Abstract
Positive fluid balance is a risk factor for mortality in critically ill patients, especially those requiring continuous renal replacement therapy (CRRT). However, the association between daily fluid balance and various organ impairments remains unclear. This study investigated the impacts of daily fluid balance prior to CRRT on organ dysfunction, as well as mortality in critically ill patients. We identified daily fluid balance between intensive care unit (ICU) admission and CRRT initiation. According to daily fluid balance, the time to CRRT initiation and the rate of organ failure based on the sequential organ failure assessment (SOFA) score were assessed. We recruited 100 patients who experienced CRRT for acute kidney injury. CRRT was initiated within 2 [0, 4] days. The time to CRRT initiation was shortened in proportion to daily fluid balance, even after the adjustment for the renal SOFA score at ICU admission (HR 1.14, P = 0.007). Based on the SOFA score, positive daily fluid balance was associated with respiratory, cardiovascular, nervous, and coagulation failure, independent of each initial SOFA score at ICU admission (HR 1.36, 1.26, 1.24 and 2.26, all P < 0.05). Ultimately, we found that positive fluid balance was related with an increase in the rate of 28-day mortality (HR 1.14, P = 0.012). Positive daily fluid balance may accelerate the requirement for CRRT, moreover, it can be associated with an increased risk of multiple organ failure in critically ill patients.
Keywords: Continuous Renal Replacement Therapy, Critically Ill Patients, Daily Fluid Balance, Organ Failure
Graphical Abstract
INTRODUCTION
Fluid therapy is essential for treating critically ill patients. A large amount of fluid may be needed to optimize hemodynamics, especially in patients with severe sepsis and septic shock (1). On the other hand, fluid is excessively administered in numerous cases, sometimes resulting in fluid accumulation, inducing tissue edema, which can predispose organ dysfunction (2). Studies have demonstrated that positive fluid balance is associated with worse outcomes, such as prolonged duration of mechanical ventilation and delayed recovery of kidney function, as well as increased mortality in critically ill patients (3,4,5). Thus, a conservative strategy of fluid management in critically ill patients is now being advocated rather than liberal fluid therapy.
Acute kidney injury (AKI) frequently occurs in critically ill patients (6,7,8), and fluid therapy is performed to maintain adequate renal perfusion in patients with or at risk of AKI (9,10). Due to the fact that the mortality rate of such patients is high, appropriate management is required for the treatment and prevention of AKI (8,11,12). However, liberal fluid therapy for kidney protection is now being challenged. Several studies have supported that liberal fluid therapy in critically ill patients with AKI might be associated with poor outcomes, so restrictive fluid therapy is suggested to improve the survival rate (3,13,14,15). Although previous studies have shown an increased mortality, there is a paucity of studies with respect to organ impairment depending on inadequate fluid balance in patients with AKI to date. In addition, most studies have focused on the respiratory system, thus, the impact of fluid balance on other organs remains uncertain.
In this study, we performed a retrospective study to investigate the influence of daily fluid balance prior to continuous renal replacement therapy (CRRT) on various organ outcomes in critically ill patients. The time to CRRT initiation and multiple organ impairment based on the sequential organ failure assessment (SOFA) score as well as mortality were evaluated according to daily fluid balance.
MATERIALS AND METHODS
Patients
Patients who underwent CRRT between April 2007 and August 2011 were recruited. A total of 140 adult patients who had received CRRT were identified. Of these, the study included AKI patients who were admitted for medical illness. Thus, we excluded 34 patients based on the following criteria: 6 were trauma admissions; 12 were postoperative admissions; and 16 had preexisting end-stage renal disease requiring dialysis. We additionally excluded 2 patients who had simultaneously received extracorporeal membrane oxygenation and 4 patients who had received a kidney transplant before admission. Ultimately, 100 patients treated with CRRT in the intensive care unit (ICU) were analyzed in the present study.
Continuous renal replacement therapy protocol
The initiation of CRRT was decided by the physician if the patients who could not bear intermittent hemodialysis due to unstable vital signs had refractory pulmonary edema, intractable hyperkalemia or metabolic acidosis, uremic symptoms including pericarditis and encephalopathy, or oliguria with progressive azotemia. A central venous catheter was inserted into the internal jugular or femoral vein. Continuous hemodiafiltration was carried out using the Prisma (Gambro, Lund, Sweden) or Prismaflex (Gambro) machines with high flux hemofilter (ST100, Gambro), and dialysate and replacement fluids (Hemosol B0, Gambro). Anticoagulation was conducted with nafamostat mesilate (SK chemicals, Seoul, Korea). The target dose of CRRT was 40 mL/kg/hour, which could be adjusted by the physician depending on the patient’s condition.
Data collection
All data were collected from electronic medical records. Baseline demographic and clinical data at ICU admission included age, sex, comorbidities, and routes and causes of admission. The data at CRRT initiation included the reasons for AKI, the time to initiate CRRT, central venous pressure, chest radiograph to identify pulmonary edema, urine output and doses of diuretics. Urinary results for fractional excretion of sodium were also recorded. To calculate the SOFA score, related parameters were obtained both at ICU admission and at CRRT initiation (16). All available intake and output data between ICU admission and CRRT initiation were collected. Intake was composed of oral and parenteral fluid administered, and output included urine, gastrointestinal losses, and drains.
Definitions
Fluid balance was computed using total intake and output data. Cumulative fluid balance was defined as the difference between total intake and total output from ICU admission to CRRT initiation. Daily fluid balance was calculated by dividing the cumulative fluid balance by the day of CRRT initiation. Patients were classified into tertiles based on daily fluid balance: group 1 included patients with a fluid balance of < 1.5 L/day; group 2 included those with a fluid balance of 1.5 to 3.0 L/day; and group 3 included those with a fluid balance of ≥ 3.0 L/day.
Failure of organ systems was evaluated to elucidate the impact depending on daily fluid balance. Organ failure was assessed based on the SOFA score excluding renal system and was defined as each SOFA score of 4.
Outcome
We firstly evaluated the time to start CRRT from ICU admission according to daily fluid balance. Next, the prevalence of organ failure was compared between the groups and the probability of organ failure depending on daily fluid balance was determined. We also evaluated the 28-day mortality according to daily fluid balance. Lastly, recovery of renal function was assessed by dialysis independency at 90 days from ICU admission.
Statistical analysis
Continuous variables are expressed as the median [interquartile range], and were compared using the Wilcoxon rank sum test or ANOVA, followed by the Turkey-Kramer method for multiple comparison. Categorical variables are expressed as a number (percentage), and were analyzed using the χ2 test. The Kaplan-Meier method was used to evaluate the CRRT implementation rate and the 28-day patient survival rate, and those were assessed by the log-rank test. Univariate and multivariate Cox regression analyses were performed to explore the hazard ratio (HR) for daily fluid balance. Multivariate analyses for the risk of organ failure were conducted with adjustment for age, sex and the SOFA score of each organ at ICU admission. On the other hand, the 28-day mortality was adjusted as follows: model 1 was adjusted for age and sex; model 2 was adjusted for the variables in model 1 plus the total SOFA score at ICU admission; and model 3 was adjusted for the variables in model 2 plus causes of AKI. All statistical analyses were performed using SPSS Statistics version 18 (IBM Corp., Armonk, NY, USA). A two-sided P value < 0.05 was considered to be significant.
Ethics statement
The study was approved by the institutional review board at Chung-Ang University Hospital, IRB number: C2013013(973). Since the study was retrospective and the subjects were de-identified, the board waived the need for written consent from patients.
RESULTS
Baseline characteristics at ICU admission according to daily fluid balance
Of the 100 included patients, there were 67 (67.0%) men and 33 (33.0%) women, and the median age was 65 [51, 73] years old. Those admitted to ICU were from either the emergency department (65.0%) or a general ward (35.0%). The most common reason for admission was infection (49.0%), followed by heart disease (17.0%).
According to daily fluid balance between ICU admission and CRRT initiation, there were 37 patients (37.0%) in group 1, 31 (31.0%) in group 2, and 32 (32.0%) in group 3. Baseline characteristics at ICU admission were compared between the groups (Table 1). The total SOFA scores at ICU admission were 9 [7, 12], 10 [6, 12] and 11 [9, 13] in group 1, 2, and 3, respectively (P = 0.251). The coagulation and renal SOFA scores differed at the time of ICU admission (P = 0.034 and 0.015, respectively).
Table 1. Baseline characteristics at ICU admission according to daily fluid balance.
| Parameters | Group 1 (n = 37) | Group 2 (n = 31) | Group 3 (n = 32) | P value |
|---|---|---|---|---|
| Age, yr | 66 [52, 78] | 66 [51, 74] | 58 [50, 70] | 0.295 |
| Sex, M:F | 24:13 | 21:10 | 22:10 | 0.938 |
| Source of admission | ||||
| Emergency room | 22 (59.5) | 19(61.3) | 24 (75) | 0.351 |
| Comorbidities | ||||
| Hypertension | 19 (51.4) | 18 (58.1) | 12 (37.5) | 0.247 |
| Congestive heart failure | 6 (16.2) | 4 (12.9) | 2 (6.3) | 0.439 |
| Diabetes | 11 (29.7) | 11 (35.5) | 10 (31.3) | 0.874 |
| Chronic kidney disease | 7 (18.9) | 1 (3.2) | 4 (12.5) | 0.139 |
| Cancer | 8 (21.6) | 8 (25.8) | 11 (34.4) | 0.485 |
| Reasons for admission | ||||
| Infection | 16 (43.2) | 18 (58.1) | 15 (46.9) | 0.457 |
| Cardiovascular | 9 (24.3) | 4 (12.9) | 4 (12.5) | 0.327 |
| Gastrointestinal | 1 (2.7) | 4 (12.9) | 5 (15.6) | 0.165 |
| Renal | 8 (21.6) | 2 (6.5) | 6 (18.8) | 0.207 |
| Others | 3 (8.1) | 3 (9.7) | 2 (6.3) | 0.882 |
| SOFA score | 9 [7, 12] | 10 [6, 12] | 11 [9, 13] | 0.251 |
| Respiratory | 2 [2, 3] | 3 [2, 3] | 2 [0, 3] | 0.102 |
| Nervous | 1 [0, 3] | 1 [0, 3] | 1 [1, 3] | 0.465 |
| Cardiovascular | 2 [0, 3] | 2 [0, 4] | 3 [0, 4] | 0.279 |
| Liver | 0 [0, 1] | 0 [0, 2] | 0 [0, 2] | 0.228 |
| Coagulation | 0 [0, 1] | 1 [0, 2] | 2 [0, 3] | 0.034 |
| Renal | 4 [2, 4] | 2 [1, 3] | 3 [2, 3] | 0.015 |
| 28-day mortality | 21 (56.8) | 21 (67.7) | 26 (81.3) | 0.094 |
Data are expressed as the median [interquartile range] or number (percentage).
Group 1 included patients with a fluid balance of < 1.5 L/day; group 2 included patients with a fluid balance of 1.5–3.0 L/day; and group 3 included patients with a fluid balance of ≥ 3.0 L/day.
SOFA, sequential organ failure assessment.
Timing of CRRT initiation from ICU admission according to daily fluid balance
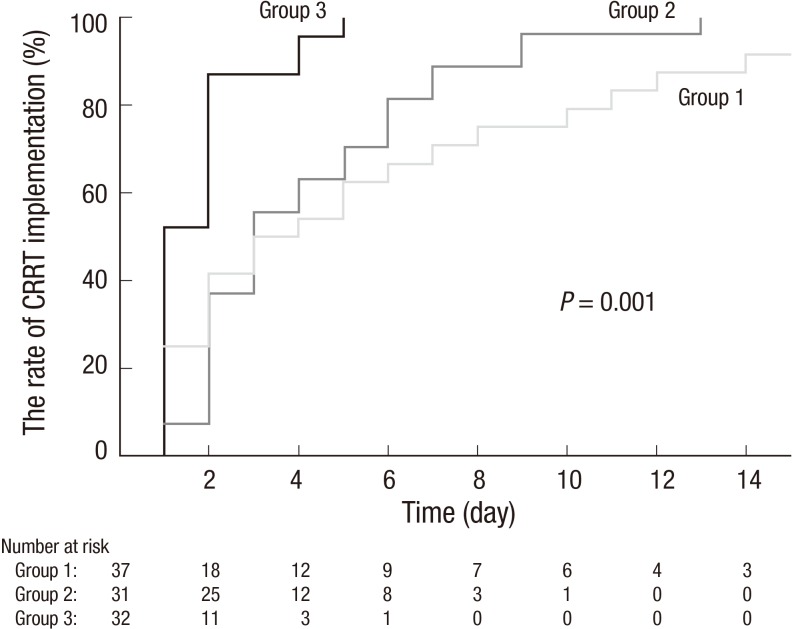
The median time to start CRRT was 2 [0, 4] days, and the causes of AKI were septic (47.0%), cardiorenal (14.0%), ischemic (9.0%), hepatorenal (14.0%), and others (16.0%) such as toxic, postrenal or unknown. Table 2 shows the comparison at CRRT initiation among three groups. The causes of AKI did not differ between the groups (P = 0.359, Table 2). The time to CRRT initiation was gradually shortened from group 1 to group 3 (P = 0.001, Fig. 1). Furthermore, positive daily fluid balance from ICU admission to CRRT initiation was related with the acceleration of the requirement of CRRT even after adjustment with age, sex, and the baseline renal SOFA score (HR 1.14, 95% confidence interval [CI] 1.04 to 1.25, P = 0.007).
Table 2. Comparisons at CRRT initiation according to daily fluid balance.
| Clinical conditions | Group 1 (n = 37) | Group 2 (n = 31) | Group 3 (n = 32) | P value |
|---|---|---|---|---|
| Causes of AKI | ||||
| Septic | 14 (37.8) | 17 (54.8) | 16 (50.0) | |
| Cardiorenal | 9 (24.3) | 3 (9.7) | 2 (6.3) | |
| Ischemic | 4 (10.8) | 3 (9.7) | 2 (6.3) | |
| Hepatorenal | 3 (8.1) | 5 (16.1) | 6 (18.8) | |
| Others | 7 (18.9) | 3 (9.7) | 6 (18.8) | 0.359 |
| Time to start CRRT, days | 1 [0, 6] | 3 [2, 6] | 1 [0, 2] | 0.003 |
| Central venous pressure, cmH2O | 14 [13, 19] | 16 [13, 19] | 18 [16, 21] | 0.249 |
| Presence of pulmonary edema, No. (%) | 21 (56.8) | 21 (67.7) | 24 (75.0) | 0.272 |
| Fractional excretion of sodium, % | 5.4 [1.8, 7.3] | 1.5 [0.6, 5.4] | 1.4 [0.3, 2.8] | 0.493 |
| Urine output, mL/hr | 10 [2, 32] | 14 [6, 29] | 11 [7, 22] | 0.912 |
| Use of diuretics, % | 16 (43.2) | 12 (38.7) | 21 (65.6) | 0.069 |
| Doses of furosemide, mg/day | 90 [80, 12] | 100 [20, 300] | 140 [90, 240] | 0.195 |
| SOFA score | 11 [8, 14] | 15 [12, 17] | 17 [13, 18] | < 0.001 |
| Respiratory | 1 [0, 4] | 3 [2, 3] | 3 [2, 4] | 0.047 |
| Nervous | 3 [2, 3] | 3 [3, 4] | 4 [3, 4] | < 0.001 |
| Cardiovascular | 2 [0, 3] | 4 [0, 4] | 4 [4, 4] | < 0.001 |
| Liver | 0 [0, 2] | 1 [0, 2] | 2 [0, 2] | 0.120 |
| Coagulation | 1 [0, 2] | 2 [1, 2] | 2 [1, 3] | 0.001 |
Data are expressed as the median [interquartile range] or number (percentage).
Group 1 included patients with a fluid balance of < 1.5 L/day; group 2 included patients with a fluid balance of 1.5–3.0 L/day; and group 3 included patients with a fluid balance of ≥ 3.0 L/day.
SOFA, sequential organ failure assessment.
Fig. 1.
Time to CRRT according to daily fluid balance in critically ill patients.
The time to start CRRT was shortened from group 1 to group 3 (P = 0.001). On day 5, the rate of CRRT implementation was 54.2%, 63.0%, and 95.7% in group 1, 2, and 3, respectively.
Group 1 included patients with a fluid balance of < 1.5 L/day; group 2 included patients with a fluid balance of 1.5–3.0 L/day; and group 3 included patients with a fluid balance of ≥ 3.0 L/day.
CRRT, continuous renal replacement therapy.
Organ dysfunction depending on daily fluid balance
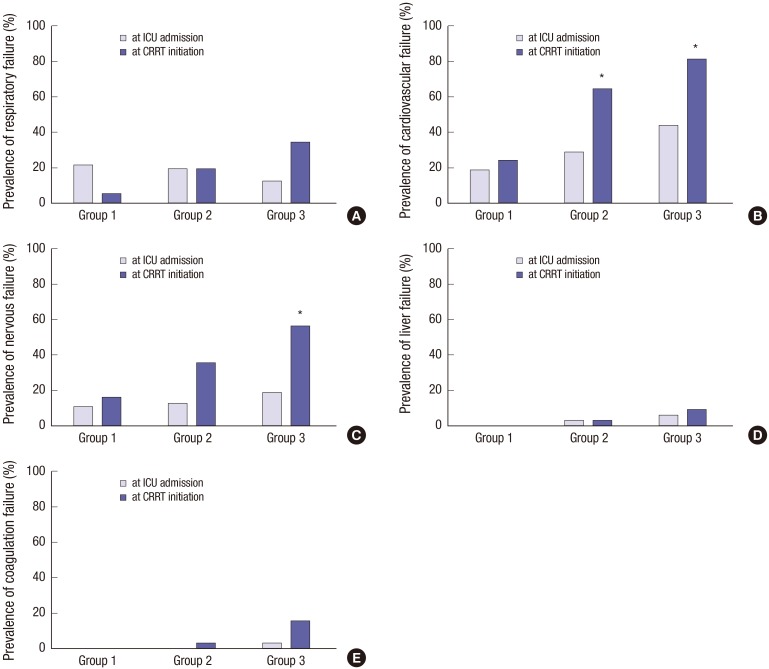
The prevalence of organ failure based on the SOFA score was compared between ICU admission and CRRT initiation (Fig. 2). The prevalence of respiratory failure was insignificantly decreased in group 1 but was increased in group 3 (P = 0.085 and 0.075, respectively). Cardiovascular failure became prevalent at CRRT initiation in group 2 and 3, in comparison with at ICU admission (P = 0.010 and 0.004). In addition, the prevalence of nervous failure was increased in group 3 (P = 0.004). However, increases in liver and coagulation failure were not significant (P = 1.000 and 0.196 in group 3).
Fig. 2.
The comparisons of the prevalence of organ failure between ICU admission and CRRT initiation.
(A) Changes of the prevalence of respiratory failure did not statistically differ. But it was slightly decreased from 21.6% to 5.4% in group 1, but was increased from 12.5% to 34.4% in group 3 (P = 0.085 and 0.075, respectively). (B) Cardiovascular failure became prevalent at CRRT initiation in groups 2 and 3, compared with those at ICU admission (from 29.0% to 64.5% and from 43.8% to 81.3%, P = 0.010 and 0.004, in group 2 and 3, respectively). (C) The nervous failure also increased from 18.8% to 56.3% in group 3 (P = 0.004). (D) The prevalence of liver failure did not change between ICU admission and CRRT initiation (from 6.3% to 9.4%, P = 1.000 in group 3). (E) Although a slight increase in coagulation failure from 3.1% to 15.6% was seen in group 3, this was not statistically significant (P = 0.196).
Group 1 included patients with a fluid balance of < 1.5 L/day; group 2 included patients with a fluid balance of 1.5–3.0 L/day; and group 3 included patients with a fluid balance of ≥ 3.0 L/day.
CRRT, continuous renal replacement therapy; ICU, intensive care unit.
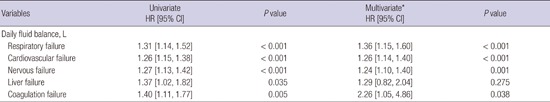
The HR for organ failure depending on daily fluid balance was determined (Table 3). The risk of respiratory failure was increased in proportion to daily fluid balance (HR 1.36, 95% CI 1.15 to 1.60, P < 0.001). We found that positive daily fluid balance was associated with an increase in the probability of cardiovascular failure, independently of age, sex, and the initial cardiovascular SOFA score at ICU admission (HR 1.26, 95% CI 1.14 to 1.40, P < 0.001). In addition, nervous and coagulation failure were related with positive daily fluid balance (HR 1.24 and 2.26, 95% CI 1.10 to 1.40 and 1.05 to 4.86, P = 0.001 and 0.038, respectively). Despite the association between liver failure and positive daily fluid balance in univariate analysis, this was not seen in multivariate analysis (P = 0.275).
Table 3. Organ failure depending on daily fluid balance prior to CRRT in critically ill patients.
| Variables | Univariate HR [95% CI] |
P value | Multivariate* HR [95% CI] |
P value |
|---|---|---|---|---|
| Daily fluid balance, L | ||||
| Respiratory failure | 1.31 [1.14, 1.52] | < 0.001 | 1.36 [1.15, 1.60] | < 0.001 |
| Cardiovascular failure | 1.26 [1.15, 1.38] | < 0.001 | 1.26 [1.14, 1.40] | < 0.001 |
| Nervous failure | 1.27 [1.13, 1.42] | < 0.001 | 1.24 [1.10, 1.40] | 0.001 |
| Liver failure | 1.37 [1.02, 1.82] | 0.035 | 1.29 [0.82, 2.04] | 0.275 |
| Coagulation failure | 1.40 [1.11, 1.77] | 0.005 | 2.26 [1.05, 4.86] | 0.038 |
CI, confidence interval; CRRT, continuous renal replacement therapy; HR, hazard ratio; ICU, intensive care unit; SOFA, sequential organ failure assessment.
*Adjusted by age, sex, and the SOFA score of each organ at ICU admission.
Twenty-eight-day mortality rate according to daily fluid balance
Included patients were followed for a median of 10 [3, 26] days from ICU admission until hospital discharge. The median length of ICU stay was 7 [3, 16] days, and the 28-day mortality rate was 68.0%. The duration of ICU stay did not differ between the groups (7 [3, 14] days in group 1, 9 [5, 26] days in group 2 and 4 [2, 14] days in group 3, P = 0.110). However, as figure 3 shows, the cumulative survival rate was decreased from group 1 to group 3 (P = 0.013). The 28-day survival rates were 41.0%, 31.6% and 18.8% in group 1, 2, and 3, respectively. In Cox regression analysis, we found that positive daily fluid balance prior to CRRT was associated with an increase in the risk of death in critically ill patients, and this was independent of age, sex, the baseline SOFA score at the time of ICU admission, and causes of AKI (HR 1.14, 95% CI 1.03 to 1.26, P = 0.012, Table 4).
Fig. 3.
Twenty-eight-day survival rate according to daily fluid balance prior to CRRT in critically ill patients.
The cumulative survival rates differed between the groups (P = 0.013). The 28-day survival rates were 41.0% in group 1, 31.6% in group 2, and 18.8% in group 3.
Group 1 included patients with a fluid balance of < 1.5 L/day; group 2 included patients with a fluid balance of 1.5–3.0 L/day; and group 3 included patients with a fluid balance of ≥ 3.0 L/day.
CRRT, continuous renal replacement therapy.
Table 4. Daily fluid balance prior to CRRT for 28-day mortality in critically ill patients.
| Daily fluid balance, L | HR [95% CI] | P value |
|---|---|---|
| Univariate | 1.11 [1.02, 1.21] | 0.014 |
| Multivariate model 1* | 1.12 [1.03, 1.22] | 0.009 |
| Multivariate model 2† | 1.14 [1.04, 1.25] | 0.007 |
| Multivariate model 3‡ | 1.14 [1.03, 1.26] | 0.012 |
CI, confidence interval; CRRT, continuous renal replacement therapy; HR, hazard ratio; ICU, intensive care unit; SOFA, sequential organ failure assessment.
*Model 1 was adjusted by age and sex; †Model 2 was adjusted by age, sex and the total SOFA score at ICU admission; ‡Model 3 was adjusted by age, sex, the total SOFA score at ICU admission and causes of AKI.
Recovery of renal function according to daily fluid balance
A total of 32 patients (16 in group 1, 10 in group 2 and 6 in group 3) who survived at 28 days were followed until 90 days to determine dialysis dependency. Of those, 13 patients (40.6%) successfully terminated CRRT and 19 patients (59.4%) were converted to intermittent hemodialysis. At 90 days from ICU admission, 18 (56.3%) had recovery of renal function, 9 (28.1%) were receiving chronic dialysis, and 5 (15.6%) had been transferred to another hospital with maintaining renal replacement therapy. The incidence of renal recovery did not differ between the groups (6 [37.5%] in group 1, 7 [70.0%] in group 2, and 5 [83.3%] in group 3, P = 0.089). The median duration of renal replacement therapy was 5 [4, 14] days in group 1, 3 [2, 11] days in group 2, and 3 [1, 11] days in group 3, respectively (P = 0.031).
DISCUSSION
We retrospectively investigated the outcomes affected by fluid accumulation in critically ill patients receiving CRRT. The present study found that the implementation of CRRT had been brought forward due to an increase in daily fluid overload prior to initiation of CRRT. Moreover, we found a significant relationship between organ failure including respiratory, nervous, cardiovascular, and coagulation systems, and daily fluid balance. The present study also confirmed that the 28-day mortality was increased in proportion to daily fluid balance prior to CRRT initiation in critically ill patients.
Since the landmark trial of early goal-directed therapy, several concerns regarding fluid accumulation have been raised, and numerous researches have dealt with the impacts of fluid balance on outcomes (4,17,18,19). Besides, fluid therapy is a major concern in patients with AKI, especially those requiring CRRT, and previous studies have demonstrated an association between fluid overload and mortality in patients who have undergone renal replacement therapy (3,15,20,21). Nevertheless, there is a lack of clinical research regarding the influence of fluid accumulation on various organ systems. Therefore, we evaluated the association between fluid balance and organ dysfunction in critically ill patient receiving CRRT and found that positive fluid balance prior to CRRT might relate the probability of organ failure at the time of CRRT initiation, although the study could not conclude whether fluid balance is a therapeutic target or just a biomarker.
The study showed that positive fluid balance shortened the time to CRRT commencement. Traditionally, liberal fluid therapy has been thought to be good for the kidneys (9,10). However, previous studies have demonstrated that positive fluid balance cannot protect the kidneys (22,23), further it may decrease the likelihood of renal recovery (3,14). Adverse effects of fluid accumulation on the kidneys may be explained by the renal compartment. Due to the fact that the kidney is an encapsulated organ, increases in renal venous pressure associated with volume expansion lead to a lower renal blood flow (24,25). This adverse effect of fluid accumulation on the kidneys has also been shown in patients with heart failure and sepsis (26,27).
Besides the kidneys, the lungs are the most affected organ by fluid overload (28). It is well-known that a conservative fluid strategy improves oxygenation and shortens the duration of mechanical ventilation in critically ill patients (4,5,29). Again, the present study emphasized the importance of restrictive fluid therapy for lung protection. As shown, the prevalence of respiratory failure between ICU admission and CRRT initiation was increased in patients with a fluid balance of ≥ 3.0 L/day, whereas it was decreased in those with a fluid balance of < 1.5 L/day. Due to the fact that mechanical ventilation is an independent risk factor of mortality (6), a conservative fluid strategy to prevent lung injury is required in critically ill patients, especially those requiring RRT.
It is noteworthy that positive fluid balance was associated with cardiovascular failure in the present study. In general, aggressive fluid therapy is performed to maintain blood pressure and to improve cardiovascular function in critically ill patients (1). However, we found that daily fluid balance was a risk factor of cardiac dysfunction. Increased venous return through vasodilation caused by fluid overload may cause cardiac chamber dilatation, increased ventricular wall stress, and functional atrioventricular valvular insufficiency (30). In another aspect, increases in intra-abdominal pressure by fluid overload may lead to reduced venous return, which results in hemodynamic deterioration (31). Because this study was retrospective, we could not conclude the causality, but the association between positive fluid balance and cardiovascular failure was independent of baseline cardiovascular dysfunction assessed by the SOFA score. Further prospective studies are needed to investigate whether positive fluid balance can aggravate the cardiovascular outcome in critically ill patients.
In the present study, there was an association between daily fluid balance and failure of the nervous system. This could be caused because the nervous SOFA score, based on the Glasgow coma scale, is influenced by intubation and use of sedation (32). Accordingly, the nervous SOFA score at the time of CRRT might be prominent in patients with excessive fluid accumulation.
In addition, the study showed increases in the failure of coagulation in patients with positive fluid balance. Although our results could not also exclude increases in platelet consumption in severely ill patients, this coagulopathy might be explained by dilutional coagulopathy, which refers to alterations in the coagulation system induced by aggressive fluid therapy in patients with shock (33). Thus, judicious uses for fluid therapy to prevent undesirable bleeding complications should be performed in critically ill patients requiring excessive fluid administration.
The present study also evaluated liver failure according to daily fluid balance. The risk of liver failure was increased in univariate analysis, but disappeared after adjustment. There are few reports regarding the association between fluid overload and hepatic failure. Since the liver is also an encapsulated organ like the kidney, liver function can be compromised by fluid overload (34). In animal models, increased intra-abdominal pressure caused impairment of hepatic perfusion and inflammatory changes (35,36). However, clinical research is required to confirm these findings.
The present study has several limitations that must be mentioned. Firstly, selection bias should be considered. Due to the fact that the study was not a randomized trial, more severe patients may need aggressive fluid management. Actually, organ failure at ICU admission, with the exception of kidneys and lungs, was more prevalent in patients administered excessive daily fluid. To find an independent impact of fluid accumulation on organ systems, we collected the severity score assessed by the SOFA score at ICU admission, and those were used to adjust the data. Consequently, we found the relationship between fluid overload and organ dysfunction, independent of the baseline severity at the time of ICU admission. Secondly, present study did not handle the data after CRRT initiation, such as doses and fluid removal of CRRT. This could influence survival rates. However, this study intended to investigate whether fluid balance prior to CRRT affects the organ dysfunction at the time of CRRT. We deduced that adverse outcomes of organ systems at the time of CRRT initiation may consequently result in worse survival rates in patients receiving CRRT. Thirdly, our small sample size limits the power of the results. However, we demonstrated significant impact of daily fluid balance on various organs, and our results give clinical implications for the treatment of critically ill patients despite a small sample size.
In conclusion, this retrospective study investigated the influence of daily fluid balance prior to CRRT on outcomes including organ failure and 28-day mortality in critically ill patients. Positive daily fluid balance may accelerate the requirement of RRT and may relate unfavorable impacts on various organs. Furthermore, these can influence poor patient survival. Therefore, judicious fluid therapy is necessary in critically ill patients prior to CRRT initiation. Further prospective controlled research is needed, to investigate whether the modification of daily fluid balance can have a beneficial effect on organ dysfunction as well as on patient survival.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Design of the study: Shin J, Kim SH. Collection of data: Han MJ, Park KH, Shin J. Analysis and interpretation of data: Shin J. Drafting the manuscript: Han MJ, Shin J. Critical revision of the manuscript: Shin J. Manuscript approval: all authors.
References
- 1.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M, Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 2.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6:107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 3.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL, Program to Improve Care in Acute Renal Disease (PICARD) Study Group Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76:422–427. doi: 10.1038/ki.2009.159. [DOI] [PubMed] [Google Scholar]
- 4.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 5.Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA. Fluid balance and weaning outcomes. Intensive Care Med. 2005;31:1643–1647. doi: 10.1007/s00134-005-2801-3. [DOI] [PubMed] [Google Scholar]
- 6.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 7.Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, Rocco M, Alessandri E, Giunta F, Michetti V, et al. Prospective multicenter study on epidemiology of acute kidney injury in the ICU: a critical care nephrology Italian collaborative effort (NEFROINT) Minerva Anestesiol. 2011;77:1072–1083. [PubMed] [Google Scholar]
- 8.Bagshaw SM, Laupland KB, Doig CJ, Mortis G, Fick GH, Mucenski M, Godinez-Luna T, Svenson LW, Rosenal T. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:R700–9. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kellum JA, Cerda J, Kaplan LJ, Nadim MK, Palevsky PM. Fluids for prevention and management of acute kidney injury. Int J Artif Organs. 2008;31:96–110. doi: 10.1177/039139880803100204. [DOI] [PubMed] [Google Scholar]
- 10.Leblanc M, Kellum JA, Gibney RT, Lieberthal W, Tumlin J, Mehta R. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care. 2005;11:533–536. doi: 10.1097/01.ccx.0000183666.54717.3d. [DOI] [PubMed] [Google Scholar]
- 11.Vaara ST, Pettilä V, Reinikainen M, Kaukonen KM, Finnish Intensive Care Consortium Population-based incidence, mortality and quality of life in critically ill patients treated with renal replacement therapy: a nationwide retrospective cohort study in Finnish intensive care units. Crit Care. 2012;16:R13. doi: 10.1186/cc11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, MacLeod AM. A prospective national study of acute renal failure treated with RRT: incidence, aetiology and outcomes. Nephrol Dial Transplant. 2007;22:2513–2519. doi: 10.1093/ndt/gfm264. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, Forfori F, Pelaia P, Rocco M, Ronco C, et al. Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care. 2013;17:R14. doi: 10.1186/cc12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12:R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RENAL Replacement Therapy Study Investigators An observational study fluid balance and patient outcomes in the randomized evaluation of normal vs. augmented level of replacement therapy trial. Crit Care Med. 2012;40:1753–1760. doi: 10.1097/CCM.0b013e318246b9c6. [DOI] [PubMed] [Google Scholar]
- 16.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, et al. Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006;34:344–353. doi: 10.1097/01.ccm.0000194725.48928.3a. [DOI] [PubMed] [Google Scholar]
- 19.Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17:R246. doi: 10.1186/cc13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silversides JA, Pinto R, Kuint R, Wald R, Hladunewich MA, Lapinsky SE, Adhikari NK. Fluid balance, intradialytic hypotension, and outcomes in critically ill patients undergoing renal replacement therapy: a cohort study. Crit Care. 2014;18:624. doi: 10.1186/s13054-014-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, et al. Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Crit Care. 2012;16:R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Biesen W, Yegenaga I, Vanholder R, Verbeke F, Hoste E, Colardyn F, Lameire N. Relationship between fluid status and its management on acute renal failure (ARF) in intensive care unit (ICU) patients with sepsis: a prospective analysis. J Nephrol. 2005;18:54–60. [PubMed] [Google Scholar]
- 23.de Oliveira FS, Freitas FG, Ferreira EM, de Castro I, Bafi AT, de Azevedo LC, Machado FR. Positive fluid balance as a prognostic factor for mortality and acute kidney injury in severe sepsis and septic shock. J Crit Care. 2015;30:97–101. doi: 10.1016/j.jcrc.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Burnett JC, Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–82. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 25.Shibagaki Y, Tai C, Nayak A, Wahba I. Intra-abdominal hypertension is an under-appreciated cause of acute renal failure. Nephrol Dial Transplant. 2006;21:3567–3570. doi: 10.1093/ndt/gfl496. [DOI] [PubMed] [Google Scholar]
- 26.Damman K, van Deursen VM, Navis G, Voors AA, van Veldhuisen DJ, Hillege HL. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol. 2009;53:582–588. doi: 10.1016/j.jacc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- 27.Legrand M, Dupuis C, Simon C, Gayat E, Mateo J, Lukaszewicz AC, Payen D. Association between systemic hemodynamics and septic acute kidney injury in critically ill patients: a retrospective observational study. Crit Care. 2013;17:R278. doi: 10.1186/cc13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2010;5:733–739. doi: 10.2215/CJN.00060110. [DOI] [PubMed] [Google Scholar]
- 29.Epstein CD, Peerless JR. Weaning readiness and fluid balance in older critically ill surgical patients. Am J Crit Care. 2006;15:54–64. [PubMed] [Google Scholar]
- 30.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kramer AA, Wijdicks EF, Snavely VL, Dunivan JR, Naranjo LL, Bible S, Rohs T, Dickess SM. A multicenter prospective study of interobserver agreement using the full outline of unresponsiveness score coma scale in the intensive care unit. Crit Care Med. 2012;40:2671–2676. doi: 10.1097/CCM.0b013e318258fd88. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber MA. Coagulopathy in the trauma patient. Curr Opin Crit Care. 2005;11:590–597. doi: 10.1097/01.ccx.0000186374.49320.ab. [DOI] [PubMed] [Google Scholar]
- 34.Firth JD, Raine AE, Ledingham JG. Raised venous pressure: a direct cause of renal sodium retention in oedema? Lancet. 1988;1:1033–1035. doi: 10.1016/s0140-6736(88)91851-x. [DOI] [PubMed] [Google Scholar]
- 35.Diebel LN, Wilson RF, Dulchavsky SA, Saxe J. Effect of increased intra-abdominal pressure on hepatic arterial, portal venous, and hepatic microcirculatory blood flow. J Trauma. 1992;33:279–282. doi: 10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Sheth RA, Arellano RS, Uppot RN, Samir AE, Goyal L, Zhu AX, Gervais DA, Mahmood U. Prospective trial with optical molecular imaging for percutaneous interventions in focal hepatic lesions. Radiology. 2015;274:917–926. doi: 10.1148/radiol.14141308. [DOI] [PMC free article] [PubMed] [Google Scholar]