Abstract
Eradication of Helicobacter pylori using first-line therapy is becoming less effective. Subjects who had been treated for H. pylori infection were prospectively enrolled through an on-line database registry from October 2010 to December 2012. Demographic data, detection methods, treatment indication, regimens, durations, compliance, adverse events, and eradication results for H. pylori infection were collected. Data of 3,700 patients from 34 hospitals were analyzed. The overall eradication rate of the first-line therapy was 73.0%. Eradication failure was significantly associated with old age, concomitant medication, and comorbidity. Regional differences in eradication rates were observed. The most common first-line therapy was proton pump inhibitor-based triple therapy (standard triple therapy, STT) for 7 days (86.8%). The eradication rates varied with regimens, being 73% in STT, 81.8% in bismuth-based quadruple therapy, 100% in sequential therapy, and 90.3% in concomitant therapy. The eradication rate in treatment-naïve patients was higher than that in patients previously treated for H. pylori infection (73.8% vs. 58.5%, P < 0.001). The overall eradication rate for second-line therapy was 84.3%. There was no statistical difference in eradication rates among various regimens. H. pylori eradication rate using STT is decreasing in Korea and has become sub-optimal, suggesting the need for alternative regimens to improve the efficacy of first-line therapy for H. pylori infection.
Keywords: Helicobacter pylori, Eradication Success, First-line Therapy, On-line Registry
Introduction
Helicobacter pylori infection affects 20%–50% of the population in the Western world and up to 80% of those in developing countries (1). H. pylori infection causes chronic gastritis and increases the risk of several upper gastrointestinal diseases including peptic ulcer disease, gastric cancer, and mucosa-associated lymphoid tissue (MALT) lymphoma (2,3).
In East Asian countries, such as Korea and Japan, where the incidence of gastric cancer is high, eradication of H. pylori has been a key strategy in controlling gastric cancer incidence. The incidence of H. pylori infection has declined in developed countries, but has remained persistently high in Korea.
The first-line therapy for H. pylori infection used world-wide as standard triple therapy (STT) comprising proton pump inhibitor (PPI) and the antibiotics amoxicillin and clarithromycin (4,5). The eradication rate with first-line therapy is disappointingly low (70%–85%). In recent decades, substantial decrease in the efficacy of STT to ≤ 80% has been observed in most countries (5,6,7). Thus, the continued use of STT as the first-line regimen has been questioned (8).
The optimal regimen reflects local preference and experience. Continuous evaluation of treatment outcomes in clinical practice should also be taken into account (9). There is no large-scale, systematic registry on H. pylori eradication in any nation.
The aims of the present study were to obtain a prospective systematic registry database on a nation-wide scale in Korea, and to sample representative Koreans in routine clinical practice to gauge H. pylori eradication in Korea. This study focused on epidemiology, efficacy, and safety of the commonly-used treatment regimens to eradicate H. pylori in Korea.
Materials and Methods
Study design
This multicenter, prospective, and observational study (cris.nih.go.kr/KCT0001390) was trial conducted from October 2010 to June 2015.The planned interim analysis was performed in the first half period, from October 2010 to December 2012.
Subjects
Patients who were confirmed to be infected by H. pylori and treated for the ensuing infection were enrolled. Patients were not eligible for the study if one of the following criteria was met: known or suspected allergy to PPIs or antibiotics, previous history of gastric surgery, pregnancy or lactation, or therapy with a PPI or antibiotics within 4 weeks of entry.
Evaluation of H. pylori infection
H. pylori infection was diagnosed in patients based on histologic evidence of H. pylori by Giemsa staining, positive rapid urease test, positive H. pylori culture test, and positive 13C-urea breath test. Patients were diagnosed as infected if any of these tests was positive.
Eradication therapy
Investigators prescribed the eradication regimen in accordance with the guidelines established by the Korean College of Helicobacter and Upper Gastrointestinal Research group. First-line STT therapy included standard-dose PPI (esomeprazole 40 mg, omeprazole 40 mg, lansoprazole 30 mg, pantoprazole 40 mg, or rabeprazole 20 mg) plus amoxicillin (1,000 mg) and clarithromycin (500 mg) twice a day for 7–14 days. Second-line therapy (PBMT) included the standard-dose twice daily PPI, 120 mg bismuth four times a day, 500 mg metronidazole three times a day, and 500 mg tetracycline four times a day for 7–14 days. Other eradication regimens including sequential therapy (SQ) or concomitant therapy (CT) were also prescribed as first-line or second-line therapy.
To evaluate the success of the eradication therapy, follow-up endoscopy with a rapid urease test and histologic examination, or a 13C-urea breath test was performed at least 4 weeks after the completion of the therapy. The eradication success for H. pylori was defined as a negative rapid urease test and histology or a negative 13C-urea breath test, which was confirmed within 6 months after H. pylori eradication therapy. Eradication failure was defined as a positive result in any of these tests. Compliance was considered to be satisfactory when drug intake exceeded 80%. Adverse events associated with treatment were also investigated.
Data collection
The electronic data management system for this study was developed by the Seoul National University Medical Research Collaborating Center using the Pharmacoepidemiology and Clinical Trial Application X (Phacta X) system. Data entry was completed by selecting appropriate icons. Data from clinical information and laboratory test results were entered into a web-based electronic case-reporting form (CRF) following the standard protocol for data-entry (www.phactax.org). The electronic CRF included variables including demographic data (e.g., age, sex, residence, smoking status, alcohol consumption, and comorbidity including hypertension, diabetes, ischemic heart disease, liver cirrhosis, chronic renal failure, and malignancy), diagnostic methods for H. pylori infection, treatment indication, regimens, durations, compliance, and treatment-related adverse events. All patients provided written informed consent to participate prior to enrollment. The study was approved by the representative Institutional Review Boards of participating hospitals in Korea.
Statistical analyses
Patient characteristics and eradication rate of H. pylori were summarized using mean and standard deviation for continuous variables and frequency or percentage for categorical variables. Comparison of continuous variable was performed using Student t-test. Fisher's exact test was performed to compare categorical variables. Age was categorized as < 30, 30–40, 40–50, 50–60, 60–70, or ≥ 70 years. Eradication success rate for the first-line therapy was evaluated by geographic regions. The standardized rates were calculated using patients receiving STT regimens as standard population within the Korean national health insurance database that covered all patients in 2010. Logistic regression analysis was used to examine the associations between H. pylori eradication therapy and each of the following factors: age, sex, residence, current smoking, alcohol consumption, medication, previous gastrointestinal disorder, comorbidity, previous H. pylori eradication, PPI, duration of treatment, treatment compliance, and treatment-related complications. A multivariate model was constructed using variables with P < 0.2 in univariate logistic regression analysis. SAS version 9.2 (SAS institute Inc., Cary, NC, USA) was used for statistical analysis. Statistical significance was considered for P < 0.05. All P values were two-sided.
Ethics statement
The protocol was approved by the institutional review board at each center (Chung-Ang University Yongsan Hospital, IRB No. 10-031-04-08). All of the patients provided written informed consent for participation in the study.
Results
Baseline demographics
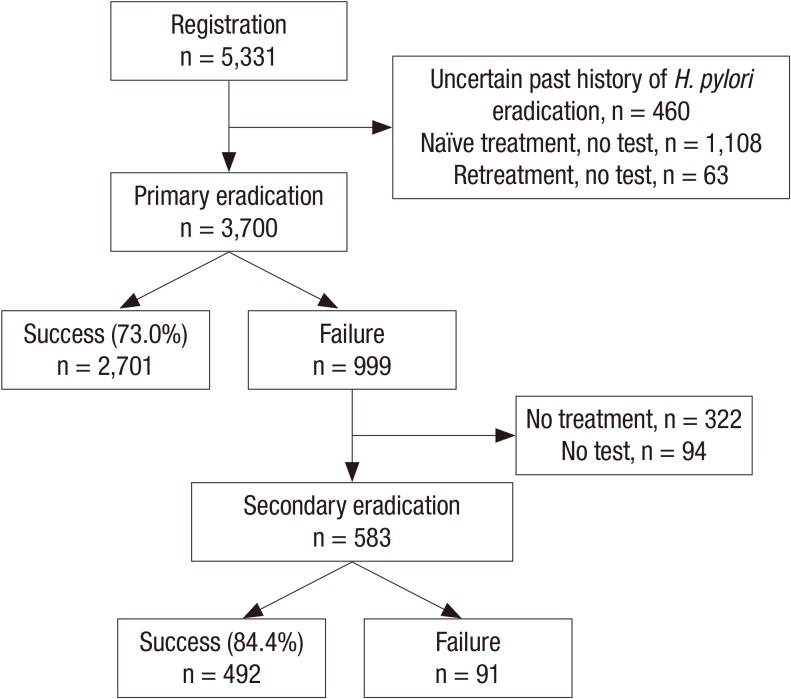
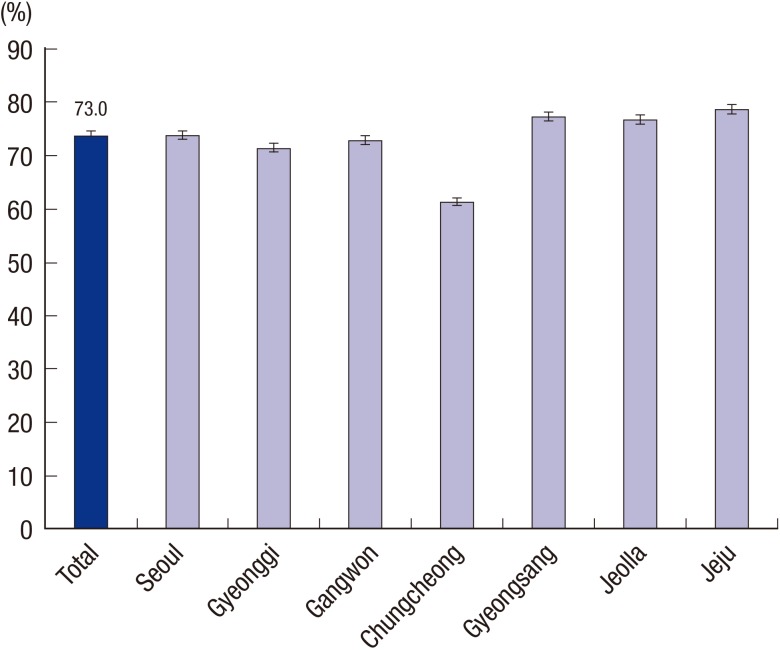
Thirty four tertiary and academic hospitals, which were representative of Korean hospitals, prospectively enrolled 5,331 patients. Of them, 3,700 were identified using their eradication results within 6 months after H. pylori eradication therapy (Fig. 1). The baseline characteristics of these patients who received first-line eradication therapy are summarized in Table 1. Eradication success in first-line therapy was achieved in 2,701 (73.0%) patients. Eradication success declined with age (P < 0.001). However, there was no significant difference (P = 0.328) in eradication success between sexes. Concerning regional differences, the eradication rate in Jeju province was the highest (76.1%) and the rate in Chugcheong province was the lowest (60.3%). The difference was significant (P < 0.001) (Fig. 2). There was no significant difference between patients with a current smoking status or currently consuming alcohol and those not currently smoking or drinking (P = 0.799 and P = 0.261, respectively). The eradication rate in patients who had taken other medications, especially non-steroidal anti-inflammatory drugs, was lower than that in those who had not (P < 0.019). Patients with previous gastrointestinal disorders or comorbidities had significantly lower eradication rates (P = 0.018 and P = 0.031, respectively). The eradication rate in patients with liver cirrhosis or chronic renal failure was significantly lower than in patients who were free of these diseases (47.0% and 52.9%, respectively). Common adverse events were diarrhea, abdominal pain, nausea, and vomiting (Table 2). Diarrhea and abdominal pain were especially common in STT (144 and 35 cases, respectively).
Fig. 1.
Helicobacter pylori eradication in first-line and second-line therapy. A total of 3,700 patients participated in the study. First-line and second-line therapy overall eradication rate was 73.0% and 84.3%, respectively.
Table 1. Baseline characteristics of 3,700 Korean patients who underwent first-line H. pylori eradication therapy.
| Parameters | Total participants with first-line therapy (n = 3,700) No. (%) | Eradication success (n = 2,701) No. (%) | P value |
|---|---|---|---|
| Age in years | 0.001 | ||
| < 30 | 144 (3.89) | 105 (72.92) | |
| 30-39 | 322 (8.70) | 251 (77.95) | |
| 40-49 | 666 (18.0) | 511 (76.73) | |
| 50-59 | 1,247 (33.7) | 917 (73.54) | |
| 60-69 | 884 (23.89) | 625 (70.7) | |
| ≥ 70 | 437 (11.81) | 292 (66.82) | |
| Sex | 0.328 | ||
| Male | 2,233 (60.35) | 1,643 (73.58) | |
| Female | 1,467 (39.65) | 1,058 (72.12) | |
| Residence | < 0.001 | ||
| Seoul | 809 (21.86) | 592 (73.18) | |
| Gyeonggi | 692 (18.7) | 496 (71.68) | |
| Gangwon | 93 (2.51) | 68 (73.12) | |
| Chungcheong | 310 (8.38) | 187 (60.32) | |
| Gyeongsang | 1,162 (31.41) | 884 (76.08) | |
| Jeolla | 276 (7.46) | 201 (72.83) | |
| Jeju | 358 (9.68) | 273 (76.26) | |
| Current smoking | 0.799 | ||
| No | 2,045 (67.6) | 1,486 (72.67) | |
| Yes | 795 (26.28) | 568 (71.45) | |
| Alcohol consumption | 0.261 | ||
| No | 1,637 (54.12) | 1,165 (71.17) | |
| Yes | 1,209 (39.97) | 894 (73.95) | |
| Medication | 0.019 | ||
| No | 1,759 (59.09) | 1,285 (73.05) | |
| Yes | 1,114 (37.42) | 781 (70.11) | |
| Aspirin | 215 (19.30) | 155 (72.09) | |
| Non-steroidal anti-inflammatory drugs | 82 (7.36) | 46 (59.76) | |
| Antiplatelet/anticoagulation agents, or steroids | 41 (3.68) | 28 (68.29) | |
| Previous GI disorder | 0.018 | ||
| No | 2,315 (77.27) | 1,697 (73.3) | |
| Yes | 567 (18.93) | 382 (67.37) | |
| Peptic ulcer | 417 (73.54) | 276 (66.19) | |
| Others | 242 (42.68) | 158 (65.29) | |
| Comorbidity | 0.031 | ||
| No | 1,743 (58.39) | 1,289 (73.95) | |
| Yes | 1,162 (38.93) | 808 (69.54) | |
| Hypertension | 721 (62.05) | 517 (71.71) | |
| Diabetes | 315 (27.11) | 227 (72.06) | |
| Ischemic heart disease | 62 (5.34) | 41 (66.13) | |
| Liver cirrhosis | 17 (1.46) | 8 (47.06) | |
| Chronic renal failure | 17 (1.46) | 9 (52.94) | |
| Malignancy | 43 (3.70) | 28 (65.12) | |
| Others | 422 (36.32) | 277 (65.64) |
Fig. 2.
Standardized Helicobacter pylori eradication rates in first-line therapy according to residence in Korea. The overall eradication rate in seven regions in Korea was 73.0%. Jeju province showed the highest eradication rate in the first-line therapy, whereas Chungcheong province had the lowest (76.1 vs. 60.3, P < 0.001).
Table 2. Eradiation success rates and related characteristics of 3,700 Korean patients who underwent the first-line H. pylori eradication therapy.
| Related parameters | Total participants with first-line therapy (n = 3,700) No. (%) |
Eradication success (n = 2,701) No. (%) |
P value |
|---|---|---|---|
| Indication for eradication | |||
| BGU | 1,083 (29.27) | 736 (67.96) | < 0.001 |
| DU | 1,057 (28.57) | 811 (76.73) | 0.001 |
| BGU+DU | 1,982 (53.57) | 1,426 (71.95) | 0.121 |
| Bleeding peptic ulcer | 14 (0.38) | 12 (85.71) | 0.377 |
| After endoscopic resection for early gastric cancer | 444 (12) | 337 (75.9) | 0.142 |
| Gastric MALT lymphoma | 39 (1.05) | 35 (89.74) | 0.018 |
| Others | 870 (23.51) | 632 (72.64) | 0.787 |
| Previous H. pylori eradication | < 0.001 | ||
| No | 3,495 (94.46) | 2,581 (73.85) | |
| Yes (within 1 yr) | 60 (1.62) | 33 (5.5) | |
| Yes (before 1 yr) | 145 (3.92) | 87 (6.0) | |
| STT | 157(76.59) | 85 (54.14) | |
| PBMT | 32 (15.61) | 26 (81.25) | |
| CT | 1 (0.49) | 1 (100) | |
| SQ | 1 (0.49) | 1 (100) | |
| Others | 14 (6.83) | 7 (50) | |
| Regimens | < 0.001 | ||
| STT | 3,550 (95.95) | 2,592 (73.01) | |
| PBMT | 33 (0.89) | 27 (81.82) | |
| CT | 31 (0.84) | 28 (90.32) | |
| SQ | 14 (0.38) | 14 (100) | |
| Others | 72 (1.95) | 40 (55.56) | |
| Durations | < 0.001 | ||
| 7 day | 3,310 (89.46) | 2,435 (73.56) | |
| 14 day | 299 (8.08) | 216 (72.24) | |
| Others | 91 (2.46) | 50 (54.95) | |
| Compliance | < 0.001 | ||
| ≥ 80% | 3,608 (97.54) | 2,653 (73.53) | |
| < 80% | 42 (1.14) | 22 (52.38) | |
| Complications | 0.626 | ||
| No | 2,967 (80.21) | 2,175 (73.31) | |
| Yes | 612 (16.55) | 437 (71.41) | |
| Abdominal pain | 73 (11.92) | 47 (64.38) | |
| Nausea, vomiting | 126 (20.59) | 74 (58.73) | |
| Diarrhea | 215 (35.13) | 159 (73.95) | |
| Others | 343 (56.05) | 249 (72.59) | |
| First-line therapy regimens | < 0.001 | ||
| STT | 3,550 (95.95) | 2,592 (73.01) | |
| PBMT | 33 (0.89) | 27 (81.82) | |
| CT | 31 (0.84) | 28 (90.32) | |
| SQ | 14 (0.38) | 14 (100) | |
| Others | 72 (1.95) | 40 (55.56) | |
| Second-line therapy regimens | 583 (100) | - | 0.327 |
| PBMT | 456 (78.22) | 391 (85.75) | |
| PAC | 10 (1.72) | 6 (60) | |
| PTM | 33 (5.66) | 29 (87.88) | |
| PLM | 2 (0.34) | 2 (100) | |
| PAM | 2 (0.34) | 2 (100) | |
| SQ | 6 (1.03) | 5 (83.33) | |
| Others | 74 (12.69) | 60 (81.08) |
CT: PPI+AMX+CLA+MTZ; PAC: Proton pump inhibitor+Amoxicillin+Clarithromycin; PAM: Proton pump inhibitor+Amoxicillin+Metronidazole; PBMT: Proton pump inhibitor+Denol+Metronidazole+Tetracyclin; PLM: Proton pump inhibitor+Levofloxacin+Metronidazole; PTM: Proton pump inhibitor+Tetracyclin+Metronidazole; SQ: PPI+AMX -> PPI+CLA+MTZ; STT: Proton pump inhibitor+Amoxicillin+Clarithromycin.
BGU, benign gastric ulcer including scar; DU, duodenal ulcer including scar; BGU+DU, both benign gastric and duodenal ulcers; CT, Concomitant therapy; SQ, Sequential therapy.
First-line H. pylori eradication
The results of H. pylori eradication after the first-line therapy are summarized in Table 2. The most common indication for H. pylori eradication was benign gastric and duodenal ulcers (53.5%). The eradication rates were significantly different among various gastroduodenal diseases. The eradication rate in patients with gastric MALT lymphoma and benign gastric ulcer was 89.7% and 67.9%, respectively (P < 0.001). The eradication success in naïve patients was higher than that in patients with past history of H. pylori eradication treatment (73.8% vs. 58.5%, P < 0.001). The most common first-line therapy was the 7-day PPI-based STT (86.8%). The eradication rates varied among different treatment regimens including STT, CT, PBMT, ST, and others (P < 0.001). The eradication rate with STT therapy was 73.0% compared to 90.3% with CT. The eradication rate of the 7-day treatment was 73.5%. Patients with good compliance to therapy had higher eradication rates than those with poor compliance (73.5% vs. 52.3%, P < 0.001). There was no significant difference in the eradication rate of patients who experienced complications (P = 0.626).
H. pylori eradication rates according to treatment regimens
The frequency of prescribing regimens in the final analysis (n = 3,700) did not differ significantly from those in the total population (n = 5,331). For the first-line therapy, the frequencies of STT were 3,550/3,700 (95.9%) in the final analysis and 5,099/5,331 (95.6%) in the total analysis. For the second-line therapy, the frequency of PBMT in the final analysis and the total analysis was 456/583 (78.2%) and 531/677 (78.4%), respectively. The H. pylori eradication rates in the first-line and second-line therapy according to treatment regimens are summarized in Table 2. For patients who had first-line therapy, most commonly STT (95.9%), the overall eradication rate was 73.0%. There was significant difference in the eradication rates according to treatment regimen: 73.0% in STT, 81.8% in PBMT, 100% in SQ, and 90.3% in CT. Table 3 shows the results of first-line H. pylori eradication rate according to the regimen and treatment duration. The most common regimen and treatment duration was STT for 7 days (3,214/3,700, 86.8%), which achieved a 74% eradication success rate. Of the 999 patients whose first-line therapy failed, 583 (58.3%) patients tried the second-line therapy. The overall eradication rate was 84.3%. However, there was no statistically significant difference (P = 0.25) in the eradication rate among various treatment regimens (Table 2).
Table 3. First-line Helicobacter pylori eradication rate according to the regimen and treatment duration.
| Duration, day | STT | PBMT | CT | SQ | Others | Total |
|---|---|---|---|---|---|---|
| 7 | 2,371/3,214 (74%) | 24/29 (83%) | 3/4 (75%) | 0/0 (0%) | 37/63 (59%) | 3310 |
| 8-13 | 32/64 (50%) | 1/1 (100%) | 0/0 (0%) | 14/14 (100%) | 1/4 (25%) | 83 |
| 14 | 188/268 (70%) | 2/3 (67%) | 25/26 (96%) | 0/0 (0%) | 1/2 (50%) | 299 |
| Other | 42373 | 0/0 (0%) | 0/1 (0%) | 0/0 (0%) | 1/3 (33%) | 8 |
| Total | 3,550 | 33 | 31 | 14 | 72 | 3,700 |
STT: Proton pump inhibitor+Amoxicillin+Clarithromycin; PBMT: Proton pump inhibitor+Denol+Metronidazole+Tetracyclin; CT: PPI+AMX+CLA+MTZ; SQ: PPI+AMX -> PPI+CLA+MTZ.
CT, Concomitant therapy; SQ, Sequential therapy.
Factors related to H. pylori eradication success
In univariate analysis, age, residential area, medication, previous gastrointestinal disorder, comorbidity, previous H. pylori eradication, PPI, duration of treatment, and compliance to treatment were related to the success of eradication (Table 4). After the adjustment for age, residential area, history of H. pylori eradication, duration of treatment, and compliance to treatment remained statistically significant. Older age was related to unfavorable outcome (P for trend = 0.0543). Chungcheong province showed a decreased success rate; eradication failure was 1.67 times more frequent than in Seoul. Previous history of H. pylori eradication treatment increased the failure rate almost twice compared to those without previous treatment. Lower compliance (< 80%) was related to 2.78 times more treatment failure than patients with high compliance (> 80%). Treatment duration longer than 7 days did not show any increased success in eradication treatment.
Table 4. Association between the outcome of eradication therapy and risk factors evaluated using logistic regression model.
| Variables | Eradication success rates No. (%) / Total | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P value | Odds Ratio (95% CI) | P value | |||
| Age in years | < 30 | 105 (72.92) / 144 | Reference | 0.002 | Reference | 0.009 |
| 30-39 | 251 (77.95) / 322 | 1.313 (0.835-2.064) | 1.337 (0.792-2.257) | |||
| 40-49 | 511 (76.73) / 666 | 1.224 (0.813-1.843) | 1.236 (0.764-2.000) | |||
| 50-59 | 917 (73.54) / 1,247 | 1.032 (0.700-1.522) | 0.995 (0.626-1.581) | |||
| 60-69 | 625 (70.7) / 884 | 0.896 (0.604-1.331) | 0.854 (0.531-1.373) | |||
| ≥ 70 | 292 (66.82) / 437 | 0.748 (0.492-1.136) | 0.753 (0.456-1.243) | |||
| Sex | Male | 1,643 (73.58) / 2,233 | Reference | 0.329 | NA | |
| Female | 1,058 (72.12) / 1467 | 0.929 (0.801-1.077) | NA | |||
| Residence | Seoul | 592 (73.18) / 809 | Reference | < 0.001 | Reference | < 0.001 |
| Gyeonggi | 496 (71.68) / 692 | 0.928 (0.739-1.164) | 0.859 (0.660-1.116) | |||
| Gangwon | 68 (73.12) / 93 | 0.997 (0.614-1.618) | 1.015 (0.560-1.841) | |||
| Chungcheong | 187 (60.32) / 310 | 0.557 (0.423-0.734) | 0.570 (0.413-0.786) | |||
| Gyeongsang | 884 (76.08) / 1,162 | 1.166 (0.949-1.432) | 1.128 (0.882-1.443) | |||
| Jeolla | 201 (72.83) / 276 | 0.982 (0.722-1.336) | 1.000 (0.712-1.405) | |||
| Jeju | 273 (76.26) / 358 | 1.177 (0.882-1.572) | 1.294 (0.948-1.766) | |||
| Current smoking | No | 1,486 (72.67) / 2,045 | Reference | 0.794 | NA | |
| Yes | 568 (71.45) / 795 | 0.941 (0.785-1.129) | NA | |||
| NA | 135 (72.97) / 185 | 1.016 (0.724-1.425) | NA | |||
| Alcohol consumption | No | 1,165 (71.17) / 1,637 | Reference | 0.264 | NA | |
| Yes | 894 (73.95) / 1,209 | 1.150 (0.973-1.359) | NA | |||
| NA | 129 (72.07) / 179 | 1.045 (0.741-1.474) | NA | |||
| Medication | No | 1,285 (73.05) / 1,759 | Reference | 0.022 | Reference | 0.012 |
| Yes | 781 (70.11) / 1,114 | 0.865 (0.733-1.021) | 0.947 (0.788-1.137) | |||
| NA | 85 (81.73) / 104 | 1.650 (0.993-2.744) | 2.500 (1.328-4.708) | |||
| Previous gastrointestinal disorder | No | 1,697 (73.30) / 2,315 | Reference | 0.019 | Reference | 0.038 |
| Yes | 382 (67.37) / 567 | 0.752 (0.617-0.917) | 0.816 (0.659-1.012) | |||
| NA | 83 (72.81) / 114 | 0.975 (0.639-1.488) | 0.595 (0.351-1.011) | |||
| Comorbidity | No | 1,289 (73.95) / 1,743 | Reference | 0.021 | NA | |
| Yes | 808 (69.54) / 1,162 | 0.804 (0.682-0.948) | NA | |||
| NA | 61 (77.22) / 79 | 1.194 (0.698-2.041) | NA | |||
| Previous H. pylori eradication | No | 2,581 (73.85) / 3,495 | Reference | < 0.001 | Reference | < 0.001 |
| Yes (within 1 yr) | 33 (55.0) / 60 | 0.433 (0.259-0.723) | 0.364 (0.207-0.640) | |||
| Yes (before 1 yr) | 87 (60.0)/145 | 0.531 (0.378-0.746) | 0.627 (0.425-0.925) | |||
| Duration in days | 7 | 2,435 (73.56) / 3,310 | Reference | < 0.001 | Reference | 0.019 |
| 14 | 216 (72.24) / 299 | 0.935 (0.718-1.218) | 0.918 (0.651-1.295) | |||
| Other | 50 (54.95) / 91 | 0.438 (0.288-0.667) | 0.520 (0.329-0.821) | |||
| Compliance | ≥ 80% | 2,653 (73.53) / 3,608 | Reference | < 0.001 | Reference | < 0.001 |
| < 80% | 22 (52.38) / 42 | 0.396 (0.215-0.729) | 0.375 (0.196-0.717) | |||
| NA | 25 (51.02) / 49 | 0.375 (0.213-0.660) | 0.390 (0.189-0.805) | |||
| Complication | No | 2,175 (73.31) / 2,967 | Reference | 0.626 | NA | |
| Yes | 437 (71.41) / 612 | 0.909 (0.749-1.103) | NA | |||
| NA | 88 (73.33) / 120 | 1.001 (0.663-1.513) | NA | |||
NA, not applicable.
Discussion
The current study reports on the establishment and interim results of a nationwide online registry in Korea aimed at tracking the eradication of H. pylori. This registry is unique globally. This interim analysis of the 5-year study was planned to review the completeness of the data to date and predict the results at an early stage.
The data supports the evidence that the H. pylori eradication rate using standard triple therapy is indeed decreasing and has become sub-optimal in Korea. The eradication rate of first-line therapy has significantly decreased to 73% since 2000, demonstrating a lower than expected efficacy of STT of H. pylori infection in Korea. This eradication rate is similar to that in other countries (7,10,11). This suggests that alternate regimens including sequential or concomitant therapy are required as soon as possible. This interim analysis underscores the urgency of the current situation in Korea.
Korean guidelines for H. pylori infection were formulated in 1998. They state that a definitive indication for H. pylori eradication is peptic ulcer including scarring, marginal zone B cell lymphoma (MALT type), and early gastric cancer after endoscopic resection (12). Since 1998, PPI-based triple therapy has been recommended as the first-line therapy for H. pylori eradication in Korea. However, since 2000, the eradication rate has been declining, falling below 80%. The Asia-Pacific Consensus Guidelines have stricter standards that require a success rate of > 80% in intention-to-treat analysis and > 90% in per-protocol analysis for a regimen to be considered suitable for first-line eradication therapy (8). In the present study, the overall eradication rate was only 73% in first-line therapy. Considering the fact that the majority (89.4%) of cases were treated with STT for 7 days, a decline in the eradication rate could imply a serious problem in the management of H. pylori infection in Korea. Fortunately, naïve patients had a significantly higher eradication rate than those with previous H. pylori infection. These results strongly suggest that more effective first-line therapy is necessary to treat H. pylori infection.
In cases where treatment fails, the consensus treatment is one or two weeks of quadruple therapy (PPI + metronidazole + tetracycline + bismuth) (12). The overall eradication rate of 84.3% in second-line therapy was consistent with the eradication rates observed in previous studies (13).
Several factors affect the eradication of H. pylori. These include antibiotic resistance, geographical area, patient age, smoking status, compliance, duration of therapy, bacterial density, Cag A, gastric acid concentration, individual response to PPI, and the presence of CYP2C19 polymorphism (8). Of these factors, clarithromycin resistance has been suspected to be the main cause of eradication failure (14,15). In Korea, 5.9% of those treated were resistant to clarithromycin before 2000. However, from 2007–2009, the rate of resistance showed a sharp increase, reaching 38.5% (10,16,17). Rates of resistance in other countries are much lower. The discrepancy can be explained by wide international and regional variations in the prevalence of resistance to antibiotics (18,19). In the present study, Jeju province showed the highest eradication rate with STT as the first-line therapy, whereas STT therapy in Chungcheong province had the lowest eradication rate (76.2% vs. 60.3%). Nonetheless, the hypothesis that there are regional differences in Korea is still under debate.
Studies have shown conflicting results in the eradication rate of elderly patients (20,21). In this study, advanced age was positively associated with treatment failure, whereas gender was not associated with treatment failure or eradication rates, which supported the results of a previous study (22). Some reports assert that smoking can increase treatment failure in H. pylori eradication as gastric blood flow and mucus secretion decrease, and as acid secretion increases (23). However, no significant association between smoking and treatment failure was observed in the present study. A previous report demonstrated an inverse relationship between alcohol consumption and H. pylori infection, which indicated alcohol consumption might facilitate eradication (24). However, no significant relationship between alcohol consumption and eradication failure was observed in this study.
Underlying chronic diseases, such as diabetes mellitus, hypertension, chronic kidney disease, chronic liver disease, and chronic lung disease, have been periodically reported to influence the outcome of H. pylori eradication therapy. However, the evidence is limited and the results are inconsistent (25). In the present study, eradication rates were significantly lower in patients with a history of peptic ulcer and other comorbidities, particularly liver cirrhosis and chronic renal failure. Therefore, this study demonstrates that eradication success is determined by various clinical factors as well as bacterial factors.
Most of recently recommended therapy regimens for H. pylori eradication are 7 days in duration. This period is associated with higher compliance and lower medical costs, while maintaining a similar eradication rate to that of longer regimens. Currently, the Korean College of Helicobacter and Upper Gastrointestinal Research group recommend a 7-day or 14-day STT. Previous studies in Korea reported that a 7-day STT was not inferior to a 10-day or a 14-day therapy. Recently, more diverse treatment regimens have being administered, including sequential and concomitant therapy, whose satisfactory eradication rates are higher than STT (26,27). Therefore, new strategies to achieve higher H. pylori eradication rates are required. Potential approaches in overcoming this problem are patient-tailored sequential or concomitant therapies coupled with prolonged treatment duration in Korea.
Although the number of cases in this study is small, the eradication success with SQ (100%) and CT (90.3%) were significantly (P < 0.001) higher when compared to STT (73.0%). PBMT for 14 days had an eradication success of 81.8%. International guidelines have not incorporated such emerging first-line therapies so far (5,28). However, more recent recommendations suggest that change is likely in the immediate future. A lot of work in developing and validating novel regimens needs to be done before recommending new therapies as first-line therapy against H. pylori infection (27,29). Adverse events were reported in 16.5% of patients, mainly mild diarrhea, nausea, and mild vomiting. The types of adverse events or severity were not in particular affected by the differences in the patients' background.
To date, this is the most comprehensive, systematic, and easily accessible online registration database tracking H. pylori eradication in Korea. This registration database will serve as a valuable resource in monitoring antibiotic resistance, H. pylori reinfection, and association of H. pylori infection with gastric carcinogenesis. This database makes it possible to construct a global network for those working in the field of H. pylori control in the future.
In conclusion, our data support the evidence that H. pylori eradication rate using STT is decreasing and has become sub-optimal in Korea, suggesting the need for alternate regimens including sequential or concomitant therapy to improve the efficacy of first-line therapy for H. pylori infection in Korea.
Footnotes
Funding: This work was supported by the Korean Research Foundation of Internal Medicine Grant 2010.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conception and design of the study: Kim JG, Lee JH. Performing the study: Kim HS, Song HJ, Chung IK, Kim GH, Kim BW, Shim KN, Jeon SW, Jung YJ, Yang CH, Kim JH, Kim TH, Kim SG, Shin WG, Kim SM, Han SW, Kim KH, Kim BJ, Lee JH, Kim JG. Analysis of the data: Park SK, Park BJ, Lee J. Writing the first draft: Kim BJ. Revision of manuscript: Kim BJ, Kim JG. Approval of the final version of the manuscript: all authors.
References
- 1.Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment Pharmacol Ther. 2011;34:1255–1268. doi: 10.1111/j.1365-2036.2011.04887.x. [DOI] [PubMed] [Google Scholar]
- 2.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 3.McColl KE. Clinical practice. Helicobacter pylori infection. N Engl J Med. 2010;362:1597–1604. doi: 10.1056/NEJMcp1001110. [DOI] [PubMed] [Google Scholar]
- 4.Kim MS, Kim N, Kim SE, Jo HJ, Shin CM, Lee SH, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Long-term follow-up Helicobacter pylori reinfection rate and its associated factors in Korea. Helicobacter. 2013;18:135–142. doi: 10.1111/hel.12018. [DOI] [PubMed] [Google Scholar]
- 5.Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, Xiao SD, Lam SK, Goh KL, Chiba T, et al. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol. 2008;23:351–365. doi: 10.1111/j.1440-1746.2008.05314.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, et al. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104. doi: 10.1186/1471-230X-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim N, Kim JJ, Choe YH, Kim HS, Kim JI, Chung IS, Korean College of Helicobacter and Upper Gastrointestinal Research Korean Association of Gastroenterology. Diagnosis and treatment guidelines for Helicobacter pylori infection in Korea. Korean J Gastroenterol. 2009;54:269–278. doi: 10.4166/kjg.2009.54.5.269. [DOI] [PubMed] [Google Scholar]
- 8.Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob Agents Chemother. 2004;48:4843–4847. doi: 10.1128/AAC.48.12.4843-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho DK, Park SY, Kee WJ, Lee JH, Ki HS, Yoon KW, Cho SB, Lee WS, Joo YE, Kim HS, et al. The trend of eradication rate of Helicobacter pylori infection and clinical factors that affect the eradication of first-line therapy. Korean J Gastroenterol. 2010;55:368–375. doi: 10.4166/kjg.2010.55.6.368. [DOI] [PubMed] [Google Scholar]
- 11.Choi YS, Cheon JH, Lee JY, Kim SG, Kim JS, Kim N, Lee DH, Kim JM, Jung HC, Song IS. The trend of eradication rates of first-line triple therapy for Helicobacter pylori infection: single center experience for recent eight years. Korean J Gastroenterol. 2006;48:156–161. [PubMed] [Google Scholar]
- 12.Gong EJ, Yun SC, Jung HY, Lim H, Choi KS, Ahn JY, Lee JH, Kim DH, Choi KD, Song HJ, et al. Meta-analysis of first-line triple therapy for Helicobacter pylori eradication in Korea: is it time to change? J Korean Med Sci. 2014;29:704–713. doi: 10.3346/jkms.2014.29.5.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgopoulos SD, Papastergiou V, Karatapanis S. Helicobacter pylori eradication therapies in the era of increasing antibiotic resistance: a paradigm shift to improved efficacy. Gastroenterol Res Pract. 2012;2012:757926. doi: 10.1155/2012/757926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung JW, Lee GH, Han JH, Jeong JY, Choi KS, Kim DH, Jung KW, Choi KD, Song HJ, Jung HY, et al. The trends of one-week first-line and second-line eradication therapy for Helicobacter pylori infection in Korea. Hepatogastroenterology. 2011;58:246–250. [PubMed] [Google Scholar]
- 16.Park JM, Hahm KB. The Korean perspective of Helicobacter pylori infection: lessons from the Japanese government's policy to prevent gastric cancer. Dig Dis. 2014;32:290–294. doi: 10.1159/000357861. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Jung SW. Helicobacter pylori eradication therapy in Korea. Korean J Gastroenterol. 2011;58:67–73. doi: 10.4166/kjg.2011.58.2.67. [DOI] [PubMed] [Google Scholar]
- 18.Saracino IM, Zullo A, Holton J, Castelli V, Fiorini G, Zaccaro C, Ridola L, Ricci C, Gatta L, Vaira D. High prevalence of primary antibiotic resistance in Helicobacter pylori isolates in Italy. J Gastrointestin Liver Dis. 2012;21:363–365. [PubMed] [Google Scholar]
- 19.Liu G, Xu X, He L, Ding Z, Gu Y, Zhang J, Zhou L. Primary antibiotic resistance of Helicobacter pylori isolated from Beijing children. Helicobacter. 2011;16:356–362. doi: 10.1111/j.1523-5378.2011.00856.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS, Graham DY, Kwon DH. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47:459–461. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 21.Hwang TJ, Kim N, Kim HB, Lee BH, Nam RH, Park JH, Lee MK, Park YS, Lee DH, Jung HC, et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J Clin Gastroenterol. 2010;44:536–543. doi: 10.1097/MCG.0b013e3181d04592. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Kim NY, Kim SJ, Baik GH, Kim GH, Kim JM, Nam RH, Kim HB, Lee DH, Jung HC, et al. Regional difference of antibiotic resistance of Helicobacter pylori strains in Korea. Korean J Gastroenterol. 2011;57:221–229. doi: 10.4166/kjg.2011.57.4.221. [DOI] [PubMed] [Google Scholar]
- 23.Kim N, Kim JM, Kim CH, Park YS, Lee DH, Kim JS, Jung HC, Song IS. Institutional difference of antibiotic resistance of Helicobacter pylori strains in Korea. J Clin Gastroenterol. 2006;40:683–687. doi: 10.1097/00004836-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Treiber G, Ammon S, Klotz U. Age-dependent eradication of Helicobacter pylori with dual therapy. Aliment Pharmacol Ther. 1997;11:711–718. doi: 10.1046/j.1365-2036.1997.00210.x. [DOI] [PubMed] [Google Scholar]
- 25.Labenz J, Leverkus F, Börsch G. Omeprazole plus amoxicillin for cure of Helicobacter pylori infection. Factors influencing the treatment success. Scand J Gastroenterol. 1994;29:1070–1075. doi: 10.3109/00365529409094890. [DOI] [PubMed] [Google Scholar]
- 26.Moayyedi P, Chalmers DM, Axon AT. Patient factors that predict failure of omeprazole, clarithromycin, and tinidazole to eradicate Helicobacter pylori . J Gastroenterol. 1997;32:24–27. doi: 10.1007/BF01213292. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki T, Matsuo K, Sawaki A, Ito H, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, et al. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;24:273–280. doi: 10.1111/j.1365-2036.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- 28.Kuepper-Nybelen J, Rothenbacher D, Brenner H. Relationship between lifetime alcohol consumption and Helicobacter pylori infection. Ann Epidemiol. 2005;15:607–613. doi: 10.1016/j.annepidem.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Lee JY, Kim N, Kim MS, Choi YJ, Lee JW, Yoon H, Shin CM, Park YS, Lee DH, Jung HC. Factors affecting first-line triple therapy of Helicobacter pylori including CYP2C19 genotype and antibiotic resistance. Dig Dis Sci. 2014;59:1235–1243. doi: 10.1007/s10620-014-3093-7. [DOI] [PubMed] [Google Scholar]
- 30.Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: past, present and future. World J Gastrointest Pathophysiol. 2014;5:392–399. doi: 10.4291/wjgp.v5.i4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, Chung WC, Kim BW, Kim SS. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World J Gastroenterol. 2015;21:351–359. doi: 10.3748/wjg.v21.i1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 33.Yoon H, Lee DH, Kim N, Park YS, Shin CM, Kang KK, Oh DH, Jang DK, Chung JW. Meta-analysis: is sequential therapy superior to standard triple therapy for Helicobacter pylori infection in Asian adults? J Gastroenterol Hepatol. 2013;28:1801–1809. doi: 10.1111/jgh.12397. [DOI] [PubMed] [Google Scholar]