Abstract
In an answer to the challenge of enzymatic instability and low oral bioavailability of proteins/peptides, a new type of drug-delivery vesicle has been developed. The preparation, based on sodium dodecyl sulfate (SDS) and β-cyclodextrin (β-CD) embedded in chitosan gel, was used to successfully deliver the model drug-insulin. The self-assembled SDS/β-CD vesicles were prepared and characterized by particle size, zeta potential, appearance, microscopic morphology and entrapment efficiency. In addition, both the interaction of insulin with vesicles and the stability of insulin loaded in vesicles in the presence of pepsin were investigated. The vesicles were crosslinked into thermo-sensitive chitosan/β-glycerol phosphate solution for an in-situ gel to enhance the dilution stability. The in vitro release characteristics of insulin from gels in media at different pH values were investigated. The insulin loaded vesicles–chitosan hydrogel (IVG) improved the dilution stability of the vesicles and provided pH-selective sustained release compared with insulin solution–chitosan hydrogel (ISG). In vitro, IVG exhibited slow release in acidic solution and relatively quick release in neutral solutions to provide drug efficacy. In simulated digestive fluid, IVG showed better sustained release and insulin protection properties compared with ISG. Thus IVG might improve the stability of insulin during its transport in vivo and contribute to the bioavailability and therapeutic effect of insulin.
Keywords: Vesicles, β-Cyclodextrin, Insulin, Self-assembly, Gel, pH-selective release
Graphical abstract
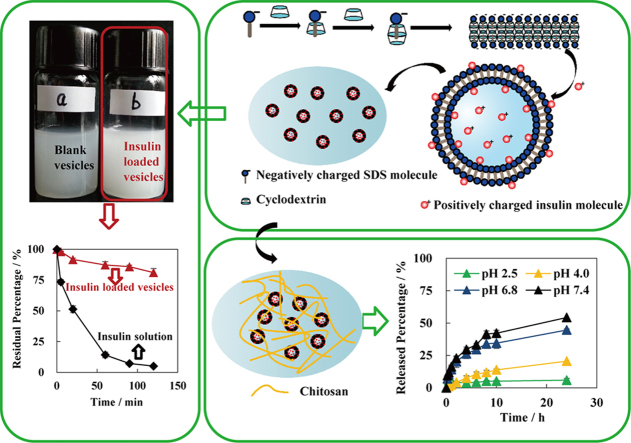
Insulin-loaded SDS/β-CD vesicles successfully protected proteins/peptides from enzymatic degradation. The vesicles were crosslinked into chitosan/β-glycerol phosphate solution to form insulin-loaded vesicles-chitosan hydrogel (IVG) with pH-selective sustained release and enzymatic protection.
1. Introduction
The ordered assemblies formatted by some amphiphilic molecules exhibiting bilayer structures, such as natural or synthetic surfactants, is a well-known phenomenon. In general, amphiphilic molecules participating in self-assembling can be natural or synthetic surfactants which result in two very different structures defined as liposomes1, 2, 3 and vesicles, respectively. They have been widely used in the delivery of small molecule drugs4, 5, 6 and macromolecular protein drugs3, 7, 8. Vesicular carriers offer the following advantages: (i) they facilitate drug absorption across the bilayer membrane, (ii) they can improve drug stability by protecting it from biological environment, resulting in better bioavailability and therapeutic efficacy, (iii) they can regulate drug delivery rate thereby promoting sustained release. The non-ionic surfactant vesicles (named niosomes) are composed of non-ionic surfactant and cholesterol or its derivatives. Niosomes have been widely studied as one type of synthetic surfactant vesicle9, 10, 11. In our research, we employed another relatively new type of synthetic surfactant vesicles consisting of anionic surfactant and cyclodextrins (CDs).
CDs have been widely used in pharmaceutical formulations12, 13 and have undergone continuous development since they were first reported by Villiers in 189114. CDs consist of identical α-d-glucopyranose units linked by α-1,4 glycosidic bonds to form a round macromolecule. CD formulations are shaped as hollow, truncated cones rather than perfect cylinders due to the chair conformation of the glucopyranose units. The bigger and smaller edges of the cone are referenced as the “head” and “tail”, respectively. The secondary hydroxyl groups form at the head, whereas the primary hydroxyl groups are seen at the tail15. The most common CDs containing 6, 7 and 8 α-d-glucopyranose units are called α-CD, β-CD and γ-CD, respectively. It has long been considered that the addition of CDs disfavors the self-assembly of surfactants in dilute solutions since the hydrophobic effect is destroyed upon the formation of the hydrophiphilic CD/surfactant inclusion complex16. However, some researchers have reported the nonamphiphilic self-assembly behavior of hydrophilic sodium dodecyl sulfate/β-CD (SDS/β-CD) complexes in water, which did not rely on the hydrophobic effect. As reported, annular-ring tubes (i.e., multilamellar tubes) formed in SDS/β-CD aqueous solution from concentration of 6%–25%, w/v (total mass concentration of SDS and β-CD). Lamellar structures were found when the concentration range extended to 25%–50%, w/v and vesicles were revealed from 4% to 6%, w/v17. In addition, vesicles and microtubes can reversibly interchange upon temperature alternation18. Herein, we only focused on the vesicle preparations and investigated the properties of vesicles used as carriers of macromolecular drugs.
As is known, insulin monotherapy can be used as early treatment for patients with type 2 diabetes mellitus or as an alternative or add-on in patients with inadequate glycemic control despite treatment with oral antidiabetic agents, diet and exercise therapy19. Because of the popularity of insulin delivery in pharmaceutical research7, 8, 20, 21, 22, 23, 24, insulin was selected as the model drug delivered by SDS/β-CD vesicles in this report.
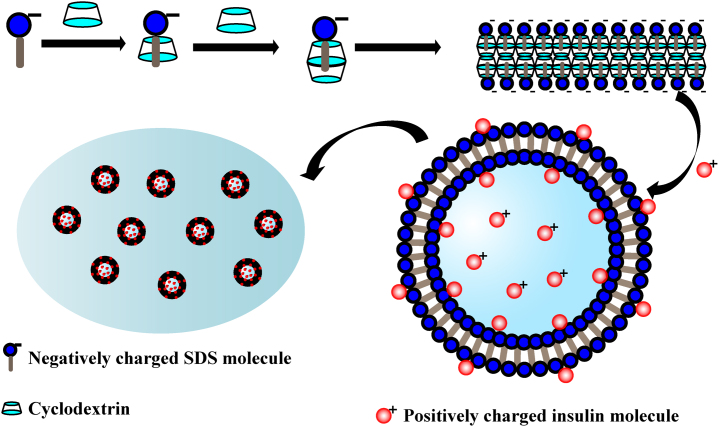
The fabrication mechanism of insulin-loaded vesicles is shown in Fig. 1. The hydrophobic carbon chain of SDS was wrapped into the β-CD cavity forming host-guest molecules complex of β-CD and SDS in a 2:1 molar ratio in the solution. The complex formed lamellar structure at high concentrations (25%–50%, w/v) but self-assembled into vesicles at lower concentrations (4%–6%, w/v) when incubated under suitable conditions17. The vesicles showed a closed hollow spherical structure by bending of the bilayer membrane. The insulin molecules were positively charged in hydrochloric acid solution (pH 2.6) with an isoelectric point of 5.35–5.45, which not only helps the drug into the interior cavity of vesicles, but also possibly combines with the negatively charged SDS through electrostatic force, thereby improving the affinity of the drug for the carrier. Since the SDS/β-CD system only exists in vesicular form in a narrow scope of concentration, the vesicles were crosslinked into thermo-sensitive chitosan/β-glycerol phosphate (β-GP) solution to prepare in-situ gel in order to further improve the stability of the vesicular system25, 26, 27.
Figure 1.
Schematic diagram of the formation of SDS/β-CD vesicles and loading of insulin.
In summary, the purposes of this study were: (i) to fabricate of a new type of protein/peptide-loaded vesicles based on SDS and β-CD, (ii) to study insulin interaction with the vesicles and (iii) to investigate the in vitro release characteristics of insulin loaded vesicles–chitosan hydrogel (IVG) in media for the pH selectivity.
2. Materials and methods
2.1. Materials
Insulin was purchased from Wanbang Jinqiao Pharmaceutical Co., Ltd. (Xuzhou, Jiangsu, China). β-CD was obtained from Anhui Sunhere pharmaceutical excipients Co., Ltd. (Anhui, China). SDS was purchased from Sigma-Aldrich Company (St. Louis, MO, USA). Pepsin (enzyme activity ≥1200 U/g), trypsin (enzyme activity ≥2500 U/mg) and β-GP (disodium salt pentahydrate) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Chitosan (molecular weight about 1000 kD) was purchased from Zhejiang Golden-Shell biological chemical Co., Ltd. (Taizhou, Zhejiang, China). Acetonitrile (HPLC grade) was obtained from Merck Co., Ltd. (Darmstadt, Germany). Other reagents were of analytical grade. Water was purified by Milli-Q system (Millipore, Bedford, MA, USA).
2.2. Preparation of vesicles
Two methods of preparation were employed, a dilution method and a direct preparation method. For the former, desired amounts of β-CD, SDS and water were weighed into a small beaker to obtain a constant SDS/β-CD molar ratio (2:1). The sample was then heated to 60 °C in water bath to obtain an isotropic solution as concentrated sample, the total concentration of β-CD and SDS in this solution was 25%, w/v. The concentrated solution was diluted 5 times with hydrochloric acid solution (pH 2.6) to prepare low concentration samples. The solutions were incubated at 25 °C for over 24 h to allow the self-assembly of SDS/β-CD blank vesicles.
For the direct preparation method, desired amounts of β-CD and SDS were weighed and transferred with hydrochloric acid solution (pH 2.6) into a small beaker to obtain a constant SDS/β-CD molar ratio (2:1). Then the sample was heated to 60 °C in water bath to obtain an isotropic solution. The total concentration of β-CD and SDS in this solution was 5%, w/v. The solutions were then incubated at 25 °C for over 24 h to allow self-assembly of SDS/β-CD blank vesicles.
Insulin-loaded vesicles were prepared by the same procedure, but insulin was firstly dissolved in hydrochloric acid solution (pH 2.6). All samples were stored at 4 °C for further use.
2.3. Characterization of vesicles
2.3.1. Particle size and zeta potential
The size and zeta potential of vesicles were measured by a dynamic light scattering (DLS) method by use of the Malvern Zetasizer Nano ZS 90 instrument (Malvern Instruments Ltd., Worcestershire, UK) at 25 °C. The scattering angle was fixed at 90°. The size distribution results were described as percentage of intensity. The polydispersity index (PDI) value was determined as a measurement of the breadth of the size distribution. The PDI value (lower than 0.3) and count rate (ranged 100–300 kcps) indicated a homogenous and monodisperse population. Zeta potential was measured by the principle of Laser Doppler electrophoresis, and higher potential indicated better stability of the system. The assays were performed in triplicate.
2.3.2. Macroscopic appearance
The macroscopic appearance of the vesicles was directly observed by the naked eye.
2.4. Entrapment efficiency
The entrapment efficiency (EE, %) of insulin in vesicles was determined by centrifugation to separate free insulin from entrapped drug. Briefly, 0.5 mL of insulin-loaded vesicles suspension was introduced into a clean tube and centrifuged at 12,000 rpm for 5 min. The precipitate was collected and diluted with sodium hydroxide solution (0.01 mol/L) in order to fully dissolve the precipitate. The solution was again centrifuged at 12,000 rpm for 5 min and the concentration of insulin in the supernatant was assayed by HPLC as described below. EE (%) was then calculated using Eq. (1).
| (1) |
where Wt and Wd were the amounts of insulin in total and loaded in the vesicles respectively.
2.5. Interaction of insulin with vesicles
Desired amount of insulin was added into suspension of blank vesicles, the amount of insulin was equal to that of insulin loaded vesicles. Then, the mixed system was incubated at 25 °C for 30 min to make sure that the insulin fully interacted with the vesicles. The particle size and zeta potential was measured by the same parameters as explained above.
2.6. In vitro pepsin degradation
The stability of the insulin in the presence of pepsin was investigated in order to validate whether the vesicles could protect insulin from the degradation. Typical incubations were described as follows. Pepsin solution (600 μg/mL, 0.72 U/mL), insulin solution, insulin loaded vesicles and the mixture of insulin and blank vesicles were preheated in a water bath at 37 °C for 15 min, after which 50 μL pepsin solution and 950 μL samples were introduced into clean tubes and vortexed quickly and placed in a water bath at 37 °C. Incubations were stopped at intervals of 0, 5, 20, 60, 90 and 120 min by withdrawing 50 μL incubated solution to a new plastic tube with addition of 100 μL ice-bathed tris buffer (pH 8.0, 1 mmol/L CaCl2 included)28. The tubes were immediately vortexed and placed on ice for 30 min and centrifuged at 12,000 rpm for 5 min. The concentration of insulin in supernatant was analyzed by HPLC as described below. The assays were performed in triplicate.
2.7. Preparation of thermo-sensitive crosslinked chitosan solutions
One mL of 65% (w/v) β-GP (in deionized water) solution was added to 3 mL of 3% (w/v) chitosan solution (in 1%, v/v, acetic acid) drop-wise, stirring continuously over ice bath. The final solution was stirred for additional 10 min to ensure complete mixing. Vesicles were embedded into the chitosan network by adding vesicles into the prepared CS-β-GP solution, mixing thoroughly at 25 °C. The resulting solution was allowed to facilitate crosslinking to completely become a gel in an incubator at 37 °C for 1 h. The resultant IVG was used immediately for drug-release experiments. Insulin solution–chitosan hydrogel (ISG) was prepared by the same procedure as a reference, simply replacing insulin vesicles with insulin solution.
2.8. In vitro drug release studies
2.8.1. Without enzymes
The gels were placed in the containers containing 50 mL of phosphate buffer solution (PBS, pH 2.5, 6.8 or 7.4) or citric acid–disodium hydrogen phosphate buffer solution (pH 4.0) at 37±1 °C with mild stirring. At designated time points (15, 30 min, 1, 2, 4, 8, 10 and 24 h), 0.5 mL of the release media was withdrawn and the same volume of fresh media incubated at 37±1 °C was added into the containers. All the samples were centrifuged at 12,000 rpm for 5 min and the concentration of insulin in supernatant was analyzed by HPLC as described below. The assays were duplicated in triplicate.
2.8.2. With enzymes
Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) with enzymes were used to mimic the gastrointestinal conditions in vivo. SGF consisted of 2.0 g NaCl in 1 L pure water with pH of 1.20±0.02 adjusted by hydrochloric acid, then pepsin was added (3.2 mg/mL) and the solution was sufficiently stirred. SIF was composed of a buffer (50 mmol/L Tris, 50 mmol/L maleate, 150 mmol/L NaCl, 5 mmol/L CaCl2), bile salts (5 mmol/L cholates) and phosphatidylcholine (1.25 mmol/L soybean lecithin)29. The final pH was adjusted to 7.50±0.02, then trypsin was added (10 mg/mL) and the solution was sufficiently stirred.
The gels were placed in the containers and 50 mL of SGF or SIF was added at 37±1 °C with mild stirring. The dissolution medium was carefully poured out after 1 h, the remaining portion of the gel was dissolved with an adequate volume of dilute hydrochloric acid solution (pH 1.2, without pepsin) under 37 °C with rapid stirring. All the samples were centrifuged at 12,000 rpm for 5 min. The concentration of insulin in supernatant was analyzed by HPLC as described below. The assays were performed in triplicate.
2.9. Determination of insulin
Insulin was assayed by a reversed-phase HPLC–UV method. The HPLC system (1260 Series, Agilent Technologies, USA) was composed of a quaternary pump, a degasser, an autosampler, a column heater and a tunable ultraviolet detector. The separation was achieved on the Vydac C18 analytical column (150 mm×4.6 mm i.d., 5 μm; Grace, USA) maintained at 35 °C. The mobile phase was composed of acetonitrile and 0.1% trifluoroacetic acid solution using gradient elution and the flow rate was set to 1.0 mL/min. The detection wavelength of the detector was set at 214 nm. The HPLC method was of good specificity and accuracy, and the linear range was 5–400 μg/mL.
3. Results and discussion
3.1. Preparation method, size and zeta potential of vesicles
The vesicles prepared by two methods were obviously different (Table 1). The standard deviation for particle size of the direct preparation method was significantly greater than those obtained from the dilution method, although mean diameters were similar. In addition, PDI values varied remarkably between the two methods. The PDI value obtained from the direct method was higher than 0.70, indicating that the system was not uniform and had poor dispersibility. On the other hand, the system prepared by the dilution method was homogeneous and well-dispersed with PDI value of 0.30. Thus, the dilution method seemed to favor formation of cyclodextrin inclusion complexes at the higher reagents concentrations, thereby promoting self-assembly into vesicles after dilution. No large differences in zeta potential were observed between samples prepared by two methods, since the amount of negatively charged SDS remained unchanged.
Table 1.
The particle size, PDI and zeta potential of vesicles prepared by different methods (n=3).
| Sample | Preparation method | Size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|---|
| Blank vesicles | Dilution | 1421±84 | 0.294±0.041 | −49.5±2.3 |
| Blank vesicles | Direct preparation | 1629±251 | 0.823±0.146 | −49.8±1.3 |
| Insulin-loaded vesicles | Dilution | 871±42 | 0.187±0.058 | −39.4±2.0 |
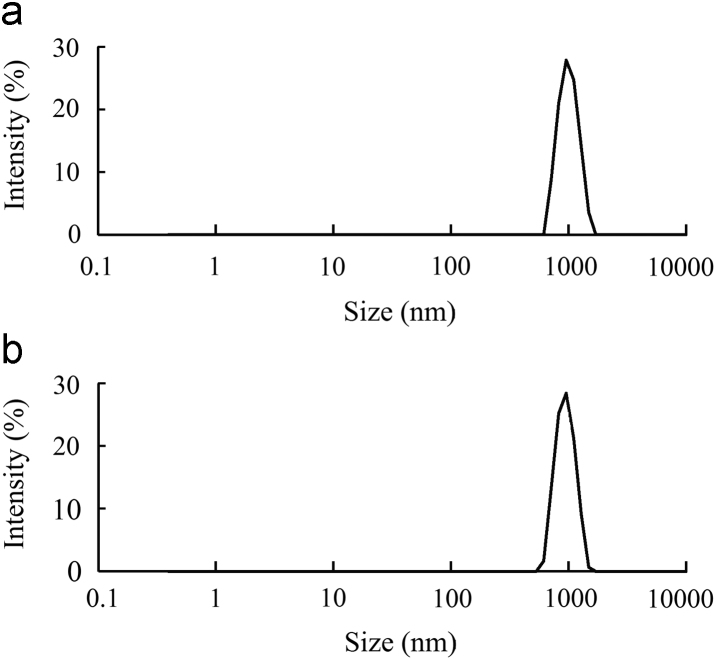
Since the preparation method showed significant impact on the particle size but almost no effect on zeta potential, the dilution method was selected for the preparation of vesicles. This method yielded narrowly-distributed particle sizes for blank and insulin-loaded vesicles (Fig. 2).
Figure 2.
Particle size distributions of blank vesicles (a) and insulin loaded vesicles (b).
3.2. Macroscopic appearance
Blank and insulin-loaded vesicles were observed macroscopically (Fig. 3). For the 5% (w/v) system of SDS/β-CD, the suspension of blank vesicles was of low turbidity at 25 °C; however, the turbidity of insulin-loaded vesicles was higher at the same temperature, indicating the existence of certain interactions between insulin and the components of vesicles.
Figure 3.

Macroscopic photos of blank vesicles (a) and insulin-loaded vesicles (b).
3.3. EE
The EE of insulin loaded SDS/β-CD vesicles prepared by dilution method was 87.62±8.56% (n=6). For comparison, the entrapment efficiency of vesicles obtained from direct preparation method was measured to be 80.58±6.79% (n=6). The drug-loading efficiency was 1.47±0.14% (n=6) for the dilution method and 1.37±0.11% (n=6) for the direct preparation method. Thus, the drug-loading efficiencies of the two methods were similar. Because of the possible electrostatic interactions in the system, the interactions of the drug and the carrier were further studied as below.
3.4. Interaction of insulin with vesicles
The insulin could be located in three possible ways in the vesicles, namely, being adsorbed on the vesicle bilayer membrane, encapsulated in the inner cavity of vesicles or both. The insulin-loaded vesicles showed smaller particle size when compared with blank vesicles. It was known that insulin was positively charged under the given conditions (pH 2.6) with an isoelectric point of 5.35–5.45. The reduction of particle size from 1421±84 nm to 871±42 nm following insulin loading might be due to the combination of partial insulin with negatively charged SDS by an electrostatic force, resulting in the shrinkage of the hollow vesicles. When insulin was physically mixed with blank vesicles, the particle size reduced from 1421±84 nm to 682±18 nm, indicating that the contractile effect caused by electrostatic interaction was stronger than that in insulin loaded vesicles. In other words, the loaded insulin interacted with SDS/β-CD vesicles not only by electrostatic force, but a part of insulin may also be wrapped inside the vesicles.
The zeta potential is a sign of the strength of attractive and repulsive forces between particles. The higher zeta potential (positive or negative) indicates the greater stability of the system. In general, if the absolute value of zeta potential was higher than 30 mV, the system could be considered in the stable category. The zeta potential of the blank and insulin loaded vesicles were about −50 mV and –40 mV, respectively, showing a change of about 10 mV after insulin loading. However, the zeta potential of the physical mixture was −9.6±1.1 mV, with a variation value between blank vesicles and the physical mixture of nearly 40 mV. These data suggest that only a small fraction of insulin was combined on the surface of vesicles by electrostatic forces and that most of the insulin was wrapped in the inner compartment of the vesicles in the case of insulin-loaded vesicles.
3.5. Stability of the particle size
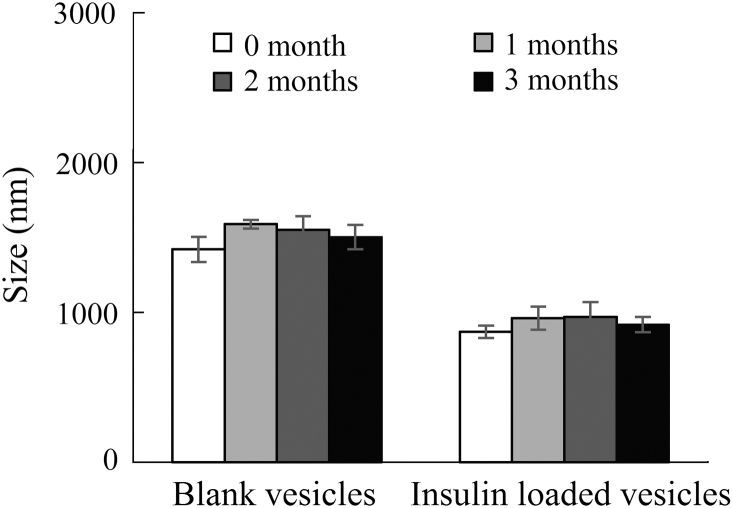
The physical stabilities of blank and insulin-loaded vesicles were evaluated by comparing the changes in the mean diameters, during their storage at 4 °C for over three months (Fig. 4). The particle sizes for both were not significantly changed and remained in acceptable range of 1420–1590 nm for the blank and 870–970 nm for the insulin-loaded one. Thus, the vesicles can be stored and remain stable at 4 °C and for extended periods.
Figure 4.
Particle sizes of the blank and insulin loaded vesicles stored at 4 °C (n=3).
3.6. In vitro pepsin degradation
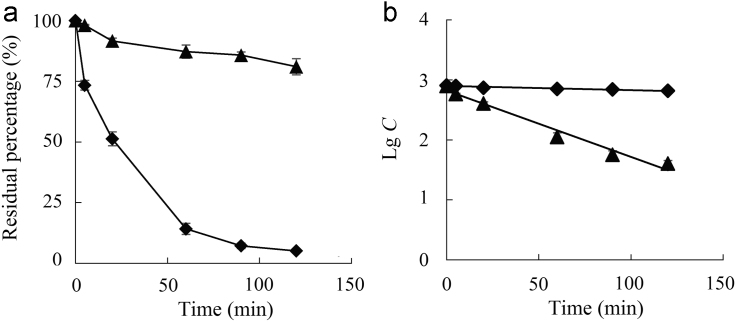
The in vitro degradation of insulin solutions and insulin-loaded vesicles were investigated in the presence of pepsin over a period of 120 min. The remaining percentages of insulin incubated with pepsin for 1 h were 14.2% and 87.2% for insulin solution and insulin-loaded vesicles, respectively (Fig. 5a). The significant decline in the insulin percentage/concentration was recorded for the first one hour of degradation process. After incubation with pepsin for 2 h, 81.0% insulin remained in vesicles. However, the residual percentage of insulin in solution was only 5.11%. The data clearly show that the vesicles successfully protected the insulin from the degradation by pepsin. The degradation of insulin followed typical first order kinetics (Fig. 5b). The first-order kinetic rate constants of solution and the vesicles were 1.61×10−3 min−1 and 2.56×10−2 min−1, respectively. Thus the half-life of insulin solution was 27.1 min in pepsin solution and then was prolonged to 432 min for insulin-loaded vesicles.
Figure 5.
The degradation profiles (a) and degradation kinetic curves (b) of insulin solution (♦) and insulin loaded vesicles in pepsin solutions (▲) (n=3).
3.7. In vitro drug release
3.7.1. Without enzymes
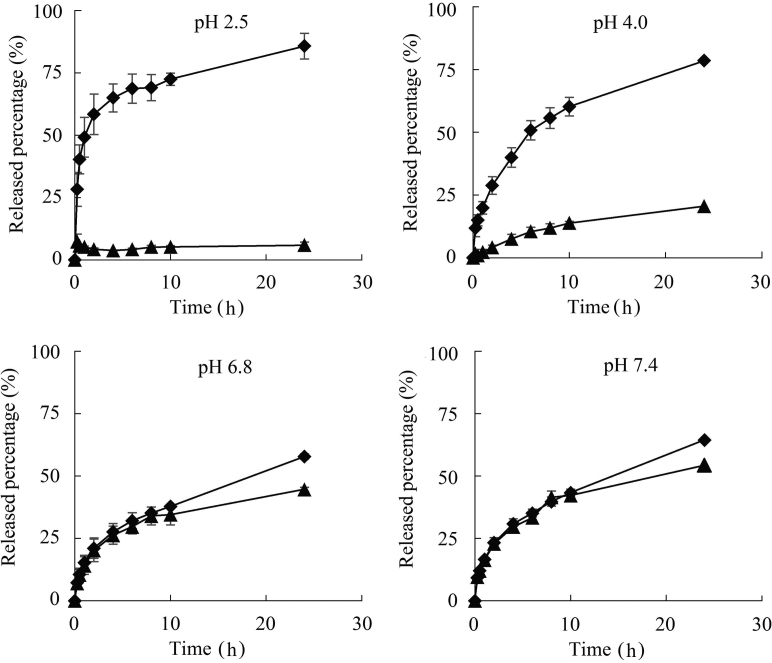
The in vitro drug release of insulin-containing gels was studied at pH 2.5, 4.0, 6.8 and 7.4 (Fig. 6), which simulated the pH profile from gastric to intestinal conditions. First of all, both ISG and IVG showed sustained-release characteristics under the all pH conditions, and continuously released insulin for 24 h. More importantly, the percentage of cumulative release at 24 h of ISG did not vary obviously across pH conditions. In contrast, IVG showed substantial differences in release at varied pH values. In an acidic environment, only a small amount of insulin was released with the remaining amounts protected from acidic environment and pepsin. In a neutral or weakly alkaline environment, insulin was quickly released to provide therapeutic effect.
Figure 6.
Cumulative release profiles of ISG (♦) and IVG (▲) under different pH conditions (n=3).
Furthermore, although no significant differences in release rates were observed between ISG and IVG in media of pH 6.8 and pH 7.4 within 10 h, ISG showed slightly slower and sustained release behavior vs. IVG between 10 and 24 h. The most significant difference observed between ISG and IVG was reflected in the release profile in the acidic environment. In release media of pH 2.5 and pH 4.0, IVG demonstrated substantially slower release as compared with ISG even from the initial period. However, it is worth mentioning that the gel would begin to dissolve within 30 min under pH conditions lower than pH 2.0. The variation of cumulative release percentages between IVG and ISG in release media of pH 2.5, 4.0, 6.8 and 7.4 were 79.9%, 57.9%, 13.4% and 10.2%, respectively. Thus, the differences between insulin release from IVG and ISG increased with decreasing pH values, indicating that the presence of vesicles changed the release behavior of insulin from the gels.
Overall, IVG showed pH-selective release of insulin in vitro, suggesting the improved stability and bioavailability of insulin from this formulation in vivo. SDS/β-CD vesicles helped the gel to achieve selective sustained release at different pH values, and the chitosan gel was effective to improve the stability of the vesicles when being diluted.
3.7.2. With enzymes
The release of insulin from gels was studied in SGF and SIF with digestive enzymes. After release in SGF and SIF for 1 h at 37 °C, the residual percentage of insulin in IVG and ISG varied considerably. The percentage of insulin remaining was 13.9% in IVG, while the content of insulin was undetectable in ISG after incubation in SGF. In SIF, the residual insulin percentages of IVG and ISG were 18.3% and 3.00% (P<0.05), respectively. This shows that IVG exhibits better sustained release and insulin-protecting properties compared with ISG in simulative digestive fluid. The gels showed faster release characteristics in the simulated gastrointestinal environment than that under in vitro conditions. This phenomenon is probably due to the presence of digestive enzymes as well as bile salts and phosphatidylcholine.
3.8. Determination of the secondary structure of insulin
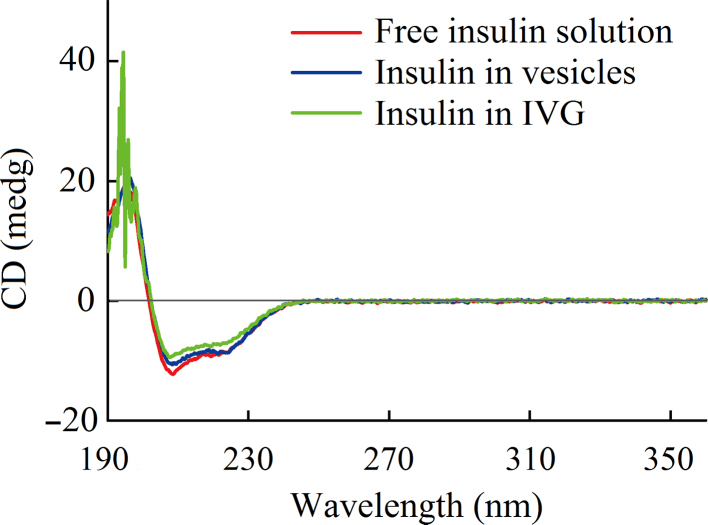
Circular dichroism (CD) was employed to characterize the secondary structures of insulin in the vesicles and IVG (Fig. 7). The CD spectra showed that all samples were very similar for typical features of α-helix structure of proteins with a positive absorption peak below 200 nm and two negative absorption peaks at 208 and 222 nm, all of similar magnitude. The characteristic peak of the β-sheet was not obvious at 215–217 nm because of the low insulin concentrations in the samples. The noise in 190–200 nm range of insulin isolated from IVG was attributed to the residual impurities or high concentration of chloride ions in the sample. Thus, the preparation conditions and the system had little effects on the conformation and the secondary structure of insulin in carriers.
Figure 7.
CD spectra of free insulin solution, insulin extracted from the vesicles and insulin isolated from gels.
4. Conclusions
In the current study, a novel type of SDS/β-CD vesicles was used to deliver protein/peptide drug with high encapsulation efficiency. The insulin-loaded vesicles showed excellent physical stability and protect insulin from proteolytic degradation. In addition, IVG made from the strategy of “drug-in-vesicles-in-gels” improved the stability of the vesicles undergoing dilution and provided selective sustained release at different pH values in vitro when compared with ISG. Specifically, only a small amount of insulin was released in acidic environments thereby protecting most of the drug from degradation. Insulin release rates gradually increased with increasing pH values. In neutral and weakly alkaline environments, insulin was quickly released to provide therapeutic effects. In SGF and SIF with enzymes, IVG preparations showed better sustained release and insulin-protecting properties compared with ISG. The results suggest that the presently-described IVG preparations might increase the stability and bioavailability of insulin in vivo.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Dawei Chen, Email: chendawei@syphu.edu.cn.
Jiwen Zhang, Email: jwzhang@simm.ac.cn.
References
- 1.Manosroi A., Khanrin P., Werner R.G., Götz F., Manosroi W., Manosroi J. Entrapment enhancement of peptide drugs in niosomes. J Microencapsule. 2010;27:272–280. doi: 10.3109/02652040903131293. [DOI] [PubMed] [Google Scholar]
- 2.González-Rodríguez M.L., Rabasco A.M. Charged liposomes as carriers to enhance the permeation through the skin. Expert Opin Drug Deliv. 2011;8:857–871. doi: 10.1517/17425247.2011.574610. [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan J., Ehrhardt C. Liposomal delivery of proteins and peptides. Expert Opin Drug Deliv. 2012;9:1489–1503. doi: 10.1517/17425247.2012.735658. [DOI] [PubMed] [Google Scholar]
- 4.Wang C.X., Li C.L., Zhao X., Yang H.Y., Wei N., Li Y.H. Pharmacodynamics, pharmacokinetics and tissue distribution of liposomal mitoxantrone hydrochloride. Acta Pharm Sin. 2010;45:1565–1569. [PubMed] [Google Scholar]
- 5.Zou W.W., Wang D.H., Sun C.Y., Han J.B., Yin Q., Yang Q.M. Characterization of vinflunine tartrate liposomes in vitro and in vivo. Acta Pharm Sin. 2011;46:1515–1519. [PubMed] [Google Scholar]
- 6.Li X., Zhang J., Wang D.K., Pan W.S. Anti-tumor activity of folate receptor targeting docetaxel-loaded membrane-modified liposomes. Acta Pharm Sin. 2013;48:1142–1147. [PubMed] [Google Scholar]
- 7.Niu M.M., Lu Y., Hovgaard L., Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomed. 2011;6:1155–1166. doi: 10.2147/IJN.S19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park S.J., Choi S.G., Davaa E., Park J.S. Encapsulation enhancement and stabilization of insulin in cationic liposomes. Int J Pharm. 2011;415:267–272. doi: 10.1016/j.ijpharm.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 9.Moghassemi S., Hadjizadeh A. Nano-niosomes as nanoscale drug delivery systems: an illustrated review. J Control Release. 2014;185:22–36. doi: 10.1016/j.jconrel.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Kumar G.P., Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery–an overview. Acta Pharm Sin B. 2011;1:208–219. [Google Scholar]
- 11.Marianecci C., Di Marzio L., Rinaldi F., Celia C., Paolino D., Alhaique F. Niosomes from 80s to present: the state of the art. Adv Coll Interface Sci. 2014;205:187–206. doi: 10.1016/j.cis.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 12.Loftsson T., Duchêne D. Cyclodextrins and their pharmaceutical applications. Int J Pharm. 2007;329:1–11. doi: 10.1016/j.ijpharm.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 13.Loftsson T., Brewster M.E. Pharmaceutical applications of cyclodextrins: basic science and product development. J Pharm Pharmacol. 2010;62:1607–1621. doi: 10.1111/j.2042-7158.2010.01030.x. [DOI] [PubMed] [Google Scholar]
- 14.Villiers A. Sur la fermentation de la fécule par l’action du ferment butyrique. Compt Rend Acad Sci. 1981;112:536–538. [Google Scholar]
- 15.Jiang L.X., Yan Y., Huang J.B. Versatility of cyclodextrins in self-assembly systems of amphiphiles. Adv Coll Interface Sci. 2011;169:13–25. doi: 10.1016/j.cis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C.C., Cheng X.H., Zhao Q., Yan Y., Wang J.D., Huang J.B. Self-assembly of nonionic surfactant tween 20@2β-CD inclusion complexes in dilute solution. Langmuir. 2013;29:13175–13182. doi: 10.1021/la403257v. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L.X., Peng Y., Yan Y., Huang J.B. Aqueous self-assembly of SDS@2β-CD complexes: lamellae and vesicles. Soft Matter. 2011;7:1726–1731. [Google Scholar]
- 18.Zhou C.C., Cheng X.H., Yan Y., Wang J.D., Huang J.B. Reversible transition between SDS@2β-CD microtubes and vesicles triggered by temperature. Langmuir. 2014;30:3381–3386. doi: 10.1021/la500010p. [DOI] [PubMed] [Google Scholar]
- 19.Kaku K., Mori M., Kanoo T., Katou M., Seino Y. Efficacy and safety of alogliptin added to insulin in Japanese patients with type 2 diabetes: a randomized, double-blind, 12-week, placebo-controlled trial followed by an open-label, long-term extension phase. Expert Opin Pharmacother. 2014;15:2121–2130. doi: 10.1517/14656566.2014.956722. [DOI] [PubMed] [Google Scholar]
- 20.Xu J., Liu C., Xu Y.N., Shan W., Liu M., Huang Y. Mechanism of cellular uptake and transport mediated by integrin receptor targeting trimethyl chitosan nanoparticles. Acta Pharm Sin. 2015;50:893–898. [PubMed] [Google Scholar]
- 21.Wong T.W. Design of oral insulin delivery systems. J Drug Target. 2010;18:79–92. doi: 10.3109/10611860903302815. [DOI] [PubMed] [Google Scholar]
- 22.Oak M., Singh J. Controlled delivery of basal level of insulin from chitosan-zinc-insulin-complex-loaded thermosensitive copolymer. J Pharm Sci. 2012;101:1079–1096. doi: 10.1002/jps.22823. [DOI] [PubMed] [Google Scholar]
- 23.Sajeesh S., Bouchemal K., Marsaud V., Vauthier C., Sharma C.P. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: an oral delivery system for insulin. J Control Release. 2010;147:377–384. doi: 10.1016/j.jconrel.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Han L.N., Zhao Y.F., Yin L.F., Li R.M., Liang Y., Huang H. Insulin-loaded pH-sensitive hyaluronic acid nanoparticles enhance transcellular delivery. AAPS Pharm Sci Tech. 2012;13:836–845. doi: 10.1208/s12249-012-9807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams E.C., Toomey R., Alcantar N. Controlled release niosome embedded chitosan system: effect of crosslink mesh dimensions on drug release. J Biomed Mater Res A. 2012;100:3296–3303. doi: 10.1002/jbm.a.34275. [DOI] [PubMed] [Google Scholar]
- 26.Kim S., Nishimoto S.K., Bumgardner J.D., Haggard W.O., Gaber M.W., Yang Y.Z. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials. 2010;31:4157–4166. doi: 10.1016/j.biomaterials.2010.01.139. [DOI] [PubMed] [Google Scholar]
- 27.Wang L.M., Stegemann J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31:3976–3985. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang C., Yin C.H., Pei Y.Y., Yu L., Cui F.Y. Study on the stability of oral insulin in gastrointestinal tract. Chin Pharm J. 2004;39:764–768. [Google Scholar]
- 29.Shangguan M.Z., Lu Y., Qi J.P., Han J., Tian Z.Q., Xie Y.C. Binary lipids-based nanostructured lipid carriers for improved oral bioavailability of silymarin. J Biomater Appl. 2014;28:887–896. doi: 10.1177/0885328213485141. [DOI] [PubMed] [Google Scholar]