Abstract
Purpose
Intranasal dexmedetomidine is an effective sedative for premedication and is regularly used to reduce preoperative tension and anxiety in children. This study aimed to assess the effect of intranasally adjunctive dexmedetomidine on perioperative sedative and analgesic requirements in adults.
Materials and Methods
Patients were randomly divided into four groups to receive preoperative administration of saline, intranasal dexmedetomidine 1 µg/kg and 2 µg/kg, and intravenous dexmedetomidine 1 µg/kg, respectively. Propofol and remifentanil were target-controlled infused to maintain intraoperative bispectral index at 45–55 and blood pressure at baseline value±20%. Sufentanil was administered to maintain postoperative visual analogue scale ≤3. Perioperative anesthetics requirements were compared using nonparametric tests.
Results
Intranasal dexmedetomidine significantly attenuated propofol requirements for anesthesia induction and maintenance in a dose-dependent manner. Patients given intranasal dexmedetomidine 2 µg/kg required less remifentanil for anesthesia maintenance. The first postoperative request for sufentanil analgesia was delayed in patients given intranasal dexmedetomidine 2 µg/kg. The anesthetics-sparing effect of intranasal dexmedetomidine was significantly weaker than intravenous dexmedetomidine at the same dose of 1 µg/kg. The incidences of adverse events, including hemodynamic instability and delayed recovery, were comparable with and without intranasal dexmedetomidine.
Conclusion
Intranasal administration of dexmedetomidine can reduce perioperative anesthetic requirements, and a dose of dexmedetomidine 2 µg/kg produces a better effect in adults. The anesthetics-sparing effect of intranasal dexmedetomidine 1 µg/kg is less than that with the same intravenous dose of dexmedetomidine.
Keywords: Dexmedetomidine; administration, intranasal; anesthetics, intravenous
INTRODUCTION
With sedative, analgesic, and anti-sympathetic effects, dexmedetomidine is increasingly and widely used as an adjuvant drug in general anesthesia. Indeed, dexmedetomidine can reduce the perioperative use of sedatives and analgesics, and stabilizes intraoperative hemodynamics.1,2 It is clinically administered by intravenous and intranasal routes. Compared to intravenous administration, intranasal treatment is easier to administer and non-invasive.3 In addition, dexmedetomidine has a molecular weight of 236.7 Da and is easily absorbed through nasal mucosa. Intranasal dexmedetomidine is regularly used as premedication to reduce preoperative tension and anxiety in children.4,5
Recently, it has been reported that intranasal dexmedetomidine is a safe and effective sedative approach during short procedures in adults.6,7,8 However, the effect of intranasally administered adjunctive dexmedetomidine on perioperative anesthetic requirements in general anesthesia remains unknown. It was reported that the sedative onset time of intranasal dexmedetomidine is 45–60 min with a peak effect at 90–105 min in adults.9 We, therefore, hypothesized that intranasal dexmedetomidine could reduce anesthetic amounts used in operations lasting between 1–2 h. In addition, we aimed to compare the effects of dexmedetomidine administered intranasally and intravenously at equal preoperative doses on perioperative anesthetics requirements in adults.
MATERIALS AND METHODS
The study was approved by the ethics committee of the Affiliated People's Hospital of Jiangsu University and registered at www.chictr.org (ChiCTR-IOC-14005537). With written informed consent, patients scheduled for elective hysterectomy were recruited. Eligible participants were 40 to 55 years old with ASA physical status I. Exclusion criteria were as follows: 1) body mass index ≥30 kg/m2; 2) history of nasal operation and nasal diseases, including rhinitis, nasal polyp, and nasosinusitis; 3) cardiovascular comorbidity, including bradycardia and atrioventricular block; 4) cardiovascular treatment, including antihypertensive medications; 5) neurological comorbidity; 6) recent use of sedative and analgesic drugs and psychotropic medications; 7) allergy to an α2-adrenergic agonist; 8) alcohol abuse.
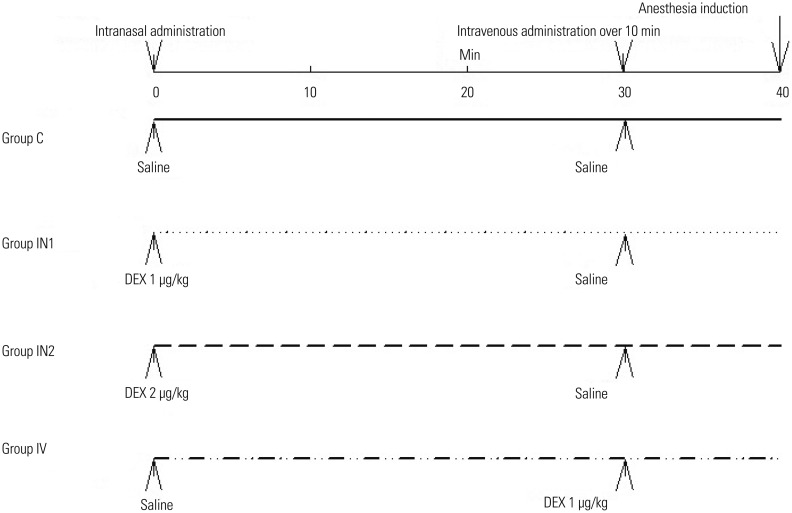
Patients were randomly divided into four groups: control (group C), intranasal dexmedetomidine 1 µg/kg (group IN1), intranasal dexmedetomidine 2 µg/kg (group IN2), and intravenous dexmedetomidine 1 µg/kg (group IV) group. Dexmedetomidine 1 µg/kg and 2 µg/kg were administered intranasally 40 min before anesthesia induction in groups IN1 and IN2, respectively. The initial concentration of dexmedetomidine was 100 µg/mL (Hengrui Medicine Co., Ltd., Lianyungang, Jiangsu, China), and intranasal drug was diluted with normal saline to a final volume of 1.6 mL. Dexmedetomidine was sprayed into both nostrils (0.8 mL per nostril). Normal saline was administered intravenously 30 min following nasal medication. Dexmedetomidine 1 µg/kg was administered intravenously 10 min before anesthesia induction in group IV. The study drug was diluted with normal saline to a final volume of 15 mL and was administered over 10 min. Normal saline was administered intranasally 30 min before intravenous medication. In group C, patients received normal saline intranasally and intravenously according to the above method (Fig. 1).
Fig. 1. Study design. DEX, dexmedetomidine; IN1, intranasal dexmedetomidine 1 µg/kg; IN2, intranasal dexmedetomidine 2 µg/kg; IV, intravenous dexmedetomidine 1 µg/kg.
No pre-anesthetic medication was given. After patients entered the operating room, nurse anesthetists were instructed to keep patients calm and to avoid unnecessary noise. An 18 G catheter was inserted in the forearm vein and Lactated Ringer's solution was infused at 8 mL/kg/h. The study was initiated after heart rate, noninvasive blood pressure, electrocardiography, pulse oxygen saturation, and bispectral index (BIS) monitoring were carried out. Atropine 0.005 mg/kg was given at a heart rate (HR) <50 bpm; phenylephrine 0.5 µg/kg was given when mean arterial pressure (MAP) reached <80% of the baseline value following intranasal administration. The Observer's Assessment of Alertness/Sedation (OAA/S) scores and BIS values were recorded before induction.
The commercial BIS-guided closed-loop target-controlled infusion (TCI) system (Slgo Medical Technology Co., Ltd., Beijing, China) of propofol was adopted for general anesthesia induction. This system integrates the BIS module (Aspect Medical Systems, Newton, MA, USA) for continuous BIS monitoring and Marsh pharmacokinetic parameters for the TCI of propofol. After entering patient information, such as sex, age, weight and height, the initial plasma propofol concentrations of groups C, IN1, IN2, and IV were set at 4.0, 3.0, 2.0, and 2.0 µg/mL, respectively, with their target BIS values at 50±5. When the BIS values decreased below 75, the closed-loop TCI system was initiated for automatic adjustment of propofol concentrations based on the dynamic BIS values, maintaining the BIS values within the target range. In addition, the system provides a real-time display of the dynamic BIS values and calculates plasma and effect-site concentrations of propofol. When the BIS reached the target values, effect-site TCI of remifentanil (Yichang Humanwell Pharmaceutical Co., Ltd., Yichang, Hubei, China) was applied via a commercially available TCI pump (slgo CP-600; Slgo Medical Technology Co., Ltd., Beijing, China) using the Minto pharmacokinetic model and adopting a constant effect-site concentration of 3.0 ng/mL. Then, intravenous administration of rocuronium 0.6 mg/kg was carried out 2 min later, and endotracheal intubation was performed 1 min thereafter. The lungs were ventilated to maintain an end-tidal carbon dioxide partial pressure (PetCO2) of 30–40 mm Hg with 100% oxygen. If HR fell below 50 bpm during anesthesia induction, atropine 0.005 mg/kg was administered intravenously; in case of MAP below 80% of the baseline value, phenylephrine 0.5 µg/kg was administered intravenously. These treatments were repeated as necessary. Upon induction completion, the TCI of remifentanil was discontinued temporally while urinary catheterization, as well as skin sterilization and draping, were initiated.
All hysterectomy procedures were performed by the same surgical team. TCI of remifentanil was restored 3 min before skin incision, with the same effect-site concentration of 3.0 ng/mL. The BIS values were maintained at 50±5 by closed-loop TCI of propofol; intraoperative MAP was maintained at baseline value ±20% by remifentanil administration. With MAP below the preset range, TCI of remifentanil was terminated and restored when MAP reached the preset range, with the level obtained at that time set as the new effect-site concentration. If BP was over the preset range, the effect-site concentration was increased by 0.5 ng/mL every 3 min till the preset MAP range was reached. When strong surgical stimuli were triggered (for instance, at the moment of skin incision, abdomen approach, and surgical exploration) and the concentration of remifentanil was altered, MAP was measured every 1 min; otherwise, it was measured every 3 min. If intraoperative MAP reached the preset range with HR below 50 bpm, atropine 0.005 mg/kg was administered; however, with normal MAP and HR over 90 bpm, esmolol 0.5 mg/kg was given; these treatments were repeated as needed. Atracurium was administered to maintain muscle relaxation at a rate of 5 µg/kg/min and stopped before closing the abdomen. Prior to skin anastomosis, propofol and remifentanil were discontinued, while sufentanil 0.3 µg/kg and ramosetron 0.3 mg were administrated.
Patients were transferred to the postanesthesia care unit (PACU) for 5 hours of postoperative observation. Patients were wakened when BIS values were restored to 70 and administrated neostigmine 0.02 mg/kg and atropine 0.01 mg/kg to antagonize muscle relaxation if necessary. Tracheal extubation was preformed after patients could obey simple commands, raise their heads for more than 5 sec, and breathe deeply on request. Oxygen inhalation, vital sign monitoring, and pain assessment were conducted postoperatively for all patients. When visual analogue scale (VAS) exceeded 3, sufentanil 0.1 µg/kg was administered, repeatedly if necessary, till VAS reached ≤3. Afterwards, patients were given a patient-controlled intravenous analgesia delivery system with sufentanil 2.0 µg/kg diluted to 100 mL, 2.0 mL boluses with a 10-minute lockout interval without continuous infusion, to maintain VAS ≤3.
Random numbers were generated using the EXCEL random number generator and allocated to each patient. Then, patients were divided into four groups in numerical order. One anesthetist applied intravenous or intranasal dexmedetomidine administration, set the initial propofol target concentration according to grouping, and turned on or off the BIS-guided propofol TCI pump. Another anesthetist, who was blinded to patient grouping and propofol TCI pump, performed anesthesia following the study protocol. Another PACU anesthetist blinded to grouping was in charge of pain assessment and analgesic administration.
The main outcome measures were effect-site concentration of propofol required to reach the target BIS value and total doses of propofol and remifentanil during anesthesia maintenance. Secondary outcome measures were time to first postoperative sufentanil supplement, postoperative total sufentanil dosage within 24 h, and number of patients that required atropine and phenylephrine during anesthesia induction.
Sample size and statistical analysis
Sample size calculation was based on the following assumptions: 1) The primary end point was propofol dosage required for maintenance of anesthesia. 2) In a previous study, the average infusion rate of propofol was 4.7±1.6 mg/kg/h in maintenance when using manual propofol and remifentanil TCI.10 3) Le Guen, et al.11 showed a 30% decrease of propofol requirements by a loading dose of 1 µg/kg dexmedetomidine followed by an infusion rate of 0.5 µg/kg/h during maintenance of anesthesia. We assumed that propofol requirements would be 20%, 20%, and 30% less in the present of intravenous dexmedetomidine 1 µg/kg and intranasal dexmedetomidine 1 and 2 µg/kg than without dexmedetomidine, respectively. 4) A two-tailed α of 0.05 and β of 0.10 required a sample size of 26 subjects per group. We thus planned to enroll 120 subjects in this study.
Data were analyzed using the SPSS statistical package, version 17.0 (SPSS Inc., Chicago, IL, USA) for Microsoft Windows. Data are expressed as means±SD or medians (with interquartile ranges, IQR) and numbers when appropriate. The significance level was set at 5% unless otherwise reported. Numerical variables were compared using parametric one-way ANOVA with least significant difference test for post hoc analysis or the nonparametric Kruskal-Wallis test followed by Mann-Whitney U with Bonferroni correction for between-group comparison after testing for normal distribution and homogeneity variance. The time to first sufentanil rescue was compared using a log-rank test with Bonferroni correction. Chi-square test and Fisher exact test were used for intergroup comparison of categorical data with Bonferroni correction. The significance level for Bonferroni correction was set at 0.0083.
RESULTS
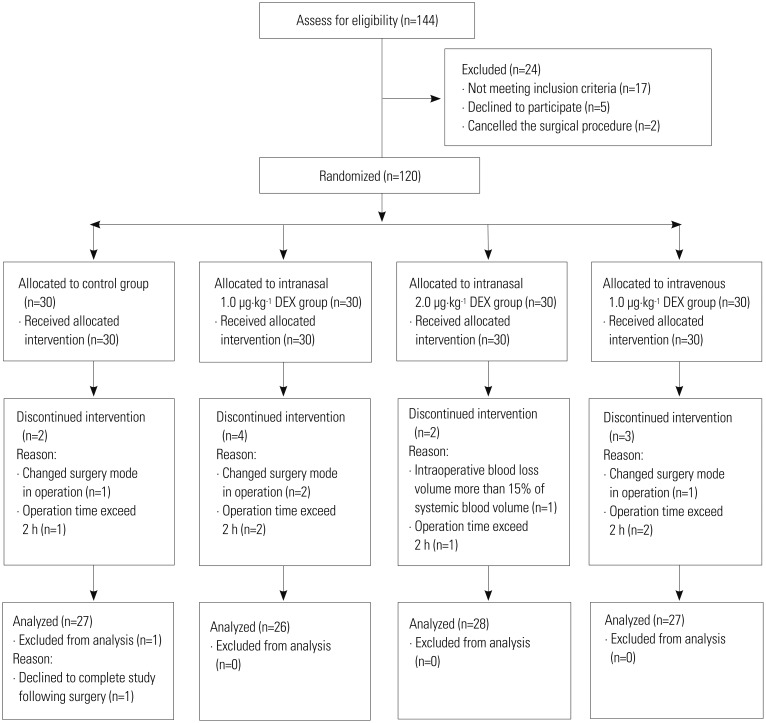
One hundred and forty-four patients were screened for eligibility, and 120 were subsequently allocated to four groups. Eleven patients with hypertension, four patients with nasal diseases, and two patients with bradycardia were excluded. A total of one hundred and eight patients completed the study (Fig. 2).
Fig. 2. Trial consort diagram. DEX, dexmedetomidine.
There were no significant differences in patient characteristics, baseline MAP and HR, baseline BIS, operation time, intraoperative urine output, and blood loss (Table 1).
Table 1. Demographic Data and Perioperative Parameters.
| Group C (n=27) | Group IN1 (n=26) | Group IN2 (n=28) | Group IV (n=27) | |
|---|---|---|---|---|
| Age (yrs) | 48±4 | 47±4 | 47±5 | 47±5 |
| Weight (kg) | 59±5 | 58±5 | 58±4 | 58±4 |
| Baseline MAP (mm Hg) | 87±7 | 84±6 | 86±6 | 88±5 |
| Baseline HR (bpm) | 77±10 | 81±7 | 81±9 | 79±10 |
| Baseline BIS | 97 (95-98) | 97 (96-98) | 97 (96-98) | 97 (96-97) |
| Operative time (min) | 103±15 | 99±15 | 102±12 | 97±14 |
| Intraoperative urine output (mL/h) | 133±39 | 128±34 | 153±46 | 145±44 |
| Blood loss (mL) | 61±28 | 48±25 | 56±27 | 66±27 |
MAP, mean arterial pressure; HR, heart rate; BIS, bispectral index; IN1, intranasal dexmedetomidine 1 µg/kg; IN2, intranasal dexmedetomidine 2 µg/kg; IV, intravenous dexmedetomidine 1 µg/kg.
Data are mean±SD or median (interquartile ranges).
Dexmedetomidine significantly decreased BIS values and OAA/S scores at 40 min after intranasal administration. The BIS values and OAA/S scores were significantly lower in patients given intranasal dexmedetomidine 2 µg/kg than intranasal dexmedetomidine 1 µg/kg. There were no significant differences in adverse hemodynamics events before anesthesia induction between the groups (Table 2).
Table 2. Effects on BIS and OAA/S and Hemodynamics of Intranasal and Intravenous Dexmedetomidine.
| Group C (n=27) | Group IN1 (n=26) | Group IN2 (n=28) | Group IV (n=27) | |
|---|---|---|---|---|
| BIS values before induction | 96 (93-97) | 90 (88-94)* | 78 (73-84)*† | 84 (82-86)*†‡ |
| OAA/S scores before induction | 5 (5-5) | 5 (4-5)* | 4 (4-4)*† | 4 (4-5)*‡ |
| Bradycardia requiring treatment | 1 | 0 | 1 | 3 |
| Hypotension requiring treatment | 0 | 0 | 1 | 0 |
BIS, bispectral index; OAA/S, observer assessment of alertness/sedation; IN1, intranasal dexmedetomidine 1 µg/kg; IN2, intranasal dexmedetomidine 2 µg/kg; IV, intravenous dexmedetomidine 1 µg/kg.
Data are median (interquartile ranges) or number.
*p<0.0083 vs. group C, †p<0.0083 vs. group IN1, ‡p<0.0083 vs. group IN2.
Anesthesia induction was quicker in groups IN2 and IV, compared to groups IN1 and C. The effect-site concentration of propofol for reaching target BIS values in groups IN1 and IN2 were significantly lower than the control group in the induction phase. In addition, the concentration of propofol in group IN2 was significantly lower than that in group IN1. Patients given intravenous dexmedetomidine required significantly less propofol concentration than the same dose of intranasal dexmedetomidine for anesthetic induction and more propofol concentration than the double dose of intranasal dexmedetomidine. More patients in group IV required treatment with atropine than the other groups. No intergroup differences were observed for numbers of patients requiring phenylephrine (Table 3).
Table 3. Induction Phase (from the Start of TCI Propofol to the End of TCI Remifentanil).
| Group C (n=27) | Group IN1 (n=26) | Group IN2 (n=28) | Group IV (n=27) | |
|---|---|---|---|---|
| Induction time (min) | 10.0 (9.0-11.0) | 9.5 (7-11) | 8 (7-10)* | 8 (8-10)* |
| Propofol concentration reaching preset BIS value (µg/mL) | 3.8 (3.6-4.0) | 2.9 (2.6-3.0)* | 1.6 (1.4-2.1)*† | 2.2 (1.7-2.4)*†‡ |
| Number of patients required atropine | 3 | 2 | 3 | 12*†‡ |
| Number of patients required phenylephrine | 2 | 1 | 2 | 2 |
TCI, target-controlled infusion; BIS, bispectral index; IN1, intranasal dexmedetomidine 1 µg/kg; IN2, intranasal dexmedetomidine 2 µg/kg; IV, intravenous dexmedetomidine 1 µg/kg.
Data are median (interquartile ranges) or number.
*p<0.0083 vs. group C, †p<0.0083 vs. group IN1, ‡p<0.0083 vs. group IN2.
There were no significant differences in anesthesia maintenance time. Significantly less propofol were required in groups IN1 and IN2 than the control group in the maintenance phase. In addition, the dosage of propofol for maintaining the target BIS value in group IN2 was significantly lower than that in group IN1. Patients given intravenous dexmedetomidine required significantly less propofol dosage than intranasal dexmedetomidine for anesthesia maintenance at the same preoperative dose and more propofol dosage than the double dose of intranasal dexmedetomidine. Significantly less remifentanil was required in groups IN2 and IV than groups C and IN1 in the maintenance phase. There were no significant differences in remifentanil requirements between groups C and IN1 (Table 4).
Table 4. Maintenance Phase (from Skin Incision to Termination of TCI Propofol and Remifentanil).
| Group C (n=27) | Group IN1 (n=26) | Group IN2 (n=28) | Group IV (n=27) | |
|---|---|---|---|---|
| Maintenance time (min) | 99±15 | 95±15 | 98±11 | 93±14 |
| Propofol dosage (mg/kg/h) | 4.00 (3.77-4.32) | 3.70 (3.35-3.90)* | 2.70 (2.50-3.22)*† | 3.30 (3.00-3.40)*†‡ |
| Remifentanil dosage (µg/kg/min) | 0.15 (0.13-0.17) | 0.14 (0.13-0.18) | 0.11 (0.07-0.15)*† | 0.11 (0.10-0.15)*† |
TCI, target-controlled infusion; IN1, intranasal dexmedetomidine 1 µg/kg; IN2, intranasal dexmedetomidine 2 µg/kg; IV, intravenous dexmedetomidine 1 µg/kg.
Data are mean±SD or median (interquartile ranges).
*p<0.0083 vs. group C, †p<0.0083 vs. group IN1, ‡p<0.0083 vs. group IN2.
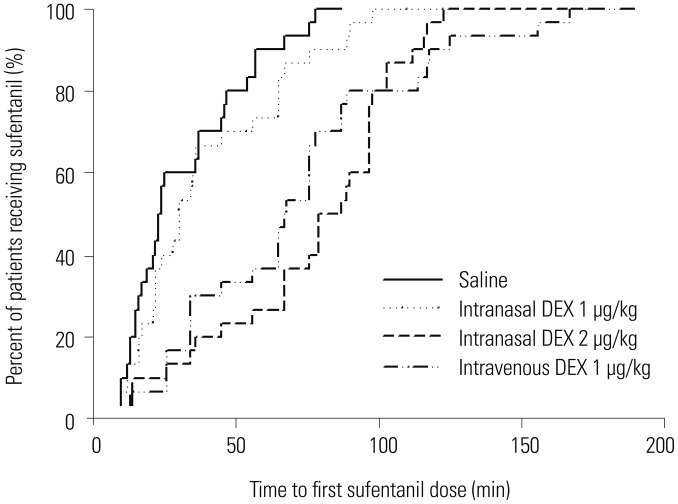
There were no significant differences in postoperative extubation time. The median time to first postoperative rescue sufentanil was significantly longer in groups IN2 and IV than groups C and IN1. There were no significant differences in delay to first postoperative sufentanil administration between groups C and IN1 (Fig. 3).
Fig. 3. Kaplan-Meier curves analyzing delay to first postoperative sufentanil rescue. DEX, dexmedetomidine.
The sufentanil requirements in the first 24 h postoperatively were significantly lower in patients given intranasal dexmedetomidine 2 µg/kg or intravenous dexmedetomidine 1 µg/kg than intranasal dexmedetomidine 1 µg/kg or saline (Table 5).
Table 5. Postoperative Phase.
| Group C (n=27) | Group IN1 (n=26) | Group IN2 (n=28) | Group IV (n=27) | |
|---|---|---|---|---|
| Extubation time (min) | 10±3 | 10±4 | 9±3 | 9±3 |
| Time to VAS >3 at rest (min) | 25 (15-50) | 35 (20-65) | 90 (65-100)*† | 75 (45-95)*† |
| Postoperative sufentanil requirements in the first 24 h (µg) | 76 (64-82) | 57 (56-88) | 45 (34-58)*† | 56 (50-57)*†‡ |
VAS, visual analogue scale; IN1, intranasal dexmedetomidine 1 µg/kg; IN2, intranasal dexmedetomidine 2 µg/kg; IV, intravenous dexmedetomidine 1 µg/kg.
Data are mean±SD or median (interquartile ranges).
*p<0.0083 vs. group C, †p<0.0083 vs. group IN1, ‡p<0.0083 vs. group IN2.
DISCUSSION
As shown above, intranasal administration of dexmedetomidine prior to anesthesia induction can reduce perioperative anesthetics requirements, and the effect of intranasal dexmedetomidine 1 µg/kg was less pronounced than that observed with the same dose of intravenous dexmedetomidine in adults.
Our study applied a BIS-guided, closed-loop TCI system, which automatically adjusts the target concentration of propofol to maintain target BIS values, making medication more objective, avoiding subjective influences from the operators, and realizing a smoother sedation depth. During induction and maintenance of anesthesia, intranasal dexmedetomidine attenuated propofol requirements for reaching predetermined BIS values in a dose-dependent manner, in accordance with previous findings focused on the sedative effect of intranasal dexmedetomidine in children. A study by Wang, et al.12 indicated intranasal dexmedetomidine 2 µg/kg induces a stronger sedative effect than 1 µg/kg in children. When dexmedetomidine was administered intranasally for procedural sedation in children undergoing diagnostic magnetic resonance imaging, the success rates of dexmedetomidine 2 µg/kg and 3 µg/kg were 60% and 86%, respectively.13,14
Previous studies indicated that intranasal and intravenous dexmedetomidine had similar pharmacological effects. Iirola, et al.15 applied both intranasal and intravenous dexmedetomidine to healthy volunteers and recorded their OAA/S scores and BIS values, finding similar areas under the curve for the OAA/S scores and BIS values within 3 h. In addition, Zhang, et al.8 demonstrated that OAA/S scores of patients receiving the same dose of dexmedetomidine intranasally and intravenously were not significantly different. As shown above, intravenous dexmedetomidine 1 µg/kg resulted in a lower dosage of propofol used during anesthesia induction and maintenance than those required with the same dose of intranasal dexmedetomidine, not corroborating the above studies. The following reasons may explain this inconsistency: first, dexmedetomidine has a unique conscious sedation effect, and it is hard to judge variations in the degree of sedation based on subjective OAA/S scores when sedation is not deep; patients need to be called and patted during OAA/S assessment, and these stimuli not only disturb the sedative state of healthy volunteers but also alter the BIS values. In addition, without muscle relaxants, BIS monitoring is easily disturbed by electromyography of the frontalis muscle.16 Here, we adjusted propofol dosages based on intraoperative BIS monitoring, providing a better reflection of the different sedation effects induced by intravenous and intranasal administrations of dexmedetomidine. Secondly, drug absorption is slow after intranasal dexmedetomidine, and the peak concentration is significantly lower than that for its intravenous counterpart. This not only delays the drug onset time but also results in a less pronounced maximum effect of intranasal vs. intravenous dexmedetomidine.15 Finally, absorption of intranasal medication is affected by many factors. Apart from mucociliary clearance rate, nasal secretion, and nasal mucosal blood flow, administration volume is also an important factor. The adequate administration volume for the nasal cavity is 0.1–0.2 mL. It is possible that part of the intranasal drug solution can flow into the pharynx and be swallowed. In the present study, we used an intravenous preparation of dexmedetomidine and the intranasal administration volume was large, which might reduce the bioavailability and pharmacological effect after intranasal treatment.
Intranasal dexmedetomidine 2 µg/kg reduced remifentanil amounts during anesthesia maintenance, while small doses of dexmedetomidine displayed no significant effect. Intravenous dexmedetomidine also showed a similar dose-effect relationship. Ngwenyama, et al.17 found that intravenous infusion of dexmedetomidine 0.5 µg/kg/h without preload did not affect the intraoperative analgesics dosage. Meanwhile, Bekker, et al.18 applied continuous infusion of dexmedetomidine 0.5 µg/kg/h with a preload of 1.0 µg/kg to patients receiving craniotomy, and intraoperative analgesics requirements were reduced. Systemic dexmedetomidine lacks robust analgesic efficacy.19,20 Our pilot study also showed that a loading dose of intravenous dexmedetomidine 1 µg/kg, followed by an infusion rate of 0.33 µg/kg/h, does not reduce intraoperative opioid requirements, irrespective of its sedative and sympatholytic properties.21 Dexmedetomidine is a potent sympatholytic agent, which can inhibit sympathetic activation following surgical stimuli. In the previous and present studies, hemodynamic criteria were used as stress response indicators to guide intraoperative opioid administration upon adjunctive DEX administration in general anesthesia.22,23 Therefore, the analgesics-sparing effect produced by dexmedetomidine may be attributed to its sympatholytic properties.
Preoperative administration of dexmedetomidine can reduce analgesic requirements until the postoperative period. Unlugenc, et al.24 found that intravenous dexmedetomidine 1.0 µg/kg prior to anesthesia induction reduced the required morphine use by 28% in abdominal operations. Dexmedetomidine's half-life is about 2 h after both intravenous and intranasal administrations;15 therefore, the effects of preoperatively intranasal and intravenous dexmedetomidine should be similar and persist until the postoperative phase. As shown above, intranasal dexmedetomidine 2.0 µg/kg delayed the first postoperative sufentanil supplement and reduced the analgesics required 24 h postoperatively.
Dexmedetomidine has central anti-sympathetic and vagal nervous activity promoting effects, which may result in hypotension and bradycardia, in particular at high doses. We found that more patients required atropine injections following intravenous dexmedetomidine 1.0 µg/kg; meanwhile, Wang, et al.25 also demonstrated that intravenous dexmedetomidine 1.0 µg/kg during TCI of propofol increases the incidence of bradycardia. However, intranasal administration of dexmedetomidine did not increase atropine requirements during anesthesia induction, which may be associated with slower absorption and lower peak concentration with intranasal administration, compared with the intravenous treatment. There are mainly two possible reasons why intravenous and intranasal dexmedetomidine do not increase vasopressors requirements. First, dexmedetomidine has a bidirectional effect upon blood pressure: when exerting an effect upon the peripheral α2 adrenoceptor, the drug will contract peripheral blood vessels and increase blood pressure. Therefore, even an intravenous load of 1.0 µg/kg dexmedetomidine will not increase the usage rate of vasopressors. Second, because propofol induces a dose-dependent decrease in blood pressure, its reduced usage induced by dexmedetomidine can compensate for the hypotension caused by the latter drug.
Dexmedetomidine produces characteristically arousable sedation, which differs markedly from other sedatives. Kasuya, et al.26 showed that at comparable BIS values, OAA/S scores were higher with dexmedetomidine than with propofol sedation. Although the median (range) of BIS was 78 (69–93) with intranasal dexmedetomidine 2 µg/kg, the median (range) of OAA/S was 4 (4–5), which is considered a shallow sedation level. In the study, the sedative level and hemodynamics parameter were recorded merely 40 min following intranasal dexmedetomidine. Further investigation was needed to explore pharmacological effect of intranasal dexmedetomidine 2 µg/kg.
Onset of intranasal dexmedetomidine is delayed compared with that of intravenous treatment, which limits its application in anesthesia, particularly during consecutive operations. In the current study, we did not emphatically compare differences in onset time between intranasal dexmedetomidine 1 and 2 µg/kg. Previous studies on children showed the onset time of sedation following intranasal administration of 1 µg/kg dexmedetomidine was 25 min, whereas the onset time of intranasal dexmedetomidine 2 and 2.5 µg/kg shortened to 15 and 13.4 min, respectively.27,28,29 We, therefore, speculate that the onset time of sedation of intranasal dexmedetomidine 2 µg/kg may be shortened in adults, although this still requires further verification.
In conclusion, both intranasal and intravenous administrations of dexmedetomidine reduced perioperative anesthetic requirements. Intranasal treatment, however, resulted in a less pronounced effect at the same dose of 1 µg/kg. Preoperatively, intranasal dexmedetomidine 2 µg/kg produces a more obvious anesthetics-sparing effect and seems to not increase the incidence of bradycardia and hypotension in adults.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kaur M, Singh PM. Current role of dexmedetomidine in clinical anesthesia and intensive care. Anesth Essays Res. 2011;5:128–133. doi: 10.4103/0259-1162.94750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcangeli A, D'Alò C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10:687–695. doi: 10.2174/138945009788982423. [DOI] [PubMed] [Google Scholar]
- 3.Grassin-Delyle S, Buenestado A, Naline E, Faisy C, Blouquit-Laye S, Couderc LJ, et al. Intranasal drug delivery: an efficient and noninvasive route for systemic administration: focus on opioids. Pharmacol Ther. 2012;134:366–379. doi: 10.1016/j.pharmthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Jia JE, Chen JY, Hu X, Li WX. A randomised study of intranasal dexmedetomidine and oral ketamine for premedication in children. Anaesthesia. 2013;68:944–949. doi: 10.1111/anae.12312. [DOI] [PubMed] [Google Scholar]
- 5.Ghali AM, Mahfouz AK, Al-Bahrani M. Preanesthetic medication in children: a comparison of intranasal dexmedetomidine versus oral midazolam. Saudi J Anaesth. 2011;5:387–391. doi: 10.4103/1658-354X.87268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung CW, Ng KF, Liu J, Yuen MY, Ho MH, Irwin MG. Analgesic and sedative effects of intranasal dexmedetomidine in third molar surgery under local anaesthesia. Br J Anaesth. 2011;107:430–437. doi: 10.1093/bja/aer164. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CW, Qiu Q, Liu J, Chu KM, Irwin MG. Intranasal dexmedetomidine in combination with patient-controlled sedation during upper gastrointestinal endoscopy: a randomised trial. Acta Anaesthesiol Scand. 2015;59:215–223. doi: 10.1111/aas.12445. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Bai X, Zhang Q, Wang X, Lu L. The safety and efficacy of intranasal dexmedetomidine during electrochemotherapy for facial vascular malformation: a double-blind, randomized clinical trial. J Oral Maxillofac Surg. 2013;71:1835–1842. doi: 10.1016/j.joms.2013.06.202. [DOI] [PubMed] [Google Scholar]
- 9.Yuen VM, Irwin MG, Hui TW, Yuen MK, Lee LH. A double-blind, crossover assessment of the sedative and analgesic effects of intranasal dexmedetomidine. Anesth Analg. 2007;105:374–380. doi: 10.1213/01.ane.0000269488.06546.7c. [DOI] [PubMed] [Google Scholar]
- 10.Liu N, Chazot T, Hamada S, Landais A, Boichut N, Dussaussoy C, et al. Closed-loop coadministration of propofol and remifentanil guided by bispectral index: a randomized multicenter study. Anesth Analg. 2011;112:546–557. doi: 10.1213/ANE.0b013e318205680b. [DOI] [PubMed] [Google Scholar]
- 11.Le Guen M, Liu N, Tounou F, Augé M, Tuil O, Chazot T, et al. Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index-guided closed-loop anesthesia: a doubleblind, placebo-controlled trial. Anesth Analg. 2014;118:946–955. doi: 10.1213/ANE.0000000000000185. [DOI] [PubMed] [Google Scholar]
- 12.Wang SS, Zhang MZ, Sun Y, Wu C, Xu WY, Bai J, et al. The sedative effects and the attenuation of cardiovascular and arousal responses during anesthesia induction and intubation in pediatric patients: a randomized comparison between two different doses of preoperative intranasal dexmedetomidine. Paediatr Anaesth. 2014;24:275–281. doi: 10.1111/pan.12284. [DOI] [PubMed] [Google Scholar]
- 13.Ambi US, Joshi C, Ganeshnavar A, Adarsh E. Intranasal dexmedetomidine for paediatric sedation for diagnostic magnetic resonance imaging studies. Indian J Anaesth. 2012;56:587–588. doi: 10.4103/0019-5049.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim M. A prospective, randomized, double blinded comparison of intranasal dexmedetomodine vs intranasal ketamine in combination with intravenous midazolam for procedural sedation in school aged children undergoing MRI. Anesth Essays Res. 2014;8:179–186. doi: 10.4103/0259-1162.134495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iirola T, Vilo S, Manner T, Aantaa R, Lahtinen M, Scheinin M, et al. Bioavailability of dexmedetomidine after intranasal administration. Eur J Clin Pharmacol. 2011;67:825–831. doi: 10.1007/s00228-011-1002-y. [DOI] [PubMed] [Google Scholar]
- 16.Vivien B, Di Maria S, Ouattara A, Langeron O, Coriat P, Riou B. Overestimation of Bispectral Index in sedated intensive care unit patients revealed by administration of muscle relaxant. Anesthesiology. 2003;99:9–17. doi: 10.1097/00000542-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ngwenyama NE, Anderson J, Hoernschemeyer DG, Tobias JD. Effects of dexmedetomidine on propofol and remifentanil infusion rates during total intravenous anesthesia for spine surgery in adolescents. Paediatr Anaesth. 2008;18:1190–1195. doi: 10.1111/j.1460-9592.2008.02787.x. [DOI] [PubMed] [Google Scholar]
- 18.Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008;107:1340–1347. doi: 10.1213/ane.0b013e3181804298. [DOI] [PubMed] [Google Scholar]
- 19.Angst MS, Ramaswamy B, Davies MF, Maze M. Comparative analgesic and mental effects of increasing plasma concentrations of dexmedetomidine and alfentanil in humans. Anesthesiology. 2004;101:744–752. doi: 10.1097/00000542-200409000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu X, Hang LH, Chen YF, Wang H, Shao DH, Chen Z. Remifentanil requirements for preventing motor response to skin incision in healthy women anesthetized with combinations of propofol and dexmedetomidine titrated to similar Bispectral Index (BIS) values. Ir J Med Sci. 2015;184:805–811. doi: 10.1007/s11845-014-1176-2. [DOI] [PubMed] [Google Scholar]
- 22.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–652. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 23.Al-Zaben KR, Qudaisat IY, Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Oweidi AS, et al. Intraoperative administration of dexmedetomidine reduces the analgesic requirements for children undergoing hypospadius surgery. Eur J Anaesthesiol. 2010;27:247–252. doi: 10.1097/EJA.0b013e32833522bf. [DOI] [PubMed] [Google Scholar]
- 24.Unlugenc H, Gunduz M, Guler T, Yagmur O, Isik G. The effect of pre-anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient-controlled morphine. Eur J Anaesthesiol. 2005;22:386–391. doi: 10.1017/s0265021505000669. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Ge S, Xiong W, Zhou P, Cang J, Xue Z. Effects of different loading doses of dexmedetomidine on bispectral index under stepwise propofol target-controlled infusion. Pharmacology. 2013;91:1–6. doi: 10.1159/000343634. [DOI] [PubMed] [Google Scholar]
- 26.Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–1815. doi: 10.1213/ANE.0b013e3181c04e58. [DOI] [PubMed] [Google Scholar]
- 27.Yuen VM, Hui TW, Irwin MG, Yao TJ, Wong GL, Yuen MK. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia. 2010;65:922–929. doi: 10.1111/j.1365-2044.2010.06453.x. [DOI] [PubMed] [Google Scholar]
- 28.Talon MD, Woodson LC, Sherwood ER, Aarsland A, McRae L, Benham T. Intranasal dexmedetomidine premedication is comparable with midazolam in burn children undergoing reconstructive surgery. J Burn Care Res. 2009;30:599–605. doi: 10.1097/BCR.0b013e3181abff90. [DOI] [PubMed] [Google Scholar]
- 29.Mekitarian Filho E, Robinson F, de Carvalho WB, Gilio AE, Mason KP. Intranasal dexmedetomidine for sedation for pediatric computed tomography imaging. J Pediatr. 2015;166:1313–1315.e1. doi: 10.1016/j.jpeds.2015.01.036. [DOI] [PubMed] [Google Scholar]