Abstract
Oak and birch trees belong to Fagales order. Specific IgE to pollen allergens of both trees are frequently found in Korea pollinosis patients. Oak trees which comprise 40% of forest area are common in Korea. However, birch trees are sparse. We compared the allergenicity of pollen extracts of white oak, sawtooth and Mongolian oaks which are prevalent species in Korea, with the pollen extract of birch. The cross-reactivity of four pollen extracts was examined with pooled sera of 12 patients by ELISA, immunoblotting and CAP inhibitions. A protein of 17 kDa, putatively homologous to a major birch allergen Bet v 1, displayed strong IgE reactivity from white oak and sawtooth oak pollen extract but not from Mongolian oak pollen. Notably, a 23-kDa protein from sawtooth and white oaks showed strong IgE reactivity and inhibited by Bet v 1. IgE binding to white oak was inhibited a maximum of 94.6% by white oak, 93.4% by sawtooth oak, 83.2% by Mongolian oak, and 68.8% by birch. Furthermore, sawtooth oak, white oak, and Mongolian oak extracts were able to inhibit up to 78.5%, 76.6% and 67.3% of IgE binding to birch extract, while birch extract itself inhibited up to 94.3%. Specific IgE to Bet v 1 was inhibited a maximum of 79.1% by sawtooth oak, 77.4% by white oak, and 72.7% by Mongolian oak, while 81.5% inhibition was shown by birch. Bet v 1 was able to partially inhibit its homologous molecules from sawtooth oak and white oak in immunoblotting. Birch pollen extract was found to be cross-reactive primarily with Bet v 1-homologous allergen from oak pollens in Korea pollinosis patients. Considering the sparseness of birch tree in Korea, oak, especially sawtooth oak may be the main cause of tree pollinosis in Korea, rather than birch.
Keywords: Cross-Reactivity, Oak Pollen, Tree Pollinosis, IgE, Korea
Graphical Abstract
Introduction
In Korea, 4.1% to 22.9% of respiratory allergic subjects with perennial or seasonal symptoms were reported to be sensitized to oak and/or birch pollen as measured by skin prick test (1,2,3,4). Thus, oak and birch have been recognized as crucial culprits of tree pollinosis in Korea. However, birch trees are scarce in Korea. Oak trees represent almost 40% of all trees in Korea; less than 1% of Korean trees are birch as described in JH Park’s PhD thesis, 2009. Furthermore, pollen extracts from trees of the order Fagales, such as birch, alder, hornbeam, chestnut, hazel, and oak, show a high degree of cross-reactivity due to homologous proteins from the pathogenesis-related proteins-10 (PR-10) family (5,6). Sensitization to PR-10 allergens is known to be an important cause of oral allergy syndrome to apple (7).
Interestingly, the sensitization rate to white oak and common silver birch reportedly increased from 6.7% (1999) to 9.6% (2008) in the southern part of Gyeonggi province in Korea (3). Pollen extracts from white oak, Quercus alba, and common silver birch, Betula verrucosa, are commonly used for the diagnosis and immunotherapy of allergy patients. However, these species are not found in Korea. In a recent study, oak pollen was shown to be one of the most commonly sensitizing allergens in Korea, although there was a significant discrepancy of the positive rates between skin prick test (22.9%) and ImmunoCAP test (9.1%) (4). Only one of the studies utilized pollen extracts prepared from locally collected in Korea, even though identification of oak species were not made (1). Therefore, it is thought to be necessary to examine the cross-reactivity between commercial pollen extracts and those from native Korean oak species.
In this study, we prepared pollen extracts and investigate the cross-reactivity from common silver birch, white oak, Mongolian oak (Q. mongolica), and sawtooth oak (Q. acutissima) because Mongolian oak and sawtooth oak are dominant in Korea (8).
Materials and Methods
Serum samples
Blood was collected after informed consent from 12 patients attending the Allergy Clinic of the Severance Hospital, Yonsei University, Seoul, Korea. Allergy was diagnosed based on history and skin prick testing. Those with ImmunoCAP results higher than 0.7 kUA/L to birch (t3) and oak (t7) were used for the study (Table 1).
Table 1. Clinical features of the oak- and birch-positive subjects.
| Subject No. | Gender/age | Symptom/Diagnosis | Sensitization profile* | sIgE to birch (kUA/L) | sIgE to oak (kUA/L) | Total IgE (kUA/L) |
|---|---|---|---|---|---|---|
| S1 | F/47 | AC, AR, OAS | t3, t7, g2, g6, d2, e5 | 85.2 (5) | 73.9 (5) | 994 |
| S2 | M/46 | AC, AR, UR | t1, t3, t7, w230, w231, d2 | 25.0 (4) | 17.4 (3) | ND |
| S3 | F/44 | AD, UR | t3, t7, t17, g5, d2, e1 | 9.45 (3) | 6.48 (3) | 534 |
| S4 | M/41 | AE, AR, UR | t3, t7, d1, d2,f12, f14 | 15.5 (3) | 12.8 (3) | 206 |
| S5 | F/36 | AR | t2, t3, t7, d1, d2 | 63.1 (5) | 23.2 (4) | 233 |
| S6 | F/40 | AS, AR, CF, DA | t3, t7, f14 | 10.8 (3) | 3.45 (2) | ND |
| S7 | F/10 | AC, AD, AR, OAS | t3, w1, d2, f1, f23, f24 | 48.7 (4) | 35.8 (4) | ND |
| S8 | F/47 | AR, AS | t3, t7, w6, w22, m1 | 5.61 (3) | 1.45 (2) | 155 |
| S9 | M/15 | AR, AS | t3, t7, m6, d2, e5 | 4.76 (3) | 3.90 (3) | ND |
| S10 | F/26 | AR, AS, UR | t2, t3, t7, g5, w6, w22 | 33.0 (4) | 39.3 (4) | 2,418 |
| S11 | M/46 | AC, AR, UR | t1, t3, t7, w230, w231, d2 | 25.0 (4) | 17.4 (3) | ND |
| S12 | M/14 | AR, OAS | t3, g5, w22, m6, d2, e5 | 90.8 (5) | 48.0 (4) | 349 |
AC, allergic conjunctivitis; AD, atopic dermatitis; AE, angioedema; AR, allergic rhinitis; AS, asthma; CF, cough; DA, drug allergy; FA, Food allergy; OAS, oral allergy syndrome; UR, urticaria.
*t1, Box-elder; t2, Grey alder; t3, Common silver birch; t7, White oak; t17, Japanese cedar; g2, Bermuda grass; g5, Rye-grass; g6, Timothy grass; w1, Common ragweed; w6, Mugwort; w22, Japanese hop; w230, Amb a 1; w231, Art v 1; d1, Dermatophagoides pteronyssinus; d2, D. farinae; e1, Cat dander; e5, Dog dander; m1, Penicillium chrysogenum; m6, Alternaria alternata; f1, Egg white; f2, Milk; f12, Pea; f14, Soybean; f23, Crab; f24, Shrimp.
Allergen extracts
Pollens of Mongolian and sawtooth oaks were collected by a plant taxonomist. After defatting with ethyl ether, allergen was extracted for 48 hours in phosphate buffered saline, pH 7.4. The extract was centrifuged at 13,000 g for 15 minutes at 4°C and the supernatant was dialyzed (cutoff 3,500 Da, Spectrum, Houston, TX, USA) against distilled water. The dialysate was syringe-filtered (0.22 μm, Millipore, Bedford, MA, USA). Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, CA, USA). White oak and common silver birch extract were purchased from Hollister-Stier Laboratories LLC (Spokane, WA, USA).
SDS-PAGE and IgE immunoblotting
Allergen extracts (20 μg) were run on 18% SDS-PAGE gel under reducing conditions and stained with Coomassie brilliant blue or transferred to a polyvinylidene difluoride membrane (Micro Separation Inc., Westborough, MA, USA). After blocking with 3% skim milk, the membrane was incubated with 1:4 diluted sera. Subsequently, it was incubated with 1:1,000 diluted alkaline phosphatase-conjugated goat anti-human IgE (Sigma-Aldrich, St. Louis, MO, USA). Nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate (Promega, Madison, WI, USA) was used for color development. For inhibition analysis, 10 μg/mL of recombinant Bet v 1a (Indoor Biotechnologies Inc., Charlottesville, VA, USA) was pre-incubated with pooled sera (1:4 diluted) overnight. IgE reactive components were detected as described above.
Inhibition ELISA and ImmunoCAP
Allergen extract (10 μg/mL) was loaded to 96 well microplate (Corning Inc., NY, USA). After blocking with 3% skim milk, 1:4 diluted serum samples that were pre-incubated with various concentrations of inhibitors were incubated. IgE antibodies were detected by incubating with biotinylated goat anti-human IgE (1:1,000) (Vector, Burlingame, CA, USA), followed by streptavidin-peroxidase (1:1,000) (Sigma-Aldrich). The color was developed using 3,3',5,5'-tetramethyl-benzidine (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA) as a substrate. The enzyme reaction was stopped by the addition of 0.5 M H2SO4. The absorbance at 450 nm was determined. Percentage of inhibition was calculated by (1–absorbance with inhibitors/absorbance without inhibitor) × 100.
To investigate the role of Bet v 1-like allergens from various oak extracts, we performed inhibition ImmunoCAP. Serum samples were pre-incubated with various concentrations (0.016 to 50 μg/mL) of inhibitors. Subsequently, IgE to Bet v 1 (t215) was measured by ImmunoCAP (Phadia, Uppsala, Sweden) following the manufacturer’s instructions. The percentage of inhibition was calculated as described above (except that the IgE concentration was used instead of absorbance).
Ethics statement
The study protocol was reviewed and approved by the institutional review board of Yonsei University Severance Hospital (4-2009-0717). Informed consent was waived by the board.
Results
IgE reactivity to birch and oak pollen extracts
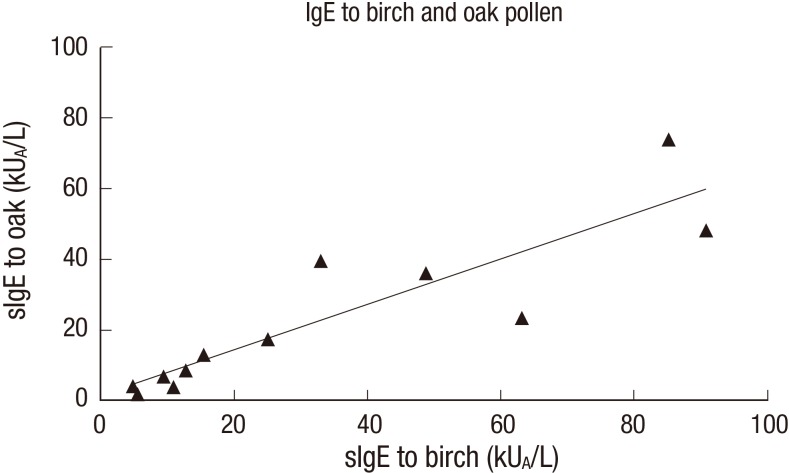
Interestingly, all the oak pollen positive serum samples were also positive to the birch pollen, suggesting the possibility of cross-reactivity (Fig. 1 and Table 1). The IgE reactivity of 12 serum samples to birch and oak were shown to be well correlated. Pearson’s correlation was calculated to be 0.89. Specific IgE (sIgE) to birch was shown to be higher than sIgE to oak.
Fig. 1.
Correlation of IgE reactivity to white oak and common silver birch pollen extract in Korean tree pollen allergy patients.
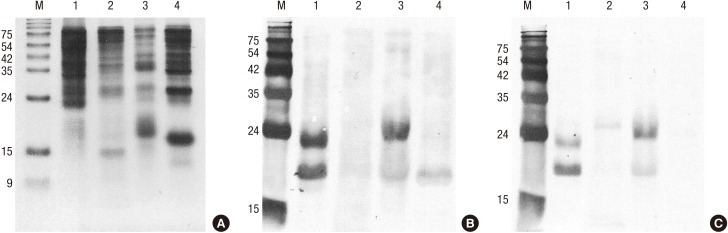
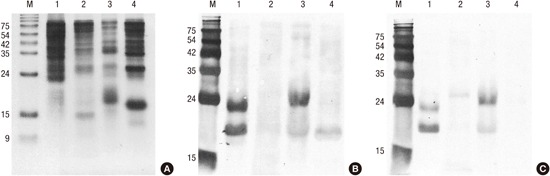
Different protein profiles were shown by SDS-PAGE analysis (Fig. 2A). White oak, sawtooth oak and common silver birch extracts showed a strong protein band of 17 kDa, putative PR-10 allergens, whereas Mongolian oak extract showed no strong protein band around 17 kDa, indicating the lower concentration of proteins of the PR-10 family in the Mongolian oak pollens than in the other pollens. IgE-binding reactivity to a 17 kDa allergen and various components of 20 to 60 kDa proteins, especially from sawtooth oak and white oak, were observed in a pooled serum (Fig. 2B). The strong IgE reactive bands were detected around 17 kDa from sawtooth oak (a putative Que ac 1), white oak (a putative Que a 1), and common silver birch (a putative Bet v 1), indicating that the PR-10 proteins are major allergens from these tree pollens. Interestingly, strong IgE binding was exhibited to about 23 kDa protein from sawtooth oak and white oak pollen extracts.
Fig. 2.
Antigenic bands of oaks and birch. (A) SDS-PAGE. Proteins (20 μg) were separated onto 18% polyacrylamide gel under reducing condition. (B) IgE immunoblot by probing IgE reactive proteins with serum from patients preincubated without inhibitor. (C) Inhibition immunoblot analysis with recombinant Bet v 1.
Lanes: M, molecular mass standard; 1, sawtooth oak (Quercus acutissima); 2, Mongolian oak (Quercus mongolica); 3, white oak (Q. alba); 4, common silver birch (Betula verrucosa).
Cross-reactivity of sawtooth oak and Mongolian oak, white oak, and common silver birch pollen extracts
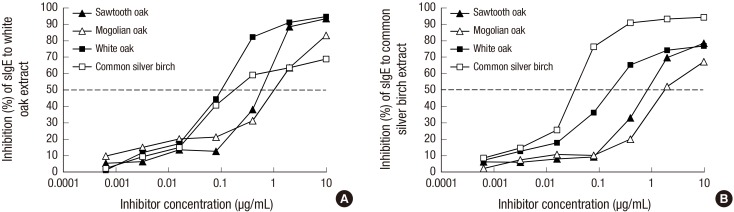
IgE reactivity to white oak was inhibited almost completely by sawtooth oak, Mongolian oak, and white oak, and profoundly by common silver birch (Fig. 3A). Maximum inhibition of IgE binding to white oak was found to be 94.6% by white oak, 93.4% by sawtooth oak, 83.2% by Mongolian oak, and 68.8% by common silver birch. Fifty percent inhibitory concentrations were 0.13 μg/mL for white oak, 0.24 μg/mL for common silver birch, 0.78 μg/mL for sawtooth oak, and 1.33 μg/mL for Mongolian oak. Maximum inhibition of IgE binding to common silver birch was 94.3% by common silver birch, 78.5% by sawtooth oak, 76.9% by white oak, and 67.3% by Mongolian oak (Fig. 3B). Fifty percent inhibition of IgE reactivity to common silver birch was obtained at an inhibitor concentration of 0.04 μg/mL for common silver birch, 0.23 μg/mL of white oak, 1.14 μg/mL of sawtooth oak, and 1.89 μg/mL of Mongolian oak.
Fig. 3.
Cross-reactivity of Fagales tree pollen extracts. (A) IgE reactivity to white oak was competitively inhibited by pre-incubation of serum with various concentrations of oak and birch. (B) IgE reactivity to common silver birch was also inhibited by oak and birch, and percentage of inhibition calculated to evaluate the cross-reactivity.
Contribution of PR-10 allergens for cross-reactivity among oaks and birch
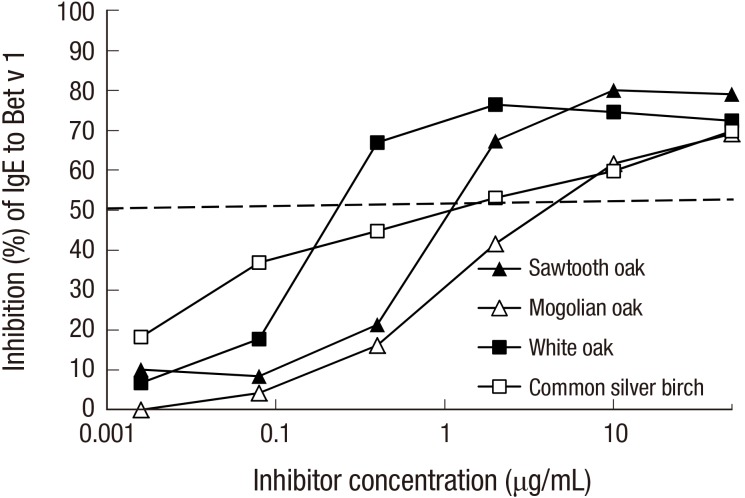
To assess the role of PR-10 allergens in cross-reactivity, we performed inhibition ImmunoCAP assays (Fig. 4). The maximum inhibition of IgE reactivity to Bet v 1 (t215) was 81.5% by common silver birch, 79.1% by sawtooth oak, 77.4% by white oak, and 72.7% by Mongolian oak. Fifty percent inhibitory concentrations were calculated to be 0.29 μg/mL for white oak, 1.39 μg/mL for sawtooth oak, 1.40 μg/mL for common silver birch, and 5.35 μg/mL for Mongolian oak pollen. In IgE immunoblotting, IgE reactivities to a putative Que ac 1 and 23 kDa protein from sawtooth oak and a putative Que a 1 and 23 kDa protein from white oak were shown to be inhibited partially by recombinant Bet v 1, while native Bet v 1 from common silver birch was completely inhibited (Fig. 2C).
Fig. 4.
Cross-reactivity by PR-10 allergens. Inhibition ImmunoCAP analysis against Bet v 1 was performed using sawtooth oak, Mongolian oak, white oak, and common silver birch pollen extracts.
Discussion
Generally, allergic subjects sensitized to oak also display IgE reactivity to birch in Korea. Oak trees are much more common than birch in Korea. Therefore, we investigated the presence of cross-reactivity between oak and birch pollen extracts. Strong correlation of sIgE to common silver birch and white oak implies probable cross-reactivity (Fig. 1). However, these two oak and birch species are not native to Korea. In this study, we prepared allergen extracts from sawtooth and Mongolian oak pollens and compared their allergenicity with common silver birch and white oak.
PR-10 allergens are known to be a main cause of cross-reactivity among pollen allergens of the tree belonging to order Fagales (9). However, protein profiles on SDS-PAGE and IgE immunoblotting showed quite different concentrations of likely PR-10 allergens (Fig. 2) in the pollen extracts. White oak showed the strongest IgE reactivity to a putative Que a 1, indicating the highest concentration of PR-10 allergen in the extract. The PR-10 allergens are known to be produced under various environmental stresses (10). Therefore, the allergen contents of the extracts might be influenced by the environmental conditions where the pollen was collected. However, the low IgE binding activity to Mongolian oak pollen was reproduced by different annual batch sources harvest at different regions. IgE immunoblot inhibition analysis with recombinant Bet v 1a was carried out to investigate the role of PR-10 allergens. A putative Que ac 1 from sawtooth oak, a putative Que a 1 from white oak were partially inhibited. Incomplete inhibition may indicate shared IgE epitopes as well as peculiar epitopes on oak pollen allergens. However, it could be the result of an inhibition with a single isoform, because there should be various isoforms of PR-10 allergens (11). We found strong IgE binding to 23 kDa allergen from sawtooth oak and white oak, and the binding was partially inhibited by Bet v 1, suggesting the allergen may also belong to the PR-10 family. Further studies are necessary to see if these are oak specific.
IgE reactivity to white oak was most strongly inhibited by white oak (0.13 μg/mL), followed by common silver birch (0.24 μg/mL), sawtooth oak (0.78 μg/mL), and finally Mongolian oak (1.33 μg/mL), at 50% inhibitory concentration (Fig. 3A). IgE reactivity to common silver birch was also strongly inhibited by white oak (0.23 μg/mL), sawtooth oak (1.14 μg/mL), and Mongolian oak (1.89 μg/mL) at 50% inhibitory concentration compared to 0.04 μg/mL of common silver birch. Furthermore, sawtooth oak (78.5%) was shown to be the strongest inhibitor of common silver birch–specific IgE. These results suggest that the stronger allergenicity of sawtooth oak compared to Mongolian oak, and sawtooth oak pollen may be the main culprit of Korean tree pollinosis. Oak species occupy about 27% of forested area and have potential to become more abundant, whereas coniferous forests are gradually decreasing in Korea (12). More specifically, sawtooth oaks are often found close at human dwellings and Mongolian oak are distributed mostly in mountainous areas (13).
IgE reactivity to Bet v 1 was also competitively inhibited by the various oak pollen extracts. Even the most potent inhibitor was white oak (0.29 μg/mL), followed by sawtooth oak (1.39 μg/mL), common silver birch (1.40 μg/mL), and Mongolian oak (5.35 μg/mL) (Fig. 3B). This result may reflect the lower concentration of Bet v 1 in the common silver birch extract used. Fifty percent inhibitory concentration, which is commonly used to assess allergenic potency (14), may reflect the concentration of PR-10 allergen in the extract. However, maximum inhibitory concentration seems to reflect the cross-reactivity. These data indicate that most of the allergen epitopes of common silver birch pollen are shared by oak pollen. Furthermore, IgE reactivity to birch extract in Korean patients is thought to be a cross-reaction, mainly to sawtooth oak.
Pan-allergens such as profilin and polcalcin may also cause cross-reactivity even though they play a minor role in tree pollen allergy (15). In addition, cross-reactive carbohydrate determinants are also known to cause in vitro IgE reactivity even though they do not induce clinical symptoms (16). Further effort is necessary to characterize the major allergens from sawtooth oak. Recombinant group 1 sawtooth oak allergens (Que ac 1) may facilitate the development of better diagnostics such as component-resolved diagnosis in East Asia area (17,18).
In conclusion, sawtooth oak showed strong cross reactivity with commercial white oak and common silver birch. PR-10 allergens were shown to contribute significantly to cross reactivity between Korean oak species and foreign tree pollens of the order Fagales. Sawtooth oaks may be major sensitizing sources of tree pollen allergy in Korea. Further studies are necessary to characterize the major allergens from Korean oak species to facilitate the development of component-resolved diagnosis, which could distinguish genuine sensitization and cross-reactivity of oak allergens.
Footnotes
Funding: This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (A092076).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Jeong KY, Park JW. Data collection: Jeong KY, Son M, Park JH, Park KH, Park HJ, Lee JH, Hong CS, Park JW. Data analysis and writing: Jeong KY. Revision: Park JW. Agreement of final manuscript and submission: all authors.
References
- 1.Kim YL, Lee SK, Oh SH, Moon BS, Park HS, Hong CS. A study of allergy skin tests with Korean pollen extracts. Yonsei Med J. 1987;28:112–118. doi: 10.3349/ymj.1987.28.2.112. [DOI] [PubMed] [Google Scholar]
- 2.Kim TB, Kim KM, Kim SH, Kang HR, Chang YS, Kim CW, Bahn JW, Kim YK, Kang HT, Cho SH, et al. Sensitization rates for inhalant allergens in Korea: a multicenter study. J Asthma Allergy Clin Immunol. 2003;23:483–493. [Google Scholar]
- 3.Lee JW, Choi GS, Kim JE, Jin HJ, Kim JH, Ye YM, Nahm DH, Park HS. Changes in sensitization rate to pollen allergens in allergic patients in the southern part of Gyeonggi province over the last 10 years. Korean J Asthma Allergy Clin Immunol. 2011;31:33–40. [Google Scholar]
- 4.Park HJ, Lee JH, Park KH, Ann HW, Jin MN, Choi SY, Lee YW, Hong CS, Park JW. A nationwide survey of inhalant allergens sensitization and levels of indoor major allergens in Korea. Allergy Asthma Immunol Res. 2014;6:222–227. doi: 10.4168/aair.2014.6.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mari A, Wallner M, Ferreira F. Fagales pollen sensitization in a birch-free area: a respiratory cohort survey using Fagales pollen extracts and birch recombinant allergens (rBet v 1, rBet v 2, rBet v 4) Clin Exp Allergy. 2003;33:1419–1428. doi: 10.1046/j.1365-2222.2003.01773.x. [DOI] [PubMed] [Google Scholar]
- 6.Wallner M, Erler A, Hauser M, Klinglmayr E, Gadermaier G, Vogel L, Mari A, Bohle B, Briza P, Ferreira F. Immunologic characterization of isoforms of Car b 1 and Que a 1, the major hornbeam and oak pollen allergens. Allergy. 2009;64:452–460. doi: 10.1111/j.1398-9995.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 7.Ebner C, Birkner T, Valenta R, Rumpold H, Breitenbach M, Scheiner O, Kraft D. Common epitopes of birch pollen and apples--studies by western and northern blot. J Allergy Clin Immunol. 1991;88:588–594. doi: 10.1016/0091-6749(91)90152-e. [DOI] [PubMed] [Google Scholar]
- 8.Kim GS, Song HK, Lee CH, Cho HJ, Lee CS. Ecological comparison of Mongolian oak (Quercus mongolica Fisch. Ex Ledeb.) community between Mt. Nam and Mt. Jeombong as a long term ecological research (LTER) site. Korean J Environ Ecol. 2011;34:75–85. [Google Scholar]
- 9.Hauser M, Asam C, Himly M, Palazzo P, Voltolini S, Montanari C, Briza P, Bernardi ML, Mari A, Ferreira F, et al. Bet v 1-like pollen allergens of multiple Fagales species can sensitize atopic individuals. Clin Exp Allergy. 2011;41:1804–1814. doi: 10.1111/j.1365-2222.2011.03866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radauer C, Lackner P, Breiteneder H. The Bet v 1 fold: an ancient, versatile scaffold for binding of large, hydrophobic ligands. BMC Evol Biol. 2008;8:286. doi: 10.1186/1471-2148-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitenbach M, Ferreira F, Jilek A, Swoboda I, Ebner C, Hoffmann-Sommergruber K, Briza P, Scheiner O, Kraft D. Biological and immunological importance of Bet v 1 isoforms. Adv Exp Med Biol. 1996;409:117–126. doi: 10.1007/978-1-4615-5855-2_16. [DOI] [PubMed] [Google Scholar]
- 12.Byun JG, Lee WK, Kim M, Kwak DA, Kwak H, Park T, Byun WH, Son Y, Choi JK, Lee YJ, et al. Radial growth response of Pinus densiflora and Quercus spp. To topographic and climatic factors in South Korea. J Plant Ecol. 2013;6:380–392. [Google Scholar]
- 13.Jeong HM, Kim HR, You YH. Growth difference among saplings of Quercus acutissima, Q. variabilis and Q. mongolica under the environmental gradients treatment. Korean J Environ Biol. 2009;27:82–87. [Google Scholar]
- 14.Jeong KY, Hong CS, Lee JS, Park JW. Optimization of allergen standardization. Yonsei Med J. 2011;52:393–400. doi: 10.3349/ymj.2011.52.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douladiris N, Savvatianos S, Roumpedaki I, Skevaki C, Mitsias D, Papadopoulos NG. A molecular diagnostic algorithm to guide pollen immunotherapy in southern Europe: towards component-resolved management of allergic diseases. Int Arch Allergy Immunol. 2013;162:163–172. doi: 10.1159/000353113. [DOI] [PubMed] [Google Scholar]
- 16.Altmann F. The role of protein glycosylation in allergy. Int Arch Allergy Immunol. 2007;142:99–115. doi: 10.1159/000096114. [DOI] [PubMed] [Google Scholar]
- 17.Movérare R, Everberg H, Carlsson R, Holtz A, Thunberg R, Olsson P, Brostedt P, Hogbom E. Purification and characterization of the major oak pollen allergen Que a 1 for component-resolved diagnostics using ImmuoCAP®. Int Arch Allergy Immunol. 2008;146:203–211. doi: 10.1159/000115888. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira F, Wolf M, Wallner M. Molecular approach to allergy diagnosis and therapy. Yonsei Med J. 2014;55:839–852. doi: 10.3349/ymj.2014.55.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]