Abstract
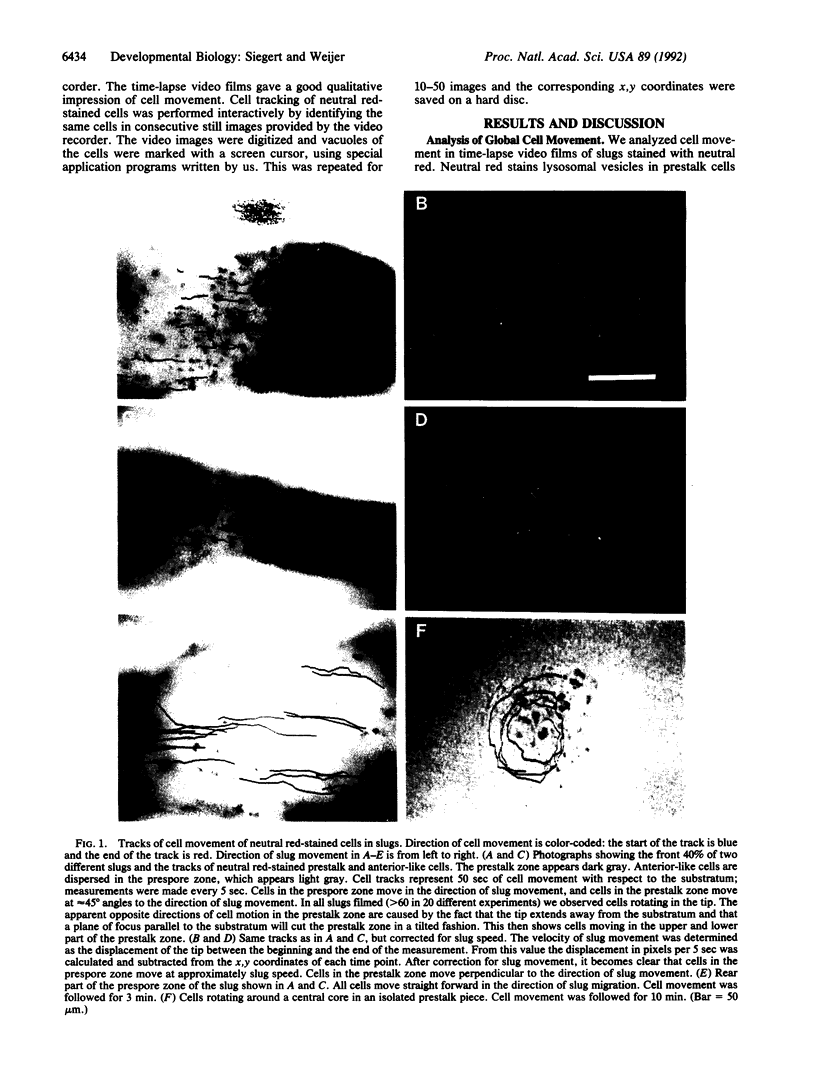
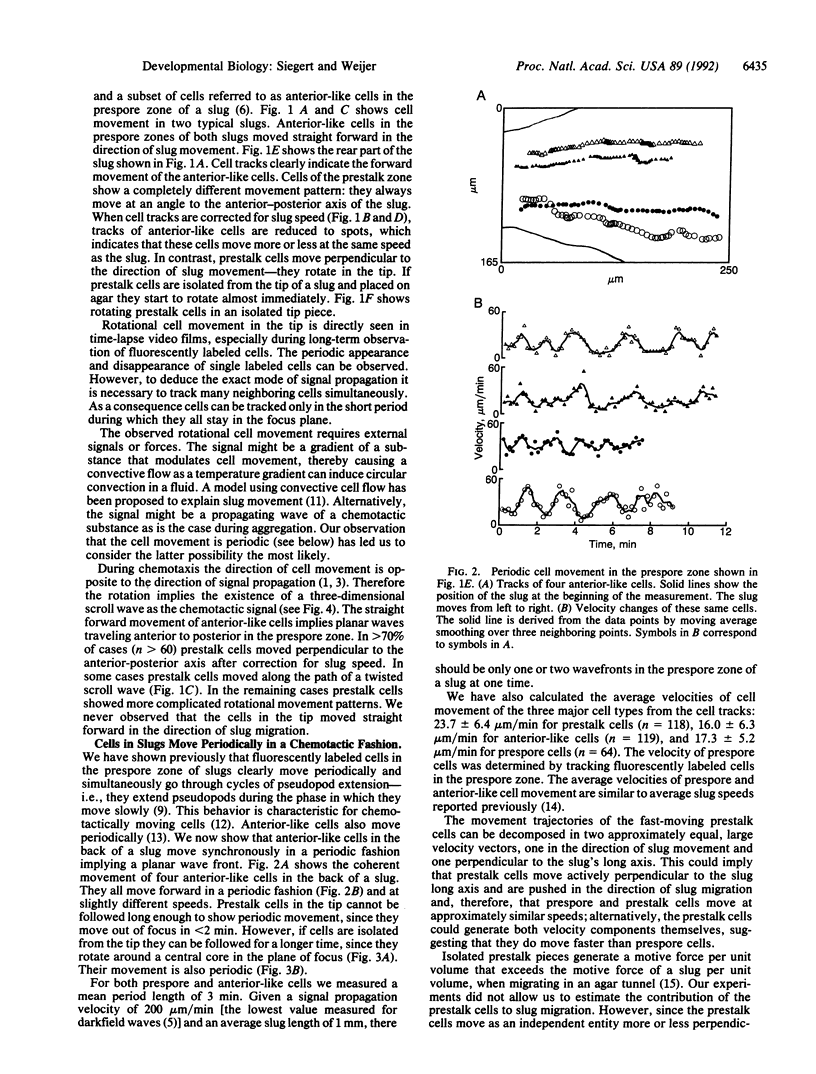
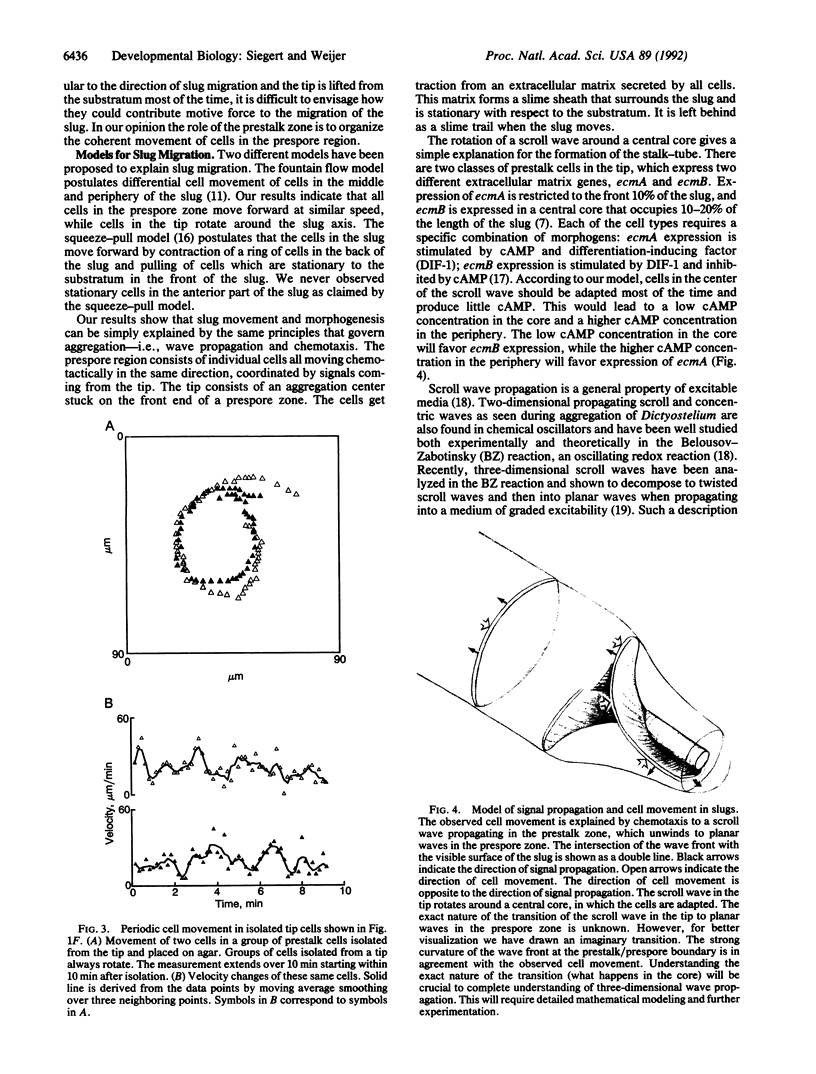
To test the hypothesis that periodic signals and chemotaxis direct later morphogenesis in Dictyostelium discoideum, we investigated cell behavior and cell movement in slugs. Trails of neutral red-stained prestalk and anterior-like cells were recorded by high-resolution digital image processing. Neutral red-stained anterior-like cells in the prespore zone of slugs move straight forward in the direction of slug migration and, furthermore, show coherent periodic cell movement. In contrast, cells in the prestalk zone move along completely different trajectories. Prestalk cells move perpendicular to the direction of slug migration; that is, they rotate around the tip. The cell movement data show that the chemotactic signal in the slug propagates as a three-dimensional scroll wave in the prestalk zone and as a planar wave in the prespore zone. The different behavior of prestalk and prespore cells is most likely caused by a difference in the oscillatory properties of the two cell types. We provide evidence that the slug stage of Dictyostelium behaves like an excitable system and that a (twisted) scroll wave organizes slug formation and migration.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcantara F., Monk M. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J Gen Microbiol. 1974 Dec;85(2):321–334. doi: 10.1099/00221287-85-2-321. [DOI] [PubMed] [Google Scholar]
- Berks M., Kay R. R. Combinatorial control of cell differentiation by cAMP and DIF-1 during development of Dictyostelium discoideum. Development. 1990 Nov;110(3):977–984. doi: 10.1242/dev.110.3.977. [DOI] [PubMed] [Google Scholar]
- Bonner J. T., Har D., Suthers H. B. Ammonia and thermotaxis: Further evidence for a central role of ammonia in the directed cell mass movements of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2733–2736. doi: 10.1073/pnas.86.8.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J., Bresnick A., Demma M., Dharmawardhane S., Eddy R., Hall A. L., Sauterer R., Warren V. Mechanisms of amoeboid chemotaxis: an evaluation of the cortical expansion model. Dev Genet. 1990;11(5-6):333–340. doi: 10.1002/dvg.1020110504. [DOI] [PubMed] [Google Scholar]
- Devreotes P. Dictyostelium discoideum: a model system for cell-cell interactions in development. Science. 1989 Sep 8;245(4922):1054–1058. doi: 10.1126/science.2672337. [DOI] [PubMed] [Google Scholar]
- Durston A. J. Pacemaker activity during aggregation in Dictyostelium discoideum. Dev Biol. 1974 Apr;37(2):225–235. doi: 10.1016/0012-1606(74)90144-4. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Vork F. A cinematographical study of the development of vitally stained Dictyostelium discoideum. J Cell Sci. 1979 Apr;36:261–279. doi: 10.1242/jcs.36.1.261. [DOI] [PubMed] [Google Scholar]
- Foerster P., Müller S. C., Hess B. Curvature and propagation velocity of chemical waves. Science. 1988 Aug 5;241(4866):685–687. doi: 10.1126/science.241.4866.685. [DOI] [PubMed] [Google Scholar]
- Inouye K., Takeuchi I. Motive force of the migrating pseudoplasmodium of the cellular slime mould Dictyostelium discoideum. J Cell Sci. 1980 Feb;41:53–64. doi: 10.1242/jcs.41.1.53. [DOI] [PubMed] [Google Scholar]
- Jermyn K. A., Williams J. G. An analysis of culmination in Dictyostelium using prestalk and stalk-specific cell autonomous markers. Development. 1991 Mar;111(3):779–787. doi: 10.1242/dev.111.3.779. [DOI] [PubMed] [Google Scholar]
- Tomchik K. J., Devreotes P. N. Adenosine 3',5'-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution--fluorography. Science. 1981 Apr 24;212(4493):443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- Van Duijn B., Inouye K. Regulation of movement speed by intracellular pH during Dictyostelium discoideum chemotaxis. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4951–4955. doi: 10.1073/pnas.88.11.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. B., Elder E. M., Sussman M. Modulation of the cAMP relay in Dictyostelium discoideum by ammonia and other metabolites: possible morphogenetic consequences. Dev Biol. 1984 Oct;105(2):377–388. doi: 10.1016/0012-1606(84)90294-x. [DOI] [PubMed] [Google Scholar]
- Williams K. L., Vardy P. H., Segel L. A. Cell migrations during morphogenesis: some clues from the slug of Dictyostelium discoideum. Bioessays. 1986 Oct;5(4):148–152. doi: 10.1002/bies.950050403. [DOI] [PubMed] [Google Scholar]