Abstract
Brain delivery of macromolecular therapeutics (e.g., proteins) remains an unsolved problem because of the formidable blood–brain barrier (BBB). Although a direct pathway of nose-to-brain transfer provides an answer to circumventing the BBB and has already been intensively investigated for brain delivery of small drugs, new challenges arise for intranasal delivery of proteins because of their larger size and hydrophilicity. In order to overcome the barriers and take advantage of available pathways (e.g., epithelial tight junctions, uptake by olfactory neurons, transport into brain tissues, and intra-brain diffusion), a low molecular weight protamine (LMWP) cell-penetrating peptide was utilized to facilitate nose-to-brain transport. Cell-penetrating peptides (CPP) have been widely used to mediate macromolecular delivery through many kinds of biobarriers. Our results show that conjugates of LMWP–proteins are able to effectively penetrate into the brain after intranasal administration. The CPP-based intranasal method highlights a promising solution for protein therapy of brain diseases.
KEY WORDS: Intranasal protein delivery, Brain targeting, Cell-penetrating peptide, Low molecular weight protamine, Blood–brain barrier
Graphical abstract
The low molecular weight protamine (LMWP) cell-penetrating peptide is utilized to mediate proteins for nose-to-brain transport. The conjugates of LMWP–proteins can effectively penetrate into the brains by noninvasive intranasal administration. The cell penetrating peptide-based intranasal method highlights a promising solution for protein therapy for brain diseases.
1. Introduction
The blood–brain barrier (BBB) poses a formidable challenge to the central nervous system (CNS) delivery of macromolecular therapeutics. In some brain diseases pathological processes often associate with changes in BBB permeability. As a good case in point, leaky blood vessels are commonly found in brain cancers, thereby contributing to the enhanced permeability and retention (EPR) effect1. In most circumstances, however, the leakage is generally limited to insignificant amounts of active macromolecules that yield little therapeutic benefit. Moreover, neurodegenerative diseases do not induce angiogenesis and thus there is no significant change in BBB permeability.
Intracranial injection is the direct but difficult way to deliver drugs into the brain. Despite the high risk of surgical operations, intracranial administration remains the primary means of direct brain drug delivery; for instance, it is the only clinically employed method for biomacromolecular drugs due to their inability to penetrate the BBB. However, the BBB is not the only problem; the difficult problem of drug diffusion across CNS compartments must also be addressed. Although intraparenchymal or CSF administration can yield a high degree of targeting, the distribution of proteins is restricted within the injection sites due largely to the hydrophilic nature and large size2. For example3, diffusion coefficients of proteins in the brain is estimated to be 10–6 cm2/s. The delay or restriction of intra-tissue diffusion may compromise the desired pharmacological efficacy. Hence, the dual barriers (i.e., BBB and intracerebral diffusion) render most efforts to deliver protein drugs into brain a failure. Therefore, the need for new techniques to overcome the BBB and deliver drugs into the CNS remains exigent.
Nasal administration using the olfactory axonal pathway from the epithelium into cerebral tissue for drug delivery has attracted scientific attention for its circumvention of BBB and ease of administration. The research focus of nose-to-brain delivery, however, has been most prominently focused on small drugs, because the tight intercellular junctions in the nasal mucous membranes normally prevent the passage of drugs with molecular weights greater than 1000 Da. Apart from the tight junction barrier, migration along olfactory axons presents an additional obstacle for the delivery of proteins because of their poor cell penetration ability. Some viruses4 and phage-display peptide sequences5 have been reported to reach the CNS via olfactory axonal transport, but so far little is known about the feasibility of the olfactory axonal delivery of macromolecular therapeutics.
Cell-penetrating peptides (CPPs), also known as protein transduction domains (PTDs), have been extensively explored for their potential application in mediating biomacromolecular drug delivery6. The CPP-based intracellular delivery has been shown to be cell-type independent7, and able to penetrate across various biobarriers (e.g., retina and neurons8, 9, blood brain barrier10, 11, intestine wall12, 13, 14, and skin15, 16) that otherwise constitute great impediments to conventional approaches of macromolecular drug delivery. Therefore, CPPs are a powerful tool for mediating protein delivery.
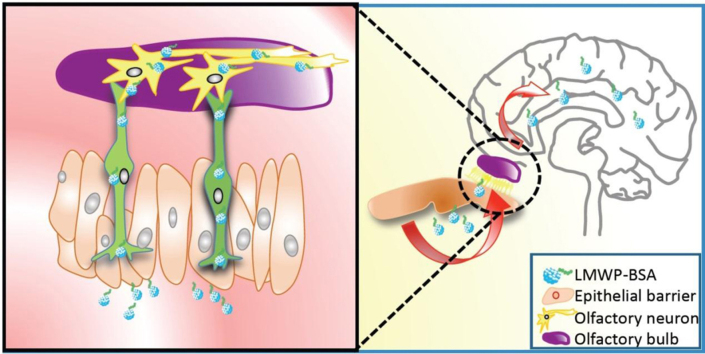
Low molecular weight protamine (LMWP) is a nature-sourced CPP with the sequence of VSRRRRRRGGRRRR, firstly identified by our laboratory from enzymatically-digested fractions of protamine. In our previous studies the ability of LMWP-mediated transcutaneous delivery has been demonstrated16, 17. More interestingly, LMWP was also found to be able to mediate nose-to-brain delivery of nanoparticles18. Therefore we were motivated to develop a nose-to-brain protein delivery system by conjugating a CPP to a protein molecule (Fig. 1).
Figure 1.
The cell-penetrating LMWP peptide-mediated protein drug from nose to brain delivery.
2. Experimental
LMWP was prepared by digestion of native protamine using an enzymatic method we reported previously19. Three model proteins, bovine serum albumin (BSA, Sigma–Aldrich), peroxidase (HRP, Sigma–Aldrich) and β-galactosidase (β-gal, Invitrogen) were used in the investigations. The proteins were conjugated to LMWP via a covalent bond. In brief, LMWP in PBS (5 mg/mL) was activated by N-succinimidyl-S-acetylthioacetate (SATA, Pierce, Fisher) at a reaction ratio of 1:3. After a 2-h incubation at room temperature (RT), the excess SATA was removed using a heparin affinity column (HiTrap, GE Healthcare). Deacylation to generate a sulfhydryl at the N-terminal of LMWP was accomplished using dydroxylamine·HCl. A maleimide-activated protein (10 mg/mL in PBS) was produced by reaction with a 3-fold molar excess of succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC, Pierce) for 30 min, and purified using a desalting column. The thiolated LMWP and maleimide-activated protein were mixed (5:1, mol/mol) and incubated for 2 h at r.t. to produce LMWP-conjugated protein via a thioether bond. Further purification of the LMWP-conjugated proteins was conducted by dialysis and heparin column as described in our previous report16. The elution obtained from the heparin column with a gradient elution of 2 mol/L NaCl solution was subject to dialysis and the final product was confirmed by protein gel electrophoresis and Q-TOF-MS.
The tested compound (LMWP–BSA) was labeled with CY5-NHS dye. BALB/c nude mice were anesthetized and placed on their backs during the experiment. Nasal administration was performed on anaesthetized mice as follows: The sample solution was introduced into the nose cavity by using a PE-50 tubing with a dose of 20 μL (10 μL per nostril). After dosing, animals were subject to in vivo imaging by using an IVIS imaging system (Caliper PerkinElmer, Hopkinton, MA, USA). After 8 h cardiac perfusion was performed with PBS and the brain tissues were collected for fluorescent imaging. The animal procedures were approved by University Committee on Use and Care of Animals (UCUCA, University of Michigan, USA) and Institutional Committee on Use and Care of Animals (ICUCA, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China).
For the intra-tissue penetration observation, the fluorescein isothiocyanate (FITC)-labeled LMWP–BSA was given to the anesthetized BALB/c mice with treatment for 3 h. The olfactory bulbs were then processed by cryosection and slices were then examined using a fluorescent microscope (Olympus BX-51).
A model protein, i.e., HRP or β-gal, with chemical linkage of LMWP was intranasally administered to the BALB/c mice. After 3 h of exposure, mice (3 per group) were killed by cardiac perfusion with PBS. Brain tissues were collected and homogenized using a BioMasher homogenizer (BioMasher, USA). After centrifugation the supernatants were subjected to the bioactivity assay. HRP activity in the tissues was detected by color development of the peroxidase substrate of tetramethylbenzidine (TMB, Kirkegaard & Perry Laboratories, USA), and β-gal using the colorimetric substrate of o-nitrophenyl-b-d-galactoside (ONPG, Sigma–Aldrich, USA) according to the manufacturers׳ instructions.
3. Results and discussion
LMWP has been utilized previously for drug delivery, including delivery of proteins16, 18, 19, 20, 21, 22, genes23, 24, 25, supramolecular assembles26, 27, and nanoparticles28. LMWP is derived from protamine (an FDA approved clinical drug), and the preclinical safety/toxicology profile of LMWP has been evaluated, displaying advantages of significantly low immunogenicity and toxic responses29, 30, 31. Furthermore, mass production of high-purity LMWP (>99%) has been established for a scale up to tens of grams per week using an enzymatic digestion method, thereby being more efficient and economically viable as compared to the chemical syntheses that are currently employed for production of all other CPPs.
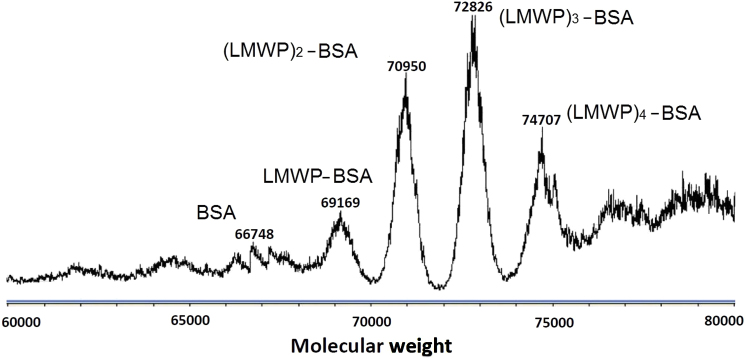
The final product of LMWP–BSA after chemical conjugation was purified by heparin column because LMWP has high affinity for the immobile heparin, thus rendering retention of LMWP–BSA in the column. After elution with 1 mol/L NaCl solution, the purity of the desalted sample was verified by gel electrophoresis (Fig. 2). Moreover, Q-TOF mass spectrometry results (Fig. 3) showed that the major product generally possessed a (2:1–3:1) molar ratio of LMWP:BSA.
Figure 2.
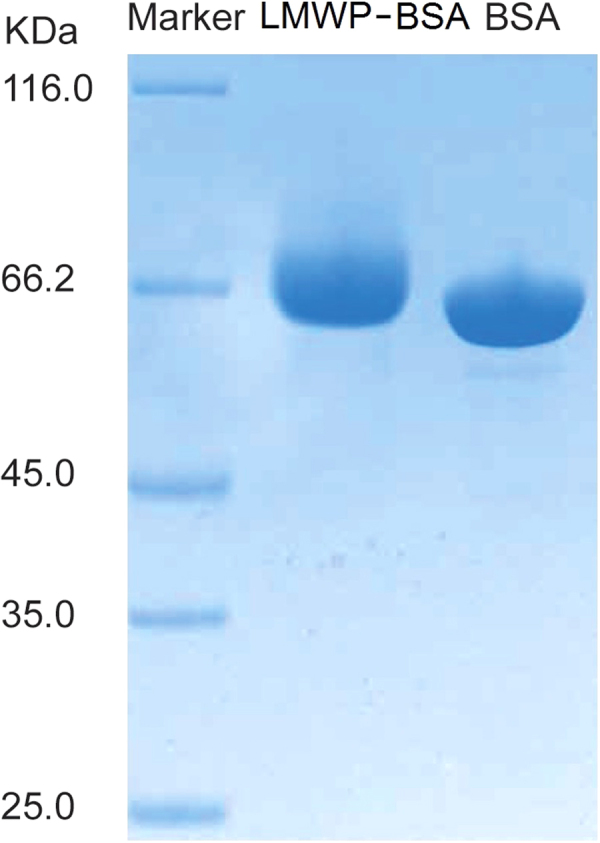
SDS-PAGE characterization of the LMWP–BSA conjugates.
Figure 3.
Q-TOF mass spectrum of the LMWP–BSA conjugates.
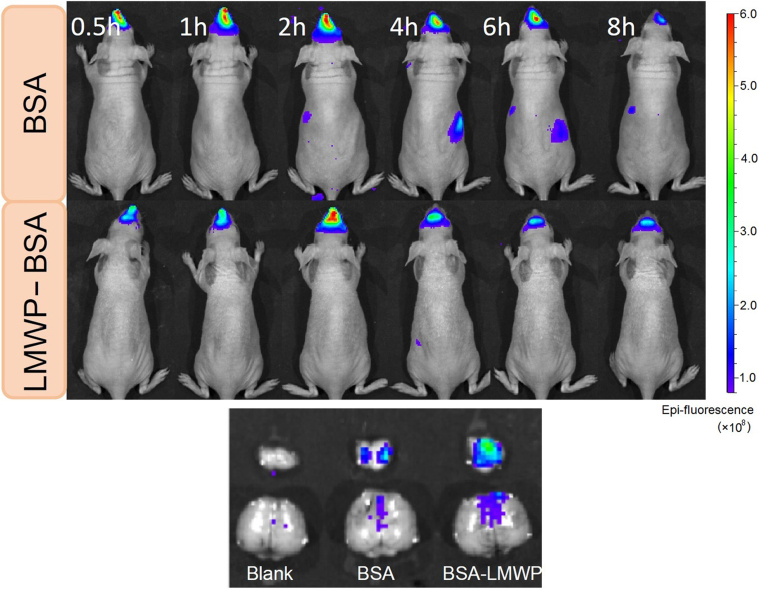
Use of CPP–protein conjugation for nasal drug delivery targeting to brain has rarely been reported. The feasibility of LMWP-mediated nasal delivery was investigated by using a CY5 dye-labeled LMWP–BSA conjugate. The biodistribution in brain was observed with an IVIS™ imaging system. The in vivo imaging revealed that both LMWP–BSA and BSA were retained in the nasal cavity during the experiment (Fig. 4, top panel). The tissue imaging showed that administered BSA was negligible in the brain. However, LMWP–BSA was delivered to the brain tissues, and exhibited significant brain distribution with higher intensity of fluorescence found in the olfactory bulbs, while showing moderate cerebral distribution (Fig. 4, bottom panel). In addition, the labeled protein was not detected in the major organs, which indicated that the intranasal absorption into circulation system was very minor. The results confirmed our speculation that LMWP would be able to overcome the mucosal tight junction barrier, axon intracellular trafficking, and intra-tissue diffusion.
Figure 4.
In vivo imaging after intranasal administration (up panel) and the fluorescence imaging of the brain tissues at experimental endpoint (bottom panel).
Diffusional limitation is a formidable hurdle for conventional protein delivery. In theory, the diffusion time is related to the square of the distance for small molecules, and therefore doubling distance results in an increase in diffusion time by 4-fold32. Significantly slower is the intra-tissue penetration of macromolecules; it may take 3 days for a 20-kDa protein to penetrate 1 mm in brain tissue3. This limited drug exposure to pathological tissues and cells usually produces insufficient therapeutic levels, thus severely hindering efficacy in protein therapy. The LMWP-mediated delivery was shown to be widespread over brain tissues. Our results were in accordance with those of other groups on the observation of extensive distribution inside brain tissues of CPP-linked proteins10, 33.
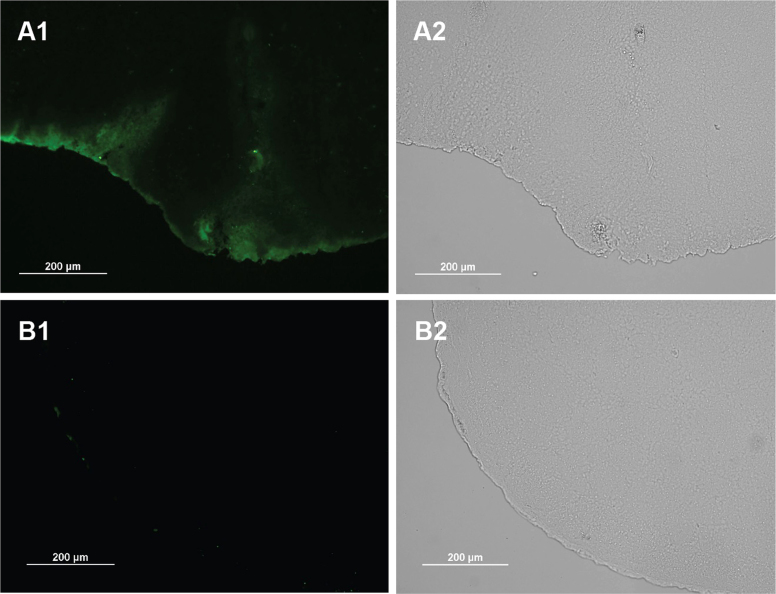
Further, the inward penetration was examined in the cryosection slices of the olfactory bulbs. As displayed in Fig. 5, more than spreading over the surface of brain, the proteins penetrated inside the tissue within 1 h, reflected by deep-migrating fluorescence. It demonstrated the success of intra-tissue penetration of LMWP-linked proteins, whereas the native proteins hardly showed any tissue diffusion ability. Overall, the intra-tissue diffusion mediated by LMWP was in a three-dimensional manner, thus enhancing the drug exposure to the targeting tissues.
Figure 5.
LMWP–BSA conjugates were intranasally administered to mice. One hour later, the olfactory bulbs were removed and processed cryosection. Slides were observed using fluorescent microscope. (A) LMWP–BSA–FITC; (B) BSA–FITC.
There is a lack of scrutiny on the mechanism of intra-brain diffusion of CPP-linked proteins. Few investigations have been conducted on the interaction of CPPs or CPP-linked proteins with the extracellular matrix, probably due to the thinking that the intercellular pathway does not play an important role in CPP-mediated delivery. Indeed, CPP-mediated penetration was shown to be by macropinocytosis in skin tissue34 and migrate deep by saturating layer by layer of cells, indicating that the transcellular route was the major pathway responsible for the CPP–drug diffusion.
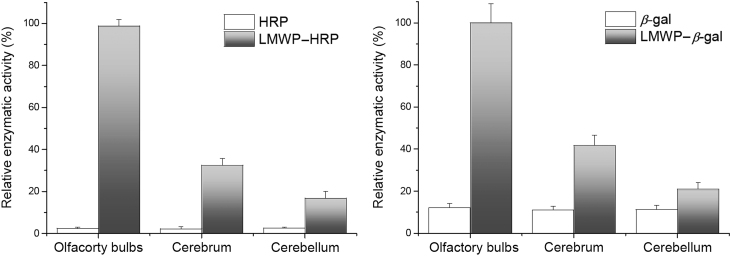
The above results demonstrated the successful nose-to-brain delivery of LMWP–BSA by a fluorescent imaging study, but it is still necessary to determine whether a biologically active protein could be intranasally delivered into CNS and retain activity. A 44-kD HRP or 540-kD β-gal was selected for this study. HRP is relatively large molecule that has no known tissue receptor35 and its brain uptake is therefore strictly controlled by the CPP-mediated mechanism. The large size of β-gal also makes it impossible to penetrate mucosal tight junctions. The biodistribution was parallel to the results of fluorescent imaging. The assay showed that enzyme activity in all the brain tissues remained at a background level for non-conjugated HRP or β-gal, whereas the high activity was detected in brain tissues for the LMWP-linked enzymes (Fig. 6). This demonstrates that the enzymes retain their functions after nose-to-brain delivery.
Figure 6.
Enzymatic activity of HPR (left panel) and β-gal (right panel). LMWP-linked proteins were represented by grey bars, and native proteins white bars. LMWP-enzymes were significantly higher than their non-modified counterparts (n=3).
The accumulation of the active enzymes was found to be the highest in the olfactory bulbs, and next was cerebrum and then cerebellum, relating to the migration distance along the route from nose to brain. Although protein drugs are particularly vulnerable to normal protein degradation pathways in the body, our results clearly showed that the test proteins retained their activity despite the remote delivery through the heterogeneous environments from nose to brain.
Endocytosis plays an important role in CPP-mediated intracellular delivery. Its mechanism involves the preferential binding with heparin sulfate glycosaminoglycans on the cell surface, which then initializes the endocytosis36. However, the intra-tissue penetration mechanisms are largely unknown, and probably occur via an extracellular pathway or layer-by-layer saturation and diffusion. There is still much work to do to elucidate the details.
It should be noted that although we did not examine neurotoxicity in this study, there are good reasons to believe the safety of this method, based on the following facts: first, the safety of use of LMWP has been confirmed in dogs28; second, daily injection of a CPP-fusion protein in mice with a dose of 1 mg/kg body weight for 14 consecutive days produced no signs of gross neurological problems or systemic distress10.
4. Conclusions
It is a long-standing problem to deliver macromolecular therapeutics into the CNS due to the BBB. The lack of effective delivery techniques largely hinders the development of many promising active macromolecules into clinically useful drugs. Up to now, merely a handful of clinical trials of have been conducted in brain protein therapy, and most employ a conventional intracranial injection procedure. On ethical grounds, it is difficult to justify such an invasive protein therapy for patients. This fact to a great extent restricts large-scale clinical trials and delays the development of protein drugs for brain diseases. Therefore there is a pressing need for novel brain delivery techniques. Nasal delivery provides a promising noninvasive solution for circumventing the BBB by a direct transport of drugs into the brain via the olfactory pathway. Although many investigations have been conducted in nose-to-brain delivery, very few of them have looked at protein drugs, of which the high hydrophilicity and large size limits their ability to: (1) penetrate through the mucosal tight junction barrier; (2) be taken up by and transported along olfactory neurons; and (3) effectively diffuse into brain tissues. We conjugated LMWP to several protein drugs and took advantage of its penetration ability to mediate nose-to-brain delivery of proteins and circumvent the BBB. Various proteins were successfully delivered intranasaly into the brain by means of conjugating to LMWP. This study demonstrates the promise of achieving a protein therapy for CNS diseases by a noninvasive, simple yet effective drug delivery system.
Acknowledgments
We thank the support from the National Basic Research Program of China (973 Program Nos. 2013CB932503 and 2014CB931900) and National Natural Science Foundation of China (Nos. 81172996, 81373357, 81422048 and 81361140344).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Victor C. Yang, Email: vcyang@umich.edu.
Yongzhuo Huang, Email: yzhuang@simm.ac.cn.
References
- 1.Matsumura Y., Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 2.Thorne R.G., Frey W.H. 2nd. Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40:907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 3.Lo E.H., Singhal A.B., Torchilin V.P., Abbott N.J. Drug delivery to damaged brain. Brain Res Brain Res Rev. 2001;38:140–148. doi: 10.1016/s0165-0173(01)00083-2. [DOI] [PubMed] [Google Scholar]
- 4.Morales J.A., Herzog S., Kompter C., Frese K., Rott R. Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med Microbiol Immunol. 1988;177:51–68. doi: 10.1007/BF00189527. [DOI] [PubMed] [Google Scholar]
- 5.Wan X.M., Chen Y.P., Xu W.R., Yang W.J., Wen L.P. Identification of nose-to-brain homing peptide through phage display. Peptides. 2009;30:343–350. doi: 10.1016/j.peptides.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca S.B., Pereira M.P., Kelley S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev. 2009;61:953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Wadia J.S., Dowdy S.F. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv Drug Deliv Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Dietz G.P.H., Kilic E., Bähr M. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-mediated protein transduction. Mol Cell Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- 9.Barnett E.M., Elangovan B., Bullok K.E., Piwnica-Worms D. Selective cell uptake of modified TAT peptide–fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest Ophthalmol Vis Sci. 2006;47:2589–2595. doi: 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- 10.Schwarze S.R., Ho A., Vocero-Akbani A., Dowdy S.F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 11.Dietz G.P.H., Bähr M. Peptide-enhanced cellular internalization of proteins in neuroscience. Brain Res Bull. 2005;68:103–114. doi: 10.1016/j.brainresbull.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Liang J.F., Yang V.C. Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun. 2005;335:734–738. doi: 10.1016/j.bbrc.2005.07.142. [DOI] [PubMed] [Google Scholar]
- 13.Kamei N., Morishita M., Eda Y., Ida N., Nishio R., Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J Control Release. 2008;132:21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Kamei N., Morishita M., Takayama K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J Control Release. 2009;136:179–186. doi: 10.1016/j.jconrel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Rothbard J.B., Garlington S., Lin Q., Kirschberg T., Kreider E., McGrane P.L. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y.Z., Park Y.S., Moon C., David A.E., Chung H.S., Yang V.C. Synthetic skin-permeable proteins enabling needleless immunization. Angew Chem Int Ed. 2010;49:2724–2727. doi: 10.1002/anie.200906153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang L.C., Lee H.F., Yang Z.Q., Yang V.C. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (I): preparation and characterization. AAPS PharmSci. 2001;3:E17. doi: 10.1208/ps030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byun Y., Chang L.C., Lee L.M., Han I.S., Singh V.K., Yang V.C. Low molecular weight protamine: a potent but nontoxic antagonist to heparin/low molecular weight protamine. ASAIO J. 2000;46:435–439. doi: 10.1097/00002480-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Byun Y., Singh V.K., Yang V.C. Low molecular weight protamine: a potential nontoxic heparin antagonist. Thromb Res. 1999;94:53–61. doi: 10.1016/s0049-3848(98)00201-1. [DOI] [PubMed] [Google Scholar]
- 20.Park Y.J., Chang L.C., Liang J.F., Moon C., Chung C.P., Yang V.C. Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: in vitro and in vivo study. FASEB J. 2005;19:1555–1557. doi: 10.1096/fj.04-2322fje. [DOI] [PubMed] [Google Scholar]
- 21.Kwon Y.M., Li Y.T., Liang J.F., Park Y.J., Chang L.C., Yang V.C. PTD-modified ATTEMPTS system for enhanced asparaginase therapy: a proof-of-concept investigation. J Control Release. 2008;130:252–258. doi: 10.1016/j.jconrel.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y.T., Kwon Y.M., Spangrude G.J., Liang J.F., Chung H.S., Park Y.J. Preliminary in vivo evaluation of the protein transduction domain-modified ATTEMPTS approach in enhancing asparaginase therapy. J Biomed Mater Res A. 2009;91A:209–220. doi: 10.1002/jbm.a.32204. [DOI] [PubMed] [Google Scholar]
- 23.Park Y.J., Liang J.F., Ko K.S., Kim S.W., Yang V.C. Low molecular weight protamine as an efficient and nontoxic gene carrier: in vitro study. J Gene Med. 2003;5:700–711. doi: 10.1002/jgm.402. [DOI] [PubMed] [Google Scholar]
- 24.Park Y.S., Huang Y.Z., Park Y.J., David A.E., White L., He H.N. Specific down regulation of 3T3-L1 adipocyte differentiation by cell-permeable antisense HIF1α-oligonucleotide. J Control Release. 2010;144:82–90. doi: 10.1016/j.jconrel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi Y.S., Lee J.Y., Suh J.S., Kwon Y.M., Lee S.J., Chung J.K. The systemic delivery of siRNAs by a cell penetrating peptide, low molecular weight protamine. Biomaterials. 2010;31:1429–1443. doi: 10.1016/j.biomaterials.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Moon C., Kwon Y.M., Lee W.K., Park Y.J., Chang L.C., Yang V.C. A novel polyrotaxane-based intracellular delivery system for camptothecin: in vitro feasibility evaluation. J Biomed Mater Res A. 2008;84:238–246. doi: 10.1002/jbm.a.31452. [DOI] [PubMed] [Google Scholar]
- 27.Moon C., Kwon Y.M., Lee W.K., Park Y.J., Yang V.C. In vitro assessment of a novel polyrotaxane-based drug delivery system integrated with a cell-penetrating peptide. J Control Release. 2007;124:43–50. doi: 10.1016/j.jconrel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suh J.S., Lee J.Y., Choi Y.S., Yu F.Q., Yang V., Lee S.J. Efficient labeling of mesenchymal stem cells using cell permeable magnetic nanoparticles. Biochem Biophys Res Commun. 2009;379:669–675. doi: 10.1016/j.bbrc.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 29.Chang L.C., Liang J.F., Lee H.F., Lee L.M., Yang V.C. Low molecular weight protamine (LMWP) as nontoxic heparin/low molecular weight heparin antidote (II): in vitro evaluation of efficacy and toxicity. AAPS PharmSci. 2001;3:E18. doi: 10.1208/ps030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee L.M., Chang L.C., Wrobleski S., Wakefield T.W., Yang V.C. Low molecular weight protamine as nontoxic heparin/low molecular weight heparin antidote (III): preliminary in vivo evaluation of efficacy and toxicity using a canine model. AAPS PharmSci. 2001;3:E19. doi: 10.1208/ps030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J.F., Zhen L., Chang L.C., Yang V.C. A less toxic heparin antagonist—low molecular weight protamine. Biochemistry (Mosc) 2003;68:116–120. doi: 10.1023/a:1022109905487. [DOI] [PubMed] [Google Scholar]
- 32.Lax A.J. Opinion: bacterial toxins and cancer—a case to answer? Nat Rev Microbiol. 2005;3:343–349. doi: 10.1038/nrmicro1130. [DOI] [PubMed] [Google Scholar]
- 33.Kilic Ü., Kilic E., Dietz G.P., Bähr M. Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke. 2003;34:1304–1310. doi: 10.1161/01.STR.0000066869.45310.50. [DOI] [PubMed] [Google Scholar]
- 34.Hou Y.W., Chan M.H., Hsu H.R., Liu B.R., Chen C.P., Chen H.H. Transdermal delivery of proteins mediated by non-covalently associated arginine-rich intracellular delivery peptides. Exp Dermatol. 2007;16:999–1006. doi: 10.1111/j.1600-0625.2007.00622.x. [DOI] [PubMed] [Google Scholar]
- 35.Dick A., Kromen W., Jüngling E., Grosskortenhaus S., Kammermeier H., Vorwerk D. Quantification of horseradish peroxidase delivery into the arterial wall in vivo as a model of local drug treatment: comparison between a porous and a gel-coated balloon catheter. Cardiovasc Intervent Radiol. 1999;22:389–393. doi: 10.1007/s002709900413. [DOI] [PubMed] [Google Scholar]
- 36.Dixon J.E., Osman G., Morris G.E., Markides H., Rotherham M., Bayoussef Z. Highly efficient delivery of functional cargoes by the synergistic effect of GAG binding motifs and cell-penetrating peptides. Proc Natl Acad Sci U S A. 2016;113:E291–E299. doi: 10.1073/pnas.1518634113. [DOI] [PMC free article] [PubMed] [Google Scholar]