Abstract
Dry powder inhalers (DPIs) offer distinct advantages as a means of pulmonary drug delivery and have attracted much attention in the field of pharmaceutical science. DPIs commonly contain micronized drug particles which, because of their cohesiveness and strong propensity to aggregate, have poor aerosolization performance. Thus carriers with a larger particle size are added to address this problem. However, the performance of DPIs is profoundly influenced by the physical properties of the carrier, particularly their particle size, morphology/shape and surface roughness. Because these factors are interdependent, it is difficult to completely understand how they individually influence DPI performance. The purpose of this review is to summarize and illuminate how these factors affect drug–carrier interaction and influence the performance of DPIs.
Abbreviations: API, active pharmaceutical ingredient; CLF, coarse lactose fines; dae, aerodynamic diameter; DPI, dry powder inhaler; ED, emission dose; ER, elongation ratio; FLF, fine lactose fines; FPF, fine particle fraction; FR, flatness ratio; Fshape, shape factor; Fsurface, surface factor; MFV, minimum fluidization velocity; PDD, pulmonary drug delivery; pMDI, pressurized metered-dose inhaler; RO, roundness
KEY WORDS: Carrier, Particle size, Morphology, Surface roughness, Performance, Dry powder inhaler
Graphical abstract
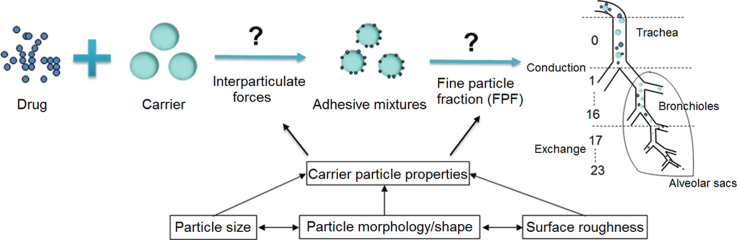
The physical properties of carrier particles used in dry powder inhalers (DPIs) particularly their size, morphology/shape, and surface roughness play a significant role in determining DPI performance by influencing the adhesion and detachment of drug–carrier adhesive mixtures. However, it is difficult to completely understand how they influence DPI performance as they are interdependent.
1. Introduction
With increases in our understanding of the physiology of the lung and related diseases, pulmonary drug delivery (PDD) is becoming an alternative choice to treat local and systemic diseases. PDD systems take a variety of forms ranging from nebulizers to inhalers1 and deliver drug directly to the site of action in the lung or to a distant site via the bloodstream. PDD possesses several distinct advantages. First, due to the high permeability, large surface area (about 100 m2) and thin adsorption membrane (0.1–0.2 µm) of the lung, and because of its excellent blood supply (5 L/min), inhalation produces rapid systemic onset almost comparable to intravenous injection2. Secondly, because the lung exhibits relatively low metabolic activity, drugs delivered via the lung are not susceptible to first pass metabolism making the lung an attractive administration route for proteins and peptides3. For these reasons, PDD is highly desirable for the treatment of patients with pulmonary diseases such as pneumonia, asthma, cystic fibrosis, chronic obstructive pulmonary disease and lung cancer.
PDD systems can be divided into three major categories viz nebulizers, pressurized metered-dose inhalers (pMDIs) and dry powder inhalers (DPIs). Nebulizers, the first type of device developed for inhalation therapy, utilize an air jet or ultrasound to convert a drug solution or suspension into fine droplets which are then inhaled by the patient over a couple of minutes4. However, nebulizers are not portable, suffer from poor reproducibility in delivering an accurate dose and are only commonly used in hospitals. Since the 1950s, portable pMDIs have been developed and have become the mainstay of asthma therapy with good patient compliance. Nevertheless, they require good coordination between actuation and inhalation and only a small fraction of drug reaches the patient׳s lungs due to the high particle exit velocity. They are also environmentally unfriendly because they require a chlorofluorocarbon propellant that depletes the ozone layer. In contrast, the DPI is propellant-free, portable, easy to operate, low-cost, and provides better formulation stability than liquid dosage forms. In particular, the development of DPIs was stimulated by the Montreal Protocol (1987) which recommended the removal of chlorofluorocarbon propellants5, 6.
DPIs consist of an active pharmaceutical ingredient (API) of suitable aerodynamic size (usually 1–5 µm) for inhalation7, contained within a device which, upon inhalation, provides sufficient deagglomeration of particles to deliver a therapeutic dose to the lungs. The main problem with particles of this small micron size is that their high surface free energy makes them stick to each other (via cohesive forces) or to any surface they encounter (via adhesive forces). As a result, they exhibit poor flowability and aerosolization performance and have a propensity to remain within the inhaler. In addition, many APIs used for treating local diseases such as asthma are highly potent and require only a low dose (200–400 µg for salbutamol and 6–12 µg for formoterol)8 which poses significant problems in relation to powder handling and accurate metering of doses. Recently, a DPI containing carrier particles as well as drug has been developed to overcome these limitations. The functions of the carrier include (1) improving flowability of drug particles to facilitate filling the DPI, (2) increasing dispersion of drug particles during emission and (3) diluting the drug to improve accurate dose delivery7.
Aerodynamic diameter (dae) is the best parameter to evaluate the ability of fine drug particles to deposit deep within the lung. It is defined (Eq. (1)) as the diameter of spherical particles of unit density that reach the same terminal velocity and deposition as the particles under investigation9:
| (1) |
where dg is the geometric diameter of the spherical particle, ρp and ρ0 represent the particle density and unit density respectively and χ is the shape factor.
This equation indicates that dae is influenced by particle size, morphology/shape and density. For porous particles with low density, dae≪dg10, and particle size has a greater effect on drug deposition than particle density. Since the amount of API in a DPI is relatively low (0.05%–10%)11, a slight change in the physical properties of the carrier has a considerable effect on DPI performance. It was also reported that carrier surface properties (e.g., surface area, morphology and roughness) play a significant role in determining interparticulate interactions, stability, ease of dispersion, and de-agglomeration12. Therefore, considerable researches have focused on particle characteristics of carriers to investigate their influence on the performance of DPIs. These important carrier characteristics are discussed below.
2. Approaches to produce DPI formulations
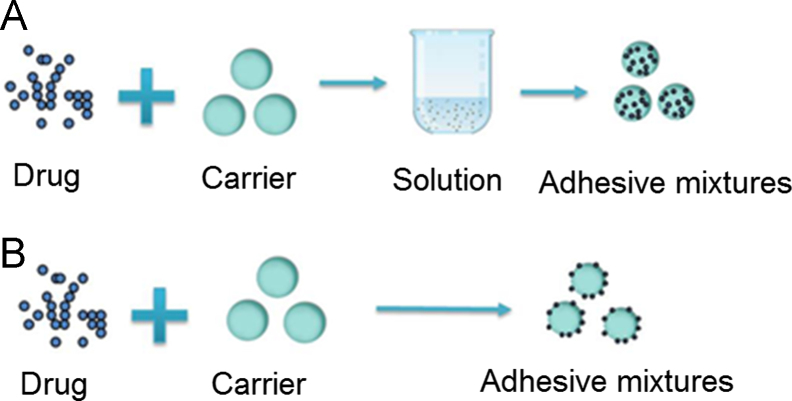
As shown in Fig. 1, there are commonly two approaches to produce a DPI formulation. One approach is to dissolve drug and carrier in a solvent and then remove solvent by spray drying or other methods (Fig. 1A). The size of the resulting particles is in the range 1–5 µm which, on inhalation, ensures the drug is deposited deep in the lung. The second approach is to combine drug and carrier via particle interactions (Fig. 1B) so that, on inhalation, drug is carried past the respiratory tree and released deep in the lung. Carriers used are commonly coarse particles with a size range of 50–200 µm13 which are designed to be swallowed after impact with the upper respiratory tract14 so that only fine drug particles are deposited deep in the lung. Due to the lack of toxicological data concerning the potential hazard of carriers to lung tissue, the number of carrier materials currently approved or certified safe by the U.S. Food and Drug Administration (FDA) remains limited so much so that most commercially available DPI formulations rely on lactose as the carrier15. Therefore, DPIs in which the API is physically combined with carrier are superior in reducing lung deposition and adverse effects of the carrier while retaining lung deposition of drug. Section 3 focuses on such physically combined DPI formulations.
Figure 1.
Two methods of combining drug and carrier for use in dry powder inhalers.
3. Interparticulate interactions
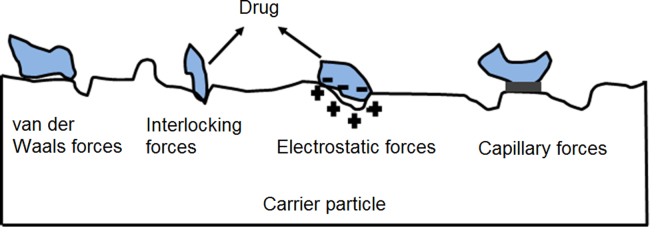
In the development of a DPI formulation, two types of interparticulate interaction should be taken into consideration viz the drug–drug cohesive force and the drug–carrier adhesive force16. The drug–carrier adhesive force fundamentally determines the performance of a DPI since excessive adhesion limits drug detachment from the carrier during aerosolization leading to poor drug dispersion17. Generally, the adhesion of a micron-sized particle to a solid surface is governed by physical forces (Fig. 2) including the van der Waals force18, interlocking force19, electrostatic force20 and capillary force21.
Figure 2.
Physical interparticulate forces between the drug and carrier.
Under conditions where particles can dissipate excess electric charge and humidity is controlled, the van der Waals force is the dominant interaction and creates a so-called ‘‘Velcro effect’’ between particles20, 22. The interlocking force is involved when drug particles fit into cavities upon intimate contact with the carrier surface23. The electrostatic force occurs when two materials with different surface charge come into contact and then separate23, 24. The capillary force is developed due to the formation of a liquid bridge between particles which is influenced by the surrounding relative humidity and varies with the type of drug used in the formulation21, 25, 26.
Overall, the contribution of these forces to particle adhesion is dependent on environmental conditions and several factors related to the particle such as its surface physical properties, mechanical properties27, area, size and solid-state nature26. Because the performance of a DPI formulation depends on dissociation of drug from carrier particles and deaggregation of drug agglomerates during aerosolization, any factor that affects the drug–carrier interaction may also affect drug delivery and deposition28. To ensure an inhaled formulation is therapeutically effective, the interactions should be strong enough to ensure homogeneity and stability during powder handling but sufficiently weak to allow the formulation to be readily dispersed18, 29. It is worth noting that the dispersion of a drug–carrier mixture is greatly influenced by the air velocity, the higher the airflow the greater the detachment of API particles from the carrier30.
Several formulation approaches have been applied to improve the aerosolization performance of drug–carrier adhesive mixtures. They generally focus on minimizing the intrinsic cohesion of the powder and/or reducing the interaction between drug and carrier. Specifically, the approaches include particle size refinement16, morphology design31 and surface modification32.
4. Mechanisms of aerosol generation and deposition
Unlike oral dosage forms, the efficiency of a DPI principally depends on the extent to which the drug particles in the formulation can be dispersed into an aerosol during inhalation33. Only free particles in the inhalatory airstream that have overcome the interparticulate forces within the bulk powder can be delivered deep into the lung. Therefore, one of the main challenges in the inhalation field is to reproducibly deliver the highest dose fraction of drug to the lung. Until now, most research into the development of DPIs has focused on improving the fine particle fraction (FPF) to generate an efficient aerosol by particle engineering of the carrier. FPF denotes the percentage relative to the total quantity of drug collected in the impactor or impinger that has a size ≤5 μm. The higher the FPF the better the aerosolization efficiency. FPF is influenced by the inhalation device34, formulation35, 36 (characteristics and downstream processability of the carrier, drug to carrier ratio), and in vitro characteristics of the aerosol37 (delivery time and rate of delivery).
However, the mechanism of aerosol generation by a DPI remains complex. The scheme of airway geometry shown in Fig. 3 indicates that the airway can be divided into two distinct zones viz the conducting zone and the respiratory zone38. The conducting zone consists of the first 16 generations beginning with the trachea (generation 0) and ending with the terminal bronchioles (generation 16). Its main function is to allow the bulk flow of air to move into and out of the lung during each breath. The respiratory zone is where gas exchange occurs. It starts at the respiratory bronchioles (generation 17) and terminates at the alveolar sacs (generation 23). In moving from trachea to alveolar sacs, there are two pronounced physical changes along the airways; the airway caliber decreases and the cross-sectional area of the airways increases as the number of airways increases12. These changes lead to variations in air flow velocity and airway surface area which have significant effects on drug deposition in the lung.
Figure 3.
Mechanisms of aerosol generation and deposition of drug in the airways for a dry powder inhaler.
According to Hickey et al.39, the aerosolization process can be roughly divided into four consecutive phases: detachment from the static powder bed, fluidization, entrainment and drug resuspension (Fig. 3). The powder bed remains static until the airflow generated by the DPI device transfers kinetic energy into the bed causing powder deaggregation. Powder fluidization is the process in which the powder mass disturbed by the airstream exhibits “fluid-like” properties40. It is primarily governed by the packing properties of the powder which are closely related to the physical properties of the particles and their interfacial interactions41. Following fluidization, the powder is entrained into the airflow42, and these two processes are critical in the ability of a DPI to generate a therapeutically effective aerosol. Drug resuspension is mainly performed by deagglomeration forces including turbulent, inertial and impacting stresses and is followed by deposition of drug in the respiratory tract39.
Typically, there are three mechanisms (Fig. 3) governing particle deposition in lung airways. (1) Inertial impaction: this involves inertial deposition of particles onto the airway surfaces and mainly occurs close to bifurcations of the large conducting airways43. Most large particles (>6 μm) are deposited in the oropharyngeal and large airways because they are unable to follow the directional changes of the inspired airstream particularly in the oropharynx and at airway bifurcations. Thus loss of drug due to inertial impaction in the oropharynx is the major hurdle to achieve lung deposition using a passive dry powder device44. (2) Gravitational sedimentation: this usually involves small particles in the size range 2–6 µm and occurs in the small conducting airways where the airflow velocity is slow12. (3) Diffusion: this involves small particles (<2 µm) for which Brownian motion is important and occurs in the small airways and alveoli where the airflow is negligible12. Overall, aerosol generation results from a competition between interparticulate interactions within the adhesive mixture and separation forces resulting from the inspiratory airflow through the inhaler.
5. Influence of carrier characteristics on aerosol performance
5.1. Particle size
It is well recognized that particle size of the carrier plays a dominant role in the aerosolization performance of carrier-based DPI formulations. However, there is, as yet, no consensus on how carrier particle size affects DPI performance. One study reported that reducing particle size improves the amount of respirable drug delivered from a DPI16 but has adverse effect on drug content uniformity and results in more drug deposited in the oropharyngeal region16. Interestingly, carrier particle size does not necessarily impact negatively on drug deposition after inhalation and in another study a higher FPF was observed45. These conflicting results could be due to the interdependence of physical properties. Whatever the case, particle size must be optimized to provide efficient aerosolization and overcome the disadvantages of small particles.
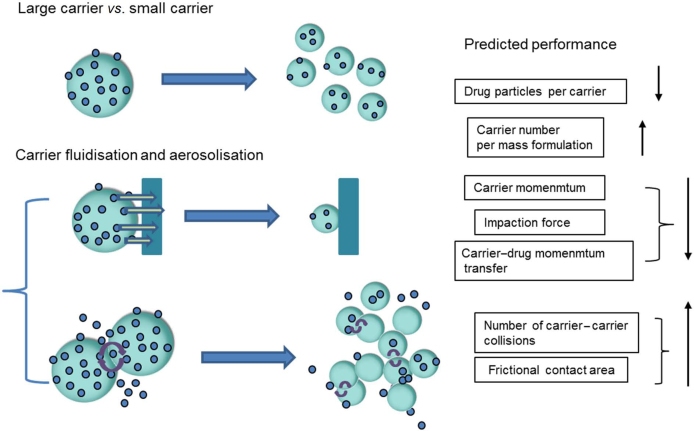
To establish the optimum particle size of aerosols, it is important to take into account the physical properties of carrier particles including their shape, surface roughness, density and geometric diameter43. The effect of these variables on aerosol performance has been studied by Ooi et al.46 using three model polystyrene spheres with d0.5 values of 82.8, 277.5 and 582.9 μm as carriers. The results showed that aerosol performance increased as particle size decreased, a finding ascribed to the decreasing particle size, including decreased number of drug particles per carrier and the increases in particle number, surface area, intercarrier adhesion and number of collisions in the powder bed during aerosolization (Fig. 4). Drug release appears to be primarily driven by the number of frictional and rotational collisions rather than conventional momentum transfer.
Figure 4.
Concurrent changes of powder fluidization and aerosolization as a function of particle size (↑ increase, ↓ decrease).
5.1.1. Particle size distribution
Particle size distribution is important in terms of aerosol quality and efficiency47. It is commonly evaluated by determining polydispersibility (PDI) which is calculated according to Eq. (2). A larger value of PDI of the carrier indicates a wider particle size distribution, and produces a more heterogeneous mixture with drug. This may lead to higher variability in lung deposition of drug upon inhalation16.
| (2) |
Measurement of particle size and particle size distribution can be conducted using sieving, optical microscopy, and laser diffraction particle size analyzer. Sieving is considered to be a rough method in determining particle size because it does not give exact measurements of any dimension of the particles48. Microscope image analysis is used for umber-weighted size measurement, while laser diffraction volume is used for weighted size measurement. On the other hand, the accuracy of size measurement by laser diffraction is affected by the particle shape and surface properties. Particles with irregular shape and rough surface morphology may lead to “overestimated” size measurements, as observed by Kaialy et al.49.
5.1.2. Role of fine carrier particles
The inclusion of a small amount of fine carrier particles (fines) in a DPI formulation is a well-researched technique to improve DPI performance. The actual definition of fines is unclear in the literature but there is agreement that fines have a small particle size compared to coarse particles10, 13, 23. It has also been suggested that fines with similar geometric size to that of the API should be used50. However, the optimum diameter of fines for use in a DPI formulation has not reached a consencus51.
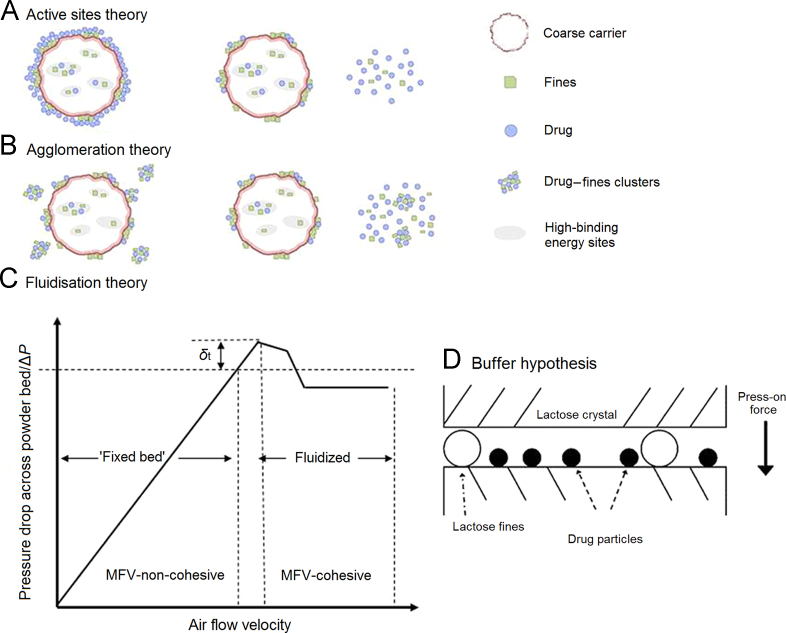
There have been numerous studies investigating how fines affect the performance of DPI formulations with contradictory results. Some studies showed that a small quantity of fines included in a powder formulation could increase the deaggregation efficiency and therefore the therapeutic efficacy of the DPI52, 53. However, Hamishehkar et al.54 demonstrated that the addition of fines with spray dried mannitol and leucine decreased both the deposition and dispersibility of microcapsules. Steckel et al.55 also reported that the presence of fines caused a decrease in FPF. Several hypotheses to explain how the presence of fines affects the aerosolization performance of a DPI have been proposed based on studies of lactose fines. These include the active-sites56, agglomeration52 and fluidization theories57, the buffer hypothesis58 and case-dependent59 theory. With the exception of case-dependent theory, these theories address the positive effects of fines on the dispersion performance of adhesive mixtures. However, the long-term safety of excipients for use as fines remains to be established and is a matter of concern to regulatory authorities51. For example, fines may cause irritation, coughing and even bronchoconstriction60 and in the case of inhaled mannitol can increase bronchial hyperresponsiveness61.
5.1.2.1. Active-sites theory
This was first introduced by Hersey62 and subsequently widely applied to understand the behavior of adhesive mixtures. The so-called “active sites”, defined as areas on the carrier surface that are more adhesive than others (Fig. 5A), are preferentially occupied by fines leaving the weaker binding sites to be occupied by drug particles23, 59. However, Grasmeijer et al.63 pointed out that a specific definition of “active sites” has never been established and that the theory is ambiguous in predicting how active sites affect the performance of a powder for inhalation. As a result, “active sites” have been newly defined based on their ability to retain drug during dispersion which depends on the physical properties of the carrier surface, formulation and dispersion conditions. A rational choice of such conditions is of utmost importance in studies concerning carrier surface site activity.
Figure 5.
Theories and hypothesis describing the effect of fine carrier particles on the aerosolization performance of a dry powder inhaler: (A) “Active sites theory”; (B) “agglomeration theory”; (C) “fluidization theory” and (D) “buffer hypothesis” (modified from Refs. 47 and 48.
5.1.2.2. Agglomeration theory
According to this theory and as shown in Fig. 5B, the improved DPI performance due to the presence of fines results from the formation of drug-fine agglomerates. These are more likely to be removed from the carrier particle surface than free API particles due to the greater aerodynamic drag force acting on agglomerates64.
5.1.2.3. Fluidization theory
As illustrated in Fig. 5C, fluidization of a DPI formulation occurs when the pressure drop across the static powder bed is equivalent to the weight of the powder40. This pressure threshold is referred to as the point of incipient fluidization or minimum fluidization velocity (MFV)42. The addition of fines improves DPI performance by increasing the tensile strength of the formulation which is directly related to interparticulate forces and the free volume of the carrier. This significantly shifts the MFV and thereby increases the aerodynamic drag force exerted to fluidize the powder bed57.
5.1.2.4. Buffer hypothesis
The commercially available brands of alpha-lactose monohydrate normally exhibit surface rugosity and carry natural fines and impurities on their surface which may influence their interaction with drug in adhesive mixtures for inhalation. In a study involving submerging lactose in ethanol–water mixtures, Dickhoff et al.58 found that submersion removed the adhering lactose fines leading to a decrease in drug particle detachment without affecting the shape or size of carrier particles. This is the basis of the buffer hypothesis (Fig. 5D) which states that the adhering lactose fines act as a buffer between colliding carrier particles and protect smaller drug particles attached to the same crystal planes from the press-on forces that cause increased drug particle detachment during inhalation58.
5.1.2.5. Case-dependent theory
Contrary to the mechanisms described above, Grasmeijer et al.59 pointed out that fines do not always improve the aerosol performance of a DPI which is determined by the formulation and dispersion conditions. They studied the effects of “fine lactose fines” (FLF) with similar size and shape as micronised budesonide on drug detachment and compared the results with those obtained using “coarse lactose fines” (CLF) at varying inhalation flow rates, drug contents and mixing orders. It was found that the presence of CLF resulted in higher detachment of drug at all flow rates and drug contents and the effects of FLF were negligible at high drug content. These results implicate two new mechanisms. First, fines below a certain size reduce dispersion performance probably by increasing the effectiveness of press-on forces or the formation of coherent fine particle networks on the carrier surface. Secondly, the CLF may weaken or prevent the formation of fine particle networks possibly through lowering tensile strength.
5.2. Morphology/shape
The morphology/shape of carrier particles exerts a dominant effect on the aerosolization performance of a DPI31. Particles with different shapes may be subject to different drag forces and terminal velocities during aerosolization which, in turn, affect their deposition in the respiratory airways47.
The effect of particle shape on aerosolization performance has been widely investigated. Kaialy et al.31 examined carriers with different morphologies to investigate the effect of elongation ratio (ER). They concluded that the higher the ER the greater the delivery of salbutamol sulphate to the lower airway regions of the lung, indicating enhanced DPI performance. However, this improvement was restricted to values below a certain “limit” since the higher the ER the more the drug remained in the inhaler device and deposited in the throat. Using lactose as a carrier, Kho et al.65 also investigated the effect of carrier particle shape in two size ranges (i.e. 50–70 μm and 14–20 μm) (Fig. 6)65 on aerosolization efficiency of drug–carrier particle blends containing amorphous nanoparticles of drug. The results revealed that lactose particles of tomahawk shape gave similar aerosolization efficiency to those with needle and pollen shape for both large and small carrier particles, a finding contrary to that of a previous study using drug microparticles66, 67, 68. Needle shaped carrier particles generally have a high ER which allows drug deposition in the small airways44 resulting in more effective drug deposition in the lung66. Pollen shaped carrier particles with low density bind drug for longer periods and thereby give higher lung deposition67, 68. According to Hassan et al.67, pollen-shaped carrier particles give a higher FPF and reduce drug loss especially at low flow rates and high drug content. However, size and surface morphology of carrier particles can have opposing effects as indicated by the fact that an increase in particle size has a mild effect on emission dose (ED) but may significantly improve the FPF while a sparse surface has negligible effect on the ED at low flow rates but improves the FPF.
Figure 6.
Scanning electron microscopy images of (A) large CL, (B) small CL, (C) large PL, (D) small PL, (E) large NL, and (F) small NL carrier particles (reproduced from Ref. 65 with permission).
As shown in Table 17, 16, 28, 31, 69, 70, shape analysis is commonly conducted using scanning electron microscopy (SEM), optical microscopy or some other imaging technique. The parameters used to quantify particle shape include ER, flatness ratio (FR), roundness (RO), shape factor (Fshape), angularity, and surface factor (Fsurface).
Table 1.
Shape descriptors and characterization methods to evaluate particle shape or morphology.
| Shape descriptor | Order rank | Equation | Determination method | Ref. |
|---|---|---|---|---|
| Elongation ratio (ER) | First | Optical microscopy or scanning electron microscopy (SEM) | 28, 69 | |
| Flatness ratio (FR) | First | Optical microscopy | 7 | |
| Roundness (RO) | — | Optical microscopy | 31 | |
| Shape factor (Fshape) | Second | Optical microscopy | 70 | |
| Angularity | Second | Optical microscopy | 16 | |
| Surface factor (Fsurface) | Third | Optical microscopy | 7 |
A, the estimated area of the particle;
Angularity, a parameter to quantify particle shape;
L, length, the maximum Feret diameter;
p, Perimeter, the estimated perimeter of particle with compensation for corners;
Pconvex, perimeter of the minimum convex boundary circumscribing the particle;
Pelipse, perimeter of fictitious equivalent ellipse which has the same area and the aspect ratio of aggregate particle;
w, width, the minimum Feret diameter;
—, there has been no rank order designated for RO.
ER and FR are considered to be first order shape descriptors71. ER is a measure of the irregularity of particles which reflects overall particle shape elongation72. A higher value of ER indicates a more elongated/irregular shape and/or a rougher surface73 and follows a similar trend as RO. Carrier particles with high ER and RO values are likely to exhibit pronounced internal friction due to their angular shape and are expected to produce different aerosolization characteristics74, 75. It has been reported that carrier particles with high ER can significantly increase the amount of drug delivered to lower airway regions of the lung but only up to a certain level. However, carrier particles with high ER are disadvantageous in DPI dose metering and processing at handling scale due to their poor flowability31. A high FR is indicative of a more flattened shape7, while a high value of ER/FR is indicative of a more elongated/less flattened particle shape. Smooth spheres and perfect cubes have ER and FR of 1.
Fshape and angularity are second order descriptors of particle shape irregularity7, 71. The value for Fshape ranges from −1 to 1 where a smooth sphere has Fshape of 170 and a smaller Fshape value indicates greater shape irregularity and/or rougher particle surface7. Particle angularity is independent of particle ER76. However, values of Fshape and ER alone may be insufficient to describe the effect of particle shape since these parameters are functions of particle orientation and contact area only which can influence the accuracy of shape assessment using microscopic image analysis70, 77. SEM is also needed to characterize qualitatively the three dimensional shape and surface morphology of carrier particles.
Fsurface is a third order shape descriptor which refers to surface roughness only7. Cubic particles with smooth surface are expected to have Fsurface of 1, and a smaller Fsurface value indicates a rougher surface.
Of these various shape descriptors of particles, ER is solely determined by their macroscopic shape while rugosity and Fshape are dependent on both macroscopic shape and surface texture. Neither rugosity nor Fshape can distinguish the surface smoothness of two particles if they differ substantially in macroscopic shape. Therefore, in order to accurately compare the surface smoothness of particles with different ER values, a new shape descriptor, Srec, was introduced by Zeng et al.28. Srec is a factor that assumes a rectangular shape and takes both Fshape and ER into consideration.
5.3. Surface roughness
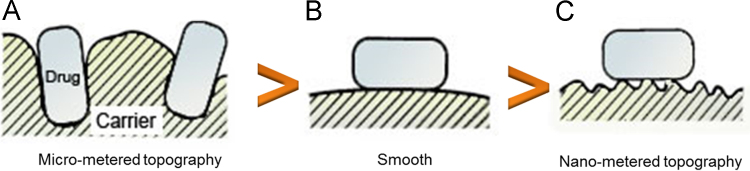
For efficient delivery of drug deep into the lung, drug particles need to be efficiently loaded onto and liberated from the carrier, processes which are largely dependent on characteristics of the contact area. Most pharmaceutical grade carriers for use in DPIs have particles with a certain amount of surface roughness67 which can be categorized into three groups (Fig. 7)78. First, carrier particles of micrometer topography generally have a large contact area for micronized drugs to adhere to, resulting in strong interaction and poor aerosol efficiency. Additionally, entrapment of drug in the cavities or valleys of the carrier surface may further reduce drug–carrier detachment. Secondly, carrier particles of nanometer topography can provide binding sites for drugs which are favorable for drug detachment from the carrier surface after inhalation thus contributing to higher FPF values7. Thirdly, carrier particles with smooth surfaces also have a large contact area for drug attachment leading to poor drug–carrier detachment. Therefore, only carrier surface irregularity of a certain scale, namely nanometer topography, provides a sufficiently low total contact area for drug to bind with weak adhesive forces78. Particles with large surface irregularity (micrometer topography) or irregularity approaching zero (smooth) bind drug with strong adhesive force.
Figure7.
Carrier particles with three different sizes of surface roughness; (A) micrometer topography, (B) smooth, and (C) nanometer topography (modified from Ref. 78).
Reports about the influence of surface roughness on aerosolization performance of a DPI are contradictory. For example, Kaialy et al.7, 79 found that rougher lactose particles showed a smaller adhesion force with salbutamol sulphate and produced enhanced DPI performance. In contrast, Flament et al.80 found a linear relationship between lactose roughness and FPF, the rougher the lactose surface the greater the adherence of terbutaline sulphate and the lower the FPF. This linear relationship can be explained as due to an increase in contact points between drug and carrier as surface roughness increases. This stabilizes the blend but leads to less drug deaggregation from carrier when the blend is carried by an airflow. Since particle size and shape also influence interparticulate forces, study of the influence of carrier surface properties on aerosol performance of a DPI becomes even more difficult. In one study, glass beads with different surface roughness but the same size and shape were used as model carriers to investigate the relationship81. The surface-modified glass beads were produced in a ball mill using different grinding materials and grinding times followed by plasma etching. The results indicate that the greater the surface roughness the higher the FPF.
These conflicting findings reveal that, in order to increase FPF, an optimum level of surface roughness is needed. They also suggest that modifying surface morphological properties is an effective way to alter particle interactions and improve aerosol performance. To date, various techniques have been employed to modify the surface roughness of carriers in order to achieve a high FPF value. These include mechanical milling11, spray drying82, 83, freeze drying47, anti-solvent precipitation from different media and under different crystallization conditions31, 84, polymer coating via spray drying85, dry mechano-fusion29, adding fine particles to fill in carrier “active sites”23, 59, and adding agents such as leucine86, magnesium stearate87, and polaxamer88 to reduce surface passivation of high surface free energy sites.
Since surface roughness is closely related to surface energy and the adhesion force between drug and carrier, its assessment using direct and indirect methods is very important (Table 27, 8, 15, 29, 70, 82, 83, 86, 89, 90, 91). Atomic force microscopy (AFM), SEM, confocal laser scanning microscopy (CLSM) and particle image analysis can directly provide data related to surface roughness. The drug–carrier adhesion force can also be measured by colloid probe microscopy and air jet sieving.
Table 2.
Characterization methods to determine surface roughness of carrier particles.
| Characterization method | Equation | Result | Ref. | |
|---|---|---|---|---|
| Direct method | Atomic force microscopy (AFM) | No significance between the surface roughness of composite carriers and regular carriers was observed due to the increased variability in sample morphology of the regular carrier. | 8 | |
| Scanning electron microscopy (SEM) | Particles produced at large scale exhibited changes not only in surface roughness but also in shape at varying outlet temperatures. A decrease in surface roughness led to a lower FPF. | 29 | ||
| Confocal laser scanning microscope (CLSM) | — | The obtained roughness profiles were consistent with results of SEM image analysis and showed distinct trends. In general, rough particles obtained at low outlet temperatures were spherical, whereas higher drying temperatures resulted in particles with multiple surface indentations and smoother surface. Different roughness was a result of underlying crystallization processes. | 15, 82, 83 | |
| Particle image analysis | All crystallized particles had rougher surfaces than the commercial lactose, leading to improved drug aerosolization performance due to lower drug–carrier adhesion forces. | 70 | ||
| Indirect method | Colloid probe microscopy | — | Median force (F0.5) value was chosen as the best descriptor of drug–carrier adhesion force. The values for polymer coated/uncoated carriers followed a rank order of PVP coated >un-coated >EC coated lactose, which showed an opposite trend of FPF. | 86, 89, 90 |
| Air depression sieving | — | It was considered as a simple method to evaluate drug–carrier adhesion force, based on the aspiration principle that the whole blend used in DPI was taken into account. The detailed procedure was presented by Le et al.91. Greater drug–carrier adhesion force was obtained under higher humidity, leading to decreased FPF. Kaialy et al.7 also used this method to evaluate the adhesion force between salbutamol sulphate and different lactose grades in the same size range, demonstrating that lactose particles with more elongated/irregular shape, and rougher surface were preferred to improve FPF. | 7, 91 | |
ConvexPerim, circumscribed particle perimeter;
l, length;
N, the number of data points in a topographical profile;
Perimeter, estimated particle perimeter;
Ra, the mean Ra values of several line profiles over the analyzed surface;
RRMS, surface root mean square roughness;
yi, the distance of asperities (i) from the center line;
Z, the deviation of all points from a plane fit to the test surface over sampling length l;
—, no equation was reported for the method.
6. Conclusions
Carrier physical properties, such as particle size, morphology, and surface roughness, play significant roles in determining DPI performance since they directly influence the adhesion and detachment of drug and carrier. Studies of the effects of these properties on drug aerosolization efficiency have given controversial results for two main reasons. First, a complete understanding of the independent influence of each property remains challenging as the properties are interdependent. Secondly, most studies have focused on one property and given less attention to others. Despite these limitations, it is clear that higher drug deposition is achieved by reducing the adhesion force of drug and carrier in mixed DPI formulations.
Acknowledgements
The work was supported by Pearl River S&T Nova Program of Guangzhou (2014J2200082).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Xin Pan, Email: pxin_1385@163.com.
Chuanbin Wu, Email: chunabin_wu@126.com.
References
- 1.Anselmo A.C., Mitragotri S. An overview of clinical and commercial impact of drug delivery systems. J Control Release. 2014;190:15–28. doi: 10.1016/j.jconrel.2014.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilcer G., Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 2010;392:1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 3.Patton J.S. Inhalation delivery of therapeutic peptides and proteins. J Aerosol Med. 1999;12:45–46. doi: 10.1089/jam.1999.12.45. [DOI] [PubMed] [Google Scholar]
- 4.Steckel H., Eskandar F. Factors affecting aerosol performance during nebulization with jet and ultrasonic nebulizers. Eur J Pharm Sci. 2003;19:443–455. doi: 10.1016/s0928-0987(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 5.Newman S.P. Metered dose pressurized aerosols and the ozone layer. Eur Respir J. 1990;3:495–497. [PubMed] [Google Scholar]
- 6.Jarabek A.M., Fisher J.W., Rubenstein R., Lipscomb J.C., Williams R.J., Vinegar A. Mechanistic insights aid the search for CFC substitutes: risk assessment of HCFC-123 as an example. Risk Anal. 1994;14:231–250. doi: 10.1111/j.1539-6924.1994.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 7.Kaialy W., Ticehurst M., Nokhodchi A. Dry powder inhalers: mechanistic evaluation of lactose formulations containing salbutamol sulphate. Int J Pharm. 2012;423:184–194. doi: 10.1016/j.ijpharm.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 8.Young P.M., Roberts D., Chiou H., Rae W., Chan H.-K., Traini D. Composite carriers improve the aerosolisation efficiency of drugs for respiratory delivery. J Aerosol Sci. 2008;39:82–93. [Google Scholar]
- 9.Hassan M.S., Lau R. Effect of particle formulation on dry powder inhalation efficiency. Curr Pharm Design. 2010;16:2377–2387. doi: 10.2174/138161210791920423. [DOI] [PubMed] [Google Scholar]
- 10.Hickey A.J. Marcel Dekker; New York: 2003. Pharmaceutical inhalation aerosol technology. [Google Scholar]
- 11.Guchardi R., Frei M., John E., Kaerger J. Influence of fine lactose and magnesium stearate on low dose dry powder inhaler formulations. Int J Pharm. 2008;348:10–17. doi: 10.1016/j.ijpharm.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 12.Lee S.L., Adams W.P., Li B.V., Conner D.P., Chowdhury B.A., Yu L.X. In vitro considerations to support bioequivalence of locally acting drugs in dry powder inhalers for lung diseases. AAPS J. 2009;11:414–423. doi: 10.1208/s12248-009-9121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zellnitz S., Redlinger-Pohn J.D., Kappl M., Schroettner H., Urbanetz N.A. Preparation and characterization of physically modified glass beads used as model carriers in dry powder inhalers. Int J Pharm. 2013;447:132–138. doi: 10.1016/j.ijpharm.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Littringer E.M., Paus R., Mescher A., Schroettner H., Walzel P., Urbanetz N.A. The morphology of spray dried mannitol particles—the vital importance of droplet size. Powder Technol. 2013;239:162–174. [Google Scholar]
- 15.Littringer E.M., Noisternig M.F., Mescher A., Schroettner H., Walzel P., Griesser U.J. The morphology and various densities of spray dried mannitol. Powder Technol. 2013;246:193–200. [Google Scholar]
- 16.Kaialy W., Alhalaweh A., Velaga S.P., Nokhodchi A. Influence of lactose carrier particle size on the aerosol performance of budesonide from a dry powder inhaler. Powder Technol. 2012;227:74–85. [Google Scholar]
- 17.Begat P., Morton D.A., Staniforth J.N., Price R. The cohesive–adhesive balances in dry powder inhaler formulations I: direct quantification by atomic force microscopy. Pharm Res-Dordr. 2004;21:1591–1597. doi: 10.1023/b:pham.0000041453.24419.8a. [DOI] [PubMed] [Google Scholar]
- 18.Singh D.J., Jain R.R., Soni P.S., Abdul S., Darshana H., Gaikwad R.V. Preparation and evaluation of surface modified lactose particles for improved performance of fluticasone propionate dry powder inhaler. J Aerosol Med Pulm D. 2015;28:254–267. doi: 10.1089/jamp.2014.1146. [DOI] [PubMed] [Google Scholar]
- 19.Telko M.J., Hickey A.J. Dry powder inhaler formulation. Respir Care. 2005;50:1209–1227. [PubMed] [Google Scholar]
- 20.Karner S., Maier M., Littringer E., Urbanetz N.A. Surface roughness effects on the tribo-charging and mixing homogeneity of adhesive mixtures used in dry powder inhalers. Powder Technol. 2014;264:544–549. [Google Scholar]
- 21.Price R., Young P.M., Edge S., Staniforth J.N. The influence of relative humidity on particulate interactions in carrier-based dry powder inhaler formulations. Int J Pharm. 2002;246:47–59. doi: 10.1016/s0378-5173(02)00359-9. [DOI] [PubMed] [Google Scholar]
- 22.Smyth H.D.C., Hickey A.J. Springer; Germany: 2011. Controlled pulmonary drug delivery. [Google Scholar]
- 23.Pilcer G., Wauthoz N., Amighi K. Lactose characteristics and the generation of the aerosol. Adv Drug Deliv Rev. 2012;64:233–256. doi: 10.1016/j.addr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Harper W.R. Laplacian Press; Morgan Hill: 1998. Contact and frictional electrification. [Google Scholar]
- 25.Das S., Larson I., Young P., Stewart P. Influence of storage relative humidity on the dispersion of salmeterol xinafoate powders for inhalation. J Pharm Sci. 2009;98:1015–1027. doi: 10.1002/jps.21500. [DOI] [PubMed] [Google Scholar]
- 26.Young P.M., Price R. The influence of humidity on the aerosolisation of micronised and SEDS produced salbutamol sulphate. Eur J Pharm Sci. 2004;22:235–240. doi: 10.1016/j.ejps.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Buckton G. Characterisation of small changes in the physical properties of powders of significance for dry powder inhaler formulations. Adv Drug Deliv Rev. 1997;26:17–27. doi: 10.1016/s0169-409x(97)00507-3. [DOI] [PubMed] [Google Scholar]
- 28.Zeng X.M., Martin G.P., Marriott C., Pritchard J. The influence of carrier morphology on drug delivery by dry powder inhalers. Int J Pharm. 2000;200:93–106. doi: 10.1016/s0378-5173(00)00347-1. [DOI] [PubMed] [Google Scholar]
- 29.Littringer E.M., Mescher A., Schroettner H., Achelis L., Walzel P., Urbanetz N.A. Spray dried mannitol carrier particles with tailored surface properties—the influence of carrier surface roughness and shape. Eur J Pharm Biopharm. 2012;82:194–204. doi: 10.1016/j.ejpb.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Yang J.C., Wu C.Y., Adams M. Three-dimensional DEM–CFD analysis of air-flow-induced detachment of API particles from carrier particles in dry powder inhalers. Acta Pharm Sin B. 2014;4:52–59. doi: 10.1016/j.apsb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaialy W., Alhalaweh A., Velaga S.P., Nokhodchi A. Effect of carrier particle shape on dry powder inhaler performance. Int J Pharm. 2011;421:12–23. doi: 10.1016/j.ijpharm.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Q.T., Qu L., Larson I., Stewart P.J., Morton D.A. Improving aerosolization of drug powders by reducing powder intrinsic cohesion via a mechanical dry coating approach. Int J Pharm. 2010;394:50–59. doi: 10.1016/j.ijpharm.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 33.Tonnis W.F., Bagerman M., Weij M., Sjollema J., Frijlink H.W., Hinrichs W.L. A novel aerosol generator for homogenous distribution of powder over the lungs after pulmonary administration to small laboratory animals. Eur J Pharm Biopharm. 2014;88:1056–1063. doi: 10.1016/j.ejpb.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 34.Otake H., Okuda T., Hira D., Kojima H., Shimada Y., Okamoto H. Inhalable spray-freeze-dried powder with l-leucine that delivers particles independent of inspiratory flow pattern and inhalation device. Pharm Res. 2016;33:922–931. doi: 10.1007/s11095-015-1838-4. [DOI] [PubMed] [Google Scholar]
- 35.Faulhammer E., Wahl V., Zellnitz S., Khinast J.G., Paudel A. Carrier-based dry powder inhalation: impact of carrier modification on capsule filling processability and in vitro aerodynamic performance. Int J Pharm. 2015;491:231–242. doi: 10.1016/j.ijpharm.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Miller D.P., Tan T., Tarara T.E., Nakamura J., Malcolmson R.J., Weers J.G. Physical characterization of tobramycin inhalation powder: I. Rational design of a stable engineered-particle formulation for delivery to the lungs. Mol Pharmaceutics. 2015;12:2582–2593. doi: 10.1021/acs.molpharmaceut.5b00147. [DOI] [PubMed] [Google Scholar]
- 37.Ziffels S., Bemelmans N.L., Durham P.G., Hickey A.J. In vitro dry powder inhaler formulation performance considerations. J Control Release. 2015;199:45–52. doi: 10.1016/j.jconrel.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 38.Patton J.S., Byron P.R. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 39.Hickey A.J., Mansour H.M., Telko M.J., Xu Z., Smyth H.D., Mulder T. Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J Pharm Sci. 2007;96:1282–1301. doi: 10.1002/jps.20916. [DOI] [PubMed] [Google Scholar]
- 40.Castellanos A., Valverde J.M., Pérez A.T., Ramos A., Watson P.K. Flow regimes in fine cohesive powders. Phys Rev Lett. 1999;82:1156. [Google Scholar]
- 41.Valverde J.M., Ramos A., Castellanos A., Watson P.K. The tensile strength of cohesive powders and its relationship to consolidation, free volume and cohesivity. Powder Technol. 1998;97:237–245. [Google Scholar]
- 42.Castellanos A. The relationship between attractive interparticle forces and bulk behaviour in dry and uncharged fine powders. Adv Phys. 2005;54:263–376. [Google Scholar]
- 43.Sahane S.P., Nikhar A.K., Bhaskaran S., Mundhada D.R. Dry powder inhaler: an advance technique for pulmonary drug delivery system. Int J Pharm Chem Sci. 2012;1:1376–1383. [Google Scholar]
- 44.Yang M.Y., Chan J.G.Y., Chan H.K. Pulmonary drug delivery by powder aerosols. J Control Release. 2014;193:228–240. doi: 10.1016/j.jconrel.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 45.Kaialy W., Hussain T., Alhalaweh A., Nokhodchi A. Towards a more desirable dry powder inhaler formulation: large spray-dried mannitol microspheres outperform small microspheres. Pharm Res. 2014;31:60–76. doi: 10.1007/s11095-013-1132-2. [DOI] [PubMed] [Google Scholar]
- 46.Ooi J., Traini D., Hoe S., Wong W., Young P.M. Does carrier size matter? A fundamental study of drug aerosolisation from carrier based dry powder inhalation systems. Int J Pharm. 2011;413:1–9. doi: 10.1016/j.ijpharm.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Kaialy W., Nokhodchi A. Freeze-dried mannitol for superior pulmonary drug delivery via dry powder inhaler. Pharm Res. 2013;30:458–477. doi: 10.1007/s11095-012-0892-4. [DOI] [PubMed] [Google Scholar]
- 48.Kaialy W., Nokhodchi A. Dry powder inhalers: physicochemical and aerosolization properties of several size-fractions of a promising alterative carrier, freeze-dried mannitol. Eur J Pharm Sci. 2015;68:56–67. doi: 10.1016/j.ejps.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Kaialy W., Nokhodchi A. Antisolvent crystallisation is a potential technique to prepare engineered lactose with promising aerosolisation properties: effect of saturation degree. Int J Pharm. 2012;437:57–69. doi: 10.1016/j.ijpharm.2012.07.064. [DOI] [PubMed] [Google Scholar]
- 50.Beilmann B., Kubiak R., Grab P., Häusler H., Langguth P. Effect of interactive ternary mixtures on dispersion characteristics of ipratropium bromide in dry powder inhaler formulations. AAPS PharmSciTech. 2007;8:E32–E39. doi: 10.1208/pt0802031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones M.D., Price R. The influence of fine excipient particles on the performance of carrier-based dry powder inhalation formulations. Pharm Res. 2006;23:1665–1674. doi: 10.1007/s11095-006-9012-7. [DOI] [PubMed] [Google Scholar]
- 52.Louey M.D., Stewart P.J. Particle interactions involved in aerosol dispersion of ternary interactive mixtures. Pharm Res. 2002;19:1524–1531. doi: 10.1023/a:1020464801786. [DOI] [PubMed] [Google Scholar]
- 53.Tee S.K., Marriott C., Zeng X.M., Martin G.P. The use of different sugars as fine and coarse carriers for aerosolised salbutamol sulphate. Int J Pharm. 2000;208:111–123. doi: 10.1016/s0378-5173(00)00553-6. [DOI] [PubMed] [Google Scholar]
- 54.Hamishehkar H., Emami J., Najafabadi A.R., Gilani K., Minaiyan M., Mahdavi H. Influence of carrier particle size, carrier ratio and addition of fine ternary particles on the dry powder inhalation performance of insulin-loaded PLGA microcapsules. Powder Technol. 2010;201:289–295. [Google Scholar]
- 55.Steckel H., Markefka P., teWierik H., Kammelar R. Functionality testing of inhalation grade lactose. Eur J Pharm Biopharm. 2004;57:495–505. doi: 10.1016/j.ejpb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Zeng X.M., Martin G.P., Tee S.-K., Marriott C. The role of fine particle lactose on the dispersion and deaggregation of salbutamol sulphate in an air stream in vitro. Int J Pharm. 1998;176:99–110. [Google Scholar]
- 57.Shur J., Harris H., Jones M.D., Kaerger J.S., Price R. The role of fines in the modification of the fluidization and dispersion mechanism within dry powder inhaler formulations. Pharm Res. 2008;25:1631–1640. doi: 10.1007/s11095-008-9538-y. [DOI] [PubMed] [Google Scholar]
- 58.Dickhoff B.H., de Boer A.H., Lambregts D., Frijlink H.W. The effect of carrier surface treatment on drug particle detachment from crystalline carriers in adhesive mixtures for inhalation. Int J Pharm. 2006;327:17–25. doi: 10.1016/j.ijpharm.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Grasmeijer F., Lexmond A.J., van den Noort M., Hagedoorn P., Hickey A.J., Frijlink H.W. New mechanisms to explain the effects of added lactose fines on the dispersion performance of adhesive mixtures for inhalation. PLoS One. 2014;9:e87825. doi: 10.1371/journal.pone.0087825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karhu M., Kuikka J., Kauppinen T., Bergström K., Vidgren M. Pulmonary deposition of lactose carriers used in inhalation powders. Int J Pharm. 2000;196:95–103. doi: 10.1016/s0378-5173(99)00450-0. [DOI] [PubMed] [Google Scholar]
- 61.Rademacher J., Ringshausen F.C. Prevention and treatment of exacerbations of non-CF bronchiectasis. In: Blasi F., Miravitlles M., editors. The spectrum of bronchial infection. European Respiratory Society; Sheffield: 2013. pp. 127–136. [Google Scholar]
- 62.Hersey J.A. Ordered mixing: a new concept in powder mixing practice. Powder Technol. 1975;11:41–44. [Google Scholar]
- 63.Grasmeijer F., Frijlink H.W., de Boer A.H. A proposed definition of the ‘activity’ of surface sites on lactose carriers for dry powder inhalation. Eur J Pharm Sci. 2014;56:102–104. doi: 10.1016/j.ejps.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 64.Jones M.D., Hooton J.C., Dawson M.L., Ferrie A.R., Price R. An investigation into the dispersion mechanisms of ternary dry powder inhaler formulations by the quantification of interparticulate forces. Pharm Res. 2008;25:337–348. doi: 10.1007/s11095-007-9467-1. [DOI] [PubMed] [Google Scholar]
- 65.Kho K., Hadinoto K. Dry powder inhaler delivery of amorphous drug nanoparticles: effects of the lactose carrier particle shape and size. Powder Technol. 2013;233:303–311. [Google Scholar]
- 66.Larhrib H., Martin G.P., Marriott C., Prime D. The influence of carrier and drug morphology on drug delivery from dry powder formulations. Int J Pharm. 2003;257:283–296. doi: 10.1016/s0378-5173(03)00156-x. [DOI] [PubMed] [Google Scholar]
- 67.Hassan M.S., Lau R. Inhalation performance of pollen-shape carrier in dry powder formulation: effect of size and surface morphology. Int J Pharm. 2011;413:93–102. doi: 10.1016/j.ijpharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 68.Larhrib H., Cespi M., Dyas M., Roberts M., Ford J. Engineered carrier with a long time of flight (TOF) to improve drug delivery from dry powder inhalation aerosols. Drug Deliv Lung. 2006;17:304–307. [Google Scholar]
- 69.Kou X., Chan L.W., Steckel H., Heng P.W. Physico-chemical aspects of lactose for inhalation. Adv Drug Deliv Rev. 2012;64:220–232. doi: 10.1016/j.addr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Kaialy W., Martin G.P., Larhrib H., Ticehurst M.D., Kolosionek E., Nokhodchi A. The influence of physical properties and morphology of crystallised lactose on delivery of salbutamol sulphate from dry powder inhalers. Colloids Surf B. 2012;89:29–39. doi: 10.1016/j.colsurfb.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 71.Kuo C.-Y., Rollings R.S., Lynch L.N. Morphological study of coarse aggregates using image analysis. J Mater Civil Eng. 1998;10:135–142. [Google Scholar]
- 72.Barrett P.J. The shape of rock particles, a critical review. Sedimentology. 1980;27:291–303. [Google Scholar]
- 73.Allen T. 4th ed. Springer; New York: 1981. Particle size measurement. [Google Scholar]
- 74.Zeng X.M., Martin G.P., Marriott C. Taylor & Francis; London: 2001. Particulate interactions in dry powder formulations for inhalation. [Google Scholar]
- 75.Zeng X.M., Martin G.P., Marriott C., Pritchard J. Lactose as a carrier in dry powder formulations: the influence of surface characteristics on drug delivery. J Pharm Sci. 2001;90:1424–1434. doi: 10.1002/jps.1094. [DOI] [PubMed] [Google Scholar]
- 76.Kuo C.Y., Freeman R. Imaging indices for quantification of shape, angularity, and surface texture of aggregates. Transport Res Rec. 2000;1721:57–65. [Google Scholar]
- 77.Hassan M.S., Lau R.W.M. Effect of particle shape on dry particle inhalation: study of flowability, aerosolization, and deposition properties. AAPS PharmSciTech. 2009;10:1252–1262. doi: 10.1208/s12249-009-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kawashima Y., Serigano T., Hino T., Yamamoto H., Takeuchi H. Effect of surface morphology of carrier lactose on dry powder inhalation property of pranlukast hydrate. Int J Pharm. 1998;172:179–188. [Google Scholar]
- 79.Kaialy W., Ticehurst M.D., Murphy J., Nokhodchi A. Improved aerosolization performance of salbutamol sulfate formulated with lactose crystallized from binary mixtures of ethanol–acetone. J Pharm Sci. 2011;100:2665–2684. doi: 10.1002/jps.22483. [DOI] [PubMed] [Google Scholar]
- 80.Flament M.-P., Leterme P., Gayot A. The influence of carrier roughness on adhesion, content uniformity and the in vitro deposition of terbutaline sulphate from dry powder inhalers. Int J Pharm. 2004;275:201–209. doi: 10.1016/j.ijpharm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Zellnitz S., Schroettner H., Urbanetz N.A. Influence of surface characteristics of modified glass beads as model carriers in dry powder inhalers (DPIs) on the aerosolization performance. Drug Dev Ind Pharm. 2015;41:1710–1717. doi: 10.3109/03639045.2014.997246. [DOI] [PubMed] [Google Scholar]
- 82.Maas S.G., Schaldach G., Littringer E.M., Mescher A., Griesser U.J., Braun D.E. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technol. 2011;213:27–35. [Google Scholar]
- 83.Littringer E.M., Mescher A., Eckhard S., Schröttner H., Langes C., Fries M. Spray drying of mannitol as a drug carrier—the impact of process parameters on product properties. Dry Technol. 2012;30:114–124. [Google Scholar]
- 84.Zeng X.M., Martin G.P., Marriott C., Pritchard J. The influence of crystallization conditions on the morphology of lactose intended for use as a carrier for dry powder aerosols. J Pharm Pharmacol. 2000;52:633–643. doi: 10.1211/0022357001774462. [DOI] [PubMed] [Google Scholar]
- 85.Traini D., Scalia S., Adi H., Marangoni E., Young P.M. Polymer coating of carrier excipients modify aerosol performance of adhered drugs used in dry powder inhalation therapy. Int J Pharm. 2012;438:150–159. doi: 10.1016/j.ijpharm.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 86.Staniforth JN, inventor; Co-Ordinated Drug Development Limited, assignee. Carrier particles for use in dry powder inhalers. US Patent 6153224. 2000 Nov 28.
- 87.Iida K., Hayakawa Y., Okamoto H., Danjo K., Luenberger H. Effect of surface layering time of lactose carrier particles on dry powder inhalation properties of salbutamol sulfate. Chem Pharm Bull. 2004;52:350–353. doi: 10.1248/cpb.52.350. [DOI] [PubMed] [Google Scholar]
- 88.Singh D.J., Parmar J.J., Hegde D.D., Menon M.D., Soni P.S., Samad A. Poloxamer coated fluticasone propionate microparticles for pulmonary delivery; in vivo lung deposition and efficacy studies. Indian J Pharm Sci. 2007;69:714–715. [Google Scholar]
- 89.Young P.M., Price R., Tobyn M.J., Buttrum M., Dey F. Investigation into the effect of humidity on drug–drug interactions using the atomic force microscope. J Pharm Sci. 2003;92:815–822. doi: 10.1002/jps.10250. [DOI] [PubMed] [Google Scholar]
- 90.Young P.M., Price R., Tobyn M.J., Buttrum M., Dey F. The influence of relative humidity on the cohesion properties of micronized drugs used in inhalation therapy. J Pharm Sci. 2004;93:753–761. doi: 10.1002/jps.10549. [DOI] [PubMed] [Google Scholar]
- 91.Le V.N.P., Thi T.H.H., Robins E., Flament M.P. Dry powder inhalers: study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13:477–484. doi: 10.1208/s12249-012-9765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]