Abstract
Exosomes are small intracellular membrane-based vesicles with different compositions that are involved in several biological and pathological processes. The exploitation of exosomes as drug delivery vehicles offers important advantages compared to other nanoparticulate drug delivery systems such as liposomes and polymeric nanoparticles; exosomes are non-immunogenic in nature due to similar composition as body׳s own cells. In this article, the origin and structure of exosomes as well as their biological functions are outlined. We will then focus on specific applications of exosomes as drug delivery systems in pharmaceutical drug development. An overview of the advantages and challenges faced when using exosomes as a pharmaceutical drug delivery vehicles will also be discussed.
Abbreviations: ALIX, ALG-2 interacting protein X; ATPase, adenosine triphosphatase; BBB, blood–brain barrier; CCK-8, cell counting kit-8; CD, cluster of differentiation; DIL, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DNA, deoxyribonucleic acid; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; EpCAM, epithelial cell adhesion molecule; ESCRT, endosomal sorting complexes required for transport; EV, extracellular vesicle; HEK293, human embryonic kidney cell line 293; HeLa, Henrietta Lacks cells; HIV, human immunodeficiency virus; HMGA2, high-mobility group AT-hook protein; Hsp, heat shock proteins; IL-6, interleukin-6; ILVs, intraluminal vesicles; kRAS, Kirsten rat sarcoma; LPS, lipopolysaccharides; MAPK-1, mitogen-activated protein kinase 1; MHC, major histocompatibility complex; miRNA, micro RNA; MPS, mononuclear phagocyte system; mRNA, messenger RNA; MVB, multi-vesicular body biogenesis; PBMC, peripheral blood mononuclear cells; PD, Parkinson’s disease; PEG, polyethylene glycol; RNA, ribonucleic acid; ROS, reactive oxygen species; RPE1, retinal pigment epithelial cells 1; siRNA, small interference RNA; TNF-α, tumor necrosis factor α; TSG101, tumor susceptibility gene 101; VPS4, vacuolar protein sorting-associated protein 4
KEY WORDS: Exosomes, Nanocarrier, Extracellular vesicles, Drug delivery systems
Graphical abstract
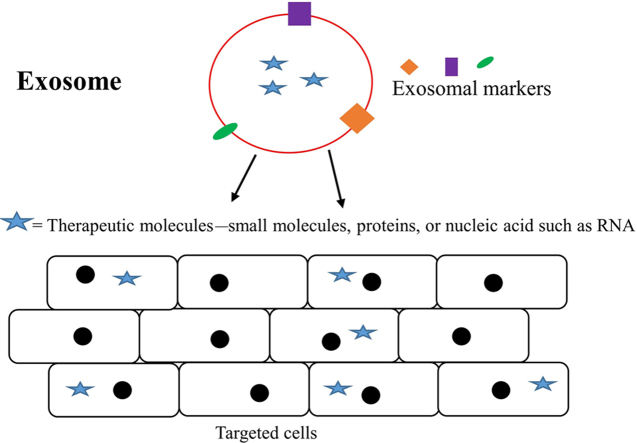
Exosomes are small vesicles secreted by various cell types and are involved in many biological functions. There is also significant evidence on critical role played by exosomes in many diseases. Here we discuss regarding their potential usage and relevance in therapeutic drug delivery.
1. Introduction
Intercellular communication is absolutely essential to cell development and the maintenance of homeostasis in multicellular organisms. These communications between cells can be localized or distant. Local communication involves direct contact between cells facilitated through communication systems, like a gap junction that connects the cytoplasm of cells adjacent to one another, allowing signaling substances to pass between the cells. On the other hand, distant intercellular communication is facilitated by molecules like hormones that send signals through circulatory system to other parts of the body. Another case of distant intercellular communication also occurs in extracellular vesicle (EV), which is a membrane-based structure. These EVs serve as vehicles to carry different types of cellular cargo—such as lipids, proteins, receptors and effector molecules—to the recipient cells1.
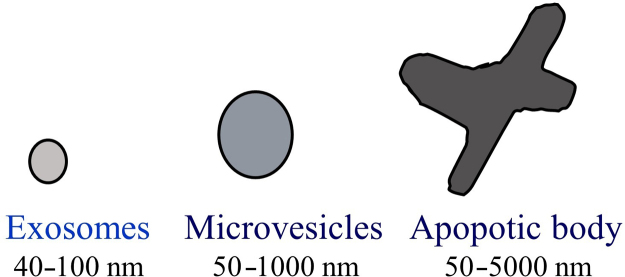
There are three types of EVs that are differentiated based on their intracellular origins: apoptotic bodies, microvesicles and exosomes2. Apoptotic bodies have a size ranging from 50 to 5000 nm and contain cellular contents such as deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and histone proteins. During apoptosis, apoptotic bodies present these contents to marcrophages, which results in cell engulfation3, 4. Other types of EVs known as microvesicles are also referred to as ectosomes, shedding vesicles, microparticles, plasma membrane-derived vesicles, and exovesicles. Microvesicles are formed through the outward budding and fission from plasma membranes, with a size ranging from 50 nm to 1000 nm. Once microvesicles are formed, they carry specific proteins and lipids and then deliver their cargoes to designated recipient cell2. The final category of EVs is exosomes, which differ from microvesicles mainly in terms of their intracellular origin and size (Fig. 1).
Figure 1.
Types of microvesicles. Exosome with sizes ranging 40–100 nm (left), microvesicles with sizes ranging 50–1000 nm (middle), apoptotic body size ranging 50–5000 nm (right).
Over the past decades, there has been extensive research carried out on exosomes. The word “exosome” was first used in 1970 by Rose Johnstone and her colleagues5. While working with maturing reticulocytes, they observed the formation of “an intracellular sac filled with small membrane-enclosed structure of nearly uniform size”. These formed intracellular vesicles and released contents outside the cell, as opposed to inside of cell (endocytosis), where external molecules are internalized into the membrane-bound structure. Hence, these intracellular-formed vesicles were named “exosome”5.
Exosomes are originated from endosomes with a smaller size, ranging from 40 to 100 nm6. They are secreted by all cell types and can be found in most body fluids, including blood, saliva, and urine. An exosome is a “nanosphere” with a bilayerd membrane, containing various types of lipids and proteins derived from the parent cell. Some of these proteins include transport proteins, heat shock proteins, proteins associated with multi-vesicular body biogenesis (MVB), and tetraspanin. In addition to proteins, exosomes are comprised of different types of lipids, such as cholesterol, sphingolipids, phosphoglycerides, ceramides, and saturated fatty acid chains7. The composition of exosomes is critical since they serve as a biomarker and provide an indication of its function in biological processes.
1.1. Formation of exosomes
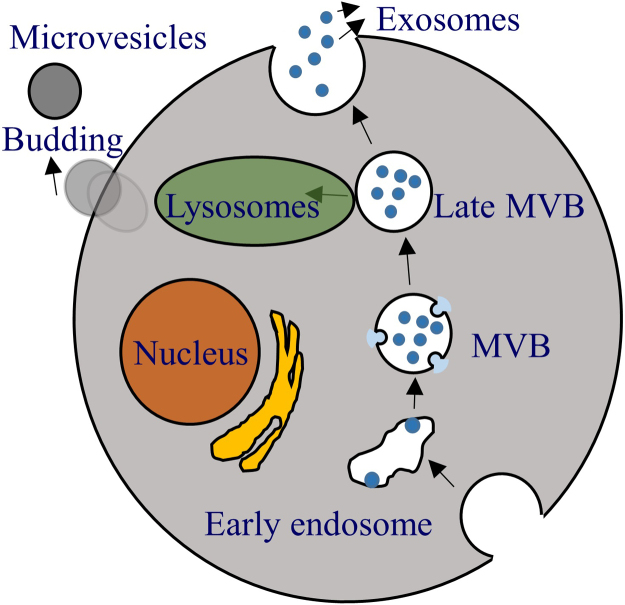
In general, the formation of exosomes consists of three different stages: (1) the formation of endocytic vesicles from plasma membrane, (2) the inward budding of the endosomal vesicle membrane resulting in MVBs that consist of intraluminal vesicles (ILVs), and (3) the fusion of these MVBs with the plasma membrane, which releases the vesicular contents, known as exosomes7 (Fig. 2). In the first stage, endocytic vesicles are formed from the plasma membrane, creating an early endosome, which is then matured into late endosomes. The limiting membrane of these late endosomes undergoes inward budding, in turn forming vesicles inside the lumen. The accumulation of these ILVs inside the late endosomes is termed as MVBs. There are two known pathways to the formation of MVBs. One pathway involves endosomal sorting complexes required for transport (ESCRT) and another pathway is ESCRT independent. These MVBs can either fuse with lysosome for degradation or fused with the plasma membrane of the cell, releasing ILVs into extracellular space and these released ILVs are exosomes8.
Figure 2.
Formation of exosome and microvesicle. Exosome is derived from endosome formed from plasma membrane. As early endosome becomes late endosomes, inward budding occurs and forms multivesicular bodies (MVB) containing numerous intraluminal vesicles (ILV). MVB can either get degraded by lysosomes or fuse with the membrane to release ILV called exosomes. Micorvesicles, on the other hand, originate from the budding of the plasma membrane.
The ESCRT consists of four soluble multi-protein complexes named ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. The complex is recruited to sort selected proteins into ILVs. ESCRT-0 is responsible for cargo clustering, which is required for the ubiquitination of endocytosed receptors9. These cargoes constitute proteins that will be incorporated into ILVs and, later, become part of the released exosomes. Tumor susceptibility gene 101 (TSG101), a component of ESCRT-I, forms a complex with the ubiquinated cargo protein and helps in the activation of ESCRT-II complex, inducing its bud formation. This complex then involves the sequestration of MVB proteins and the recruitment of the de-ubiquitination enzyme to remove the ubiquitin from the cargo proteins before sorting them into ILVs. In the last stage, ESCRT-III gets disassembled by vacuolar protein sorting-associated protein 4 (VPS4) adenosine triphosphatase (ATPase)10, 11.
Studies have shown that molecules that inhibit ESCRT can also inhibit exosome secretion. For example, four independent studies have demonstrated that inhibiting the HRS member of ESCRT-0 resulted in decreased exosome secretion in various cell types, including Henrietta Lacks cells (HeLa)—a lineage of cancer human epithelial cells, mouse dentritic cells, human embryonic kidney cell line 293 (HEK293), and squamous carcinoma cells12, 13, 14, 15. Other studies have shown that the depletion of TSG101 also reduced exosome secretion in tumor cells and in immortalized retinal pigment epithelial cells 1(RPE1)12, 16. In addition, several studies have shown the formation of exosomes despite the inhibition of ESCRT, indicating the existence of an ESCRT independent pathway. This independent mechanism involves molecules such as lipids, tetraspanins, or heat shock proteins17. It has been proposed that lipids such ceramides induce the inward curvature of the limiting membrane of MVBs to form ILVs. Other lipids associated with the formation of exosomes through ESCRT independent mechanisms are cholesterol and phosphophatidic acid. Besides lipids, proteins such as tetraspanins have also been associated with cargo sorting for exosomes18 and the incorporation of melanosomal proteins into ILVs in an ESCRT-independent manner19.
1.2. Isolation and characterization of exosomes
Exosomes are isolated in cell culture studies using several different techniques. The most common isolation technique is differential centrifugation, whereby large particles and cell debris in the culture medium are separated using centrifugal force between 200–100,000×g and the exosomes are separated from supernatant by the sedimenting exosomes at 100,000×g20, 21, 22. However, this technique is not precise in purifying exosomes and, thus, the resulting exosome extractions tend to contain aggregates of cellular proteins and particulates from the cell culture medium23. In addition, this technique is time consuming and requires specialized equipment. Exosome purity can be improved, however, by centrifuging the samples using flotation density gradient centrifugation with sucrose or Optiprep, which results in highly purified and enriched exosomes1, 24.
Another common technique for exosome isolation is the monoclonal antibody based method. Antibodies against exosome-associated antigens—such as cluster of differentiation (CD) molecules CD63, CD81, CD82, CD9, epithelial cell adhesion molecule (EpCAM), and Ras-related protein (Rab5)—are used for separation1, 24. The antibodies can be immobilized in different media conditions and combined with magnetic beads, chromatographic matrix, plates, and microfluidic devices for separation24. A drawback to this technique, however, is that non-exosomes vesicles that carry the antigens also bind to the antibody, reducing the purity of the extracted exosomes1. Ultrafiltration is another way to isolate exosomes based on their size differences. This method is less time consuming compared to ultracentrifugation and does not require special equipment7. One alternative method for isolating exosomes based on size is using high performance liquid chromatography. Exosomes prepared by this technique provide highly purified exosomes. However, this technique also requires expensive equipment1, 7. Overall, there is no single ideal isolation and purification method that is highly selective for exosomes.
Exosomes can be characterized based on their size, protein content, and lipid content. Exosomes are sphere-shaped structures with sizes between 40–100 nm and are much smaller compared to other systems, such as a microvesicle, which has a size range from 100–500 nm. Several methods can be used to characterize exosomes, including flow cytometry, nanoparticle tracking analysis, dynamic light scattering, western blot, mass spectrometry, and microscopy techniques25. Exosomes can also be characterized and marked based on their protein compositions, with intergrins and tetraspanins being the two most abundant proteins found in exosomes. Other protein markers include TSG101, ALG-2 interacting protein X (ALIX), flotillin 1, and cell adhesion molecules. Similar to proteins, lipids are major components of exosomes and can be utilized to characterize them.
1.3. Composition of exosomes
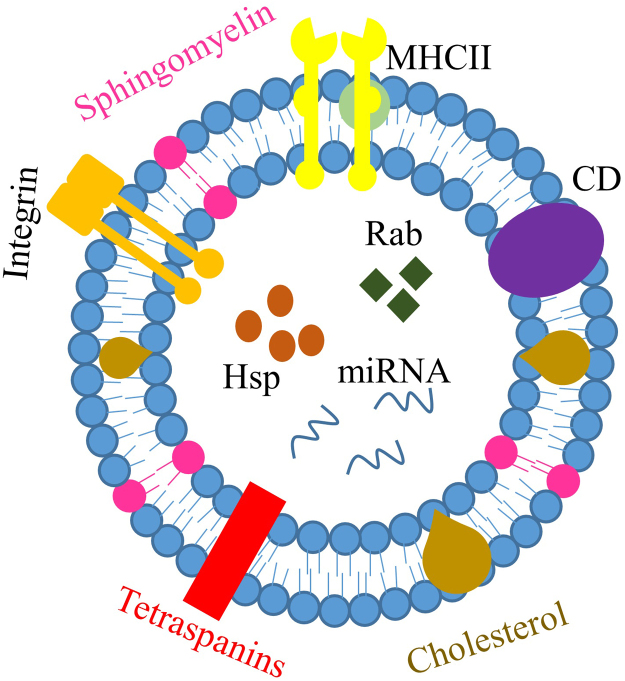
The nanospherical membrane-type structure of exosomes is formed with a bilayer of lipids. It is also composed of various types of lipids and proteins (Table 1) that are derived from the parent cells from which the exosome is formed (Fig. 3). According to ExoCarta, an exosome database, there are currently around 8000 proteins and 194 lipids known to be associated with exosomes24.
Table 1.
Summary and examples of exosomal compositions.
| Exosome composition | Example |
|---|---|
| Proteins | Heat shock proteins: Hsp70, Hsp90 |
| Membrane transport and fusion proteins: GTPase, annexins, flotillin | |
| Tetraspanins: CD9, CD63, CD81, CD82 | |
| Thrombospondin | |
| ALIX | |
| TSG101 | |
| Lipids | Sphingomyelin |
| Phosphatidylcholine | |
| Phosphatidylethanolamine | |
| Phosphatidylserine | |
| GM3 | |
| Phosphatidylinositol | |
| Nucleic acids | mRNA |
| miRNA | |
| Non-coding RNA |
Figure 3.
Composition of exosomes. Exosomes are composed of various types of proteins, such as major histocompatibility complex (MHC)-II, integrin, cluster of differentiation (CD), tetraspanins, heat shock protein (Hsp), Ras-related protein (Rab), etc. Exosomes also contain various types of lipids, such as sphingomyelin and cholesterol. Lastly, exosomes are found to contain nucleic acid, including miRNA, mRNA and non-coding RNAs.
Since exosomes originate from the intracellular component “endosome”, they contain proteins such as heat shock proteins (Hsp70 and Hsp90), membrane transport and fusion proteins (GTPases. Annexins and flotillin), and tetraspanins (CD9, CD63, CD81, and CD82)24. Among these proteins, heat shock proteins, annexins, and proteins of the Rab family are abundantly detected in exosomes and are involved in their intracellular assembly and trafficking. Tetraspanins, a family of transmembrane proteins, are another protein commonly detected in exosomes. In a cell, tetraspanins mediate fusion, cell migration, cell–cell adhesion, and signaling. However, in exosomes, their function remains largely unknown2. Another abundant protein found in exosomes is integrins, which are adhesion molecules that facilitate cell binding to the extracellular matrix. In exosomes, integrins are involved in adhering the vesicles to their target cells26. All these proteins have been used as positive markers to detect the presence of exosomes. Other proteins associated with exosomes include thrombospondin, CD55, CD59, lactadherin, ALIX, and TSG1012, 27. These different types of proteins become incorporated into exosomes during exosome formation and serve as cargo for cell–cell communication.
Besides proteins, exosomes are also rich in lipids, with different types of exosomes containing different types of lipids. The lipid bilayer of exosomes is mainly constituted of cell plasma membrane types of lipids such as sphingomyelin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, monosialotetrahexosylganglioside (GM3), and phosphatidylinositol28. Sphingomyeline and GM3 are responsible for determining the exosome׳s rigidity29 while phosphatidylserine is expressed on the plasma membrane of exosomes through different types of phospholipid transportation enzymes. It is involved in docking the outer proteins, allowing the signaling and fusion of the exosome to the plasma membrane30. Other types of lipids that can form exosomes are cholesterol, ceramide, and phosphoglycerides, along with saturated fatty-acid chains. Additional constituents of exosomes include nucleic acids such as micro RNA (miRNA), messenger RNA (mRNA), and non-coding RNAs24.
1.4. Biological functions of exosomes
Exosomes are secreted by many different types of cells through biological fluids, including synovial fluid, breast milk, blood, urine, saliva, amniotic liquid, and in-blood serum7, suggesting that they play a major role in intercellular communication and triggering physiological responses. Exosomes are found first to be involved in removing unnecessary proteins during the cell maturation process31, 32. Depending on the origin of the exosomes, they have different functions. For example, studies have found that exosomes derived from antigen presenting cells have the ability to express major histocompatibility complex (MHC) class I and II molecules on the cell surface, which helps in activating CD8+ and CD4+ T-cells to induce specific immune responses26, 33, 34. By carrying prostaglandins, exosomes secreted by platelets are involved in the inflammatory response18. Besides exchanging proteins and lipids between recipient cells, exosomes are shown to carry a nucleic acid type of cargo, with exosomes secreted by mast cells containing mRNA and small RNA, which then gets transferred to targeted recipient cells and translated into the recipient cell35. Glioblastoma cells also secret exosomes containing mRNA, miRNA, and angiogentic proteins to microvascular endothelial cells, stimulating angiogenesis36. In general, the functions of exosomes facilitate cell-to-cell communication in a target-specific manner throughout the body. Currently, it is known that exosomes are involved in many biological processes, including the maturation of erythrocytes, the elimination of unnecessary proteins and RNA37, antigen presentation in immune responses38, coagulation, inflammation, and angiogenesis7. Exosomes are also found to have the ability to transfer horizontal miRNA6. These processes are facilitated through the exosome׳s cargo function, with each exosome׳s composition based on the source of the parental cell, from which it is derived.
Besides involving normal biological process, exosomes are found to be associated with many common diseases. In cancer, for example, exosomes are found to induce a pro-tumoral microenvironment by transferring mRNA and protein, such as mutant Kirsten rat sarcoma (kRAS) viral oncogene protein and c-Met oncoprotein, to distant sites, thus promoting angiogenesis, thrombosis, and tumor cell proliferation39, 40, 41. In neurodegenerative disease, such as Parkinson׳s disease (PD), exosomes are found to be responsible for transporting misfolded proteins from unhealthy neurons to nearby cells, thus spreading the disease from cell to cell42. Exosome involvement is also observed in other diseases, such as myocardial infraction and human immunodeficiency virus (HIV), a type of cardiovascular disease and infectious disease, respectively.
Given that exosomes can be isolated from almost any cell, are involved in cell-to-cell communication, and participate in both normal and pathobiological mechanisms, there have been extensive studies exploiting their use both as diagnostics and therapeutics. For example, exosomes are used to detect tumors in patients with prostate, breast, and ovarian cancers43, 44, 45. In infectious disease, they carry and express infectious RNA and proteins, which allows for the diagnosis of infectious diseases as well as the detection of active and latent forms of intracellular infection24. In terms of therapeutics, exosomes are shown to have the ability to induce tissue regeneration by delivering growth factors, proteins, miRNA, mRNA, non-coding RNA, and lipids. For example, in a myocardial infarction and kidney injury model, exosomes derived from stem cells and endothelial progenitor cells have been able to regenerate cardiac tissue and neovascularization.
2. Exosomes as drug delivery vehicle
Currently, the most preferred drug delivery systems are liposomes and polymeric nanoparticles. A liposome is a synthetic vesicle with a phospholipid membrane that self-assembles into various sizes and shapes in an aqueous environment46. Polymeric nanoparticles are drug delivery systems that help in the entrapment, encapsulation, or attachment of drug molecules47. Both of these delivery systems have been used to deliver different types of drug molecules, including anti-cancer drugs, anti-fungal drugs, and analgesics. However, the ability of an ideal liposome to evade the host immune system with a long circulating capability, with stability, and without toxicity still remains elusive48. On the other hand, polymeric nanoparticles may have better stability than liposomal systems, but their biocompatibility and long-term potential safety remain a concern49. In these cases, exosomes or exosome mimetics50—with many of the desirable features of an ideal drug delivery system, such as a long circulating half-life, the intrinsic ability to target tissues, biocompatibility, and minimal or no inherent toxicity issues51—appear to be a superior choice, overcoming the limitations observed with the majority of liposomal or polymeric drug delivery systems52. Table 2 summarizes the studies conducted using exosomes as drug delivery system.
Table 2.
Summary of studies conducted using exosomes as drug delivery system.
| Research | Aim | Ref. (Author, Year) |
|---|---|---|
| Small molecules | Using exosomes to improve delivery of curcumin | Sun, 201053 |
| Delivery of doxorubicin into tumor tissue | Tian, 201454 | |
| Deliver paclitaxel and doxorubicin across blood–brain barrier | Yang, 201555 | |
| Protein | Deliver catalase across BBB to treat PD | Haney, 201556 |
| Nucleic Acids | ||
| siRNA | Human exosomes to deliver siRNA into T cells | Wahlgren, 201257 |
| Exosomes to deliver siRNA into HeLa & Fibrosarcoma cell line (HT1080 cells) | Shtam, 201358 | |
| Delivery of siRNA using endothelial derived exosomes | Banizs, 201459 | |
| miRNA | Exosome to antitumor miRNA into breast cancer cells | Ohno, 201360 |
2.1. Exosomes as drug delivery vehicles for small molecules
Extensive research has been done using exosomes as vehicles for therapeutic drug delivery. One study involved the use of exosomes to deliver curcumin and treat an inflammatory disease53. Curcumin is a natural polyphenol found in the rhizomes of turmeric and it has anti-inflammatory, antineoplastic, antioxidant, and chemo-preventive properties61, 62, 63. Exosomes are employed to form a complex with curcumin for the purpose of enhancing curcumin׳s effectiveness. Clinical trials have also shown its efficacy and safety for cancer patients64. However, its low solubility due to its hydrophobic nature and preferential interaction with lipid membranes reduces its bioavailability65.
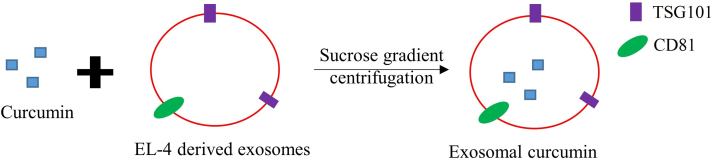
In this study, curcumin was incorporated into exosomes by mixing curcumin with murine tumor cell line (EL-4) derived exosomes and subjecting it to a sucrose-gradient centrifugation. Exosomal protein markers such as TSG101 and CD81 were used to specifically identify the exosome curcumin complex (Fig. 4). Various experiments were then carried out to show the incorporation of curcumin into the exosome while increasing its solubility, stability, and bioavailability. The anti-inflammatory activity of exosomal curcumin was also assessed both in vitro and in vivo. In vitro, marcophages treated with exosomal curcumin produced significantly fewer inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) compared to macrophages treated with curcumin alone, indicating that exosomal curcumin enhances anti-inflammatory activity. In vivo, an lipopolysaccharides (LPS)-induced septic shock animal model was used, and mice treated with exosomal curcumin showed significant survival compared to mice treated with only curcumin. Finally, the study showed that exosomal curcumin decreased the number of CD11b+Gr-1+ cells, a type of cell that increases a response to LPS-induced septic shock in the lungs and leads to acute lung inflammation53. In general, this study showed the ability of exosomes to carry highly hydrophobic drugs, such as curcumin, enhancing their anti-inflammatory effects.
Figure 4.
Formation of exosomal curcumin. Curcumin was incorporated into murine tumor cell line (EL-4), derived exosomes and isolated using sucrose gradient centrifugation.
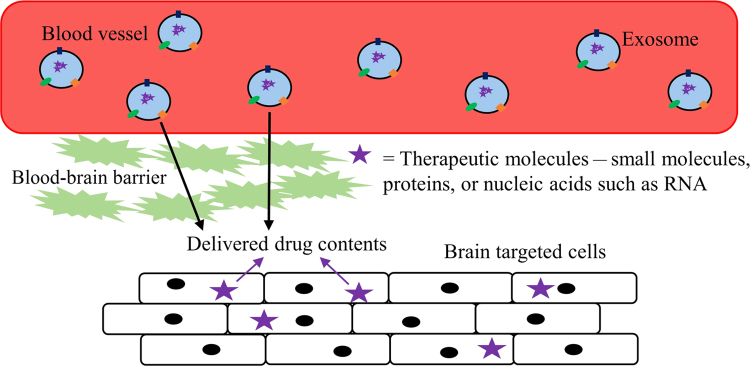
Besides enhancing the properties of drugs, exosomes are also employed to carry small molecular drugs across the blood–brain barrier (BBB). Indeed, 98% of potent central nervous system drugs cannot cross the BBB and their conceptual efficacy shown in labs have not been successful in clinical trials66. Many Nano-formulations have been employed to solve the problems associated with the permeability of drugs across the BBB. However, other problems, such as nano-toxicity and rapid drug clearance by the mononuclear phagocyte system (MPS), have also been observed67. To compensate for these complications, polyethylene glycol (PEG) has been introduced to decrease MPS drug uptake. However, this resulted in reduced interaction between target cells, consequently decreasing drug distribution in the brain68, 69. In this case, exosomes, a natural product of the body’s own cells, can be tailored to cross the BBB, thus improving drug transport to the brain by decreasing MPS drug clearance (Fig. 5).
Figure 5.
Ability of exosome and its drug contents to cross the blood–brain barrier.
Meanwhile, research has been carried out encapsulating anticancer drugs such as paclitaxel and doxorubicin into exosomes, showing the potential of exosomes for brain delivery across the BBB and explaining their transport mechanisms, using zebrafish as an animal model55. In that study, exosomes were isolated from various cell lines, including gliobastoma astrocytoma U-87 MG, endothelial bEND.3, neuroectodermal tumor PFSK-1, and glioblastoma A-172, using Invitrogen® total exosome RNA and a protein isolation kit. Exosomes were loaded with rhodamine 123 and paclitaxel or doxorubicin through mixing and incubation. Experiments were then performed to characterize the isolated exosomes, the cellular uptake of the exosomes containing rhodamine 123, and the cytotoxicity of the delivered anticancer drugs within the exosomes of U-87 MG and bEND.3 cells. In vivo, the ability of exosomes to deliver drugs across the BBB was examined by injecting bEND.3-derived exosomes loaded with rhodamine 123 and doxorubicin, or paclitaxel, in zebrafish embryos. At the end of the experiments, brain tissue was examined for the presence of rhodamine 123 fluorescence. The results undeniably showed the drug׳s distribution in the brain region of the zebrafish embryos, suggesting the ability of exosomes to deliver drugs across the BBB. In subsequent experiments, a primary brain cancer model was developed using zebrafish and anticancer drugs loaded within and without exosomes, which were compared. The data showed significant therapeutic efficacy in the zebrafish brain model treated with exosomes loaded doxorubicin compared to doxorubicin alone. Overall, the results obtained from the study showed the potential of exosomes to deliver small molecule drugs across the BBB to treat both brain cancers and neurological disorders.
Since these exosomes were small and native to the animals, the researchers were able to avoid phagocytosis, fuse with the cell membrane, and bypass the engulfment by lysosomes. The fact that exosomes are a natural product of the body resulted in a low immune response. Most chemotherapeutic agents, such as doxorubicin, have low solubility and toxicity due to nonspecific tissue targeting and a short half-life, which leads to the poor efficacy of the drugs. Another study was performed using exosomes to deliver the antitumor agent doxorubicin into a mouse tumor tissue model both in vitro and in vivo54. In this study, exosomes were isolated and purified from pEGFP-C1-RVG-Lamp2b plasmid that contained immature mouse dendritic cells through centrifugation and ultrafiltration. The isolated exosomes were characterized using nanoparticle tracking analysis loaded with doxorubicin through electroporation. The ability of exosomes to deliver doxorubicin was tested by facilitating the fusion of encapsulated exosomes with a human breast cancer MDA-MB-231 cells. Fluorescence measurements and overlap clearly showed successful exosome delivery into the cells. The inhibition of in vitro cancer proliferation was measured by treating MDA-MB-231 with these exosomes and determining cell viability using cell counting kit-8 (CCK-8) assays. The results showed that cell inhibition by doxorubicin encapsulated exosomes was similar to inhibition using free doxorubicin, indicating the anticancer ability of doxorubicin encapsulated exosomes. The ability of exosomes to specifically deliver doxorubicin to tumor tissue and inhibit tumor growth in vivo was analyzed by injecting the fluorescent exosomes. The data showed an accumulation of doxorubicin containing exosomes specifically at the targeted organ and a major suppression of tumor growth. Collectively, the results confirmed the ability of exosomes to target and deliver doxorubicin efficiently.
2.2. Exosomes as drug delivery vehicles for proteins
In addition to delivering small molecules, exosomes are also used to deliver large molecules such as proteins. A recent study, for example, showed that exosomes loaded with the antioxidant protein catalase was successfully delivered across the BBB, resulting in an improved disease state in PD56. PD is associated with inflammation in the brain, microglia activation, and the secretion of reactive oxygen species (ROS)69, 70, 71. Patients with PD are shown to have lower levels of antioxidant enzymes like catalase and superoxide dismutase, which helps in oxidative stress and the inhibition of neurodegeneration72, 73, 74. Catalase is an enzyme that can deactivate one million free radicals per second per molecule in a single cycle of catalytic reaction56. Like many other drugs, the delivery of catalase across the BBB is a hurdle, but delivery of catalase to the brain using exosomes seems a promising option for PD therapy.
In this study, catalase was incorporated into exosomes using multiple methods: incubation at room temperature with or without the use of saponin permeabilization, freeze–thaw cycles, sonication, and extrusion procedures. Analysis by western blot showed that sonication and extrusion techniques resulted in the most efficient incorporation of catalase into exosomes. To confirm the delivery of catalase, exosomes were labeled with lipophilic fluorescent dye and incubated with a cell line derived from a pheochromocytoma of the rat adrenal medulla (PC12 cells). Confocal images confirmed the uptake of DIL (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate)-labeled exosomes in PC12 cells. The elimination of ROS in in vitro–activated macrophages was also observed when exosomal catalase was added, suggesting the successful exosomal delivery of catalase and the neutralization of ROS. Further visualization experiments were performed to understand the transportation of exosomes in inflamed brain tissues in a PD mouse model using confocal microscopy. Results showed the wide distribution of exosomes in the brain, indicating the ability of exosomes to target and deliver their cargo to the brain tissue. Overall, this study showed various methods used to isolate exosomes and the ability of exosomes to carry catalase across BBB in the treatment of PD.
2.3. Exosomes as drug delivery vehicles for nucleic acids
It has also been shown that exosomes naturally carry nucleic acids, such as DNA and RNA, to targeted cells, inducing genetic modifications in both biological and pathogenic processes. These features of exosomes became a major interest in treatment strategies involving genetic therapy, and studies using exosomes as drug delivery systems have been conducted to deliver therapeutic genetic materials that alter gene expression in certain diseases and improve genetic therapy.
2.3.1. Small interference RNA (siRNA)
In genetic therapy, siRNA is used to disrupt genes of interest. However, these siRNA have low stability and tend to degrade quickly in the systemic circulation. Exosomes, however, can act as therapeutic vehicles that help in the protection and delivery of siRNA to the targeted cells. However, determining the utility of exosomes to deliver exogenous siRNA still requires much more investigation and understanding. Several studies have been conducted to test the usefulness of exosomes as therapeutic vehicles to successfully deliver exogenous genetic material and their post-delivery functionality, and the first published study, conducted by Alvarez-Erviti et al.75, used exosomes to deliver siRNA to the brains of mice. After this first study, another group examined the delivery of siRNA to T cells and monocytes using human exosomes57. There is a significant shortage of therapeutic delivery vehicles for genetic materials that are safe, efficient, and target specific. The purpose of that study was to find a method to deliver vectors in gene therapy. Exosomes were used because they have the natural ability to deliver RNA from cell to cell and are non-immunogenic to the host. The purpose of the study was mainly to find a suitable method to deliver vectors in gene therapy
In the study, exosomes were isolated using differential centrifugation from various cell types, including the peripheral blood of healthy donors, lung cancer cells TB-177, and HeLa cells. Then, siRNA was introduced into exosomes using two different methods: chemical transfection and electroporation. The results showed that chemical transfection was unsuitable and the data were inconclusive. Analysis by western and northern blotting, confocal microscopy, and flow cytometry suggested the successful introduction of siRNA into exosomes. Plasma exosomes were transfected with Alexa Fluor 488-tagged siRNA against mitogen-activated protein kinase 1 (MAPK-1) using electrophoresis and then co-cultured with monocytes and lymphocytes isolated from the peripheral blood of healthy donors. siRNA florescence by exosomes was detected in the cytoplasm of the recipient cells. Flow cytometry confirmed the effective delivery of siRNA into peripheral blood mononuclear cells (PBMC). Additionally, immunoblotting was performed to evaluate the ability of delivered siRNA with exosome to have caused post-transcriptional gene-silencing in recipient cells. Results showed a decrease in MAPK-1 expression by exosomal siRNA, indicating down regulation of the specific gene and successful gene silencing. And the research clearly showed that exosomes can be used as delivery vehicles in genetic therapy57.
A similar in vitro study also showed the ability of exosomes to deliver siRNA to human cells. RAD51 (a eukaryote gene protein that assists in repair of DNA double strand breaks) has previously been shown as a potential target to repress the growth of abnormally proliferating cells in cancer76. In an in vitro study, exosomes-delivered siRNA against RAD51 was used, and its functionality after delivery to human cells was demonstrated. Further, exosomes were isolated from HeLa cells by ultracentrifugation and loaded with Alexa-flour 488-labeled siRNA using chemical treatment and then further co-cultured with recipient cells (HeLa and HT1080 cells). The successful delivery efficiency of siRNA by exosomes was confirmed by confocal microscopy and flow cytometry. The study also investigated whether delivered siRNA were efficient and whether they down-regulated the expression of RAD51 and RAD52. Experimental results from western blot analysis showed a considerable reduction in RAD51 and RAD52 protein levels, indicating successful down-regulation of this specific gene58. Largely, these results showed the potential of exosomes as therapeutic vehicles by keeping the cargoes they carry functional. However, exosomes derived from different cell types have slightly different compositions and functions. Exosomes derived from endothelial cells are associated with vascular inflammation and atherosclerosis. However, little is known about their ability to deliver exogenous contents. Given this context, another study has investigated the use of endothelial exosomes to deliver siRNA to endothelial cells59. Exosomes were isolated from endothelial cells by combining filtration and ultracentrifugation techniques, and their interactions with endothelial cells were evaluated. Exosomes were then loaded with siRNA using electroporation, and the functionality of endothelial exosomes to deliver siRNA to endothelial cells was tested. Exosomes loaded with siRNA were incubated with luciferase-expressing endothelial cells. siRNA was designed to silence a vector encoding for the production of luciferase (pGL2) in transiently-transfected endothelial cells with pGL2. The results showed significantly lower luciferase expression in endothelial exosomes carrying siRNA compared to the control groups. This indicates that endothelial exosomes can also deliver exogenous agents to cells in vitro and are functional at the targeted site.
2.3.2. miRNA
miRNA is short form of non-coding RNA and with non-protein nucleotides found in eukaryotic cells. These miRNAs bind to complementary sequences on targeted mRNA and control post-transcriptional gene expression77, 78. Exosomes are known to naturally carry miRNA and, hence, it is logical to use exosomes as a therapeutic vehicle to deliver miRNA to targeted cells. Ohno et al.60, for example, conducted a study using exosomes to deliver miRNA that targeted epidermal growth factor receptor (EGFR) in breast cancer cells. Various human tumors originated from epithelial showed an elevated level of EGFR expression, suggesting the possibility of using EGFR ligand as cancer drug targets79. There has also been evidence suggesting that miRNA, such as let-7a, functions as a tumor suppressor and inhibits the growth of cancer by reducing RAS and high-mobility group AT-hook protein (HMGA2) expression. In the study, epidermal growth factor (EGF) and EGFR-specific peptide (GE11), which binds specifically to EGFR, were incorporated onto the surfaces of exosomes that carried let-7a in order to deliver let-7a to EGFR-expressing cancer tissue60. To create GE11- and EPF-positive exosomes, a pDisplay vector encoding GE11 or EGF was transfected into HEK-293 cells. Exosomes were then isolated from the cloned cells by differential centrifugation. The expression of GE11 or EGF on the surface of the exosomes was examined using anti-hemagglutinin and western blot analysis. Fluorescence-activated cell sorting and anti-Myc-tag antibodies were also used to confirm the presence of GE11 or EGF on the surface of the exosomes. The ability of GE11- or EGF-positive exosomes to bind to EGFR in various breast cancer cell lines, including HCC70, HCC1954 and MCF-7, were then tested. Exosomes were labeled with PKH67 dye to show the engulfment of exosomes into the recipient cells. Various experiments were then conducted to verify that the exosomes were taken up by an EGFR-dependent mechanism. These results showed that the modified exosomes were taken into recipient cells. In addition, the researchers assessed whether GE11- or EGF-positive exosomes affected cell growth in vitro by performing cell proliferation assays. They found that GE11-positive exosomes did not stimulate EGFR signaling like EGF did and, thus, GE-11 positive exosomes might be more suitable than EGF-positive exosomes.
In in vivo experiments, lethal-7 gene (let-7a) was introduced into GE11 exosomes by the lipofection method. Then, GE11 exosomes containing let-7a were intravenously injected into tumor-bearing mice. Since previous studies showed let-7a inhibited tumor growth by reducing the expression level of RAS and HMGA2, the expressions of these genes were examined in injected tumor-bearing mice, using real-time reverse transcription-PCR analysis, immunoblotting, and immunostaining. The results showed that let-7a delivered by GE11-exosomes strongly inhibited the expression of HMGA2 in cancer cells. This indicated that exosomes do successfully deliver their cargo to the target cells, showing promising characteristics for drug delivery60.
2.4. Major advantages and disadvantages
Many of the newer drug candidates such as proteins and nucleic acids are highly unstable inside in vivo environment posing significant challenges to successful therapeutic outcomes. Given the problems associated with many of the current nanoparticulate delivery systems, exosomes as a mimic of “nature's delivery systems” allows for delivery of these biological molecules. Because of the inherent small size and nature׳s own cellular product these vehicles can avoid phagocytosis or degradation by macrophages and can also circulate for extended periods of time within the body. Unlike typical nanoparticulate systems such as liposomes or polymeric nanoparticles, exosomes can potentially avoid the endosomal pathway and lysosomal degradation, and also deliver cargoes directly into the cytoplasm. By virtue of avoiding the endosomal pathway transfection efficiency for molecules such as siRNA can be enhanced80. They are naturally stable and have inherent targeting properties depending on the composition of the exosomes. One of the significant advantages of these drug delivery vehicles also includes their ability to cross BBB2, 81. Inadequate understanding of exosomes nature and the role in overall health and disease condition make it complicated to predict long-term safety and therapeutic effect. In vivo trafficking, biological fate and their impact on targeted organs need to be understood more thoroughly. There exist many challenges in a clear understanding of exosomes with regard to therapeutic cargo loading and assembly for drug delivery.
Currently, there is no distinct optimal purification technique for isolation of exosomes with high purity82. The isolation methods yield low quantities of exosomes and their large scale production for clinical studies and post drug approval is expensive83. It is highly likely that future clinical use demands hybrid type of exosome designs84, and when combined with therapeutic cargos they may show undesirable effects. If such systems are designed their clinical efficacy and safety parameters needs to be thoroughly characterized. To functionalize exosomes ligands are attached to the surface through chemical conjugation and these active targeting molecular combinational product and methods needs to be investigated. Integrative studies using therapeutic cargo and functional exosomes or hybrid exosomes mimetic are becoming very critical. Even though extensive biology is already known, exosomes comprise heterogeneous components and may show immunogenicity (immunostimulatory or immunospressive) effects based on nature of parental donor cells. Exosomes involvement in tumor progression or help membrane antigens release that help in tumor growth is a huge concern. Exosomes that carry caspase-3 may also inhibit cell death by apoptosis or enhance tumor cell survival by preventing chemotherapeutics drug accumulation51. One of the approaches is to design artificial exosomes or exosome mimetics that has the ability to overcome the potential disadvantages such as unwanted immune reactions50.
3. Conclusions
Exosomes provide an enormous promise and a fresh therapeutic area for delivery of different synthetic and biological molecules in cellular therapy. Exosomes as drug delivery vehicles offer a major advantage as there is no unwanted accumulation or homing of exosomes in the liver and (or) avoid the first pass metabolic effect, before reaching target sites. The well characterized exosomes along with long-term safety and natural ability to carry intercellular nucleic acids and therapeutic molecules, across membranes difficult to cross such as BBB, would have major practical significance. Before these drug delivery systems become a therapeutic reality, component characterization and immune reactions need to be clearly understood.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Lai R.C., Yeo R.W.Y., Tan K.H., Lim S.K. Exosomes for drug delivery—a novel application for the mesenchymal stem cell. Biotechnol Adv. 2013;31:543–551. doi: 10.1016/j.biotechadv.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Kooijmans S.A., Vader P., van Dommelen S.M., van Solinge W.W., Schiffelers R.M. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiller M., Bekeredjian-Ding I., Heyder P., Blank N., Ho A.D., Lorenz H.M. Autoantigens are translocated into small apoptotic bodies during early stages of apoptosis. Cell Death Differ. 2008;15:183–191. doi: 10.1038/sj.cdd.4402239. [DOI] [PubMed] [Google Scholar]
- 4.Gregory C.D., Pound J.D. Microenvironmental influences of apoptosis in vivo and in vitro. Apoptosis. 2010;15:1029–1049. doi: 10.1007/s10495-010-0485-9. [DOI] [PubMed] [Google Scholar]
- 5.Johnstone R.M. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis. 2005;34:214–219. doi: 10.1016/j.bcmd.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Simons M., Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Qin J., Xu Q. Functions and applications of exosomes. Acta Pol Pharm. 2014;71:537–543. [PubMed] [Google Scholar]
- 8.Urbanelli L., Magini A., Buratta S., Brozzi A., Sagini K., Polchi A. Signaling pathways in exosomes biogenesis, secretion and fate. Genes. 2013;4:152–170. doi: 10.3390/genes4020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katzmann D.J., Odorizzi G., Emr S.D. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 10.Février B., Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Raiborg C., Rusten T.E., Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 12.Colombo M., Moita C., van Niel G., Kowal J., Vigneron J., Benaroch P. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 13.Tamai K., Tanaka N., Nakano T., Kakazu E., Kondo Y., Inoue J. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399:384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 14.Gross J.C., Chaudhary V., Bartscherer K., Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino D., Kirkbride K.C., Costello K., Clark E.S., Sinha S., Grega-Larson N. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrami L., Brandi L., Moayeri M., Brown M.J., Krantz B.A., Leppla S.H. Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin. Cell Rep. 2013;5:986–996. doi: 10.1016/j.celrep.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Heijnen H.F.G., Schiel A.E., Fijnheer R., Geuze H.J., Sixma J.J. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and α-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 19.van Niel G., Charrin S., Simoes S., Romao M., Rochin L., Saftig P. The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denzer K., Kleijmeer M.J., Heijnen H., Stoorvogel W., Geuze H.J. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci. 2000;113:3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 21.Lamparski H.G., Metha-Damani A., Yao J.-Y., Patel S., Hsu D.-H., Ruegg C. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 22.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 23.Simpson R.J., Mathivanan S. Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. J Proteom Bioinform. 2012;5:2. [Google Scholar]
- 24.Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Batrakova E.V., Kim M.S. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Théry C., Duban L., Segura E., Véron P., Lantz O., Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 27.Kalani A., Tyagi A., Tyagi N. Exosomes: mediators of neurodegeneration, neuroprotection and therapeutics. Mol Neurobiol. 2014;49:590–600. doi: 10.1007/s12035-013-8544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subra C., Laulagnier K., Perret B., Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Parolini I., Federici C., Raggi C., Lugini L., Palleschi S., De Milito A. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccin A., Murphy W.G., Smith O.P. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21:157–171. doi: 10.1016/j.blre.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone R.M., Adam M., Hammond J., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 32.Johnstone R.M., Mathew A., Mason A.B., Teng K. Exosome formation during maturation of mammalianand avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 33.Morelli A.E., Larregina A.T., Shufesky W.J., Sullivan M.L.G., Stolz D.B., Papworth G.D. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 34.De Toro J., Herschlik L., Waldner C., Mongini C. Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol. 2015;6:203. doi: 10.3389/fimmu.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 36.Skog J., Würdinger T., van Rijn S., Meijer D.H., Gainche L., Curry W.T. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laulagnier K., Grand D., Dujardin A., Hamdi S., Vincent-Schneider H., Lankar D. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572:11–14. doi: 10.1016/j.febslet.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 38.Aharon A., Microparticles Brenner B. thrombosis and cancer. Best Pract Res Clin Haematol. 2009;22:61–69. doi: 10.1016/j.beha.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Kucharzewska P., Christianson H.C., Welch J.E., Svensson K.J., Fredlund E., Ringnér M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webber J., Steadman R., Mason M.D., Tabi Z., Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 41.Rana S., Malinowska K., Zöller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghidoni R., Benussi L., Binetti G. Exosomes: the Trojan horses of neurodegeneration. Med Hypotheses. 2008;70:1226–1227. doi: 10.1016/j.mehy.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Nilsson J., Skog J., Nordstrand A., Baranov V., Mincheva-Nilsson L., Breakefield X.O. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corcoran C., Friel A.M., Duffy M.J., Crown J., O׳Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin Chem. 2011;57:18–32. doi: 10.1373/clinchem.2010.150730. [DOI] [PubMed] [Google Scholar]
- 45.Li J.H., Sherman-Baust C.A., Tsai-Turton M., Bristow R.E., Roden R.B., Morin P.J. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244. doi: 10.1186/1471-2407-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sercombe L., Veerati T., Moheimani F., Wu S.Y., Sood A.K., Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agrawal U., Sharma R., Gupta M., Vyas S.P. Is nanotechnology a boon for oral drug delivery? Drug Discov Today. 2014;19:1530–1546. doi: 10.1016/j.drudis.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 48.Raemdonck K., Braeckmans K., Demeester J., De Smedt S.C. Merging the best of both worlds: hybrid lipid-enveloped matrix nanocomposites in drug delivery. Chem Soc Rev. 2014;43:444–472. doi: 10.1039/c3cs60299k. [DOI] [PubMed] [Google Scholar]
- 49.Li C., Zhang J., Zu Y.J., Nie S.F., Cao J., Wang Q. Biocompatible and biodegradable nanoparticles for enhancement of anti-cancer activities of phytochemicals. Chin J Nat Med. 2015;13:641–652. doi: 10.1016/S1875-5364(15)30061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aryani A., Denecke B. Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol Neurobiol. 2016;53:818–834. doi: 10.1007/s12035-014-9054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turturici G., Tinnirello R., Sconzo G., Geraci F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: advantages and disadvantages. Am J Physiol Cell Physiol. 2014;306:C621–C633. doi: 10.1152/ajpcell.00228.2013. [DOI] [PubMed] [Google Scholar]
- 52.Rani S., Ritter T. The exosome-a naturally secreted nanoparticle and its application to wound healing. Adv Mater. 2015 doi: 10.1002/adma.201504009. Dec 17. Available from: 10.1002/adma.201504009. [DOI] [PubMed] [Google Scholar]
- 53.Sun D.M., Zhuang X.Y., Xiang X.Y., Liu Y.L., Zhang S.Y., Liu C.R. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tian Y.H., Li S.P., Song J., Ji T.J., Zhu M.T., Anderson G.J. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 55.Yang T.Z., Martin P., Fogarty B., Brown A., Schurman K., Phipps R. Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio Rerio. Pharm Res. 2015;32:2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haney M.J., Klyachko N.L., Zhao Y.L., Gupta R., Plotnikova E.G., He Z.J. Exosomes as drug delivery vehicles for Parkinson׳s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wahlgren J., Karlson T.D.L., Brisslert M., Sani F.V., Telemo E., Sunnerhagen P. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shtam T.A., Kovalev R.A., Varfolomeeva E.Y., Makarov E.M., Kil Y.V., Filatov M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:1186–1196. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Banizs A.B., Huang T., Dryden K., Berr S.S., Stone J.R., Nakamoto R.K. In vitro evaluation of endothelial exosomes as carriers for small interfering ribonucleic acid delivery. Int J Nanomed. 2014;9:4223–4230. doi: 10.2147/IJN.S64267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravindran J., Prasad S., Aggarwal B.B. Curcumin and cancer cells: how many ways can curry kill tumor cells selectively? AAPS J. 2009;11:495–510. doi: 10.1208/s12248-009-9128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anand P., Sundaram C., Jhurani S., Kunnumakkara A.B., Aggarwal B.B. Curcumin and cancer: an “old-age” disease with an “age-old” solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Dhillon N., Aggarwal B.B., Newman R.A., Wolff R.A., Kunnumakkara A.B., Abbruzzese J.L. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 65.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: problemsand promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 66.Pardridge W.M. Drug transport across the blood–brain barrier. J Cereb Blood Flow Metab. 2012;32:1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peng Q., Zhang S., Yang Q., Zhang T., Wei X.Q., Jiang L. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;34:8521–8530. doi: 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida K., Burton G.F., McKinney J.S., Young H., Ellis E.F. Brain and tissue distribution of polyethylene glycol-conjugated superoxide dismutase in rats. Stroke. 1992;23:865–869. doi: 10.1161/01.str.23.6.865. [DOI] [PubMed] [Google Scholar]
- 69.Veronese F.M., Caliceti P., Schiavon O., Sergi M. Polyethylene glycol-superoxide dismutase, a conjugate in search of exploitation. Adv Drug Deliv Rev. 2002;54:587–606. doi: 10.1016/s0169-409x(02)00029-7. [DOI] [PubMed] [Google Scholar]
- 70.Ebadi M., Srinivasan S.K., Baxi M.D. Oxidative stress and antioxidant therapy in Parkinson׳s disease. Prog Neurobiol. 1996;48:1–19. doi: 10.1016/0301-0082(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 71.Wu D.C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson׳s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ambani L.M., Van Woert M.H., Murphy S. Brain peroxidase and catalase in Parkinson disease. Arch Neurol. 1975;32:114–118. doi: 10.1001/archneur.1975.00490440064010. [DOI] [PubMed] [Google Scholar]
- 73.Riederer P., Sofic E., Rausch W.D., Schmidt B., Reynolds G.P., Jellinger K. Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 74.Abraham S., Soundararajan C.C., Vivekanandhan S., Behari M. Erythrocyte antioxidant enzymes in Parkinson’s disease. Indian J Med Res. 2005;121:111–115. [PubMed] [Google Scholar]
- 75.Alvarez-Erviti L., Seow Y., Yin H.F., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 76.Shtam T.A., Varfolomeeva E.Y., Semenova E.V., Filatov M.V. Role of human RAD51 recombinase in the cycle checkpoint and survival of a cell. Cell Tissue Biol. 2008;2:463–467. [PubMed] [Google Scholar]
- 77.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 78.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woodburn J.R. The epidermal growth factor receptor and its inhibition in cancer therapy. Pharmacol Ther. 1999;82:241–250. doi: 10.1016/s0163-7258(98)00045-x. [DOI] [PubMed] [Google Scholar]
- 80.Mehrotra N., Tripathi R.M. Short interfering RNA therapeutics: nanocarriers, prospects and limitations. IET Nanobiotechnol. 2015;9:386–395. doi: 10.1049/iet-nbt.2015.0018. [DOI] [PubMed] [Google Scholar]
- 81.Tran T.-H., Mattheolabakis G., Aldawsari H., Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160:46–58. doi: 10.1016/j.clim.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Petersen K.E., Manangon E., Hood J.L., Wickline S.A., Fernandez D.P., Johnson W.P. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Anal Bioanal Chem. 2014;406:7855–7866. doi: 10.1007/s00216-014-8040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taylor D.D., Shah S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 2015;87:3–10. doi: 10.1016/j.ymeth.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 84.Rana S., Yue S.J., Stadel D., Zöller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44:1574–1584. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]