Abstract
Intermittent Pneumatic Compression (IPC) device has been used to prevent venous thromboembolism (VTE). This study investigated the effectiveness of IPC device. We evaluated incidences of deep vein thrombosis (DVT) and pulmonary embolism (PE) in total hip arthroplasty (THA) patients after use of IPC device, and compared with historical incidences from our institution. We applied IPC device in 741 patients who underwent 870 elective primary THAs from January 2010 to December 2013, DVT was detected in 3 patients (0.3%) by sonography, and one (0.1%) of them was symptomatic. Symptomatic PE occurred in 1 patient (0.1%) and there were no cases of fatal PE. The incidence of symptomatic DVT was significantly lower than the historical control (P = 0.042). The IPC is a safe and effective prophylaxis of VTE after primary THA in Korea.
Keywords: Intermittent Pneumatic Compression, Venous Thromboembolism, Deep Vein Thrombosis, Pulmonary Embolism, Total Hip Arthroplasty
Graphical Abstract
INTRODUCTION
Deep vein thrombosis (DVT) and pulmonary embolism (PE) are well known complications after total hip arthroplasty (THA). In western countries, the incidence of symptomatic venous thromboembolism (VTE) after THA is about 3% among patients who do not receive any thromboprophylaxis (1,2). VTEs are frequently asymptomatic and imaging studies have detected DVT and PE in 40% to 79% and in 7% to 30%, respectively, among THA patients without pharmaco-prophylaxis (3,4,5,6). The American Academy of Orthopaedic Surgeons (AAOS) (7) , the American College of Chest Physicians (ACCP) (6), and the National Institute for Health and Clinical Excellence (NICE) (8), have recommended routine use of medical thromboprophylaxis, However, pharmacologic agents such as antiplatelet drugs, low-molecular-weight heparin, or warfarin are associated with complications, including bleeding, hematoma formation and infection of surgical site (9).
In East Asian countries, the occurrence of symptomatic VTE after THA is rare even without any thromboprophylaxis (10,11,12). As the dietary pattern of East Asians is westernizing, the incidence of diseases such as incidence of myocardial infarction and some cancers have increased (13). Although the incidence of VTE is low in East Asia, VTE frequently leads to serious morbidity and mortality. Although routine chemoprophylaxis is not recommended in all patients, a prophylaxis is necessary to prevent postoperative VTE after THA even in East Asian patients.
Intermittent pneumatic compression (IPC) devices have been utilized to manage swelling since the early 1950s (14). These devices enhance venous drainage and prevent venous stasis from the lower extremities, which improves blood flow velocity 50% to 250% in the femoral vein (15). IPC devices also enhance fibrinolytic activities, and reduce the risk of DVT (16,17).
Mechanical prophylaxis with use of IPC device carries little risk of bleeding or hematoma formation on postoperative patients. However, the relative effectiveness of IPC as a prophylaxis against thrombosis after THA is still unclear. Previously, we have reported low incidence rates of DVT and PE in East Asian patients after THA without thromboprophylaxis (18). The incidence of fatal PE, symptomatic PE, and symptomatic DVT was 0%, 0.1%, and 0.8%, respectively.
The purpose of this study was to evaluate the incidence of asymptomatic and symptomatic VTEs after the use of IPC device in a consecutive cohort who underwent primary THA and determined the effectiveness of this device by comparing the incidence of symptomatic VTE with previous data without prophylaxis as a historical control.
MATERIALS AND METHODS
Inclusion of patients
From January 2010 to December 2013, 876 primary THAs (746 patients) were done at our department. In these consecutive patients we used IPC for mechanical prophylaxis of VTE. Five patients (six hips) who had been treated with warfarin were excluded: one patient (one hip) had focal segmental glomerulosclerosis, which is a risk factor of thromboembolism, one patient (one hip) had coronary disease, one patient (one hip) had cerebrovascular disease, and two patients (three hips) had a history of a previous thromboembolic event. Of the remaining 870 hips (741 patients), 79 patients (87 hips) took aspirin-containing compounds or other antiplatelet medication before surgery. Because their medications were discontinued 5-7 days before surgery, these patients were not excluded (19,20). Thus, 741 patients: 344 women and 397 men were the subjects of this study. Their mean age was 51.7 years (range, 16-89 years) and mean body mass index was 24.4 kg/m2 (range, 11.7-39.1 kg/m2) at the time of the operation. The most common diagnosis for THA was osteonecrosis of the femoral head (454 hips, 52.3%) (Table 1).
Table 1. Demographics of patients.
| Parameters | No. of hips |
|---|---|
| No. of patients | 741 (870 hips) |
| Male:Female | 397 (395 hips):344 (475 hips) |
| Age, yr | 51.7 (range, 16-89) |
| Body mass index, kg/m2 | 24.4 (range, 11.7-39.1) |
| Diagnosis | |
| ONFH | 454 |
| Secondary osteoarthritis | 295 |
| Primary osteoarthritis | 73 |
| Hip fracture | 41 |
| Miscellaneous | 7 |
ONFH, osteonecrosis of femoral head.
Surgery and postoperative management
All THAs were done by two senior surgeons. Regional anesthesia was used in 787 hips and general anesthesia in 83 hips. The posterolateral approach was used in 840 hips, and the combined anterior and posterior approach was used in 30 hips. Cementless fixation was used for the acetabular cup and femoral stem in all patients. The mean operation time was 126 minutes (range, 50-390 minutes).
All patients had mechanical prophylaxis using an IPC device (Kendall Express 9525 SCD: Covidien, Dublin, Ireland). This device consisted of a garment which is fitted to the calf and thigh. The garment was inflated by a pump and deflated every 35 to 45 seconds according to venous refilling time of the patient. The inflated pressure was 45 mmHg in ankle, 40 mmHg in calf and 30 mmHg in thigh.
The IPC was applied in the calf and thigh of both legs for all the time during the postoperative immobilization. Although the actual working time of IPC depends on the patient’s compliance, most of our patients used IPC device all days and nights. The application of IPC was continued until patients were mobilized to a wheelchair or could walk with an assistive device and encourage to apply when patient stay in bed even after mobilization.
Thigh-length anti-embolic stockings were also applied for all the times after operation and the patients were encouraged to do ankle dorsiflexion and plantar flexion while in bed during the hospitalization. We did not use any pharmacologic prophylaxis such as low molecular weight heparin or rivaroxaban.
On postoperative day 1 or 2, closed suction drainage was removed and patients were mobilized to a wheelchair. On postoperative day 3, patients walked with restricted weight bearing and with the use of assistive devices (wheelchair, walker, crutches, or cane). As walking ability improved, the assistive devices were changed as determined appropriate by a physical therapist. The mean length of hospital stay was 12.5 days (range, 5-30 days).
DVT and PE evaluation
After the operation, we routinely monitored patients for clinical signs of DVT including pain and tenderness in the calf or thigh, swelling or erythema of the surgically treated limb, and a positive Homan sign. On postoperative day 5 or 6, all patients were routinely assessed by duplex ultrasonography for DVT of the lower extremities. If PE was suspected, ventilation/perfusion scan and pulmonary CT angiography were done.
After discharge, patients were followed routinely at 6 weeks, 3 months, and 6, 9, and 12 months, and every 6 months thereafter with a specific attention given to the development of DVT or PE. Patients, who did not return, were contacted and the evaluations were completed by a telephone interview by two nurses and one private locator.
Most deaths attributable to PE related to surgery reportedly occur within 3 months and any death of unknown cause that occurs within 3 months of surgery is considered to be the result of PE (1,21,22,23). We confirmed the fatal PE, if present, from the death certificate.
To determine risk factors for VTE, age, gender, body mass index (BMI), administration of aspirin, type of anesthesia, operation time, blood loss, approach, and duration of postoperative immobilization in bed were compared between the VTE group and the non-VTE group.
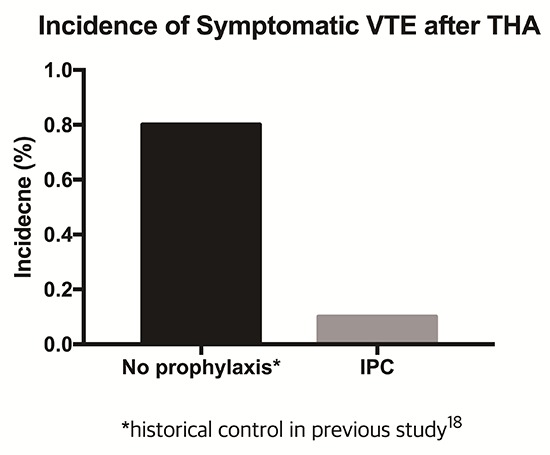
To evaluate the effectiveness of the IPC device for prophylactic treatment, we compared the incidences of symptomatic DVT and PE with a previous study (18) that reported incidences of symptomatic VTE and PE after primary THA without DVT prophylaxis. At that time, we only used thigh length anti-embolic stockings. The incidence of fatal PE, symptomatic PE, and symptomatic DVT was 0%, 0.1% (1/992), and 0.8% (8/992), respectively, in that study. With the exception of IPC application, all surgical procedures and postoperative care were done in the same manner between the current series and the control. Basic characteristics of patients of current study were similar with those of previous study (Table 1).
Statistical analysis
We used Fisher’s exact test for categorized data and the Mann-Whitney U test for continuous data. Statistical analyses were conducted with the SPSS for Windows statistical package (version 16.0; SPSS, Chicago, IL, USA).
Ethics statement
The design and protocol of this study were reviewed and approved by the institutional review board in Seoul National University Bundang Hospital (L-2014-781), which waived informed consents of patients.
RESULTS
Asymptomatic DVT was detected in 2 patients (0.2%) by sonography on postoperative day 5. And one (0.1%) of patient had swelling in the operated leg on postoperative day 5 and diagnosed with DVT next day. Symptomatic PE occurred in 1 patient (0.1%). This patient was a 35-year-old man, who had underlying urticarial vasculitis. He had dyspnea and anterior chest pain on postoperative day 17. Lung scan showed defects in the right upper and lower lobes, and CT angiography showed occlusions in lobar branches of the right pulmonary artery even he was not detected by duplex ultrasonography on postoperative day 5. Four patients, who had DVT or PE, were treated with intravenous heparin followed by oral warfarin for 2-12 months (Table 2).
Table 2. Patients with venous thromboembolism after primary total hip arthroplasty.
| Patient | Gender | Age, yr | Diagnosis | VTE | Predisposing factors |
|---|---|---|---|---|---|
| 1 | M | 35 | Osteonecrosis | PE* | Urticarial vasculitis |
| 2 | F | 66 | Osteoarthritis | Ipsilateral DVT* | No |
| 3 | F | 71 | Osteonecrosis | Ipsilateral DVT | End-stage renal disease |
| 4 | F | 65 | Septic hip Sequelae | Ipsilateral DVT | Prolonged immobilization |
VTE, venous thromboembolism; PE, pulmonary embolism; DVT, deep vein thrombosis.
*symptomatic.
No complications associated with IPC device were observed in this series. Two patients died of causes unrelated to the operation within 6 months after surgery. In the remaining 739 patients, the latest follow-up evaluations were done between 6 to 57 months (mean, 23.8 months) after THA. No patient had a fatal PE during the follow-up.
The incidence of symptomatic DVT (0.1%, 1/870) after the use of IPC device in the current series was lower than that of the historical control (0.8%, 8/992) (P = 0.042) (18). The incidence of symptomatic PE was similar with that (0.1%) of the control study.
In the univariate comparisons, age (P = 0.014) and duration of immobilization (P = 0.067) had P values less than 0.1 (Table 3). In multivariate analysis, the duration of immobilization was associated with the occurrence of DVT (OR, 2.47; 95% CI, 1.490-5.262; P = 0.000). The R 2 coefficient for the current model was 0.406 that would explain the variation of the outcome to the extent of 40.6%.
Table 3. Potential risk factors for VTE.
| Factors | VTE | Without VTE | P value |
|---|---|---|---|
| Gender | |||
| Male | 1 | 396 | 0.342 |
| Female | 3 | 341 | |
| Age, yr | 67.3 ± 3.0 | 51.6 ± 14.9 | 0.014 |
| BMI, kg/m2 | 25.6 ± 6.1 | 24.4 ± 3.6 | 0.797 |
| Operation time, min | 142.5 ± 69.8 | 126.1 ± 40.5 | 0.978 |
| Blood loss, mL | 1,050 ± 526.0 | 772.0 ± 479.1 | 0.221 |
| Duration of immobilization, day | 7.5 ± 5.5 | 3.9 ± 1.4 | 0.067 |
| Aspirin or antiplatelet | 1.000 | ||
| No | 4 | 779 | |
| Yes | 0 | 87 | |
| Anesthesia | 1.000 | ||
| Regional | 4 | 783 | |
| General | 0 | 83 |
VTE, venous thromboembolism; BMI, body mass index.
DISCUSSION
In our THA patients, there was no fatal PE, and incidences of symptomatic PE and symptomatic DVT were quite low after IPC prophylaxis. The incidence of symptomatic DVT was significantly lower than that of historical control from our institution without prophylaxis. IPC device-related complications including compartment syndrome (24) and bullous skin lesion (25) have been reported. However, none of our patients had IPC related complications.
Sugano et al. (26) retrospectively reviewed 3,016 patients who underwent hip surgery including 2,648 primary THA, 298 revisions THA, and 70 pelvic or femoral osteotomies. There were no cases of fatal pulmonary embolism, 1 symptomatic PE and 4 symptomatic DVTs. However, they did not evaluate asymptomatic DVT and used national registry data of Japan. Although the sample size of their study was large, their study alone was not a sufficient to draw a reliable conclusion from their study alone. We found that IPC after THA could reduce the rate of DVT and PE from the previous studies (Table 4).
Table 4. Incidence of venous thromboembolism after primary total hip arthroplasty.
| Study | No. of THA | No. of DVT (%) | No. of PE (%) | Prophylaxis |
|---|---|---|---|---|
| Piovella et al. (27) | 121 | 31 (25.6)* | Not described | No |
| Leizorovicz et al. (28) | 408 | 4 (1.0) | 0 (0) | No |
| Kang et al. (18) | 992 | 8 (0.8) | 1 (0.1) | No |
| Sugano et al. (26) | 2,648 | 4 (0.2) | 1 (0) | IPC |
| The present study | 870 | 3 (0.3)* | 1 (0.1) | IPC |
THA, total hip arthroplasty; DVT, deep vein thrombosis; PE, pulmonary embolism; IPC, Intermittent Pneumatic Compression.
*Including asymptomatic VTE.
There are several limitations in our study. First, our study was not a randomized controlled trial (RCT). However, RCT was not feasible because informed consent could not be obtained from a consecutive cohort of patients, and controls were unethical to our institute. Thus, the incidences of the current study were compared with a historical control, which were obtained from our institution. Basic characteristics of patients were similar, and surgical procedures and postoperative care were done in the same manner between the current series and the control. Second, sample size was not large enough. The sample size for historical comparison of symptomatic DVT is about 1,400 by calculation power 0.8. Third, the incidence of symptomatic PE of the current study was similar with that (0.1%) of the control study. The incidence of PE is quite low in East Asian patients. The sample size of our study might be not enough to discriminate the difference of PE incidence between IPC applied patients and controls. Fourth, our results cannot be generalized to western patients.
Thromboprophylactic agents can pose risks of internal bleeding risk, hematoma, operative site bleedings, and other complications (9). Considering risks and benefits of medical prophylaxis, we recommend IPC device without anticoagulant drugs as a safe and effective thromboprophylaxis for elective THA in our patient population.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study concept and design: Jo WL, Lee YK, Koo KH. Coordinated sampling and data collection: Ha YC, Kang BJ. Supportive data collection: Jo WL, Lee YK. Writing the manuscript: Jo WL, Lee YK. Critical review of the manuscript: Ha YC, Koo KH. Approval of final manuscript: all authors.
References
- 1.Warwick D, Williams MH, Bannister GC. Death and thromboembolic disease after total hip replacement. A series of 1162 cases with no routine chemical prophylaxis. J Bone Joint Surg Br. 1995;77:6–10. [PubMed] [Google Scholar]
- 2.Warwick D. New concepts in orthopaedic thromboprophylaxis. J Bone Joint Surg Br. 2004;86:788–792. doi: 10.1302/0301-620x.86b6.15085. [DOI] [PubMed] [Google Scholar]
- 3.Coventry MB, Nolan DR, Beckenbaugh RD. “Delayed” prophylactic anticoagulation: a study of results and complications in 2,012 total hip arthroplasties. J Bone Joint Surg Am. 1973;55:1487–1492. [PubMed] [Google Scholar]
- 4.Clagett GP, Anderson FA, Jr, Heit J, Levine MN, Wheeler HB. Prevention of venous thromboembolism. Chest. 1995;108:312S–34S. doi: 10.1378/chest.108.4_supplement.312s. [DOI] [PubMed] [Google Scholar]
- 5.Miric A, Lombardi P, Sculco TP. Deep vein thrombosis prophylaxis: a comprehensive approach for total hip and total knee arthroplasty patient populations. Am J Orthop. 2000;29:269–274. [PubMed] [Google Scholar]
- 6.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW American College of Chest Physicians. Prevention of venous thromboembolism: American college of chest physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman JR, Pensak MJ. Prevention of venous thromboembolic disease after total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95:1801–1811. doi: 10.2106/JBJS.L.01328. [DOI] [PubMed] [Google Scholar]
- 8.Kakkar VV, Stringer MD. Prophylaxis of venous thromboembolism. World J Surg. 1990;14:670–678. doi: 10.1007/BF01658824. [DOI] [PubMed] [Google Scholar]
- 9.Leijtens B, Kremers van de Hei K, Jansen J, Koëter S. High complication rate after total knee and hip replacement due to perioperative bridging of anticoagulant therapy based on the 2012 ACCP guideline. Arch Orthop Trauma Surg. 2014;134:1335–1341. doi: 10.1007/s00402-014-2034-4. [DOI] [PubMed] [Google Scholar]
- 10.Klatsky AL, Armstrong MA, Poggi J. Risk of pulmonary embolism and/or deep venous thrombosis in Asian-Americans. Am J Cardiol. 2000;85:1334–1337. doi: 10.1016/s0002-9149(00)00766-9. [DOI] [PubMed] [Google Scholar]
- 11.White RH, Zhou H, Gage BF. Effect of age on the incidence of venous thromboembolism after major surgery. J Thromb Haemost. 2004;2:1327–1333. doi: 10.1046/j.1538-7836.2004.00848.x. [DOI] [PubMed] [Google Scholar]
- 12.White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93:298–305. doi: 10.1160/TH04-08-0506. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen HN, Fujiyoshi A, Abbott RD, Miura K. Epidemiology of cardiovascular risk factors in Asian countries. Circ J. 2013;77:2851–2859. doi: 10.1253/circj.cj-13-1292. [DOI] [PubMed] [Google Scholar]
- 14.Brush BE, Heldt TJ. A device for relief of lymphedema. J Am Med Assoc. 1955;158:34–35. doi: 10.1001/jama.1955.02960010000009. [DOI] [PubMed] [Google Scholar]
- 15.Morris RJ, Woodcock JP. Evidence-based compression: prevention of stasis and deep vein thrombosis. Ann Surg. 2004;239:162–171. doi: 10.1097/01.sla.0000109149.77194.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Comerota AJ, Chouhan V, Harada RN, Sun L, Hosking J, Veermansunemi R, Comerota AJ, Jr, Schlappy D, Rao AK. The fibrinolytic effects of intermittent pneumatic compression: mechanism of enhanced fibrinolysis. Ann Surg. 1997;226:306–313. doi: 10.1097/00000658-199709000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kessler CM, Hirsch DR, Jacobs H, MacDougall R, Goldhaber SZ. Intermittent pneumatic compression in chronic venous insufficiency favorably affects fibrinolytic potential and platelet activation. Blood Coagul Fibrinolysis. 1996;7:437–446. doi: 10.1097/00001721-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kang BJ, Lee YK, Kim HJ, Ha YC, Koo KH. Deep venous thrombosis and pulmonary embolism are uncommon in East Asian patients after total hip arthroplasty. Clin Orthop Relat Res. 2011;469:3423–3428. doi: 10.1007/s11999-011-1979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Price MJ. Monitoring platelet function to reduce the risk of ischemic and bleeding complications. Am J Cardiol. 2009;103:35A–9A. doi: 10.1016/j.amjcard.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AT, Skinner JA, Kakkar VV. Antiplatelet treatment for thromboprophylaxis: a step forward or backwards? BMJ. 1994;309:1213–1215. doi: 10.1136/bmj.309.6963.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber O, Bounameaux H, Borst F, Rohner A. Postoperative pulmonary embolism after hospital discharge. An underestimated risk. Arch Surg. 1992;127:310–313. doi: 10.1001/archsurg.1992.01420030076014. [DOI] [PubMed] [Google Scholar]
- 22.Wroblewski BM, Siney PD, White R. Fatal pulmonary embolism after total hip arthroplasty. Seasonal variation. Clin Orthop Relat Res. 1992:222–224. [PubMed] [Google Scholar]
- 23.Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI, Schwartz JS, Thompson BT, Popovich J, Jr, Hobbins TE, et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326:1240–1245. doi: 10.1056/NEJM199205073261902. [DOI] [PubMed] [Google Scholar]
- 24.Werbel GB, Shybut GT. Acute compartment syndrome caused by a malfunctioning pneumatic-compression boot. A case report. J Bone Joint Surg Am. 1986;68:1445–1446. [PubMed] [Google Scholar]
- 25.Won SH, Lee YK, Suh YS, Koo KH. Extensive bullous complication associated with intermittent pneumatic compression. Yonsei Med J. 2013;54:801–802. doi: 10.3349/ymj.2013.54.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugano N, Miki H, Nakamura N, Aihara M, Yamamoto K, Ohzono K. Clinical efficacy of mechanical thromboprophylaxis without anticoagulant drugs for elective hip surgery in an Asian population. J Arthroplasty. 2009;24:1254–1257. doi: 10.1016/j.arth.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Piovella F, Wang CJ, Lu H, Lee K, Lee LH, Lee WC, Turpie AG, Gallus AS, Planès A, Passera R, et al. Deep-vein thrombosis rates after major orthopedic surgery in Asia. An epidemiological study based on postoperative screening with centrally adjudicated bilateral venography. J Thromb Haemost. 2005;3:2664–2670. doi: 10.1111/j.1538-7836.2005.01621.x. [DOI] [PubMed] [Google Scholar]
- 28.Leizorovicz A, Turpie AG, Cohen AT, Wong L, Yoo MC, Dans A, SMART Study Group Epidemiology of venous thromboembolism in Asian patients undergoing major orthopedic surgery without thromboprophylaxis. The SMART study. J Thromb Haemost. 2005;3:28–34. doi: 10.1111/j.1538-7836.2004.01094.x. [DOI] [PubMed] [Google Scholar]


