar spine BMD during the open label phase (6 to 12 month period); 2.75% (95% CI, 1.8%, 3.6%) compared with 4.1% in the double-blind phase. The later increase is consistent with the increase in the FREEDOM study sub-analysis of 3.9%. The increase in BMD in the double-blind placebo phase in Korean subjects receiving denosumab in the open-label phase resulted in a higher average BMD at the initiation of denosumab, potentially reducing the remodelling space available to demonstrate increased BMD, thereby contributing to the smaller apparent increase vs. placebo observed during the 6-month open-label phase.17,18 Available evidence from other trials of denosumab suggests that this trend in continued efficacy with continuing treatment is consistent among different studies in a variety of different populations.
The present study is limited by the sample size and duration. Data are needed on long-term efficacy and safety in this Korean population. This study duration is not long enough to evaluate fracture risk, although BMD complemented with bone turnover markers are accepted as surrogate endpoints for assessment of fracture risk in clinical studies to confirm the efficacy of osteoporosis pharmacotherapy.18,19 The lack of an active comparator for the double-blind or open-label phase is also a limitation of this study.
This study demonstrates that denosumab has beneficial effects on the measures of osteoporosis in Korean postmenopausal women.
ACKNOWLEDGEMENTS
The authors would like to thank all investigators, Chan-Soo Shin, Sung-Kil Lim, Jung-Min Koh, Dong Jin Chung, Yoon-Sok Chung, Moo-Il Kang, In-Ju Kim, Yong-Ki Min, Han-Jin Oh, and Il Hyung Park, for their work conducting the study and interacting with the patients, and acknowledge Han-Kyu Lee, MD, MS, for his review and assistance with this manuscript.
Funding
This study was sponsored by GlaxoSmithKline. GlaxoSmithKline was involved in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Grace H. Lee, PharmD and John Romankiewicz, PharmD, of Scientific Therapeutics Information, Inc, Madison, New Jersey, provided editorial assistance, which was funded by GlaxoSmithKline.
Disclosure statement and conflicts of interest
Jung-Min Koh, Dong Jin Chung, Yoon-Sok Chung, Moo-Il Kang, In-Ju Kim, Yong-Ki Min, Han-Jin Oh, and Il Hyung Park declare no financial conflict of interest. Yil-Seob Lee, Barbara Kravitz, Brian Waterhouse, Antonio Nino, and Lorraine A. Fitzpatrick are employees of GlaxoSmithKline and receive stock/stock options in the company.
Footnotes
The 6-month placebo-controlled study was presented as a poster at the 2013 Annual Meeting of the American Society for Bone and Mineral Research in Baltimore, Maryland on October 5, 2013.
The authors have no financial conflicts of interest.
References
- 1.Choi YJ, Oh HJ, Kim DJ, Lee Y, Chung YS. The prevalence of osteoporosis in Korean adults aged 50 years or older and the higher diagnosis rates in women who were beneficiaries of a national screening program: the Korea National Health and Nutrition Examination Survey 2008-2009. J Bone Miner Res. 2012;27:1879–1886. doi: 10.1002/jbmr.1635. [DOI] [PubMed] [Google Scholar]
- 2.Kim JW, Jeon YJ, Baek DH, Kim TN, Chang JS. Percentage of the population at high risk of osteoporotic fracture in South Korea: analysis of the 2010 Fifth Korean National Health and Nutrition Examination survey data. Osteoporos Int. 2014;25:1313–1319. doi: 10.1007/s00198-013-2595-z. [DOI] [PubMed] [Google Scholar]
- 3.Mithal A, Ebeling P, Kyer CS. The Asia-pacific regional audit: epidemiology, costs & burden of osteoporosis in 2013s. International Osteoporosis Foundation; [accessed on 2014 January 23]. Available at: http://www.iofbonehealth.org/sites/default/files/media/PDFs/Regional%20Audits/2013-Asia_Pacific_Audit_0_0.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–625. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 6.Papapoulos S, Chapurlat R, Libanati C, Brandi ML, Brown JP, Czerwiński E, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701. doi: 10.1002/jbmr.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bone HG, Chapurlat R, Brandi ML, Brown JP, Czerwinski E, Krieg MA, et al. The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the FREEDOM extension. J Clin Endocrinol Metab. 2013;98:4483–4492. doi: 10.1210/jc.2013-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 9.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–610. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO scientific group on the assessment of osteoporosis at primary health care level: summary meeting report; Brussels, Belgium, 5-7 May 2004. [accessed on 2013 December 9]. Available at: http://www.who.int/chp/topics/Osteoporosis.pdf.
- 11.Pitale S, Thomas M, Rathi G, Deshmukh V, Kumar P, Reddy S, et al. Effects of denosumab in postmenopausal women with osteoporosis from India (abstract) Indian J Endocrinol Metab. 2013;17(Suppl1):S373–S394. doi: 10.4103/2230-8210.146871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T, Matsumoto T, Sugimoto T, Shiraki M. Dose-response study of denosumab on bone mineral density and bone turnover markers in Japanese postmenopausal women with osteoporosis. Osteoporos Int. 2012;23:1131–1140. doi: 10.1007/s00198-011-1786-8. [DOI] [PubMed] [Google Scholar]
- 13.Chesnut CH, 3rd, Silverman S, Andriano K, Genant H, Gimona A, Harris S, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–276. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- 14.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Matsumoto T, Sugimoto T, Hosoi T, Miki T, Gorai I, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT) J Clin Endocrinol Metab. 2014;99:2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim SK, Kung AW, Sompongse S, Soontrapa S, Tsai KS. Vitamin D inadequacy in postmenopausal women in Eastern Asia. Curr Med Res Opin. 2008;24:99–106. doi: 10.1185/030079908x253429. [DOI] [PubMed] [Google Scholar]
- 17.Delmas PD. Markers of bone turnover for monitoring treatment of osteoporosis with antiresorptive drugs. Osteoporos Int. 2000;11(Suppl 6):S66–S76. doi: 10.1007/s001980070007. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto J, Takeda T, Sato Y, Uzawa M. Determinants of one-year response of lumbar bone mineral density to alendronate treatment in elderly Japanese women with osteoporosis. Yonsei Med J. 2004;45:676–682. doi: 10.3349/ymj.2004.45.4.676. [DOI] [PubMed] [Google Scholar]
- 19.Lau EMC, Sambrook P, Seeman E, Leong KH, Leung PC, Delmas P. Guidelines for diagnosing, prevention and treatment of osteoporosis in Asia. APLAR J Rheumatol. 2006;9:24–36. [Google Scholar]