Abstract
Biodegradable polyamines have long been studied as potential recombinant viral gene vectors. Spermine (SPE) is an endogenous tetra-amine with excellent biocompatibility yet poor gene condensation capacity. We have previously synthesized a polyspermine based on SPE and poly(ethylene glycol) (PEG) diacrylate (SPE-alt-PEG) for enhanced transfection performance, but the synthesized SPE-alt-PEG still lacked specificity towards cancer cells. In this study, folic acid (FA) was incorporated into SPE-alt-PEG to fabricate a targeted gene delivery vector (FA-SPE-PEG) via an acylation reaction. FA-SPE-PEG exhibited mild cytotoxicity in both cancer cells and normal cells. FA-SPE-PEG possessed higher transfection efficiency than PEI 25 K and Lipofectamine® 2000 in two tested cancer cell lines at functional weight ratios, and its superiority over untargeted SPE-alt-PEG was prominent in cells with overexpressed folate receptors (FRs). Moreover, in vivo delivery of green fluorescent protein (GFP) with FA-SPE-PEG resulted in highest fluorescent signal intensity of all investigated groups. FA-SPE-PEG showed remarkably enhanced specificity towards cancer cells both in vivo and in vitro due to the interaction between FA and FRs. Taken together, FA-SPE-PEG was demonstrated to be a prospective targeted gene delivery vector with high transfection capacity and excellent biocompatibility.
Keywords: Gene therapy, Targeted gene delivery, Lung cancer, Non-viral gene vector, Folic acid, Folate receptor, Polyspermine, Biocompatibility
Graphic abstract
FA-SPE-PEG was a folate receptor (FR)–targeted gene vector based on biodegradable ester bond. FA-SPE-PEG showed mild cytotoxicity towards multiple cell lines, and it manifested excellent transfection efficiency in vitro, especially in FR-overexpressed cells. In vivo study indicated that FA-SPE-PEG could efficiently deliver gene to target sites.
1. Introduction
Gene therapy has been investigated for decades as a therapeutic approach for the treatment of various diseases related with genetic disorders, including cancers1, 2, 3, 4. The success of gene therapy relies on the accurate delivery of the therapeutic gene to tumor sites. The success of this approach necessitates of an efficient and safe gene vector5, 6. Recombinant viral vectors have been widely used for gene delivery due to the inherent ability and relatively high transfection efficiency. However, the inability to deliver large genes and the immunogenic properties of these vectors remain to be addressed7, 8, 9. In contrast, non-viral vectors have large delivery capacity, low immunogenicity, and flexible structures, making them excellent alternatives to viral vectors2, 4, 10, 11.
Cationic polymers manifest excellent ability to condense and protect genes via electrostatic interactions. Polyethylenimine (PEI) has become a gold standard of non-viral vectors because of its high transfection efficiency in various cell lines12, 13. However, because of its high molecular weight, PEI shows severe cytotoxicity and poor biocompatibility, thereby limiting its clinical application14, 15. To resolve these obstacles, other biocompatible polymers, especially endogenous polyamines, have been developed as non-viral vectors for the safe and efficient delivery of therapeutic genes16.
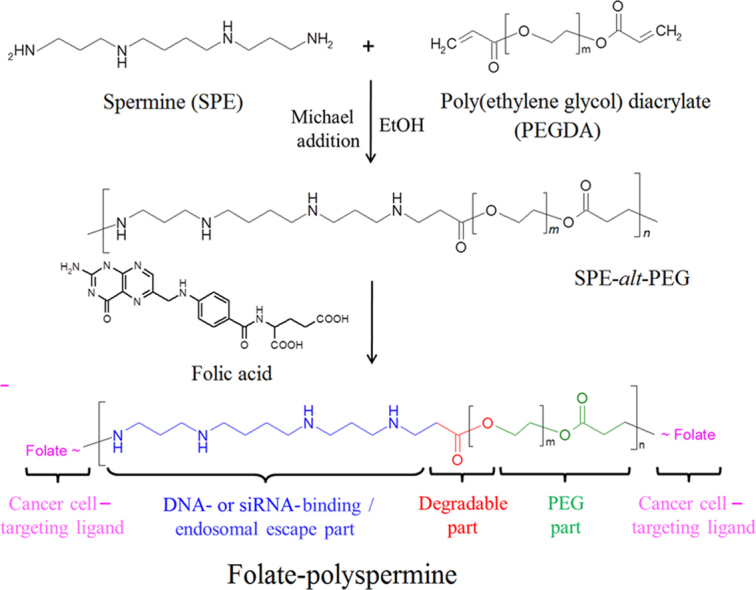
Spermine (SPE) is an endogenous tetra-amine participating in eukaryotic cell metabolism17. Despite its prominent biocompatibility, SPE exhibits poor transfection efficiency. The transfection performance of SPE could be modified by crosslinking its primary and secondary amine groups with acrylate or hydroxyl groups, resulting in biodegradable polyspermines18, 19, 20, 21. In our previous study, we prepared a polyspermine based on SPE and poly(ethylene glycol) (PEG) diacrylate (SPE-alt-PEG) for the aerosol gene delivery20. SPE-alt-PEG was shown to be a safe and efficient gene vector for the lung cancer therapy, but a facilitated specificity would be necessary for a better treatment outcome. To enhance delivery specificity, it is essential to introduce a targeting ligand onto SPE-alt-PEG. Folate receptor (FR) is abundantly expressed on the membrane of epithelial carcinomas, yet FR expression on normal cells is rather limited22, 23. The interaction between folic acid (FA) and FRs could not only lead to a better specificity, but also reinforce the internalization rate. Therefore, we chose FA as a targeting ligand for gene delivery and fabricated folate-conjugated SPE-alt-PEG (FA-SPE-PEG).
FA-SPE-PEG was synthesized by an acylation reaction between the carboxyl groups of FA and the amino groups of SPE-alt-PEG. The physicochemical properties of FA-SPE-PEG and its complexes with DNA were investigated, and the cytotoxicity of FA-SPE-PEG was compared with SPE and PEI 25K in cancer cells as well as normal cells. In addition, in vitro transfection efficiency as well as targeting ability of FA-SPE-PEG was investigated in cells with or without overexpressed FRs. Moreover, the in vivo transfection efficiency was elucidated via a green fluorescent protein (GFP) expression assay in BALB/c mice. FA-SPE-PEG is an ester-based polyamine which could be degraded by both acidic tumor microenvironment and endogenous esterase. This biodegradability led to mild cytotoxicity, low immunogenicity, and modified biocompatibility7, 24. The specific interaction between FA and FRs endowed FA-SPE-PEG with excellent targeting capacity towards cancer cells, further attenuating toxicity to normal cells. Altogether, it is evident to consider FA-SPE-PEG as an efficient and safe vector for targeted gene delivery in tumor therapy.
2. Materials and methods
2.1. Materials
PEG diacrylate, SPE, FA, anhydrous ethanol, and branched PEI 25K were purchased from Sigma (St. Louis, MO, USA). The pGL3 vector (5.3 kb) with a luciferase gene driven by an SV40 promoter and enhancer, and the Cell Titer 96® Aqueous One Solution Reagent were purchased from Promega (Madison, WI, USA). pEGFP-N2 (4.7 kb) encoding a GFP and driven by an immediate early promoter of CMV was purchased from Clontech Laboratories (Palo Alto, CA, USA). The plasmids were propagated in Escherichia coli, extracted by the alkaline lysis technique, and purified using a QIAGEN kit (Chatsworth, CA, USA). All other chemicals were of reagent grade.
2.2. Preparation and characterization of FA-SPE-PEG and FA-SPE-PEG/DNA complexes
FA-SPE-PEG was synthesized by two separated steps (Fig. 1A). Firstly, SPE-alt-PEG was synthesized by a previously reported Michael addition reaction. The preparation details were demonstrated in our former study20.
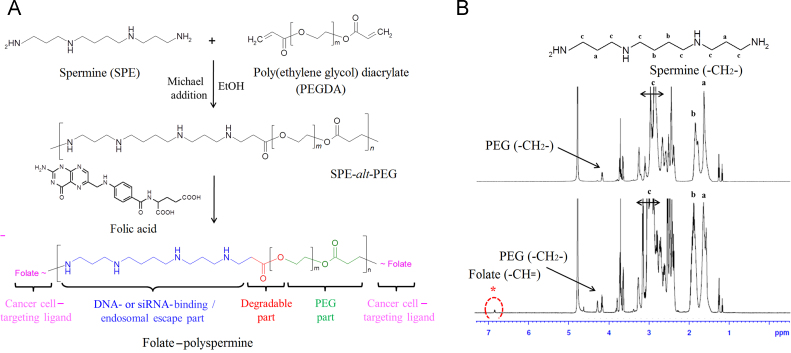
Figure 1.
Synthesis and the composition confirmation of FA-SPE-PEG. (A) Proposed reaction scheme for FA-SPE-PEG. (B) Representative 1H NMR spectrum of FA-SPE-PEG in D2O: (a) δ 1.6 and (b) δ 1.8 ppm (–CH2–, SPE); (c) δ 2.7–3.2 ppm (–NH–CH2–, SPE); δ 4.1–4.3 ppm (–CH2–, PEG); (*) δ 7.8 ppm (methylene, FA).
FA-SPE-PEG was prepared by coupling FA onto SPE-alt-PEG via DCC/NHS (dicyclohexylcarbodiimide/N-hydroxysuccinimide) chemistry according to a previous study25. Briefly, 0.16 mmol FA was dissolved in 3.0 mL anhydrous DMSO, and then activated by DCC and NHS. The molar ratio of FA, DCC, and NHS was 1:2:2. The mixture was stirred at room temperature for 18 h, and then centrifuged to remove the byproduct, dicyclohexyl urea. SPE-alt-PEG was then added to the reaction mixture of activated FA, unreacted DCC and NHS. The reaction was continued at room temperature for 6 h under nitrogen atmosphere. The crude product was first dialyzed (MWCO=1000 Da) against DMSO for 3 days to remove uncoupled FA, and thereafter against distilled water for 2 days. The synthesized FA-SPE-PEG was lyophilized and stored at –20 °C. The composition of FA-SPE-PEG was confirmed by 1H NMR.
All FA-SPE-PEG/DNA complexes were freshly prepared before use. The DNA condensation and protection abilities of FA-SPE-PEG were confirmed by electrophoresis according to Jiang et al.26. All the complexes were prepared by gently mixing pDNA (pGL3-control, 0.1 μg) with equal volume of FA-SPE-PEG solution at different N/P ratios, and the complexes were incubated at room temperature for 30 min. To evaluate the DNA protection and release abilities of FA-SPE-PEG, 1 μL of DNase I (2 U) or phosphate-buffered saline (PBS) in DNase/Mg2+ digestion buffer (50 mmol/L Tris–HCl, pH 7.6 and 10 mmol/L MgCl2) was added to 4 μL complex solution (0.1 μg pDNA with 1.0 μg FA-SPE-PEG) or to naked pDNA, the mixture was then incubated at 37 °C with shaking at 100 rpm for 30 min. To inactivate DNase, all samples were treated with 4 μL EDTA (250 mmol/L) for 10 min and mixed with 1% sodium dodecyl sulfate (SDS, pH 7.2) to a final volume of 15 μL. The samples were further incubated for 2 h, and the electrophoresis was performed in 1% agarose gel with Tris-acetate-EDTA running buffer at 50 V for 1 h.
The morphologies of the FA-SPE-PEG/DNA complexes were observed by EF-TEM (LIBRA 120, Carl Zeiss, Germany). The average particle size and surface charge of the complexes were determined by dynamic light scattering (DLS) spectrophotometer (ELS8000, Otsuka Electronics, Osaka, Japan) at 90° and 20°, respectively.
2.3. Cell culture, cell viability and transfection efficiency in vitro
A549 (human lung carcinoma, ATCC, Manassas, VA, USA) cells, KB (human epidermal carcinoma, Korean Cell Line Bank, Seoul, South Korea) cells, KB cells with overexpressed folate receptors (KB/F cells), and 16HBE (immortalized human bronchial epithelial) cells were incubated in RPMI 1640 (Gibco BRL, Paris, France), supplemented with 10% fetal bovine serum (HyClone, Logan, UT), streptomycin at 100 μg/mL, and penicillin at 100 U/mL. All the cells were incubated at 37 °C in humidified 5% CO2 atmosphere.
The in vitro cytotoxicity FA-SPE-PEG was evaluated by Cell Titer 96® Aqueous One Solution Proliferation Assay (MTS assay, Promega, Madison, WI, USA) according to a previously reported method26. Briefly, A549 and 16HBE cells were seeded into 96-well plates at an initial density of 1×104 cells/well in 0.2 mL growth media. After incubation for 18 h, the growth media were replaced with serum-free media containing FA-SPE-PEG of different concentrations. Cells treated without FA-SPE-PEG were used as a control group, and cells treated with PEI 25 K and SPE were used as parallel control groups. After incubation for another 24 or 72 h, the media was changed for growth media containing 20 μL Cell Titer 96® Aqueous One Solution Reagent. The cells were further incubated for 3 h, and the absorbance at 570 nm was measured with an ELISA plate reader (GLR 1000, Genelabs Diagnostics, Singapore) to represent the viability of the cells.
The in vitro transfection efficiency and the targeting capacity of FA-SPE-PEG were evaluated by luciferase assay (Promega, Madison, WI, USA). Briefly, A549 and KB/F cells were seeded into 24-well plates at an initial density of 1.5×105 cells/well. After incubation for 18 h, the media were changed for serum-free growth media containing FA-SPE-PEG/pGL3 (1 μg pGL3) or SPE/pGL3 complexes at different weight ratios. Cells treated with naked pGL3, SPE-alt-PEG/pGL3, PEI 25 K/pGL3 and Lipofectamine® 2000/pGL3 were used as control groups. After transfection for 4 h, the media were replaced with fresh growth media containing 10% serum and the cells were incubated for 24 h at 37 °C. The luciferase assay was conducted according to the manufacturer׳s protocol.
To investigate the buffering capacity of FA-SPE-PEG, cells were treated with 200 nmol/L bafilomycin A1 for 10 min before the assay. Then the transfection efficiency was evaluated as described above. To assess the targeting ability of FA-SPE-PEG, luciferase assay described above was carried out in KB cells and KB/F cells.
The superiority of FA-SPE-PEG as an efficient gene vector was further evaluated by GFP expression assay. Briefly, KB/F cells were seeded into confocal dishes at a primary density of 1×105 cells/dish in 1.5 mL growth media. After incubation for 18 h, cells were treated with serum-free media containing FA-SPE-PEG/GFP (1 μg GFP) or PEI 25 K/GFP complexes at functional weight ratio. Cells treated with only serum-free media were set as a control. After incubation for 4 h, the media were changed with fresh growth media containing 10% serum and the cells were incubated for 24 h at 37 °C. After washing with PBS for 3 times, cells were fixed with 4% formaldehyde for 20 min and dyed with DAPI for another 20 min. Thereafter, cells were washed with PBS for 3 times and rinsed with 1 mL PBS-glycerin buffer (50%, v/v). The synthesized samples were directly observed using a confocal laser scanning microscope (CLSM, MRC-1024, Bio-Rad, UK). Composite images were made by overlapping the images of the individual channels.
2.4. Transfection efficiency study in vivo
All animal experiments were conducted under protocols approved by the Guidelines for the Care and Use of Laboratory Animals of China Pharmaceutical University. Six-week-old female BALB/c mice (Breeding and Research Center, Seoul National University, Korea) were kept with temperature and relative humidity of 23±2 °C and 50±20%, respectively, in a 12 h light–dark cycle. The mice were placed in a nose-only exposure chamber for the aerosol delivery of FA-SPE-PEG/GFP complexes. Mice treated with PBS were used as a blank control, and mice treated with naked GFP or PEI 25 K/GFP complexes were used as parallel control groups. All mice were sacrificed 48 h after aerosol delivery, and the lungs were collected for GFP analysis. The lungs were fixed in 4% paraformaldehyde at room temperature and embedded in Tissue-Tek OCT (Sakura, Torrance, CA, USA). Lung cryosections (10 μm) were prepared by a microtome (Leica, Nussloch, Germany) and the GFP signal was detected by a Zeiss LSM510 confocal microscope (Carl Zeiss, USA).
2.5. Statistical analysis
All data were presented as mean values±standard deviation. The unpaired t-test was used to determine the statistical significance of differences among groups. P<0.05 was considered as significant.
3. Results and discussion
We described the synthesis of SPE-alt-PEG in our previous study20. SPE-alt-PEG is a hydrophilic ester-based polyspermine with a molecular weight of 5.76 kDa and a polydispersity index of 1.13. The chemical composition of the product was confirmed by 1H NMR. In the present study, we introduced a targeting ligand, FA, into SPE-alt-PEG, for a better targeting capacity towards cancer cells overexpressing folate receptors.
3.1. Preparation and characterization of FA-SPE-PEG and FA-SPE-PEG/DNA complexes
FA-SPE-PEG was synthesized via the amide linkage between the carboxyl group of FA and the amino groups of SPE-alt-PEG. Consistent with our former study, 1H NMR analysis showed proton peaks of SPE (–CH2–) at δ 1.6–1.8 ppm and the peaks of PEG diacrylate (–CH2–) at δ 4.1–4.3 ppm, indicating the successful synthesis of SPE-alt-PEG (Fig. 1B). In addition, proton peaks at δ 7.8–7.9 ppm in the 1H NMR spectra belonged to the methylene groups of FA, further confirming the identity of FA-SPE-PEG. The content of coupled FA was 4.3% (mol/mol), calculated by assigning the protons of methylene groups at δ 4.6 ppm.
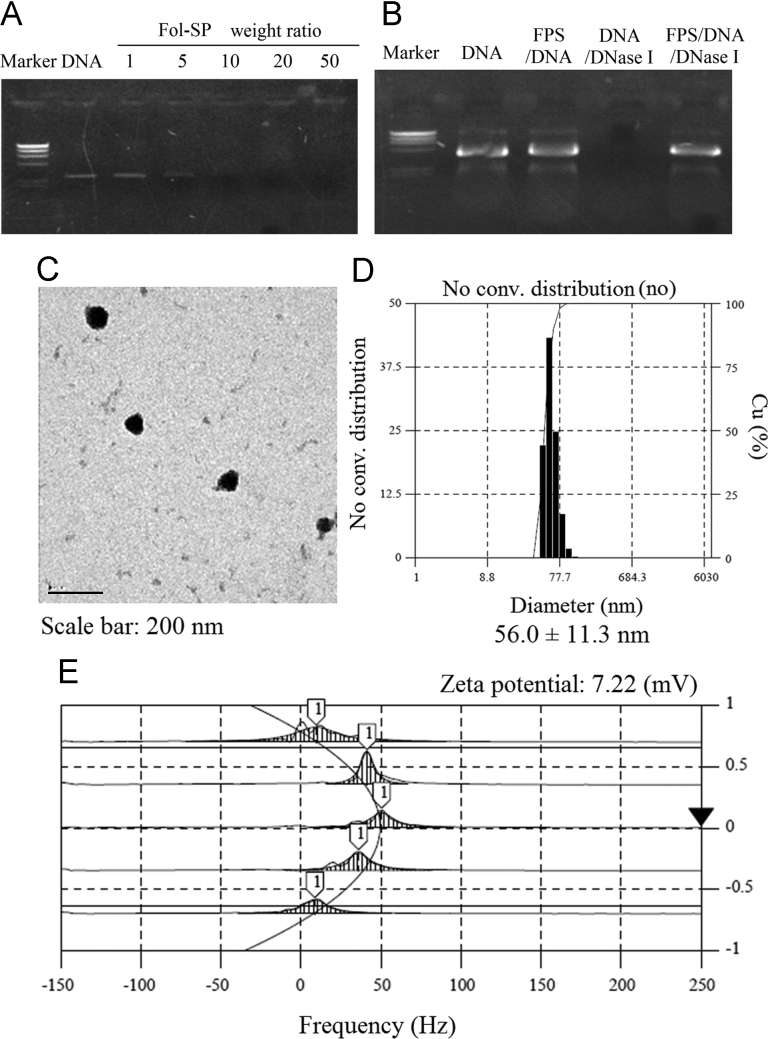
One major prerequisite for an efficient gene vector lies in a high gene affinity. The gene condensation ability of FA-SPE-PEG was assessed by agarose gel electrophoresis. Fig. 2A shows that FA-SPE-PEG has clear condensation ability towards DNA at a weight ratio of 5, and that complete retardation of DNA migration was observed when the weight ratio reached 10. Since efficient gene delivery also calls for protection of DNA from degradation, we evaluated whether FA-SPE-PEG could protect DNA against nucleases. As shown in Fig. 2B, DNA condensation by FA-SPE-PEG was effectively protected upon the addition of nuclease compared to naked DNA. SPE possesses poor gene affinity despite high content of primary and secondary amine groups, which is reported due to its relatively low molecular weight. For better gene affinity and targeting ability, we crosslinked SPE with PEG and FA. Both the higher molecular weight and abundant amine groups of FA-SPE-PEG contributed to its facilitated gene affinity and protective ability17, 26, 27. Moreover, the ester bonds in FA-SPE-PEG could be easily degraded by esterase, which further guaranteed its potential as a valid vector for the efficient and safe delivery of gene.
Figure 2.
Characterization of FA-SPE-PEG/pDNA complexes. (A) Agarose gel electrophoresis of FA-SPE-PEG/pDNA (pGL3-control) complexes at various weight ratios. The complexes were loaded onto 1% agarose gel with EtBr (0.1 μg/mL) and run with Tris–acetate EDTA (TAE) buffer at 100 V for 40 min. (B) Protection and release assay of DNA. Electrophoresis was performed in 1% agarose gel with TAE running buffer for 1 h at 50 V. (C) EF-TEM images of FA-SPE-PEG/pDNA complexes at functional weight ratio (scale bar: 200 nm). (D) Particle size distribution assay. (E) Particle surface charges of FA-SPE-PEG/pDNA at functional weight ratio.
It is reported that the enhanced permeability and retention (EPR) effect is a major mechanism involved in the targeting delivery of nanoparticles, which makes physical properties such as particle size and surface potential important factors in the assessment of gene vectors19, 26. As shown in Fig. 2C and D, FA-SPE-PEG/DNA complexes were uniform spheres with an average diameter of 56.0±11.3 nm as determined by energy-filtered (EF)-TEM-TEM. FA-SPE-PEG/DNA complexes were slightly positive-charged according to the DLS results in Fig. 2E. Positive surface potential contributed to higher cellular uptake through electrostatic interactions between nanoparticles and negatively charged cell membrane. Furthermore, the relatively low surface charges (compared to other commonly used cationic vectors) also led to better stability and less cytotoxicity, both of which further validate the safety of FA-SPE-PEG as a gene vector28.
3.2. Cell viability and transfection efficiency studies in vitro
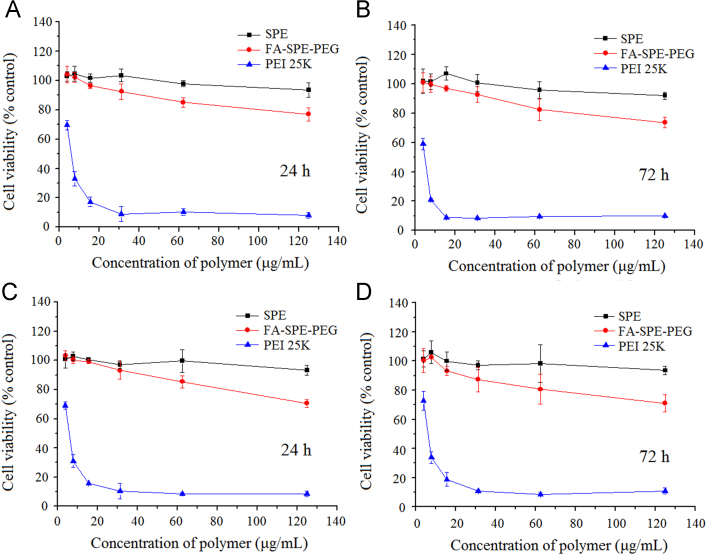
Safety issues of non-viral gene vectors have remained a significant challenge to be addressed in clinical application. High positive charges not only brought about excellent capacity to condense and protect genes, but also led to severe cytotoxicity29, 30, 31. We have previously showed SPE-alt-PEG to be a biocompatible gene vector with low cytotoxicity at functional concentrations. In this study, we investigated whether FA-SPE-PEG was as biocompatible as SPE-alt-PEG. Results of MTS assay indicated that FA-SPE-PEG exhibited similar cytotoxicity to that of SPE, both of which were remarkably lower than the cytotoxicity of PEI 25 K. As shown in Fig. 3, cells treated with FA-SPE-PEG manifested relatively high viability even at the highest administrated concentration (125 μg/mL) after incubation for different periods of time (viability of A549 cells: 76.95% for 24 h and 73.56% for 72 h; viability of 16HBE cells: 70.62% for 24 h and 70.97% for 72 h). On the contrary, cells treated with PEI 25 K showed drastically decreased viability even at low concentrations. The mild cytotoxicity of FA-SPE-PEG was mainly assigned to the endogenous SPE and the biodegradable ester bond. Furthermore, PEG also partially shielded the excessive positive charges, avoiding the most severe cellular damage17, 27. These results strongly suggest that FA-SPE-PEG can serve as a biocompatible gene vector.
Figure 3.
Cytotoxicity of FA-SPE-PEG at various concentrations in different cell lines: (A) 24 h cytotoxicity in A549 cells; (B) 72 h cytotoxicity in A549 cells; (C) 24 h cytotoxicity in 16HBE cells; (D) 72 h cytotoxicity in 16HBE cells (mean±SD, n=3).
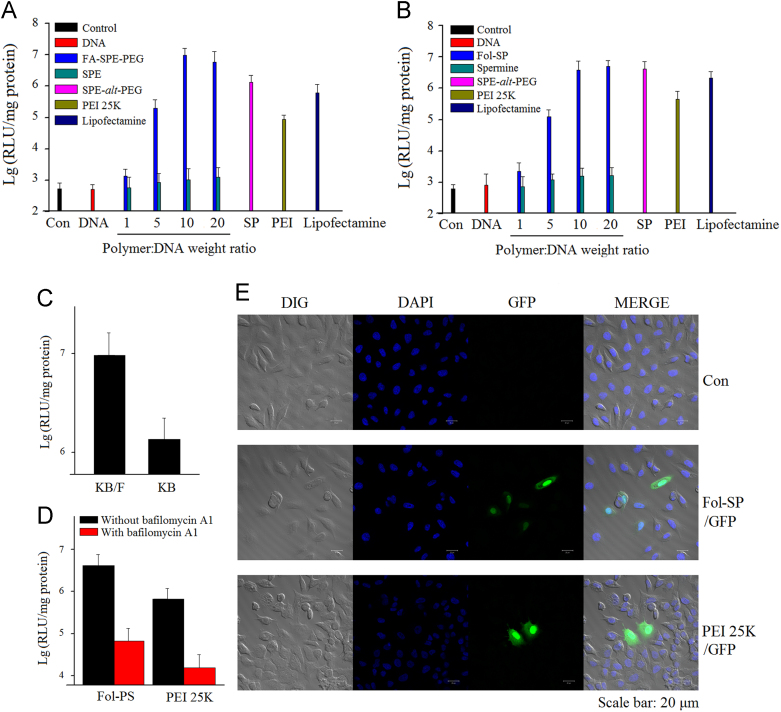
Luciferase assays were carried out in KB/F cells (FR-overexpressed), KB cells, and A549 cells to investigate the in vitro transfection efficiency and targeting ability of FA-SPE-PEG. As shown in Fig. 4A and B, the transfection efficiency of FA-SPE-PEG was higher than SPE-alt-PEG, PEI 25 K and Lipofectamine® at all weight ratios in KB/F cells and A549 cells. Noteworthy, the transfection efficiency of FA-SPE-PEG in KB/F cells was higher than the untargeted SPE-alt-PEG when the weight ratio reached 10 (7.07 times higher at weight ratio of 10 and 4.35 times higher at weight ratio of 20). Besides, the higher transfection efficiency of FA-SPE-PEG in KB/F cells than that in KB cells further reconfirmed the targeting capacity of FA-SPE-PEG towards FRs (Fig. 4C). The specific interaction between FA and FRs facilitated the internalization rate of gene complexes, which subsequently led to higher transfection efficiency32, 33, 34. Apart from high entry rates into cells, endosome escape also played a key role in the transfection of gene. The buffering capacity of FA-SPE-PEG was investigated to assess its endosome escape ability. Results in Fig. 4D demonstrated that the transfection efficiency of FA-SPE-PEG decreased sharply with the addition of bafilomycin A1 (a proton pump inhibitor), indicating that the proton sponge effect is very important in the transfection process35. The superiority of FA-SPE-PEG for gene targeting delivery was also confirmed intuitively by the expression of GFP via CLSM (Fig. 4E). Higher GFP signal intensity was observed in cells treated with FA-SPE-PEG/GFP. Although GFP signal intensity could also be observed in cells treated with PEI 25 K, cells in this group exhibited severe morphological changes. Taken with the results of in vitro cytotoxicity study, these results suggest that FA-SPE-PEG could serve as a safe and efficient non-viral vector for the targeting delivery of genes.
Figure 4.
In vitro transfection efficiency and targeting ability studies of FA-SPE-PEG. Transfection efficiency in (A) KB/F cells and (B) A549 cells by FA-SPE-PEG/pGL3 at various weight ratios (mean±SD, n=3). (C) Buffering capacity study of FA-SPE-PEG/pGL3 in KB/F cells (mean±SD, n=3). Bafilomycin A1 (200 nmol/L) diluted in DMSO were put into wells. After incubation for 10 min, transfection solution was added into wells for 4 h. Then the cells were incubated in growth medium for 24 h. (D) Targeting capacity of FA-SPE-PEG/pGL3 in KB/F cells and KB cells (mean±SD, n=3). (E) GFP expression after KB/F cells were treated with FA-SPE-PEG/GFP and PEI 25K/GFP complexes. Composite images were made by overlapping the images of the individual channels.
3.3. Transfection efficiency study in vivo
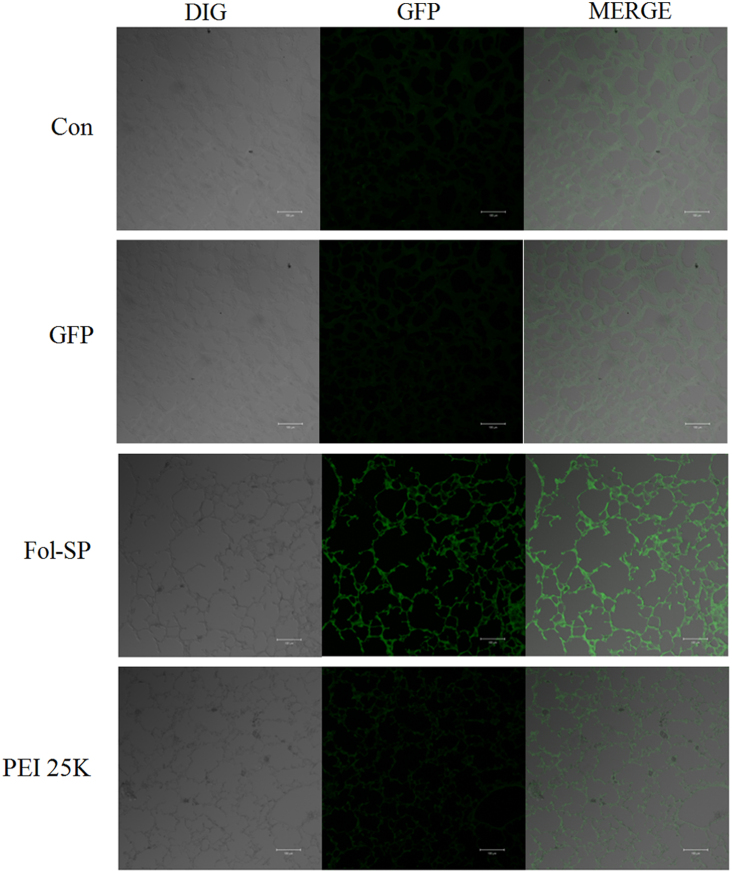
Based on the results of in vitro studies above, the targeted gene delivery capacity of FA-SPE-PEG was reconfirmed in vivo via an aerosol delivery approach. Gene delivery through the aerosol approach remains a potential method for gene therapy of lung cancer, which presents a non-invasive and instant access to lungs18, 20, 27. After aerosol delivery of PBS, naked GFP, FA-SPE-PEG/GFP complexes, and PEI 25K/GFP complexes, the fluorescent signal intensity of GFP was detected to represent the transfection efficiency in vivo. It could be observed in Fig. 5 that the GFP signal was most dominant in FA-SPE-PEG/GFP treated group, consistent with the results of in vitro transfection studies. The higher signal intensity of GFP validated that FA-SPE-PEG showed enhanced transfection vs. PEI 25K as a targeting gene delivery vector. In addition, the lack of obvious lesions in tissues further confirmed the excellent biocompatibility of FA-SPE-PEG for in vivo gene delivery. We previously showed the in vivo tumor-inhibiting effects of gene therapy in lung cancer using non-targeting SPE-alt-PEG20, so it is rational to deduce that FA-SPE-PEG would act as a valid vector for the targeting delivery of therapeutic genes. The excellent biocompatibility and high transfection efficiency of FA-SPE-PEG in vitro and in vivo strongly suggest this ligand to be an important prospective targeting vector for the gene therapy of cancer.
Figure 5.
In vivo transfection study after aerosol administration. Observation of the GFP expression level after aerosol delivery of FA-SPE-PEG/GFP complexes at a functional weight ratio to BALB/c mice (magnification: 200×, scale bar: 100 μm).
4. Conclusions
In this study, we successfully synthesized a novel FR-targeted, ester-based polyspermine, FA-SPE-PEG, and evaluated its potential as a vector for the targeted delivery of therapeutic genes. FA-SPE-PEG possessed prominent capacities to condense DNA as well as to protect DNA from the degradation by nucleases. FA-SPE-PEG/DNA complexes exhibited appropriate physicochemical properties for gene targeting delivery. The mild cytotoxicity and favorable transfection efficiency of FA-SPE-PEG insured its prospects as a valid gene vector. Moreover, results from transfection efficiency studies in vitro and in vivo validated that FA-SPE-PEG manifested high specificity towards FR-overexpressed tumor cells, which further enhance the potential of FA-SPE-PEG for the targeted delivery of therapeutic genes. Altogether, FA-SPE-PEG not only facilitated the cellular uptake of therapeutic genes, but also showed strongly reduced toxicity towards normal tissues when compared with other cationic gene vectors. In conclusion, FA-SPE-PEG holds the prospects to serve as a biocompatible and efficient vector for targeted gene delivery in tumor therapy.
Acknowledgement
We thank the National Natural Science Foundation of China (Grant Nos. 81573369, 21301191, 81570696 and 31270985), the Natural Science Foundation of Jiangsu Province, China (Grant Nos. BK20130661 and BK20140659) and the Research and Innovation Project of Jiangsu Province (Grant No. KYLX15_0640).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Yang Z.R., Wang H.F., Zhao J., Peng Y.Y., Wang J., Guinn B.A. Recent developments in the use of adenoviruses and immunotoxins in cancer gene therapy. Cancer Gene Ther. 2007;14:599–615. doi: 10.1038/sj.cgt.7701054. [DOI] [PubMed] [Google Scholar]
- 2.Dass C.R., Choong P.F.M. Non-viral methods for gene transfer towards osteosarcoma therapy. J Drug Target. 2007;15:184–189. doi: 10.1080/10611860701231547. [DOI] [PubMed] [Google Scholar]
- 3.Merdan T., Kopeček J., Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54:715–758. doi: 10.1016/s0169-409x(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Libutti S.K. New horizons for cancer gene therapy. Cancer Gene Ther. 2014;21:1. doi: 10.1038/cgt.2013.80. [DOI] [PubMed] [Google Scholar]
- 5.Benns J., Kim S. Tailoring new gene delivery designs for specific targets. J Drug Target. 2000;8:1–12. doi: 10.3109/10611860009009205. [DOI] [PubMed] [Google Scholar]
- 6.Jiang H.L., Kwon J.T., Kim E.M., Kim Y.K., Arote R., Jere D. Galactosylated poly(ethylene glycol)-chitosan-graft-polyethylenimine as a gene carrier for hepatocyte-targeting. J Control Release. 2008;131:150–157. doi: 10.1016/j.jconrel.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Wang Z.J., Gemeinhart R.A. Progress in microrna delivery. J Control Release. 2013;172:962–974. doi: 10.1016/j.jconrel.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulis N.M., Willmarth N.E., Song D.K., Feldman E.L., Imperiale M.J. Intraneural colchicine inhibition of adenoviral and adeno-associated viral vector remote spinal cord gene delivery. Neurosurgery. 2003;52:381–387. doi: 10.1227/01.neu.0000044459.24519.3e. [DOI] [PubMed] [Google Scholar]
- 9.Trepel M., Grifman M., Weitzman M.D., Pasqualini R. Molecular adaptors for vascular-targeted adenoviral gene delivery. Hum Gene Ther. 2000;11:1971–1981. doi: 10.1089/10430340050143408. [DOI] [PubMed] [Google Scholar]
- 10.Ramamoorth M., Narvekar A. Non viral vectors in gene therapy—an overview. J Clin Diagn Res. 2015;9:GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H.Y., Jiang Y.F., Peng H.G., Chen Y.Z., Zhu P.Z., Huang Y.Z. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev. 2015;81:142–160. doi: 10.1016/j.addr.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Boussif O., Lezoualc׳h F., Zanta M.A., Mergny M.D., Scherman D., Demeneix B. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park T.G., Jeong J.H., Kim S.W. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–486. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Kunath K., von Harpe A., Fischer D., Petersen H., Bickel U., Voigt K. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 15.Godbey W.T., Wu K.K., Mikos A.G. Poly(ethylenimine) and its role in gene delivery. J Control Release. 1999;60:149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 16.Drake C.R., Aissaoui A., Argyros O., Serginson J.M., Monnery B.D., Thanou M. Bioresponsive small molecule polyamines as noncytotoxic alternative to polyethylenimine. Mol Pharm. 2010;7:2040–2055. doi: 10.1021/mp9002249. [DOI] [PubMed] [Google Scholar]
- 17.Allen J.C. Biochemistry of the polyamines. Cell Biochem Funct. 1983;1:131–140. doi: 10.1002/cbf.290010302. [DOI] [PubMed] [Google Scholar]
- 18.Jiang H.L., Hong S.H., Kim Y.K., Islam M.A., Kim H.J., Choi Y.J. Aerosol delivery of spermine-based poly(amino ester)/Akt1 shRNA complexes for lung cancer gene therapy. Int J Pharm. 2011;420:256–265. doi: 10.1016/j.ijpharm.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.H., Kim Y.K., Arash M.T., Hong S.H., Lee J.H., Kang B.N. Galactosylation of chitosan-graft-spermine as a gene carrier for hepatocyte targeting in vitro and in vivo. J Nanosci Nanotechnol. 2012;12:5178–5184. doi: 10.1166/jnn.2012.6376. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.K., Cho C.S., Cho M.H., Jiang H.L. Spermine-alt-poly(ethylene glycol) polyspermine as a safe and efficient aerosol gene carrier for lung cancer therapy. J Biomed Mater Res A. 2014;102:2230–2237. doi: 10.1002/jbm.a.34905. [DOI] [PubMed] [Google Scholar]
- 21.Alex S.M., Rekha M.R., Sharma C.P. Spermine grafted galactosylated chitosan for improved nanoparticle mediated gene delivery. Int J Pharm. 2011;410:125–137. doi: 10.1016/j.ijpharm.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 22.Sun W.J., Jiang T.Y., Lu Y., Reiff M., Mo R., Gu Z. Cocoon-like self-degradable DNA nanoclew for anticancer drug delivery. J Am Chem Soc. 2014;136:14722–14725. doi: 10.1021/ja5088024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang B.F., Xing L., Cui P.F., Wang F.Z., Xie R.L., Zhang J.L. Mitochondria apoptosis pathway synergistically activated by hierarchical targeted nanoparticles co-delivering siRNA and lonidamine. Biomaterials. 2015;61:178–189. doi: 10.1016/j.biomaterials.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Köllmer M., Buhrman J.S., Tang M.Y., Gemeinhart R.A. Arginine-rich, cell penetrating peptide–anti-microRNA complexes decrease glioblastoma migration potential. Peptides. 2014;58:83–90. doi: 10.1016/j.peptides.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Y.K., Choi J.Y., Yoo M.K., Jiang H.L., Arote R., Je Y.H. Receptor-mediated gene delivery by folate-PEG-baculovirus in vitro. J Biotechnol. 2007;131:353–361. doi: 10.1016/j.jbiotec.2007.07.938. [DOI] [PubMed] [Google Scholar]
- 26.Jiang H.L., Kim Y.K., Arote R., Nah J.W., Cho M.H., Choi Y.J. Chitosan-graft-polyethylenimine as a gene carrier. J Control Release. 2007;117:273–280. doi: 10.1016/j.jconrel.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Jiang H.L., Xu C.X., Kim Y.K., Arote R., Jere D., Lim H.T. The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graft-polyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway. Biomaterials. 2009;30:5844–5852. doi: 10.1016/j.biomaterials.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Ashwell G., Morell A.G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41:99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- 29.Moghimi S.M., Symonds P., Murray J.C., Hunter A.C., Debska G., Szewczyk A. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Hunter A.C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Wong K., Sun G.B., Zhang X.Q., Dai H., Liu Y., He C.B. PEI-g-chitosan, a novel gene delivery system with transfection efficiency comparable to polyethylenimine in vitro and after liver administration in vivo. Bioconjug Chem. 2006;17:152–158. doi: 10.1021/bc0501597. [DOI] [PubMed] [Google Scholar]
- 32.Lee D., Lockey R., Mohapatra S. Folate receptor-mediated cancer cell specific gene delivery using folic acid-conjugated oligochitosans. J Nanosci Nanotechnol. 2006;6:2860–2866. doi: 10.1166/jnn.2006.465. [DOI] [PubMed] [Google Scholar]
- 33.He Z.Y., Yu Y.Y., Zhang Y., Yan Y.D., Zheng Y., He J. Gene delivery with active targeting to ovarian cancer cells mediated by folate receptor α. J Biomed Nanotechnol. 2013;9:833–844. doi: 10.1166/jbn.2013.1587. [DOI] [PubMed] [Google Scholar]
- 34.Lai W.F., Lin M.C. Folate-conjugated chitosan-poly(ethylenimine) copolymer as an efficient and safe vector for gene delivery in cancer cells. Curr Gene Ther. 2015;15:472–480. doi: 10.2174/1566523215666150812120347. [DOI] [PubMed] [Google Scholar]
- 35.Freeman E.C., Weiland L.M., Meng W.S. Modeling the proton sponge hypothesis: examining proton sponge effectiveness for enhancing intracellular gene delivery through multiscale modeling. J Biomater Sci Polym Ed. 2013;24:398–416. doi: 10.1080/09205063.2012.690282. [DOI] [PMC free article] [PubMed] [Google Scholar]