Abstract
Due to the ability of the blood–brain barrier (BBB) to prevent the entry of drugs into the brain, it is a challenge to treat central nervous system disorders pharmacologically. The development of nanotechnology provides potential to overcome this problem. In this review, the barriers to brain-targeted drug delivery are reviewed, including the BBB, blood–brain tumor barrier (BBTB), and nose-to-brain barrier. Delivery strategies are focused on overcoming the BBB, directly targeting diseased cells in the brain, and dual-targeted delivery. The major concerns and perspectives on constructing brain-targeted delivery systems are discussed.
KEY WORDS: Brain targeting, Nanoparticles, Dual targeting, Intranasal delivery, Blood–brain barrier
Graphical abstract
Several barriers are faced in brain-targeted delivery. To overcome these barriers and deliver drugs and imaging probes to the brain, various strategies have been developed for overcoming the blood–brain barrier (BBB), directly targeting diseased brain cells, and dual targeting delivery. Although significant progress has been made, there are still many issues to be resolved.
1. Introduction
In the past two decades, nanotechnology has been developing quickly and is widely used in disease diagnosis and treatment. Various kinds of nanoparticle (NP)-based drug delivery systems have been constructed using emerging novel nanomaterials. These NPs include liposomes, dendrimers, micelles, polymer nanoparticles and inorganic nanoparticles1, which can carry therapeutic drugs or imaging probes and deliver them to target site. NPs provide many benefits, including improving the solubility, protecting cargoes from digestion by enzymes, elevating targeting efficiency and enhancing cellular internalization. Therefore, NPs have gained increasing attention in the field of medicine and biology.
Currently, disorders in central nervous system (CNS) with significant consequences and attention include Alzheimer׳s disease (AD), Parkinson׳s disease (PD) and brain tumors2. However, the diagnosis and treatment of CNS disorders is far from impressive, owing to the restriction by blood–brain barrier (BBB) of drug transport into the brain3, with almost 100% of the macromolecular drugs and over 98% of the small-molecule drug candidates unable to enter the brain4. Other than nutrients, only small lipophilic molecules (<500 Da) can effectively cross the BBB and reach an efficacious brain concentration5. To conquer the BBB and deliver diagnostic and therapeutic drugs to the brain, various kinds of strategies have been developed using NPs as carriers.
In this review, the biology of the BBB and other neural barriers that restrict brain-targeted drug delivery are described. Recent advances in brain-targeted drug delivery are discussed which focus on NPs-based strategies. Lastly, the shortage of recent studies and potential future approaches are discussed.
2. Physical barriers in brain targeting
2.1. BBB
The BBB is the most important barrier in brain-targeted delivery. It was first discovered by Ehrlich in 1885, who found that intravenously injected dye could stain most organs except the brain6, 7. The BBB is composed of several kinds of cells, including brain capillary endothelial cells (BCECs), pericytes, astrocytes and neuronal cells8, 9. BCECs are the main component of BBB. Different from peripheral endothelial cells, BCECs possess several specific characteristics9. Most importantly, the continuous tight junctions between the BCECs prevent paracellular transport of compounds from blood to brain9. These tight junctions also result in extremely high transendothelial electrical resistance (TEER) between the blood and brain and the passive diffusion of compounds is considerably restricted8, 10. Despite the restriction on passive diffusion and paracellular transport from blood to brain, there are various kinds of transporters or carriers that can mediate the uptake to brain or extrusion from brain9, 11, 12 of various substances, as discussed in Section 3.
2.2. Blood–brain tumor barrier (BBTB)
In brain tumors, especially in advanced brain tumors, the BBB is compromised in the core but is integral in the surrounding area13, 14. For example, the distribution of erlotinib in the U87 tumor core is 4.69-fold higher than that in the brain around the tumor core15. However, drug distribution to brain tumor is more restricted than is found with peripheral tumors. In a metastatic breast tumor-bearing mouse model, the lapatinib concentration in lung metastasis tissue is 5.15-fold higher than that in brain metastasis16. It is assumed that the BBTB restricted the distribution of drugs from blood to brain tumor2, 17, 18. Compared with blood tumor barriers in peripheral tumors, the BBTB exhibits a smaller pore size and expresses a higher level of drug efflux pumps, such as P-glycoprotein, multidrug-resistance-associated proteins, and breast-cancer resistance protein19, 20, 21, 22, 23.
2.3. Nose to brain barrier
The anatomy, physiology and brain delivery route of the nasal cavity have been well reviewed24, 25. Basically, two parts of the nasal cavity, the respiratory region and the olfactory region, are responsible for drug absorption into brain or blood. Through the respiratory region mucosa some compounds can enter the systemic circulatory system and subsequently cross the BBB to brain, while some can be directly transported to brain via the trigeminal nerve pathway or lamina propria adsorption from perivascular and lymphatic spaces25. Through the olfactory mucosa compounds can be transported into the olfactory bulbs and then into cerebrospinal fluid through lamina propria absorption, olfactory nerves, lymphatic and perivascular spaces, and the trigeminal nerve pathway. Among these pathways, the olfactory mucosa pathway is the most rapid, and thus it is the main pathway that mediates drug delivery from the nasal cavity to the brain. Nonetheless, the volume that can be intranasally administered is very small (25–200 μL), which can limit the drug dose and the concentration of drug transported into brain. The nasal cilial clearance further diminishes the absorption time of drug in the nasal cavity and drug metabolism and secretion can also inhibit the drug transfer into the brain25.
3. Strategies to overcome the BBB
To deliver drugs to the brain, the BBB is the first barrier. Researchers have developed various kinds of strategies to overcome or bypass the BBB, including penetrating through BBB by cellular internalization, opening BBB and intranasal delivery2.
3.1. Penetrating through BBB
Although the BBB is intact, there are many receptors and carriers that are overexpressed on the BBB (Table 1)26, 27, 28, 29, 30, 31, 32, 33, 34, 35, which can mediate the transport of specific ligands and their cargoes. Additionally, the membrane of the BBB is negatively-charged and shows high affinity with positively-charged compounds, which could also trigger the internalization by cells. Thus these kinds of ligands could mediate the penetration of NPs through the BBB.
Table 1.
Transporters of the blood–brain barrier (BBB).
| Receptor-mediated transport | Active efflux-mediated transport | Transporter-mediated transport |
|---|---|---|
| Transferrin receptor26 | Adenosine triphosphate-binding cassette (ABC) transporter, subfamily B, member 1 (P-glycoprotein)31 | Glucose transporter, member 133 |
| Insulin receptor12 | ABC transporter, subfamily C12 | Large neutral amino acid29 |
| Transporter, member 134 | ||
| Low-density lipoprotein receptor– related protein27 | ABC transporter, subfamily G, member 229 | Cationic amino acid transporter, member 112 |
| Nicotinic acetylcholine receptor28 | Organic anion transporter12 | Monocarboxylic acid transporter, member 112 |
| Insulin-like growth factor receptor12 | Organic anion-transporting polypeptide29 | Concentrative nucleoside transporter12 |
| Diphtheria toxin receptor12 | Glutamic acid, amino acid transporter32 | Choline transporter35 |
| Scavenger receptor call B type29 | Taurine transporter29 | Nucleobase transporter29 |
| Leptin receptor12 | ||
| Neonatal Fc receptor30 |
3.1.1. Receptor-mediated transportation
On the BBB many receptors are overexpressed, including the transferrin (Tf) receptor, insulin receptor, low-density lipoprotein receptor–related protein, nicotinic acetylcholine receptor, insulin-like growth factor receptor, diphtheria toxin receptor, scavenger receptor call B type, leptin receptor and the neonatal Fc receptor12, 30. These receptors can specifically bind with corresponding ligands and trigger internalization into cells. Thus, the corresponding ligands could be functionalized onto NPs to mediate their transport through BBB. Due to the specificity of the interaction between receptors and ligands, the receptor-mediated transport has been the most commonly used and successful strategy to deliver NPs to brain through BBB.
The Tf receptor, which consists of two 90 kD subunits, is a transmembrane glycoprotein that is overexpressed on brain endothelial cells and serves to mediate the brain delivery of iron. It is the most widely evaluated receptor in BBB targeting delivery26. Tf, the specific protein of Tf receptor, was functionalized onto various kinds of NPs to improve brain delivery36, 37. In these studies the Tf-modified NPs (Tf-NPs) showed better brain capillary endothelial cell affinity and could deliver significantly more cargo to the brain than did unmodified NPs. For example, doxorubicin, a first-line chemotherapeutic, was loaded into Tf-NPs and they showed significantly better anti-brain tumor effect, with median survival time 70% longer than that of the doxorubicin solution-treated brain tumor-bearing rats28. Tf-modified magnetic silica poly(lactic-co-glycolic acid) (PLGA) NPs also showed the ability to target delivery both doxorubicin and paclitaxel to brain tumors38.
Although use of endogenous ligands allowed improved brain targeting delivery of NPs, the endogenous ligands may bind with the receptors and inhibit the binding efficiency of ligand-modified NPs, thus diminishing the brain-targeting delivery efficiency. To avoid this problem, antibodies against these receptors were developed. The binding site of antibodies to the receptors was different from that of ligands with receptors, and thus ligand competition could be avoided. OX26 is an antibody that can specifically recognize the Tf receptor. Pang et al.39 conjugated OX26 onto NPs for brain-targeted delivery of NC1900, a peptide for neurodegenerative disorders. The concentration of OX26-NPs in brain tissue at 2 h after intravenous injection was 2.62-fold higher than that of unmodified NPs. As a result, NC1900-loaded OX26-NPs showed the best treatment outcome for AD bearing rats as determined by a water maze learning task using scopolamine-induced learning and memory impairment rats. Anti-Tf receptor antibody RI7217 and anti-Tf receptor single chain antibody fragment (scFv) also showed the ability to mediate NP transport across the BBB to improve drug delivery and gene transfection40, 41. Additionally, antibody to insulin receptor, 83–14 mAb, possessed nearly 10-fold higher BBB penetrative effect than the anti-Tf receptor antibody42. Thus Dieu et al.43 conjugated 83–14 mAb onto the surface of NPs for brain-targeted drug delivery. In vitro results showed that the brain endothelial cells could effectively take up 83–14 mAb modified NPs, which could be inhibited by excess of 83–14 mAb.
Similarly, lactoferrin (Lf) is a mammalian cationic iron-binding glycoprotein that belongs to the Tf family, and could bind with Lf receptor which was overexpressed on BBB44. Results showed the brain targeting effect of Lf was superior to Tf and OX2645. The accumulation of Lf-conjugated NPs (Lf-NPs) in brain was 2.98-fold higher than that of NPs46, while it was even 1.96-fold higher than that of Tf-NPs47; thus Lf-NPs might be a better brain-targeting drug delivery system than Tf-NPs. Hu et al.48 utilized Lf-NPs to deliver urocortin (a peptide drug) to brain for PD treatment. The results demonstrated that urocortin-loaded Lf-NPs attenuated considerably the striatum lesion caused by 6-hydroxydopamine in rats as determined by a behavioral test. Immunohistochemistry and transmitter contents results further demonstrated that treatment with urocortin-loaded Lf-NPs could prevent the loss of contents of the transmitters in brain, which was similar to that in brain from normal rats and significantly better than that of a control group and an unmodified NPs group. Modification of Lf onto polymersomes also showed enhanced brain accumulation, which could deliver more S14G-humanin (a peptide drug) to protect rat brain from learning and memory impairment induced by amyloid β25–3549. These results demonstrated that Lf could serve as an active BBB targeting ligand to improve drug delivery to the brain.
However, protein ligands and antibodies possess several disadvantages, including poor stability, high immunogenicity, large molecular weight and high cost in production, which limit the application of these ligands. To avoid these problems, peptide-based ligands have gained increasing attention. To generate peptide ligands there are two common strategies: redesign from protein ligands and selection from a peptide library2.
CDX is a peptide that was constructed using computer-assisted redesign from the loop II region of candoxin, a ligand for the nicotinic acetylcholine receptor (nAchR)50. Although the binding affinity of CDX with nAchR is lower than that of candoxin, it could still considerably improve the uptake of NPs by BCECs. After loading with paclitaxel, CDX-modified NPs showed a better anti-brain tumor effect, with a median survival time of 27 days, which was significantly longer than that of untreated NPs (with a median survival time of 20 days). Rabies virus glycoprotein peptide (RVG29) is derived from the rabies virus glycoprotein which can also bind with nAchR28 and it could also enhance the drug delivery to brain51, 52. The apparent permeability coefficient for the RVG-modified poly(mannitol-co-PEI) vector was 1.51×10−4 cm/s, which was 2.23-fold higher than the vector without RVG modification53. In vivo, the RVG-modified vector effectively delivered GADPH siRNA and BACE1 siRNA into the brain, while the gene knockdown efficiency was significantly higher than that of the unmodified vector. Pepstatin A is a peptide that can specific bind with P-gp on the BBB. Yu et al.54 attached it to NPs to quantitate the level of P-gp, which contributes to pharmacoresistance in refractory epilepsy. This strategy can noninvasively image the status of P-gp using magnetic resonance imaging (MRI) and fluorescent imaging after loading corresponding probes into the NPs.
Phage display also can select a peptide from peptide library that can bind a specific receptor or cells50. Using this method, T7 peptide (HAIYPRH) was modified and selected for specific affinity with the Tf receptor through sequential rounds of negative and positive selection55. Kuang et al.56 decorated T7 peptide onto dendrimers to deliver DNA for gene therapy of brain cancer. The modification with T7 significantly enhanced cellular uptake of the dendrimers by the BCEC. The gene delivery efficiency could be decreased with an excess of Tf, indicating that the uptake of T7-modified dendrimers was mediated by the Tf receptor. After intravenous injection, T7-modified dendrimers yielded 1.7-fold higher gene expression in brain, demonstrating that T7 could act as an effective brain targeting ligand. The T7 peptide was also used for delivering photosensitizer-loaded gold NPs to brain tumor57. In vivo imaging demonstrated that the T7 modification could enhance brain accumulation of photosensitizer 6-fold higher than that obtained with unmodified gold NPs.
Aptamers are another kind of small molecular ligand that can recognize specific receptors on the BBB to improve brain targeted delivery. Cheng et al.58 used an in vivo systematic evolution of ligands by exponential enrichment (SELEX) to find aptamers that could bind to and penetrate the BBB. The selected A15 aptamer could effectively be taken up by bEnd.3 cells with high intensity and distribute into whole brain. However, no published study used the aptamer for mediating NP transport through the BBB.
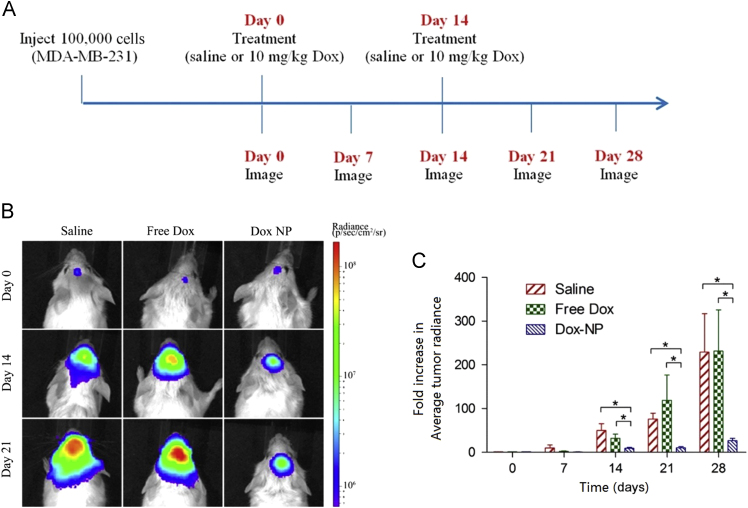
There are also other ligands that could recruit proteins in plasma which can bind with specific receptors. Apolipoproteins, including apolipoprotein A (ApoA) and apolipoprotein E (ApoE) are serum proteins that can be transported into brain through low-density lipoprotein receptors which are highly expressed on BBB59, 60. Thus ApoA, ApoE, and the peptide derived from ApoE showed the ability to mediate NPs transfer into brain61, 62, 63, 64, 65. Interestingly, non-ionic surfactants could promote the adsorption of ApoE onto the surface of NPs66. For example, polysorbate 80, an FDA-approved injectable surfactant, could adsorb ApoE in serum when it was conjugated onto NPs, and polysorbate 80-coated NPs have been evaluated as a brain targeting delivery system by many groups67, 68, 69, 70. Martins et al.69 evaluated the brain targeting efficiency of polysorbates 60 and 80 for enhancing brain targeting of NPs. Although the plasma area under curve (AUC) of polysorbate 60–coated NPs was 1.18-fold higher than that of polysorbate 80–coated NPs, the brain AUC of polysorbate 80–coated NPs was 1.77-fold higher than that of polysorbate 60–coated NPs, indicating that polysorbate 80 may be a better surfactant for brain targeting. Jiang׳s group67 found that the brain targeting efficiency of polysorbate 80–coated NPs was influenced by the particle size. After comparing NPs with particle sizes of 70, 170, 220 and 345 nm, results showed that the 70 nm polysorbate 80–coated NPs delivered cargoes to brain most effectively. Recently, Shalviri et al.71 designed polysorbate 80–coated NPs for the delivery of doxorubicin, fluorescent dye and gadolinium (Gd) to image and treat brain metastasis72. Results showed that the accumulation of polysorbate 80-coated NPs was greatly higher than that of unmodified NPs as demonstrated by MRI, fluorescent whole body imaging and fluorescent distribution in brain slices. Consequently, doxorubicin-loaded polysorbate 80–coated NPs caused considerably higher activation of caspase-3 expression and apoptosis of brain tumor cells, resulting in a significantly smaller extent of metastasis and longer survival time (Fig. 1)72.
Figure 1.
Inhibition of brain tumor growth in NRG-SCID mice. (A) Treatment schedule with saline (200 μL), doxorubincin (Dox) (10 mg/kg; 200 μL), or Dox-loaded NPs (10 mg/kg Dox; 200 μL). Treatments were administered on day 0 and 14. (B) In vivo images of brain tumor bioluminescence. (C) Fold increase in the average tumor radiance as measured by in vivo bioluminescence imaging. Data presented as mean±SEM (n=5 for saline; n=7 for free Dox and Dox-NP). Statistical significance of P<0.05 denoted by asterisk (*). This study demonstrated that polysorbate 80 modification could enhance the brain-targeted delivery of NPs, resulting in an improved anti-brain tumor effect. Reprinted from Ref. 72 with permission of the copyright holder, American Chemical Society. Dox, doxorubicin; NP, nanoparticle.
3.1.2. Transporter-mediated transport
Most nutritive materials that the brain requires are supplied by the blood. Thus transporters for these nutritive materials including hexose transporters, amino acid transporters and monocarboxylate transporters (Table 1) are usually overexpressed on the BBB and can be used for brain targeted delivery12, 73.
Glutathione is an endogenous tripeptide that shows antioxidant properties. The glutathione transporter is highly expressed on the BBB, and thus researchers conjugated it onto liposomes to deliver various drugs to brain74. Using a fluorescent probe as a tracker, intravenous injection of glutathione-modified liposomes could cause 4-fold higher accumulation in brain than unmodified liposomes75. Using glutathione-modified liposomes as a drug delivery system, several drugs have been delivered into the brain76, 77. For example, doxorubicin-loaded glutathione-modified liposomes were under evaluation in clinical trial. A protein drug, β amyloid–binding llama single domain antibody fragments (VHH-pa2H), could be loaded into the glutathione-modified liposomes78. After intravenous injection, the concentration in brain of APP/PS1 transgenic mice was over 10-fold higher than that of the free drug. This system is promising because of the safety, convenience of large-scale production, and controllable pharmacokinetics of liposomes. The human applicability of glutathione conjugates also contributes to the attraction of the glutathione-modified liposomes79.
The choline transporter is also highly expressed on the BBB because the brain needs choline to synthesize the cholinergic neurotransmitter acetylcholine35. 11C-choline has been used to diagnose brain tumors80, which demonstrated the choline could penetrate BBB. However, as choline was not suitable for modification, Li et al.81 developed several bis-quaternary ammonium compounds with high affinity for the choline transporter. To select the best ligand for the choline transporter, the inhibitory effect on the uptake of choline chloride by BCECs was determined, and the compound with the best inhibitory effect was selected as a ligand to be modified onto dendrimers. An in vitro study determined that modification with a choline derivative allowed more efficient uptake by BCEC compared with unmodified dendrimers. After loading with plasmid DNA, the brain from choline-derivative-modified dendrimer-treated mice showed higher gene expression, demonstrating that the choline derivative was an effective BBB targeted delivery ligand. The same drug delivery system was also used for delivering MRI contrast Gd and codelivery of DNA and doxorubicin, both of which showed excellent brain targeted delivery efficiency82, 83.
3.1.3. Adsorptive-mediated transportation
Adsorptive-mediated brain targeting is practicable because the negatively charged BBB can interact with positively charged drug delivery systems on the basis of the electrostatic effect12. Cationic proteins and peptides, such as cationized bovine serum albumin (CBSA), could be used for adsorptive-mediated brain targeted drug delivery.
CBSA was prepared from bovine serum albumin through cationization. After decorating onto NPs, CBSA could significantly improve cellular uptake by BCECs, with fluorescent intensity 2-fold higher than that of unmodified NPs after 4 h incubation84. Additionally, the modification with CBSA could increase not only the uptake by BCECs but also the penetrating efficiency through an in vitro BBB model. The apparent BBB permeability of CBSA-NPs was 2.7- to 9.0-fold higher than that of unmodified NPs, while increasing the surface density of CBSA from 33/NP to 372/NP increased the BBB permeability from 2.7 to 9.0 85. In vivo, the accumulation in brain of CBSA-modified NPs (CBSA-NPs) was 1.6-fold higher than that of unmodified NP86. After loading NC-1900, an active compound for treating of AD, CBSA-NPs treatment considerably improved memory impairments to normal level, while the unmodified NPs only caused modest improvement87. This kind of drug delivery system was also used to deliver the tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) gene and aclarubicin to the brain88, 89. Encapsulation into CBSA-NPs led to 2.6- and 2.7-fold higher aclarubicin concentration in the brain tumor compared with unmodified NPs at 1 and 24 h, respectively, after intravenous injection88. The CBSA-NPs also showed higher TRAIL protein expression in brain tumor compared with unmodified NPs when loading with TRAIL gene89. Several times treatment of aclarubicin or TRAIL gene–loaded CBSA-NPs greatly prolonged the median survival time of brain cancer-bearing mice, which was 1.4-fold longer than that of unmodified NPs.
Additionally, cationized immunoglobulins, cationized monoantibodies and histone have brain targeting properties via a similar mechanism12. However, poor selectivity is the inherent shortcoming of adsorptive-mediated targeting because most biological membranes are negatively charged. Due to this reason, adsorptive-mediated targeting is rarely used in systemic administration.
3.1.4. Cell penetrating peptide (CPP)-related transport
CPPs were first discovered in 1988 from HIV and characterized with the ability to facilitate cargo transport across cell membranes90, 91. As a negatively charged membrane, the BBB also showed affinity for CPPs. Thus several groups utilized CPPs for BBB penetration. Qin et al.92 showed that modification of transactivating-transduction (TAT) onto liposomes (TAT-liposomes) could enhance accumulation in brain, which was 2.54-fold higher than that of unmodified liposomes. Other CPPs, such as octaarginine, was also demonstrated to have BBB targeting delivery potential, and the brain targeting efficiency was positively related to the positive charge of the CPPs93. Another study showed the TAT-conjugated chitosan NPs improved gene delivery to brain94. TAT-modified gold NPs could deliver Gd3+ and doxorubicin to glioma with better efficiency than Gd3+ and free doxorubicin95. However, the interaction between CPPs and the cell membrane was mainly considered unspecific96. Although recently one study said their penetration through cell membrane was mediated by fatty acids in the membranes97, this would not provide the CPPs with cell selectivity. Therefore, CPP modification improved BBB penetration as well as accumulation in other organs, such as liver and spleen, leading to an increased risk of drug-originating side effects98. For this reason CPP-modified NPs are rarely used in brain targeting.
To avoid this disadvantage, a bi-ligand was developed by conjugating CPPs with a specific targeting ligand, providing the CPP with cell selectivity. Additionally, the bi-ligand also could overcome receptor saturation which restricts the internalization efficiency of ligand-modified NPs99. Sharma et al.100, 101 conjugated Tf with poly-l-arginine to form bi-ligand Tf-PR. In vitro, the bEnd.3 cell uptake of Tf-PR-modified liposomes (Tf-PR-liposomes) was significantly higher than that of Tf modified liposomes (Tf-Liposomes) and poly-arginine-modified liposomes (PR-Liposomes). Importantly, the transfer percentage through bEnd.3 monolayers of Tf-PR-liposomes was about 19% after 8 h incubation, which was considerably higher than that of Tf-liposomes (about 13%) and PR-liposomes (about 10%)100. In vivo, the brain targeting effect of Tf-PR-liposomes was approximately 2-fold higher than the Tf-Liposomes. As a result, gene expression in the brains of mice treated with gene-loaded Tf-PR-liposomes was 1.66-fold higher than that of Tf-Liposomes101.
Liu et al.102 also conjugated octaarginine with c(RGDfK) peptide to form bi-ligand RRGD (Fig. 2). The bEnd.3 cell uptake of RRGD modified liposomes (RRGD-liposomes) was 30- and 2-fold higher than that of octaarginine modified liposomes (R-liposomes) and c(RGDfK)-modified liposomes (RGD-liposomes). The same trend was also observed in bEnd.3 monolayer penetration study. Consequently, the RRGD-liposomes showed the highest accumulation in brain tumor. After loading with paclitaxel, RRGD-liposomes could extend the median survival time of brain tumor bearing mice from 26 to 48 days, which was 1.33- and 1.26-fold longer than that of mice treated with R-liposomes and RGD-liposomes, respectively.
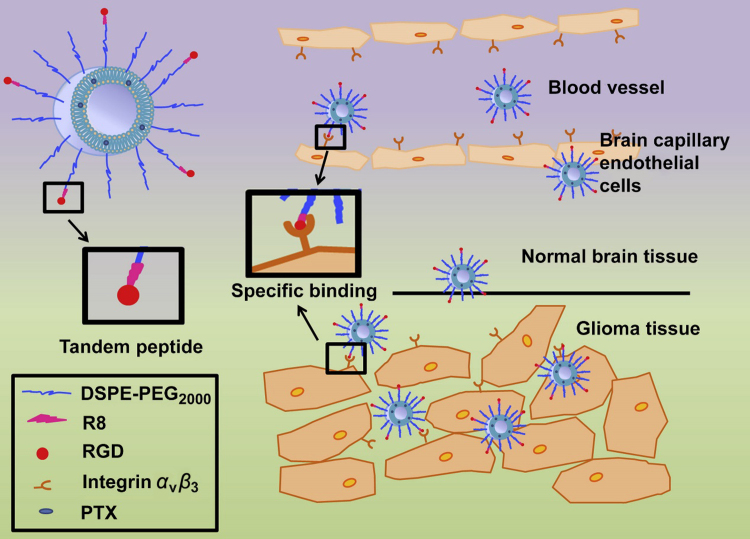
Figure 2.
Schematic illustration of paclitaxel (PTX)-loaded R8-RGD-modified liposomes (PTX-R8-RGD-lipo). Liposomes could specifically bind to integrin αvβ3 receptors expressed on brain capillary endothelial cells and be transported across the blood–brain barrier (BBB) through a synergistic effect. Liposomes could accumulate in the glioma tissue selectively, penetrate into the core region of tumor and release drug. Reprinted from Ref. 102 with permission of the copyright holder, Elsevier, Amsterdam.
3.2. Temporarily open the BBB
Since BBB is the main barrier that restricts the transportation from blood to brain, temporarily opening the BBB to enlarge the pore size could allow compounds or NPs to directly diffuse into brain. Temporarily opening the BBB could be achieved by several physical and pharmacological methods.
3.2.1. Chemical compounds enhanced BBB permeability
Borneol, a widely used messenger drug in traditional Chinese medicine, can enhance the permeability of various membranes including mucosa, skin and the BBB103. Zhang et al.104 evaluated the effect of oral administration of borneol on brain targeted delivery of NPs. Co-incubation BCECs with borneol and NPs could significantly enhance the cellular uptake. In vivo, the brain targeting index of co-administration versus that of administrating NPs solely was 1.86, suggesting the borneol could significantly enhance the brain accumulation of NPs. After loading with huperzine A, the memory of AD mice treated with borneol and NPs was considerably better than those of mice treated with NPs solely, which was demonstrated by a Morris water maze experiment. Similarly, alkylglycerols could open the BBB and facilitate the brain delivery of drugs. Toman et al.105 modified alkylglycerol onto dextran-graft-poly(lactic acid) NPs (PLA-DEX-OX4) for brain targeted delivery. The penetration through bEnd.3 monolayer of PLA-DEX-OX4 was higher than that of the unmodified NPs and free dextran. Unfortunately, there is no significant difference, suggesting that the efficiency of alkylglycerol modification was sufficient. Since these compounds could only open the BBB above a certain concentration, the BBB would return to intact status when the blood concentration of these compounds was lower than the threshold. Therefore, the dose and administration schedule must be well-optimized.
3.2.2. Receptor-involved changing of tight junctions
Since tight junctions play an important role in keeping the integrity of BBB and prohibiting the entry of molecules from blood to brain, modification of the tightness of tight junctions is an applicable method to improve the permeability of BBB.
As G-protein-coupled receptors, adenosine receptors have four subtypes: A1, A2A, A2B and A3106. Adenosine receptors have been considered as promising therapeutic targets in many disorders including CNS disorders. Many agonists and antagonists of adenosine receptors have been discovered with therapeutic effect107. Recently, it was pointed that activation of A1 and A2A adenosine receptors could elevate the permeability of BBB in vivo. One of the agonists of A2A, lexiscan, also could improve the penetration of macromolecules through BBB. Incubating lexiscan with bEnd.3 cell monolayers could decrease the TEER and diminish the expression of occludin, caudin-5 and zonula occludens-1 (ZO-1), which were all essential for keeping tight junction integrity108. Thus agonists of adenosine receptors could be used for brain targeting drug delivery.
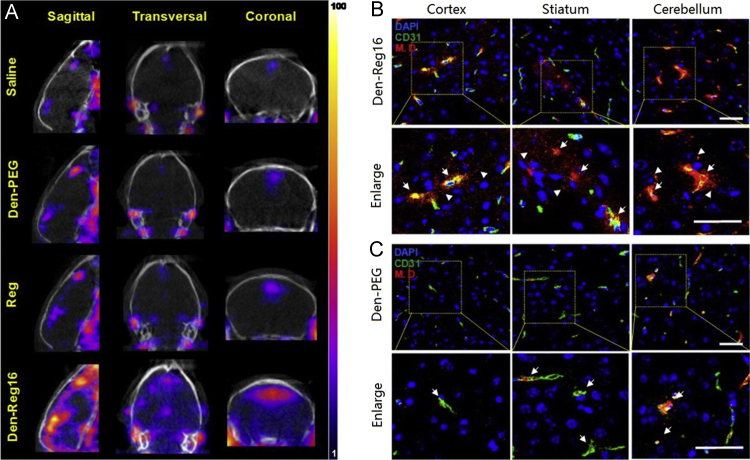
However, the application of these agonists was restricted by their short blood circulation time. Gao et al.109 conjugated lexiscan onto dendrimers to form nanoagonists (NAs) to improve the brain-targeted delivery of several model drugs. One of the nanoagnoists, Den-Reg16 (conjugating 16 of lexiscan molecules onto 1 dendrimer), showed 7.7-fold higher binding affinity with the A2A adenosine receptor than unmodified lexiscan. In vitro, the expression of ZO-1 in untreated bEnd.3 monolayers was continuously aligned on the cell–cell interface, while Den-Reg16 treatment led to discontinued expression even in absence of ZO-1, resulting in 68% reduction of TEER and 17.6-fold increase of permeability of the model drug (1 mg/mL, 45 kDa). Compared with lexiscan treatment, Den-Reg16 treatment still caused 4.6-fold higher permeability of bEnd.3 monolayers. In vivo, the model drug accumulation in the brains of mice pretreated with Den-Reg16 was 6.8- and 3.6-fold higher than that of saline with lexiscan. The increase in brain targeted delivery of model drug was also determined by SPECT/CT (Fig. 3)109, suggesting that the nanoagonists were promising adjuvants for improving BBB permeability.
Figure 3.
Brain drug delivery via nanoagonist (NA)-mediated adenosine receptor signaling. (A) In vivo SPECT/CT images of mouse brain when radioactive model drug (3.7×107 Bq/mouse) was injected at 30 min postinjection (PI) of 10 nmol NA, regadenoson (Reg), or saline via i.v. Confocal fluorescence microscopic images of brain sections presenting cortex, striatum, and cerebellum areas when model drug was injected at 30 min PI of dendrimer (Den)-Reg16 (B) or Den-PEG (C). CD31 immunofluorescence indicating brain vasculature is displayed in green; model drug is displayed in red and DAPI-stained nuclei are blue. Yellow areas present the co-localized vessel and model drugs. Arrows point to the leaky vessels, and arrowheads point to the extravasated model drug. Scale bar, 50 μm. This study demonstrated that the administration of nanoagonists could temporarily open BBB and improve the brain-targeted delivery of several model drugs. Reprinted from Ref. 109 with permission of the copyright holder, American Chemical Society. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.3. Focused ultrasound
Focused ultrasound (FUS) can provide reversible BBB disruption-enhanced permeability by concentrating acoustic energy to a focal spot, which could be used for brain-targeted drug delivery66, 110. To improve the BBB disruption and minimize damage to surrounding normal brain cells, many kinds of gas microbubbles were used as cavitation nuclei to focus and convert the acoustic energy into mechanical power. In this strategy, MRI is often combined with FUS to direct the FUS energy and monitor the local temperature elevation66.
The FUS has been used to enhance brain delivery of various NPs. Etame et al.111 evaluated the distribution of gold NPs in two hemispheres while one hemisphere was treated with FUS and the other one was not. The concentration of gold NPs in the sonicated hemisphere was 3.36 times higher than that of control hemisphere. Treatment of doxorubicin-loaded liposomes with FUS enhanced the median survival time of brain tumor-bearing mice from 23 days (control) and 27 days (treatment with doxorubicin loaded liposomes only) to 35 days, while 3 of 8 mice survived over 140 days112.
Combining FUS with other targeting methods could further elevate the brain accumulation of drugs and NPs. For example, combining FUS with magnetic targeting could further improve the brain accumulation of magnetic NPs, which was 16.3-fold higher than FUS alone113. Combing FUS with a targeting ligand modification also could further elevate brain-targeted drug delivery. Compared with the combination of FUS and doxorubicin-loaded liposomes, combination of FUS with atherosclerotic plaque-specific peptide-1-modified liposomes increased the tumor-to-contralateral brain ratios of drug from 2.1 to 3.8.
Although the toxicity of FUS to brain is considered minor and neurotoxicity was not observed in a recent study114, the clinical application of this method still needs to be viewed cautiously115. Repeated application of FUS and microbubbles to open the BBB over a long term (4–20 months) indicated lack of edema in the majority of the cases116. But in a neurotoxicity test through quantitative cognitive testing of visual, cognitive, motivational, and motor function, a significant increase of reaction time during the task was observed on the day of FUS and microbubbles application. Fortunately, the reaction time returned to baseline within 4–5 days after the procedure, demonstrating the safety of this method.
3.3. Intranasal delivery to bypass the BBB
As noted above, there is a route to deliver drugs or NPs directly from the nasal cavity to the brain, and thus intranasal delivery is considered a promising strategy in brain-targeted drug delivery. Drug solutions could be directly used for intranasal administration and many absorption promoters or modulators have been developed to improve the intranasal delivery, which has been well reviewed by Lisbeth Illum117. To protect the drugs from damage by enzymes and further elevate the delivery efficiency, nanotechnology was also widely used in intranasal delivery25.
3.3.1. Unmodified NPs
Biocompatible NPs could not only protect the cargoes from damage by enzymes in the nasal cavity but also protect the mucosa and nasal cilia from toxicity caused by the drugs. Jiang׳s group118 encapsulated nimodipine in microemulsions for intranasal drug delivery. The AUC of nimodipine in brain cerebrum and cerebrospinal fluid after intranasal administration of a microemulsion was 13.8-fold and 159-fold higher than that of intravenous administration. Remarkably, the nimodipine microemulsion did not cause ciliotoxicity at a dose much higher than the treatment dose118. Intranasal administration of H102 peptide–loaded NPs resulted in 1.6 to 2.9-fold higher concentration in different parts of brain compared with intranasal administration of H102 peptide solution119. Consequently, treatment of an AD mouse model was considerably improved as characterized by the Morris water maze test and activity of choline acetyltransferase and acetylcholinesterases. Additionally, zidovudine-loaded solid lipid microparticles showed the ability to intranasally deliver drug to cerebrospinal fluid with 6-fold higher efficiency compared with zidovudine solution120. Direct nose-to-brain transport was 61% for diazepam-loaded NPs, while the number for diazepam solution was only 1%121. RNA-encapsulated NPs also demonstrated enhanced gene expression in brain after intranasal administration122. These studies suggest that NPs are superior in intranasal drug delivery than solutions. However, the unmodified NPs lack cell internalization efficiency and olfactory mucosa targeting ability, which could be improved by specific ligand modification.
3.3.2. Agglutinant-mediated transport
It has been pointed out that the expression of some saccharide groups, such as N-acetylglucosamine and l-fucose, on the olfactory mucosa was considerably higher than that of respiratory mucosa25. Thus, agglutinins could be used for enhancing intranasal brain delivery because these agglutinins could bind with the saccharide groups and trigger endocytosis.
Jiang׳s group first modified wheat germ agglutinin (WGA) onto PLGA NPs (WGA-NPs) for intranasal delivery of cargoes25, 123. Using coumarin-6 as a fluorescent tracker, it showed the AUC of WGA-NPs in various brain tissues (olfactory bulb, olfactory tract, cerebrum and cerebellum) was approximately 2-fold higher than that of unmodified NPs. Importantly, the nasal ciliotoxicity of WGA-NPs and unmodified NPs was negligible as demonstrated by cilial movement, morphology and integrity. WGA-NPs were used for delivering vasoactive intestinal peptide (VIP), a peptide for AD treatment. The intact VIP in brain delivered by WGA-NPs was as high as 11.48% ID h/g tissue, which was 7.74- and 2.17-fold higher than intranasal administration of VIP solution and unmodified NPs, respectively123. The better delivery effect was attributed to the higher affinity of WGA-NPs for olfactory mucosa and better penetrating ability through olfactory mucosa. Intranasally administered WGA-NPs could reach the CNS via the olfactory pathway and the trigeminal nerve pathway using extracellular transport along these nerves124. Consequently, the VIP-loaded WGA-NPs showed a better AD treatment outcome than the unmodified NPs and VI solution123. Besides WGA, Ulex europeus agglutinin I (UEA I), Solanum tuberosum lectin and odorranalectin was also demonstrated to improve intranasal delivery of NPs125, 126, 127.
3.3.3. CPP mediated transport
CPPs can enhance transport through endothelial cells of nasal cavity and therefore improve intranasal delivery. Direct co-administration of CPP with macromolecules could considerably enhance the brain delivery. For example, penetratin enabled intranasally administrated insulin to distribute in regions of the brain distal to the nasal cavity, including the cerebral cortex, cerebellum, and brain stem128. Encapsulating insulin into CPP-modified NPs could deliver 6% of insulin into brain, which was much higher than that of unmodified NPs129. TAT-modified micelles could also elevate the brain delivery of drugs. Taki et al.130 encapsulated camptothecin into TAT-modified micelles. After intranasal administration, the median survival time of glioma bearing mice was prolonged significantly from 18.2 to 32.6 days, which was much longer than that obtained with unmodified micelles (22 days).
4. Directly targeting diseased cells in brain
4.1. Brain tumor targeting delivery
In brain tumor, especially in malignant brain tumor, the BBB is compromised in brain tumors14, and thus NPs can reach brain tumor directly through blood circulation and EPR effect, although the EPR effect is weak compared with peripheral tumors20.
4.1.1. Passively targeting delivery
Our group showed that docetaxel-loaded nanoemulsion and albumin NPs could passively target brain tumors, resulting in prolonged median survival time relative to docetaxel injection131, 132. Lapatinib-loaded albumin NPs also showed enhanced anti-brain tumor effect as compared to lapatinib tablets133. Guo et al.134 co-loaded tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and doxorubicin into PEGylated liposomes, which could prolong the median survival time of brain tumor-bearing mice from 32 to 48 days. NPs formed by assembly of poly(β-amino ester) with siRNA were able to effectively knockdown gene expression in 91% of glioma cells without significant cytotoxicity. At a low concentration of siRNA (4 nmol/L), the poly(β-amino ester) NPs showed higher gene knockdown efficiency than commercial lipofectamine 2000 at relative high concentration (20 nmol/L siRNA)135.
4.1.2. Aptamer-mediated targeted delivery
To further improve brain tumor targeting effectiveness, ligands could be modified onto the surface of NPs. The AS1411 aptamer was discovered by SELEX to bind with nucleolin, a brain tumor-overexpressed receptor136, 137. AS1411-modified NPs (AsNPs) showed about 2-fold higher uptake by brain tumor cells than that of unmodified NPs138. In vivo, paclitaxel-loaded AsNPs delivered significantly more paclitaxel to tumor tissue than did the unmodified NPs, effectively reducing tumor growth (81.68% slower than controls) and greatly prolonging the median survival time of brain tumor-bearing mice (72% longer than controls)138. Another aptamer, GMT-8 aptamer, also showed the ability to enhance brain tumor-targeted delivery of NPs and improve its anti-brain tumor effect139.
4.1.3. Peptide-mediated targeted delivery
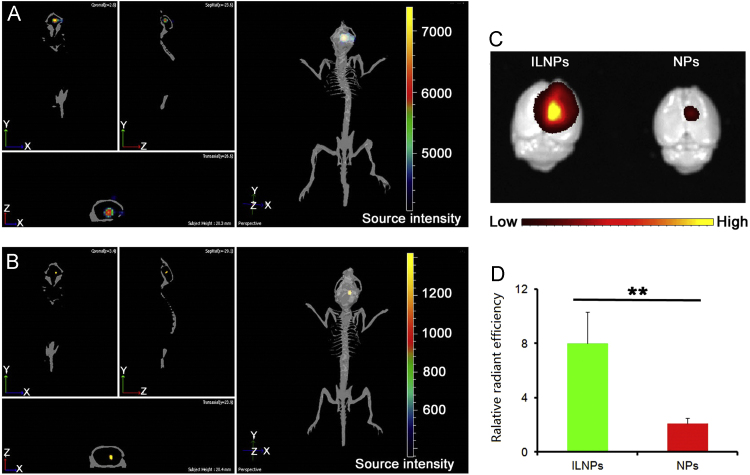
Several peptides also have been utilized for improving brain tumor targeting delivery. As a peptide derived from interlulin-13 protein, interlukin-13 peptide (IL-13p) could specifically bind IL-13Rα2, a receptor specifically overexpressed in brain tumor over normal brain tissue140. Fillmore et al.141 demonstrated that modification of IL-13p onto metallofullerenes could considerably increase uptake by brain tumor cells. Our lab also showed that IL-13p-modified NPs (ILNPs) could significantly and specifically elevate the uptake by brain tumor cells rather than macrophage cells, resulting in better cell selectivity. In vivo the brain tumor accumulation of ILNPs was 3.81-fold higher than that of unmodified NPs (Fig. 4)142. Consequently, docetaxel-loaded ILNPs showed better antitumor effect than unmodified NPs. The median survival time of brain tumor bearing mice treated with ILNPs was 1.83-fold longer and the tumor weight was 68.6% lower than that of controls142, 143, 144. Other groups also demonstrated that the brain tumor targeting ability of IL-13p through the modification of it onto polymer NPs and mesoporous silica–coated graphene nanosheet145, 146.
Figure 4.
In vivo and ex vivo imaging. (A) Fluorescent imaging of glioma-bearing mice 2 h after administered DiR-loaded IL-13p-modified NPs (ILNPs). (B) Fluorescent imaging of glioma-bearing mice 2 h after administration of DiR-loaded NPs. (C) Ex vivo imaging of brains 2 h after administration of DiR-loaded ILNPs and NPs. (D) Semi-quantitative results of fluorescence intensity of gliomas, **P<0.01 vs. control. This study demonstrated that modification with IL-13p could specifically enhance the brain tumor–targeting delivery of NPs. Reprinted from Ref. 142 with permission of the copyright holder, Huile Gao.
RGD also is a peptide that is widely used in brain tumor targeting delivery because its target, integrin receptor αvβ3, is overexpressed on brain tumor cells and especially on brain tumor neovessels147. In vitro, RGD modification elevated the uptake by brain tumor cells of dendrimers and upconversion NPs148, 149. In vivo, RGD-modified NPs delivered significantly more paclitaxel to the brain tumor, resulting in higher tumor growth inhibition and longer median survival time of brain tumor–bearing mice compared with unmodified NPs150. It was demonstrated that the RGD-modified NPs accumulated more rapidly in brain tumor and possessed higher permeability from vessels into tumor parenchyma compared with the untargeted NPs151. After loading with small hairpin (sh)RNA, the RGD-modified polyethylenimine-coated gold nanorods (RDG) led to a 70% reduction of protein expression in U87 glioma cells, which was 1.88-fold higher than obtained with particles without RGD modification152. These studies demonstrate that RGD modification could effectively conquer the BBTB.
VTW peptide (VTWTPQAWFQWV), selected from a 12-mer library using phage display, showed high binding affinity to U87 brain tumor cells153. SWDIAWPPLKVP peptide was selected for affinity to A172 brain tumor cells by phage display154. RGERPPR is a tumor-penetrating peptide that could target neuropilin-1 on brain tumor cells155. Heat shock protein (Hsp) 70 can target CD40 that is overexpressed on brain tumor cells156. Chlorotoxin is a 36-amino acid long peptide that can target matrix matalloproteinase-2 in brain tumors157. After anchoring to NPs, these peptides could effectively enhance the genes, drugs and imaging probe delivery to brain tumor cells158, 159, 160, 161, 162, 163, 164. For example, chlorotoxin-coupled lipid NPs effectively delivered anti-miR-21 oligonucleotides to glioma and promoted miR-21 silencing, resulting in decreased tumor cell proliferation and improved animal survival165.
Another effective strategy is modification of NPs with two ligands that target different part of brain tumors. Our laboratory modified NPs with RGD and IL-13p to target both brain tumor neovessels and brain tumor cells166, 167, 168. In an endothelial cell and brain tumor cell cocultured model, RGD modification selectively elevated the uptake of NPs by endothelial cells, IL-13p modification selectively elevated the uptake by brain tumor cells while dual modification enhanced uptake by both cells. In vivo immunofluorescent imaging also demonstrated the specific targeting ability of RGD and IL-13p. Because RGD and IL-13p dual modified NPs could deliver docetaxel to both neovasculature and tumor cells of brain tumor, they showed better anti-brain tumor effect than the single ligand modified NPs, suggesting it was a promising strategy to target more than one cell type in a tumor.
4.2. Other diseased brain cell targeting
Cerebral amyloid angiopathy (CAA) is characterized by the accumulation of amyloid beta (Aβ) proteins within the walls of brain blood vessels169. Thus drug could directly target to Aβ through the blood circulation without the need for penetrating through BBB. Agyare et al.170 modified F(ab′)2 fragment of anti-amyloid antibody, IgG4.1 (pF(ab′)24.1) directly onto cyclophosphamide loaded theranostic NPs. In vivo the pF(ab′)24.1 modified NPs could effectively target cerebrovascular amyloid and reduce pro-inflammatory cytokine production by the Aβ-challenged BBB endothelium, which was better than that obtained with unmodified NPs.
5. BBB and diseased cell dual targeted delivery
Although brain targeting delivery systems could enhance the distribution of drugs in brain, the distribution of NPs in brain after penetrating through BBB or after bypassing the BBB is a big concern. If the NPs unselectively distribute in the whole brain, the improvement of treatment outcome caused by elevated drug concentration might accompany even worse side effect to CNS2. Additionally, as one of the most important organs, brain is more sensitive to toxicants. Thus an ideal brain targeting drug delivery system should possess two characteristics: (1) effective penetration of the BBB or bypassing BBB, (2) selectively targeting diseased cells while minimizing the distribution into normal brain cells. To achieve these two goals, dual brain targeting drug delivery systems were developed. Normally, the “dual brain targeting” means there are ligands for brain targeting and ligands for diseased cells in one drug delivery system. If a ligand could target both brain and diseased brain cells, ligand-modified drug delivery system also can be considered as dual brain targeting drug delivery system.
5.1. Two ligand modification for two targets
To demonstrate the possibility, we constructed a kind of angiopep-2 and EGFP–EGF1 dual modified NPs (AENPs) for specifically targeting neuroglial cells in normal brain because the low-density lipoprotein receptor–related protein (LRP) (receptor of angiopep-2) is overexpressed on the BBB, and thus could bind with EGFP-EGF1, which is overexpressed on neuroglial cells while minimally expressed on endothelial cells171, 172, 173. In vitro, the angiopep-2 modification specifically increased cellular uptake of NPs by bEnd.3 cells (a generally used cell line to represent BCECs) rather than neuroglial cells, while the EGFP-EGF1 modification specifically improved cellular uptake by neuroglial cells rather than bEnd.3 cells. Consequently, the dual modification could improve cellular uptake by both kinds of cells. As a result, AENP could not only target to brain but also specifically colocalize with neuroglial cells as demonstrated by in vivo fluorescent imaging and immunofluorescence of brain slices172. These results demonstrated that the dual targeting delivery system could indeed enhance both brain targeting and cell selectivity in brain.
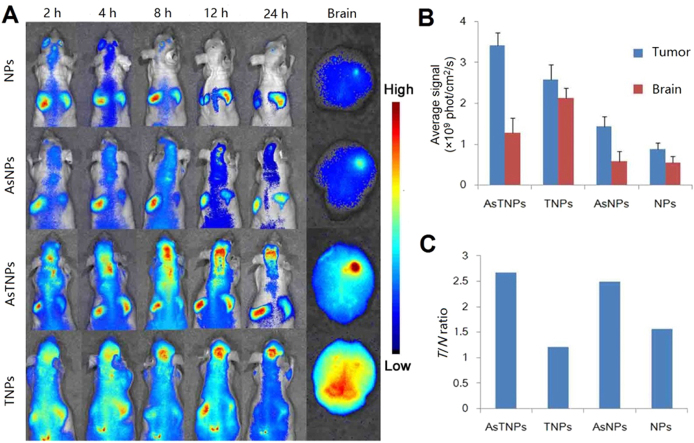
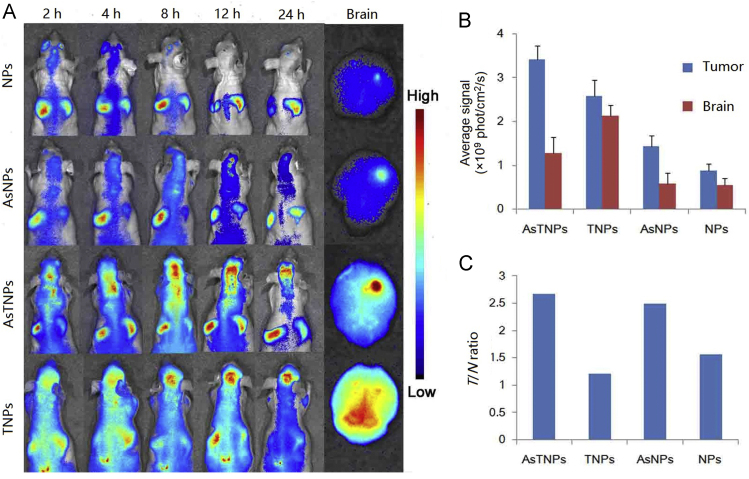
This strategy was used for treatment of several brain disorders. TGN peptide was selected by phage display and showed good BBB-targeting efficiency, and AS1411 aptamer is a brain tumor targeting ligand which has been discussed above136, 174. We functionalized these two ligands onto NPs for brain tumor dual targeting drug delivery175. In vitro, the constructed system, AsTNPs, could penetrate through the BBB model and then be taken up by brain tumor cells, with a significantly higher penetration ratio and uptake intensity than the single ligand-modified NPs176. In vivo, the accumulation of AsTNPs in brain tumor was 4.91-fold higher than that of unmodified NPs (Fig. 5)175. More importantly, to evaluate the glioma-selective distribution in brain, the intensity ratio of glioma to brain (T/N ratio) was evaluated. It was discovered that although TGN modification could enhance the glioma intensity resulting from BBB penetration, the T/N ratio of TGN modified NPs was only 1.2, which was even lower than unmodified NPs (T/N ratio was 1.6). In contrast, T/N ratio of AsTNPs was as high as 2.6, suggesting that dual modification could not only improve the accumulation in brain tumor but also the selectivity in brain. Consequently, a low dose of docetaxel-loaded AsTNPs could effectively prolong the median survival time of brain tumor–bearing mice from 17 to 32 days, while at this dose, the unmodified NPs and free docetaxel showed no therapeutic effect175. This study clearly demonstrated the superiority of dual targeting drug delivery systems to traditional brain targeting drug delivery systems.
Figure 5.
(A) The in vivo imaging of DiR-loaded NPs, AS1411 conjugated NPs (AsNPs), AsTNPs and TGN modified NPs (TNP) in the brain glioma-bearing nude mice at several time points with ex vivo imaging of the brain at 24 h. (B) Brain and glioma fluorescent intensity at 24 h. (C) The T/N ratio of the brains 24 h after treatment with different formulations. This study demonstrated that the dual targeting delivery strategy could not only increase the brain targeting efficiency but also improve the selectivity to brain tumor cells. Reprinted from Ref. 175 with permission of the copyright holder, Elsevier, Amsterdam.
This strategy was also used in AD treatment. The accumulation and aggregation of Aβ forms the amyloid plaque, which is the dominant characteristic of AD177. Approximately 96% of the total Aβ is Aβ1–42, the most toxic isoform that possesses high tendency toward aggregation178. Thus Aβ1–42 is a promising target for treatment of AD. QSH peptide (QSHYRHISPAQV) was selected using mirror-image phage display against Aβ1–42179. Both in mice and humans, QSH showed high binding affinity with Aβ1–42180. Therefore, Zhang et al.181 conjugated QSH and TGN onto NPs (TQNPs) for dual targeted delivery to amyloid plaque of AD mice. Compared with TGN-modified NPs (TNPs which could only penetrate through BBB), TQNPs delivered 1.62-fold and 1.84-fold higher amounts of fluorescent probe and H102 peptide (a therapeutic peptide for AD), respectively, to the brain hippocampus, where the Aβ1–42 was pre-implanted to establish an AD mouse model181, 182. This indicated that modification with QSH considerably improved the selectivity of NPs in brain when they penetrated BBB as mediated by TGN peptide. Consequently, the spatial learning and memory of the AD model mice in the H102-loaded TQNPs group, as demonstrated by Morris water maze experiment, were significantly improved compared with the AD control group as well as the other treatment group including the H102-loaded TNPs group. The nerve cell damage caused by Aβ accumulation in hippocampus was significantly attenuated by treatment of H102 loaded TQNPs, which was similar to a healthy brain. These results demonstrated that the dual brain targeting delivery system could considerably improve the treatment outcome of AD, which is better than traditional brain targeting drug delivery system, and thereby represents a promising direction in this field.
There are many studies developing various kinds of dual ligands–modified drug delivery systems, which all showed better drug delivery efficiency than the single ligand-modified drug delivery systems, including B6 peptide (for Tf receptor) and RGD peptide (for integrin receptor)183, Tf and RGD184, 185, Tfn and tamoxifen (to inhibit MDR protein)186, WGA and Tf187, d-manno-pyranoside and Tf188, cationic bovine serum albumin and mannose189, angiopep-2 and RGD190, angiopep-2 and tLyP-1 (for neuropilin-1 receptor)191, des-octanoyl ghrelin (for Tf receptor) and folate (for folate receptor)192, Tf and folate193, mannose-vitamin E derivative (for glucose transporters) and dequalinium-lipid derivative (for adsorptive mediated transportation)194, OX26 and chlorotoxin195, lactoferrin and folate196, c(RGDyK) and folate197, phosphatidic acid (for Aβ binding) and an ApoE-derived peptide198, Tf and vitamin E199. However, these studies focused mainly on the enhanced cell selectivity and internalization by multivalent effect200 rather than the ability to conquer different barrier sequentially.
5.2. Fusion proteins and peptides
Fusion proteins and peptides could be used for dual targeting because they can combine the active domains of two ligands into one ligand. Pardridge׳s group201 developed many fusion proteins to produce therapeutic monoclonal antibodies with BBB-penetrating ability. However, these proteins were mostly used as drugs directly rather than targeting ligands in the reported studies.
5.3. One ligand for one target on two site
In addition to fusion proteins or peptides, some ligands can directly target two sites because their receptors or transporters are overexpressed on both BBB and diseased brain cells, such as LRP and Tf receptor202, 203, 204.
Angiopep-2 (TFFYGGSRGKRNNFKTEEY, molecular weight 2.4 kDa) belongs to a family called angiopep, which was derived from the Kunitz domain of aprotinin205. Angiopep-2 shows high binding affinity with LRP, and thus it could act as a dual-targeting ligand to deliver NPs that penetrate BBB and target brain tumors. Xin et al.206 demonstrated that angiopep-2 modification enhanced BBB penetration and distribution of NPs in brains of normal mice. In brain tumor–bearing mice angiopep-2 modification could not only increase the distribution in brain but also the distribution in brain tumor207. Consequently, the median survival time of brain tumor–bearing mice treated with paclitaxel-loaded angiopep-2-modified NPs lasted from 22 to 37 days, which was significantly longer than that obtained with unmodified NPs27. The angiopep-2 modification also could elevate gene expression in brain when using liposomes as gene delivery vectors208. Recently, there were several studies using angiopep-2 as a dual targeting ligand to successfully deliver inorganic NPs, such as gold NPs and upconversion nanoprobes, to brain tumor for diagnosis or treatment209, 210. Lactoferrin could also target to LRP. Lactoferrin modification could enhance the uptake of NPs by both bEnd.3 cells and C6 glioma cells. In vivo, the doxorubicin concentration in glioma of mice treated with lactoferrin-modified NPs was almost 4-fold higher than that of unmodified NPs211. These studies from different groups demonstrated that the dual targeting delivery using a single ligand is a promising strategy for brain, especially brain tumor, targeted delivery.
In addition to receptors, there also are some transporters overexpressed on both the BBB and in brain tumor cells. d-Glucose transporter protein (GLUT) shows particularly high concentration in brain microvessels, about 100-fold higher than the Tf receptor30, 212. P-aminophenyl-α-d-mannopyranoside, a substrate of GLUT1 and GLUT3, showed the ability of improving brain accumulation of liposomes213, 214. Additionally, GLUT is also over-expressed on brain tumor cells33. Thus, Jiang et al.215 utilized 2-deoxy-d-glucose as dual targeting ligand for brain tumor-targeted delivery. Both the bEnd.3 monolayer transportation and brain tumor cell uptake of NPs were significantly elevated after 2-deoxy-d-glucose modification. In vitro, the brain tumor accumulation of 2-deoxy-d-glucose-modified NPs was considerably higher than that of unmodified NPs, resulting in greatly longer median survival time of brain tumor-bearing mice. Large amino acid transporter 1 (LAT1) also is overexpressed on both BBB and glioma cells, thus both glutamate and phenylalanine could effectively improve the delivery efficiency of NPs to glioma through dual targeting strategy34, 216.
5.4. Combining intranasal administration and diseased cell targeting
Combining intranasal delivery and diseased brain cell targeting ligand is another strategy to construct a dual targeting delivery system. Histological studies showed that the lactoferrin receptor was overexpressed on the apical surface of respiratory epithelia cells, brain endothelial cells and neurons, and the overexpression of lactoferrin receptor in the CNS was associated with age-related neurodegenerative diseases217, 218. Thus, Liu et al.219 modified lactoferrin onto NPs to intranasally deliver a short neuropeptide (NAP) to the brain of AD-bearing mice. The AUC of lactoferrin-modified NPs in hippocampus was 2.23-fold higher than that of unmodified NPs. Consequently, the memory of AD mice treated with NAP-loaded lactoferrin-modified NPs was significantly better than that of mice treated with unmodified NPs. The neuron density in the hippocampus was also considerably higher than that of mice treated with unmodified NPs.
5.5. Combining FUS and diseased cell targeting
As a method to open the BBB, FUS also could be combined with brain tumor cell-targeting NPs. Vascular endothelial growth factor A (VEGF-A) can bind with and activate brain tumor endothelial cells overexpressing VEGF receptor tyrosine kinase type R2 (VEGF-R2) to regulate brain tumor angiogenesis220. Although FUS combination with 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) loaded microbubbles could effectively deliver BCNU to brain, the modification of microbubbles with VEGF-A ligand combined with FUS further improved the brain concentration from 24.66 to 45.97 μg/g tissue. Consequently, the brain tumor growth speed of combination treatment (from day 6 to day 34 increased 5.02 times) was greatly lower than that of FUS combined with BCNU loaded unmodified microbubbles (from day 6 to day 34 increased 18.54 times), and the median survival time also increased by 121%221.
5.6. Overview of the dual targeting delivery strategies
Because more than one barrier is often faced in targeting delivery of drugs to brain disorders, dual targeting delivery strategies was developed, showing better BBB penetrating efficiency and higher drug accumulation in diseased brain tissues, and superior to BBB targeting and diseased brain cells targeting strategy alone. However, these systems are still lacking. The ligand intensity, especially the intensity ratio between two ligands, needs to be optimized. Additionally, we have no idea about the transport pathways to diseased brain cells after the systems penetrate through BBB.
6. Major concerns in constructing brain-targeted delivery systems
6.1. Potential neurotoxicity of the NPs
CNS is highly protected from exposure to xenobiotics and toxic substances by the BBB66. The brain targeting delivery system is a double-edged sword because it improves the drug concentration in diseased cells of brain as well as the drug and NPs exposure to normal brain, which may result in elevated side effect. In recent years, the neurotoxicity of NPs and nanomaterials has gained increasing attention. BBB targeting delivery system unselectively improved the concentration of systems in the whole brain, which may lead to unfavorable side effect. Therefore, dual brain targeting drug delivery systems will be the future direction in brain targeting delivery field and should gain increasing attention.
6.2. Drug release during transport of the drug delivery systems
Although the drug delivery systems, and especially the dual targeting drug delivery systems, enhanced the targeting effectiveness for diseased brain cells and improved the therapeutic outcome of drug treatment, the drug-loaded delivery systems also distributed to normal tissues, which may cause drug-originated adverse effects. Additionally, drug release in the blood circulation would diminish the drug concentration in the target site. Thus a desirable drug delivery system should keep most drug bound during delivery procedures and in normal tissues, and quickly release drugs when they arrive at the target site. To address this goal, our group constructed a pH-sensitive dual targeting delivery system and the model drug, doxorubicin was anchored using a hydrazone bond211. The system could target brain tumors because of the angiopep-2 modification. In brain tumor tissue the doxorubicin could be specifically released because of the hydrolysis of hydrazone at low pH222. Shao et al.223 fabricated NPs with a cross linker to coat the drug-containing core, which effectively prevented the drug from release during the delivery procedure. When the system reached the targeted tumor the linker was de-shielded by the highly concentrated glutathione (GSH) in tumor cells224.
6.3. The homogeneity of brain targeting drug delivery systems
In the drug delivery systems there are many factors that can affect the targeting efficiency, such as particle size, surface properties, ligand properties, and ligand density. Through optimization researchers could improve brain targeting drug delivery systems. For example, Pang et al.39 found the optimized number of OX26 conjugated per polymersome was 34 because more OX26 led to quicker clearance from blood and less OX26 led to poor targeting ability. However, this number means that the average density of OX26 on polymersomes was 34. It is hard, if not impossible, to ensure that every particle was homogeneously conjugated with 34 OX26 molecules. The lack of homogeneity may diminish the targeting efficiency of these systems.
Taking particle size as another example: The particle size of NPs considerably affects the in vivo behavior and distribution of NPs. Larger sized NPs tend to be trapped by lung and smaller sized NPs tend to be eliminated by kidney. Particle size also affected BBB permeability and diseased cell permeability. For example, the cutoff pore size of the U87 brain tumor model is 7–100 nm20, which means drug delivery systems with size higher than 100 nm would have difficulty accessing U87 brain tumors. Another study showed the gold NPs at 70 nm possessed the best penetration effect through bEnd.3 monolayers among 20–110 nm gold NPs225. Additionally, the particle size can affect the elimination and penetration in diseases tissues, such as tumor226, 227, 228, 229. To address this problem, some kinds of size changeable NPs were developed, which showed both better tumor retention and tumor penetration229, 230, 231. However, there are still other problems. For most biodegradable NPs, the size range is wide, for example, “mean particle size is 200 nm” is only a statistical description, which may actually refer to “a mixture of particles that range from 50 nm to 400 nm”. The response to physical barriers of the mixture of particles was hard to predict and control. Recently, Particle Replication in Non-wetting Templates (PRINT®) emerged with the ability to produce uniform NP232, which may partly solve the above problems. However, no ligand modified PRINT particles were prepared until early 2016.
6.4. The protein corona may hinder targeted delivery
A protein corona is formed as soon as NPs are introduced into biological fluids233. The protein corona is formed by serum proteins which can be divided into opsonin and dyopsonin due to their different role in the alteration of the blood circulation time of NPs234. In addition to the alteration of the distribution of NPs, the protein corona may cover the targeting ligand and hinder the specific reaction between ligands and their targets. For example, incubating Tf-modified NPs with serum-containing culture medium considerably decreased the specific interaction between Tf and Tf receptor on cells235. A consistent result was provided by Mirshafiee et al.236 using bicyclononyne and azide as model interaction. Unfortunately, most studies on brain targeted drug delivery systems did not evaluate the formation and influence of protein corona.
6.5. The off-target potential of brain-targeted delivery
The basis of ligand-modified NPs for brain-targeted delivery is that these ligands can specifically react with their receptors or transporters and these receptors and transporters are highly and specifically expressed on the BBB and/or brain diseased cells. As discussed above, the protein corona may attenuate the specific reaction between ligand and receptor/transporter. In addition, the expression of receptors/transporters in other tissues could also lead to off-target effects. Unfortunately, most, if not all, of the receptors/transporters also expressed on normal cells. For example, the Tf receptor is overexpressed on BBB and brain tumor cells, but it is expressed on almost all cells at various levels237. Therefore, it is hard to avoid off-target effects when using endogenous receptors/transporters as a target. However, labeling target cells with exogenous ligand as a target may avoid the off-target effect. 9-Azido sialic acid could be incorporated into brain because the metabolism of brain cells requires a high level of sialic acid. The systemic administration of an alkyne-functionalized biotin probe could then be labeled onto brain cells due to the azide-alkyne click reaction, resulting in specific imaging of brain sialoglycans in living animals238.
7. Perspectives
CNS disorders are difficult to treat pharmacologically due to the protection of the brain by the BBB. Brain-targeted drug delivery has gained increasing attention. Due to the barriers in delivering drugs to diseased brain tissues and cells, constructing brain-targeted drug delivery systems is the most promising strategy to address this natural defense.
To achieve this purpose, several basic concerns should be addressed in the future: (1) the materials should be biodegradable and able to be eliminated from brain, which would provide the brain-targeted drug delivery systems with biological safety; (2) a uniform preparation method should be developed to make the NPs more homogenous and predictable; (3) the factors that influence in vivo behavior of NPs should be well elucidated and evaluated, which is fundamental for constructing brain-targeted drug delivery systems; (4) the targeting efficiency is far from satisfactory, and considerable improvement should be made before their clinical application.
Overall, tremendous development has occurred in brain targeting during past two decades. Although it is still in development, it will play an increasingly important role in treating CNS disorders.
Acknowledgments
The work was funded by the National Natural Science Foundation of China (Nos. 31571016 and 81402866).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Srikanth M., Kessler J.A. Nanotechnology-novel therapeutics for CNS disorders. Nat Rev Neurol. 2012;8:307–318. doi: 10.1038/nrneurol.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao H.L., Pang Z.Q., Jiang X.G. Targeted delivery of nano-therapeutics for major disorders of the central nervous system. Pharm Res. 2013;30:2485–2498. doi: 10.1007/s11095-013-1122-4. [DOI] [PubMed] [Google Scholar]
- 3.Wohlfart S., Gelperina S., Kreuter J. Transport of drugs across the blood–brain barrier by nanoparticles. J Control Release. 2012;161:264–273. doi: 10.1016/j.jconrel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Pardridge W.M. Drug targeting to the brain. Pharm Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 5.Pardridge W.M. CNS drug design based on principles of blood–brain barrier transport. J Neurochem. 1998;70:1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 6.Alyautdin R., Khalin I., Nafeeza M.I., Haron M.H., Kuznetsov D. Nanoscale drug delivery systems and the blood–brain barrier. Int J Nanomedicine. 2014;9:795–811. doi: 10.2147/IJN.S52236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eyal S., Hsiao P., Unadkat J.D. Drug interactions at the blood–brain barrier: fact or fantasy? Pharmacol Ther. 2009;123:80–104. doi: 10.1016/j.pharmthera.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhowmik A., Khan R., Ghosh M.K. Blood brain barrier: a challenge for effectual therapy of brain tumors. Biomed Res Int. 2015;2015:320941. doi: 10.1155/2015/320941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posadas I., Monteagudo S., Ceña V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine (London) 2016;11:833–849. doi: 10.2217/nnm.16.15. [DOI] [PubMed] [Google Scholar]
- 10.Butt A.M., Jones H.C., Abbott N.J. Electrical resistance across the blood–brain barrier in anaesthetized rats: a developmental study. J Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao H.L., Jiang X.G. Progress on the diagnosis and evaluation of brain tumors. Cancer Imaging. 2013;13:466–481. doi: 10.1102/1470-7330.2013.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo L.R., Ren J.F., Jiang X.G. Perspectives on brain-targeting drug delivery systems. Curr Pharm Biotechnol. 2012;13:2310–2318. doi: 10.2174/138920112803341770. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal S., Sane R., Oberoi R., Ohlfest J.R., Elmquist W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev Mol Med. 2011;13:e17. doi: 10.1017/S1462399411001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolburg H., Noell S., Fallier-Becker P., Mack A.F., Wolburg-Buchholz K. The disturbed blood–brain barrier in human glioblastoma. Mol Aspects Med. 2012;33:579–589. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal S., Manchanda P., Vogelbaum M.A., Ohlfest J.R., Elmquist W.F. Function of the blood–brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;41:33–39. doi: 10.1124/dmd.112.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taskar K.S., Rudraraju V., Mittapalli R.K., Samala R., Thorsheim H.R., Lockman J. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29:770–781. doi: 10.1007/s11095-011-0601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bronger H., König J., Kopplow K., Steiner H.H., Ahmadi R., Herold-Mende C. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood–tumor barrier. Cancer Res. 2005;65:11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- 18.Groothuis D.R. The blood–brain and blood–tumor barriers: a review of strategies for increasing drug delivery. Neuro Oncol. 2000;2:45–59. doi: 10.1093/neuonc/2.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts W.G., Delaat J., Nagane M., Huang S., Cavenee W.K., Palade G.E. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol. 1998;153:1239–1248. doi: 10.1016/s0002-9440(10)65668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim R., Palazzo C., Evrard B., Piel G. Nanocarriers for the treatment of glioblastoma multiforme: current state-of-the-art. J Control Release. 2016;227:23–37. doi: 10.1016/j.jconrel.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Mohri M., Nitta H., Yamashita J. Expression of multidrug resistance-associated protein (MRP) in human gliomas. J Neurooncol. 2000;49:105–115. doi: 10.1023/a:1026528926482. [DOI] [PubMed] [Google Scholar]
- 22.Fattori S., Becherini F., Cianfriglia M., Parenti G., Romanini A., Castagna M. Human brain tumors: multidrug-resistance P-glycoprotein expression in tumor cells and intratumoral capillary endothelial cells. Virchows Arch. 2007;451:81–87. doi: 10.1007/s00428-007-0401-z. [DOI] [PubMed] [Google Scholar]
- 23.Sarin H., Kanevsky A.S., Wu H., Sousa A.A., Wilson C.M., Aronova M.A. Physiologic upper limit of pore size in the blood–tumor barrier of malignant solid tumors. J Transl Med. 2009;7:51. doi: 10.1186/1479-5876-7-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djupesland P.G., Messina J.C., Mahmoud R.A. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5:709–733. doi: 10.4155/tde.14.41. [DOI] [PubMed] [Google Scholar]
- 25.Wu H.B., Hu K.L., Jiang X.G. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin Drug Deliv. 2008;5:1159–1168. doi: 10.1517/17425247.5.10.1159. [DOI] [PubMed] [Google Scholar]
- 26.Qian Z.M., Li H.Y., Sun H.Z., Ho K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 27.Xin H.L., Sha X.Y., Jiang X.Y., Zhang W., Chen L.C., Fang X.L. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials. 2012;33:8167–8176. doi: 10.1016/j.biomaterials.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 28.Kumar P., Wu H.Q., McBride J.L., Jung K.E., Kim M.H., Davidson B.L. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 29.Pardridge W.M. blood–brain barrier delivery. Drug Discov Today. 2007;12:54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T.T., Li W., Meng G.M., Wang P., Liao W.Z. Strategies for transporting nanoparticles across the blood–brain barrier. Biomater Sci. 2016;4:219–229. doi: 10.1039/c5bm00383k. [DOI] [PubMed] [Google Scholar]
- 31.Cannon R.E., Peart J.C., Hawkins B.T., Campos C.R., Miller D.S. Targeting blood–brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci U S A. 2012;109:15930–15935. doi: 10.1073/pnas.1203534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu S.H., Wen C.J., Al-Suwayeh S.A., Chang H.W., Yen T.C., Fang J.Y. Physicochemical characterization and in vivo bioluminescence imaging of nanostructured lipid carriers for targeting the brain: apomorphine as a model drug. Nanotechnology. 2010;21:405101. doi: 10.1088/0957-4484/21/40/405101. [DOI] [PubMed] [Google Scholar]
- 33.Gorin F., Harley W., Schnier J., Lyeth B., Jue T. Perinecrotic glioma proliferation and metabolic profile within an intracerebral tumor xenograft. Acta Neuropathol. 2004;107:235–244. doi: 10.1007/s00401-003-0803-1. [DOI] [PubMed] [Google Scholar]
- 34.Li L., Di X.S., Zhang S.W., Kan Q.M., Liu H., Lu T.S. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf B Biointerfaces. 2016;141:260–267. doi: 10.1016/j.colsurfb.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 35.Geldenhuys W.J., Allen D.D. The blood–brain barrier choline transporter. Cent Nerv Syst Agents Med Chem. 2012;12:95–99. doi: 10.2174/187152412800792670. [DOI] [PubMed] [Google Scholar]
- 36.Pang Z.Q., Gao H.L., Yu Y., Guo L.R., Chen J., Pan S.Q. Enhanced intracellular delivery and chemotherapy for glioma rats by transferrin-conjugated biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem. 2011;22:1171–1180. doi: 10.1021/bc200062q. [DOI] [PubMed] [Google Scholar]
- 37.Pang Z.Q., Gao H.L., Yu Y., Chen J., Guo L.R., Ren J.F. Brain delivery and cellular internalization mechanisms for transferrin conjugated biodegradable polymersomes. Int J Pharm. 2011;415:284–292. doi: 10.1016/j.ijpharm.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 38.Cui Y.N., Xu Q.X., Chow P.K., Wang D.P., Wang C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34:8511–8520. doi: 10.1016/j.biomaterials.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 39.Pang Z.Q., Lu W., Gao H.L., Hu K.L., Chen J., Zhang C.L. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Control Release. 2008;128:120–127. doi: 10.1016/j.jconrel.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Kim S.S., Rait A., Kim E., Pirollo K.F., Nishida M., Farkas N. A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival. ACS Nano. 2014;8:5494–5514. doi: 10.1021/nn5014484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvati E., Re F., Sesana S., Cambianica I., Sancini G., Masserini M. Liposomes functionalized to overcome the blood–brain barrier and to target amyloid-β peptide: the chemical design affects the permeability across an in vitro model. Int J Nanomedicine. 2013;8:1749–1758. doi: 10.2147/IJN.S42783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu D., Yang J., Pardridge W.M. Drug targeting of a peptide radiopharmaceutical through the primate blood–brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J Clin Invest. 1997;100:1804–1812. doi: 10.1172/JCI119708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dieu L.H., Wu D.L., Palivan C.G., Balasubramanian V., Huwyler J. Polymersomes conjugated to 83−14 monoclonal antibodies: in vitro targeting of brain capillary endothelial cells. Eur J Pharm Biopharm. 2014;88:316–324. doi: 10.1016/j.ejpb.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Fillebeen C., Descamps L., Dehouck M.P., Fenart L., Benaïssa M., Spik G. Receptor-mediated transcytosis of lactoferrin through the blood–brain barrier. J Biol Chem. 1999;274:7011–7017. doi: 10.1074/jbc.274.11.7011. [DOI] [PubMed] [Google Scholar]
- 45.Ji B., Maeda J., Higuchi M., Inoue K., Akita H., Harashima H. Pharmacokinetics and brain uptake of lactoferrin in rats. Life Sci. 2006;78:851–855. doi: 10.1016/j.lfs.2005.05.085. [DOI] [PubMed] [Google Scholar]
- 46.Hu K.L., Li J.W., Shen Y.H., Lu W., Gao X.L., Zhang Q.Z. Lactoferrin-conjugated PEG-PLA nanoparticles with improved brain delivery: in vitro and in vivo evaluations. J Control Release. 2009;134:55–61. doi: 10.1016/j.jconrel.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Ye Y.J., Sun Y., Zhao H.L., Lan M.B., Gao F., Song C. A novel lactoferrin-modified β-cyclodextrin nanocarrier for brain-targeting drug delivery. Int J Pharm. 2013;458:110–117. doi: 10.1016/j.ijpharm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 48.Hu K.L., Shi Y.B., Jiang W.M., Han J.Y., Huang S.X., Jiang X.G. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: preparation, characterization and efficacy in Parkinson׳s disease. Int J Pharm. 2011;415:273–283. doi: 10.1016/j.ijpharm.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 49.Yu Y., Jiang X.G., Gong S.Y., Feng L., Zhong Y.Q., Pang Z.Q. The proton permeability of self-assembled polymersomes and their neuroprotection by enhancing a neuroprotective peptide across the blood–brain barrier after modification with lactoferrin. Nanoscale. 2014;6:3250–3258. doi: 10.1039/c3nr05196j. [DOI] [PubMed] [Google Scholar]
- 50.Zhan C.Y., Li B., Hu L.J., Wei X.L., Feng L.Y., Fu W. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew Chem Int Ed Engl. 2011;50:5482–5485. doi: 10.1002/anie.201100875. [DOI] [PubMed] [Google Scholar]
- 51.Huo H., Gao Y.K., Wang Y., Zhang J.H., Wang Z.Y., Jiang T.Y. Polyion complex micelles composed of pegylated polyasparthydrazide derivatives for siRNA delivery to the brain. J Colloid Interface Sci. 2015;447:8–15. doi: 10.1016/j.jcis.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y., An S., Li J.F., Kuang Y.Y., He X., Guo Y.B. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer׳s disease mice. Biomaterials. 2016;80:33–45. doi: 10.1016/j.biomaterials.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 53.Park T.E., Singh B., Li H.S., Lee J.Y., Kang S.K., Choi Y.J. Enhanced BBB permeability of osmotically active poly(mannitol-co-PEI) modified with rabies virus glycoprotein via selective stimulation of caveolar endocytosis for RNAi therapeutics in Alzheimer׳s disease. Biomaterials. 2015;38:61–71. doi: 10.1016/j.biomaterials.2014.10.068. [DOI] [PubMed] [Google Scholar]
- 54.Yu X.R., Wang J.H., Liu J.S., Shen S., Cao Z.L., Pan J.W. A multimodal pepstatin A peptide-based nanoagent for the molecular imaging of P-glycoprotein in the brains of epilepsy rats. Biomaterials. 2016;76:173–186. doi: 10.1016/j.biomaterials.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.H., Engler J.A., Collawn J.F., Moore B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur J Biochem. 2001;268:2004–2012. doi: 10.1046/j.1432-1327.2001.02073.x. [DOI] [PubMed] [Google Scholar]
- 56.Kuang Y.Y., An S., Guo Y.B., Huang S.X., Shao K., Liu Y. T7 peptide–functionalized nanoparticles utilizing RNA interference for glioma dual targeting. Int J Pharm. 2013;454:11–20. doi: 10.1016/j.ijpharm.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 57.Dixit S., Novak T., Miller K., Zhu Y., Kenney M.E., Broome A.M. Transferrin receptor–targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale. 2015;7:1782–1790. doi: 10.1039/c4nr04853a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng C.S., Chen Y.H., Lennox K.A., Behlke M.A., Davidson B.L. In vivo SELEX for identification of brain-penetrating aptamers. Mol Ther Nucleic Acids. 2013;2:e67. doi: 10.1038/mtna.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dehouck B., Dehouck M.P., Fruchart J.C., Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood–brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dehouck B., Fenart L., Dehouck M.P., Pierce A., Torpier G., Cecchelli R. A new function for the LDL receptor: transcytosis of LDL across the blood–brain barrier. J Cell Biol. 1997;138:877–889. doi: 10.1083/jcb.138.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Re F., Cambianica I., Zona C., Sesana S., Gregori M., Rigolio R. Functionalization of liposomes with ApoE-derived peptides at different density affects cellular uptake and drug transport across a blood–brain barrier model. Nanomedicine. 2011;7:551–559. doi: 10.1016/j.nano.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 62.Zensi A., Begley D., Pontikis C., Legros C., Mihoreanu L., Büchel C. Human serum albumin nanoparticles modified with apolipoprotein A-I cross the blood–brain barrier and enter the rodent brain. J Drug Target. 2010;18:842–848. doi: 10.3109/1061186X.2010.513712. [DOI] [PubMed] [Google Scholar]
- 63.Tamaru M., Akita H., Nakatani T., Kajimoto K., Sato Y., Hatakeyama H. Application of apolipoprotein E–modified liposomal nanoparticles as a carrier for delivering DNA and nucleic acid in the brain. Int J Nanomedicine. 2014;9:4267–4276. doi: 10.2147/IJN.S65402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jose S., Sowmya S., Cinu T.A., Aleykutty N.A., Thomas S., Souto E.B. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur J Pharm Sci. 2014;63:29–35. doi: 10.1016/j.ejps.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 65.Tamaru M., Akita H., Kajimoto K., Sato Y., Hatakeyama H., Harashima H. An apolipoprotein E modified liposomal nanoparticle: ligand dependent efficiency as a siRNA delivery carrier for mouse-derived brain endothelial cells. Int J Pharm. 2014;465:77–82. doi: 10.1016/j.ijpharm.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 66.Wong H.L., Wu X.Y., Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv Drug Deliv Rev. 2012;64:686–700. doi: 10.1016/j.addr.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Gao K.P., Jiang X.G. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int J Pharm. 2006;310:213–219. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 68.Tian X.H., Lin X.N., Wei F., Feng W., Huang Z.C., Wang P. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int J Nanomedicine. 2011;6:445–452. doi: 10.2147/IJN.S16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martins S., Sarmento B., Nunes C., Lucio M., Reis S., Ferreira D. Brain targeting effect of camptothecin-loaded solid lipid nanoparticles in rat after intravenous administration. Eur J Pharm Biopharm. 2013;85:488–502. doi: 10.1016/j.ejpb.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 70.Wilson B., Lavanya Y., Priyadarshini S.R., Ramasamy M., Jenita J.L. Albumin nanoparticles for the delivery of gabapentin: preparation, characterization and pharmacodynamic studies. Int J Pharm. 2014;473:73–79. doi: 10.1016/j.ijpharm.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 71.Shalviri A., Chan H.K., Raval G., Abdekhodaie M.J., Liu Q., Heerklotz H. Design of pH-responsive nanoparticles of terpolymer of poly(methacrylic acid), polysorbate 80 and starch for delivery of doxorubicin. Colloids Surf B Biointerfaces. 2013;101:405–413. doi: 10.1016/j.colsurfb.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 72.Li J., Cai P., Shalviri A., Henderson J.T., He C.S., Foltz W.D. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood–brain barrier targeting brain metastases of breast cancer. ACS Nano. 2014;8:9925–9940. doi: 10.1021/nn501069c. [DOI] [PubMed] [Google Scholar]
- 73.Wei X.L., Chen X.S., Ying M., Lu W.Y. Brain tumor–targeted drug delivery strategies. Acta Pharm Sin B. 2014;4:193–201. doi: 10.1016/j.apsb.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaillard P.J., Visser C.C., Appeldoorn C.C.M., Rip J. Enhanced brain drug delivery: safely crossing the blood–brain barrier. Drug Discov Today: Technol. 2012;9:e155–e160. doi: 10.1016/j.ddtec.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 75.Rip J., Chen L.D., Hartman R., van den Heuvel A., Reijerkerk A., van Kregten J. Glutathione PEGylated liposomes: pharmacokinetics and delivery of cargo across the blood–brain barrier in rats. J Drug Target. 2014;22:460–467. doi: 10.3109/1061186X.2014.888070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gaillard P.J., Appeldoorn C.C., Rip J., Dorland R., van der Pol S.M., Kooij G. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J Control Release. 2012;164:364–369. doi: 10.1016/j.jconrel.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 77.de Boer M., Visser C.C., Gaillard P.J. Upcycling drugs for brain-related diseases: a sustainable future for targeted drug delivery. Ther Deliv. 2013;4:435–438. doi: 10.4155/tde.13.7. [DOI] [PubMed] [Google Scholar]
- 78.Rotman M., Welling M.M., Bunschoten A., de Backer M.E., Rip J., Nabuurs R.J. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer׳s disease. J Control Release. 2015;203:40–50. doi: 10.1016/j.jconrel.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 79.Gaillard P.J., Visser C.C., Appeldoorn C.C., Rip J. Targeted blood-to-brain drug delivery-10 key development criteria. Curr Pharm Biotechnol. 2012;13:2328–2339. doi: 10.2174/138920112803341815. [DOI] [PubMed] [Google Scholar]
- 80.Tan H.B., Chen L.M., Guan Y.H., Lin X.T. Comparison of MRI, F-18 FDG, and 11C-choline PET/CT for their potentials in differentiating brain tumor recurrence from brain tumor necrosis following radiotherapy. Clin Nucl Med. 2011;36:978–981. doi: 10.1097/RLU.0b013e31822f68a6. [DOI] [PubMed] [Google Scholar]
- 81.Li J.F., Zhou L., Ye D.Y., Huang S.X., Shao K., Huang R.Q. Choline-derivate-modified nanoparticles for brain-targeting gene delivery. Adv Mater. 2011;23:4516–4520. doi: 10.1002/adma.201101899. [DOI] [PubMed] [Google Scholar]
- 82.Li J.F., Huang S.X., Shao K., Liu Y., An S., Kuang Y.Y. A choline derivate-modified nanoprobe for glioma diagnosis using MRI. Sci Rep. 2013;3:1623. doi: 10.1038/srep01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J.F., Guo Y.B., Kuang Y.Y., An S., Ma H.J., Jiang C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials. 2013;34:9142–9148. doi: 10.1016/j.biomaterials.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 84.Lu W., Zhang Y., Tan Y.Z., Hu K.L., Jiang X.G., Fu S.K. Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J Control Release. 2005;107:428–448. doi: 10.1016/j.jconrel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 85.Lu W., Wan J., She Z.J., Jiang X.G. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J Control Release. 2007;118:38–53. doi: 10.1016/j.jconrel.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 86.Xu F., Lu W., Wu H.B., Fan L., Gao X.L., Jiang X.G. Brain delivery and systemic effect of cationic albumin conjugated PLGA nanoparticles. J Drug Target. 2009;17:423–434. doi: 10.1080/10611860902963013. [DOI] [PubMed] [Google Scholar]
- 87.Xie Y.L., Lu W., Jiang X.G. Improvement of cationic albumin conjugated pegylated nanoparticles holding NC-1900, a vasopressin fragment analog, in memory deficits induced by scopolamine in mice. Behav Brain Res. 2006;173:76–84. doi: 10.1016/j.bbr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 88.Lu W., Wan J., Zhang Q., She Z.J., Jiang X.G. Aclarubicin-loaded cationic albumin-conjugated pegylated nanoparticle for glioma chemotherapy in rats. Int J Cancer. 2007;120:420–431. doi: 10.1002/ijc.22296. [DOI] [PubMed] [Google Scholar]
- 89.Lu W., Sun Q., Wan J., She Z.J., Jiang X.G. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 90.Bechara C., Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587:1693–1702. doi: 10.1016/j.febslet.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 91.Sawant R., Torchilin V. Intracellular delivery of nanoparticles with CPPs. Methods Mol Biol. 2011;683:431–451. doi: 10.1007/978-1-60761-919-2_31. [DOI] [PubMed] [Google Scholar]
- 92.Qin Y., Chen H.L., Yuan W.M., Kuai R., Zhang Q.Y., Xie F.L. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int J Pharm. 2011;419:85–95. doi: 10.1016/j.ijpharm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 93.Qin Y., Zhang Q.Y., Chen H.L., Yuan W.M., Kuai R., Xie F.L. Comparison of four different peptides to enhance accumulation of liposomes into the brain. J Drug Target. 2012;20:235–245. doi: 10.3109/1061186X.2011.639022. [DOI] [PubMed] [Google Scholar]
- 94.Malhotra M., Tomaro-Duchesneau C., Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34:1270–1280. doi: 10.1016/j.biomaterials.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 95.Cheng Y., Dai Q., Morshed R.A., Fan X.B., Wegscheid M.L., Wainwright D.A. Blood–brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10:5137–5150. doi: 10.1002/smll.201400654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao H.L., Zhang Q.Y., Yu Z.Q., He Q. Cell-penetrating peptide-based intelligent liposomal systems for enhanced drug delivery. Curr Pharm Biotechnol. 2014;15:210–219. doi: 10.2174/1389201015666140617092552. [DOI] [PubMed] [Google Scholar]
- 97.Herce H.D., Garcia A.E., Cardoso M.C. Fundamental molecular mechanism for the cellular uptake of guanidinium-rich molecules. J Am Chem Soc. 2014;136:17459–17467. doi: 10.1021/ja507790z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koren E., Torchilin V.P. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–393. doi: 10.1016/j.molmed.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 99.Kibria G., Hatakeyama H., Ohga N., Hida K., Harashima H. Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release. 2011;153:141–148. doi: 10.1016/j.jconrel.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Sharma G., Modgil A., Sun C., Singh J. Grafting of cell-penetrating peptide to receptor-targeted liposomes improves their transfection efficiency and transport across blood–brain barrier model. J Pharm Sci. 2012;101:2468–2478. doi: 10.1002/jps.23152. [DOI] [PubMed] [Google Scholar]
- 101.Sharma G., Modgil A., Layek B., Arora K., Sun C., Law B. Cell penetrating peptide tethered bi-ligand liposomes for delivery to brain in vivo: biodistribution and transfection. J Control Release. 2013;167:1–10. doi: 10.1016/j.jconrel.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y.Y., Ran R., Chen J.T., Kuang Q.F., Tang J., Mei L. Paclitaxel loaded liposomes decorated with a multifunctional tandem peptide for glioma targeting. Biomaterials. 2014;35:4835–4847. doi: 10.1016/j.biomaterials.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 103.Bhatia S.P., McGinty D., Letizia C.S., Api A.M. Fragrance material review on l-borneol. Food Chem Toxicol. 2008;46 doi: 10.1016/j.fct.2008.06.054. suppl 11:S81–4. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L., Han L.M., Qin J., Lu W.Y., Wang J.X. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG–PLGA nanoparticles to the brain. Pharm Res. 2013;30:2560–2572. doi: 10.1007/s11095-013-1055-y. [DOI] [PubMed] [Google Scholar]
- 105.Toman P., Lien C.F., Ahmad Z., Dietrich S., Smith J.R., An Q. Nanoparticles of alkylglyceryl-dextran-graft-poly(lactic acid) for drug delivery to the brain: preparation and in vitro investigation. Acta Biomater. 2015;23:250–262. doi: 10.1016/j.actbio.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 106.Jacobson K.A., Gao Z.G. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sachdeva S., Gupta M. Adenosine and its receptors as therapeutic targets: an overview. Saudi Pharm J. 2013;21:245–253. doi: 10.1016/j.jsps.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carman A.J., Mills J.H., Krenz A., Kim D.G., Bynoe M.S. Adenosine receptor signaling modulates permeability of the blood–brain barrier. J Neurosci. 2011;31:13272–13280. doi: 10.1523/JNEUROSCI.3337-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao X.H., Qian J., Zheng S.Y., Changyi Y.Z., Zhang J.P., Ju S.H. Overcoming the blood–brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist. ACS Nano. 2014;8:3678–3689. doi: 10.1021/nn5003375. [DOI] [PubMed] [Google Scholar]
- 110.Zhang F., Xu C.L., Liu C.M. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089–2100. doi: 10.2147/DDDT.S79592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Etame A.B., Diaz R.J., O׳Reilly M.A., Smith C.A., Mainprize T.G., Hynynen K. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomedicine. 2012;8:1133–1142. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aryal M., Vykhodtseva N., Zhang Y.Z., Park J., McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood–tumor and blood–brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu H.L., Hua M.Y., Yang H.W., Huang C.Y., Chu P.C., Wu J.S. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci U S A. 2010;107:15205–15210. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aryal M., Vykhodtseva N., Zhang Y.Z., McDannold N. Multiple sessions of liposomal doxorubicin delivery via focused ultrasound mediated blood–brain barrier disruption: a safety study. J Control Release. 2015;204:60–69. doi: 10.1016/j.jconrel.2015.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vykhodtseva N., McDannold N., Hynynen K. Progress and problems in the application of focused ultrasound for blood–brain barrier disruption. Ultrasonics. 2008;48:279–296. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen H., Chen C.C., Acosta C., Wu S.Y., Sun T., Konofagou E.E. A new brain drug delivery strategy: focused ultrasound-enhanced intranasal drug delivery. PLoS One. 2014;9:e108880. doi: 10.1371/journal.pone.0108880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Illum L. Nasal drug delivery-recent developments and future prospects. J Control Release. 2012;161:254–263. doi: 10.1016/j.jconrel.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 118.Zhang Q.Z., Jiang X.G., Jiang W.M., Lu W., Su L.N., Shi Z.Q. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int J Pharm. 2004;275:85–96. doi: 10.1016/j.ijpharm.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 119.Zheng X.Y., Shao X.Y., Zhang C., Tan Y.Z., Liu Q.F., Wan X. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer׳s disease. Pharm Res. 2015;32:3837–3849. doi: 10.1007/s11095-015-1744-9. [DOI] [PubMed] [Google Scholar]
- 120.Dalpiaz A., Ferraro L., Perrone D., Leo E., Iannuccelli V., Pavan B. Brain uptake of a zidovudine prodrug after nasal administration of solid lipid microparticles. Mol Pharm. 2014;11:1550–1561. doi: 10.1021/mp400735c. [DOI] [PubMed] [Google Scholar]
- 121.Sharma D., Sharma R.K., Sharma N., Gabrani R., Sharma S.K., Ali J. Nose-to-brain delivery of PLGA-diazepam nanoparticles. AAPS PharmSciTech. 2015;16:1108–1121. doi: 10.1208/s12249-015-0294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Baba M., Itaka K., Kondo K., Yamasoba T., Kataoka K. Treatment of neurological disorders by introducing mRNA in vivo using polyplex nanomicelles. J Control Release. 2015;201:41–48. doi: 10.1016/j.jconrel.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 123.Gao X.L., Wu B.X., Zhang Q.Z., Chen J., Zhu J.H., Zhang W.W. Brain delivery of vasoactive intestinal peptide enhanced with the nanoparticles conjugated with wheat germ agglutinin following intranasal administration. J Control Release. 2007;121:156–167. doi: 10.1016/j.jconrel.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 124.Liu Q.F., Shen Y.H., Chen J., Gao X.L., Feng C.C., Wang L. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm Res. 2012;29:546–558. doi: 10.1007/s11095-011-0641-0. [DOI] [PubMed] [Google Scholar]
- 125.Wen Z.Y., Yan Z.Q., Hu K.L., Pang Z.Q., Cheng X.F., Guo L.R. Odorranalectin-conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson׳s disease following intranasal administration. J Control Release. 2011;151:131–138. doi: 10.1016/j.jconrel.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 126.Wu H.B., Li J.X., Zhang Q.Z., Yan X.L., Guo L.R., Gao X.L. A novel small odorranalectin-bearing cubosomes: preparation, brain delivery and pharmacodynamic study on amyloid-β–-treated rats following intranasal administration. Eur J Pharm Biopharm. 2012;80:368–378. doi: 10.1016/j.ejpb.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 127.Chen J., Zhang C., Liu Q.F., Shao X.Y., Feng C.C., Shen Y.H. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: in vivo and in vitro evaluations. J Drug Target. 2012;20:174–184. doi: 10.3109/1061186X.2011.622396. [DOI] [PubMed] [Google Scholar]
- 128.Kamei N., Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release. 2015;197:105–110. doi: 10.1016/j.jconrel.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 129.Yan L., Wang H.Y., Jiang Y.F., Liu J.H., Wang Z., Yang Y.X. Cell-penetrating peptide-modified PLGA nanoparticles for enhanced nose-to-brain macromolecular delivery. Macromolecul Res. 2013;21:435–441. [Google Scholar]
- 130.Taki H., Kanazawa T., Akiyama F., Takashima Y., Okada H. Intranasal delivery of camptothecin-loaded Tat-modified nanomicells for treatment of intracranial brain tumors. Pharmaceutics. 2012;5:1092–1102. doi: 10.3390/ph5101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao H.L., Pang Z.Q., Pan S.Q., Cao S.J., Yang Z., Chen C. Anti-glioma effect and safety of docetaxel-loaded nanoemulsion. Arch Pharm Res. 2012;35:333–341. doi: 10.1007/s12272-012-0214-8. [DOI] [PubMed] [Google Scholar]
- 132.Gao H.L., Cao S.J., Yang Z., Zhang S., Pang Z.Q., Jiang X.G. Preparation, characterization and anti-glioma effects of docetaxel-loaded, lipoprotein-like nanoparticles. J Biomed Nanotech. 2015;11:2137–2147. doi: 10.1166/jbn.2015.2076. [DOI] [PubMed] [Google Scholar]
- 133.Gao H.L., Wang Y.C., Chen C., Chen J., Wei Y., Cao S.L. Incorporation of lapatinib into core–shell nanoparticles improves both the solubility and anti-glioma effects of the drug. Int J Pharm. 2014;461:478–488. doi: 10.1016/j.ijpharm.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 134.Guo L.R., Fan L., Pang Z.Q., Ren J.F., Ren Y.L., Li J.W. TRAIL and doxorubicin combination enhances anti-glioblastoma effect based on passive tumor targeting of liposomes. J Control Release. 2011;154:93–102. doi: 10.1016/j.jconrel.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 135.Kozielski K.L., Tzeng S.Y., De Mendoza B.A., Green J.J. Bioreducible cationic polymer-based nanoparticles for efficient and environmentally triggered cytoplasmic siRNA delivery to primary human brain cancer cells. ACS Nano. 2014;8:3232–3241. doi: 10.1021/nn500704t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ireson C.R., Kelland L.R. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 137.Hovanessian A.G., Soundaramourty C., El Khoury D., Nondier I., Svab J., Krust B. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS One. 2010;5:e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guo J.W., Gao X.L., Su L.N., Xia H.M., Gu G.Z., Pang Z.Q. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 139.Gao H., Qian J., Yang Z., Pang Z., Xi Z., Cao S. Whole-cell SELEX aptamer-functionalised poly(ethyleneglycol)-poly(ε-caprolactone) nanoparticles for enhanced targeted glioblastoma therapy. Biomaterials. 2012;33:6264–6272. doi: 10.1016/j.biomaterials.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 140.Mintz A., Gibo D.M., Slagle-Webb B., Christensen N.D., Debinski W. IL-13Rα2 is a glioma-restricted receptor for interleukin-13. Neoplasia. 2002;4:388–399. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fillmore H.L., Shultz M.D., Henderson S.C., Cooper P., Broaddus W.C., Chen Z.J. Conjugation of functionalized gadolinium metallofullerenes with IL-13 peptides for targeting and imaging glial tumors. Nanomedicine (London) 2011;6:449–458. doi: 10.2217/nnm.10.134. [DOI] [PubMed] [Google Scholar]
- 142.Gao H.L., Yang Z., Zhang S., Cao S.J., Pang Z.Q., Jiang X.G. Ligand modified nanoparticles increases cell uptake, alters endocytosis and elevates glioma distribution and internalization. Sci Rep. 2013;3:2534. doi: 10.1038/srep02534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao H.L., Yang Z., Zhang S., Cao S.J., Pang Z.Q., Yang X. Glioma-homing peptide with a cell-penetrating effect for targeting delivery with enhanced glioma localization, penetration and suppression of glioma growth. J Control Release. 2013;172:921–928. doi: 10.1016/j.jconrel.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 144.Gao H.L., Zhang S., Yang Z., Cao S.J., Jiang X.G., Pang Z.Q. In vitro and in vivo intracellular distribution and anti-glioblastoma effects of docetaxel-loaded nanoparticles functioned with IL-13 peptide. Int J Pharm. 2014;466:8–17. doi: 10.1016/j.ijpharm.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 145.Wang B.Y., Lv L.Y., Wang Z.Y., Zhao Y., Wu L., Fang X.L. Nanoparticles functionalized with Pep-1 as potential glioma targeting delivery system via interleukin 13 receptor α2–mediated endocytosis. Biomaterials. 2014;35:5897–5907. doi: 10.1016/j.biomaterials.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 146.Wang Y., Wang K.Y., Zhao J.F., Liu X.G., Bu J., Yan X.Y. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J Am Chem Soc. 2013;135:4799–4804. doi: 10.1021/ja312221g. [DOI] [PubMed] [Google Scholar]
- 147.Adachi Y., Lakka S.S., Chandrasekar N., Yanamandra N., Gondi C.S., Mohanam S. Down-regulation of integrin αvβ3 expression and integrin-mediated signaling in glioma cells by adenvirus-mediated transfer of antisense urokinase-type plasminogen activator receptor (uPAR) and sense p16 genes. J Biol Chem. 2001;276:47171–47177. doi: 10.1074/jbc.M104334200. [DOI] [PubMed] [Google Scholar]
- 148.Waite C.L., Roth C.M. PAMAM-RGD conjugates enhance siRNA delivery through a multicellular spheroid model of malignant glioma. Bioconjug Chem. 2009;20:1908–1916. doi: 10.1021/bc900228m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jin J.F., Xu Z.H., Zhang Y., Gu Y.J., Lam M.H., Wong W.T. Upconversion nanoparticles conjugated with Gd3+-DOTA and RGD for targeted dual-modality imaging of brain tumor xenografts. Adv Healthc Mater. 2013;2:1501–1512. doi: 10.1002/adhm.201300102. [DOI] [PubMed] [Google Scholar]
- 150.Zhan C.Y., Gu B., Xie C., Li J., Liu Y., Lu W.Y. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143:136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 151.Miura Y., Takenaka T., Toh K., Wu S.R., Nishihara H., Kano M.R. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood–brain tumor barrier. ACS Nano. 2013;7:8583–8592. doi: 10.1021/nn402662d. [DOI] [PubMed] [Google Scholar]
- 152.Wang F.H., Zhang W.J., Shen Y.Y., Huang Q., Zhou D.J., Guo S.R. Efficient RNA delivery by integrin-targeted glutathione responsive polyethyleneimine capped gold nanorods. Acta Biomater. 2015;23:136–146. doi: 10.1016/j.actbio.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 153.Wu C.X., Lo S.L., Boulaire J., Hong M.L., Beh H.M., Leung D.S. A peptide-based carrier for intracellular delivery of proteins into malignant glial cells in vitro. J Control Release. 2008;130:140–145. doi: 10.1016/j.jconrel.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 154.Wang D.Y., Li W.B., Zhang H., Mao Q.W., Xia H.B. A targeting peptide improves adenovirus-mediated transduction of a glioblastoma cell line. Oncol Rep. 2014;31:2093–2098. doi: 10.3892/or.2014.3065. [DOI] [PubMed] [Google Scholar]
- 155.Wild J.R.L., Staton C.A., Chapple K., Corfe B.M. Neuropilins: expression and roles in the epithelium. Int J Exp Pathol. 2012;93:81–103. doi: 10.1111/j.1365-2613.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wischhusen J., Schneider D., Mittelbronn M., Meyermann R., Engelmann H., Jung G. Death receptor-mediated apoptosis in human malignant glioma cells: modulation by the CD40/CD40L system. J Neuroimmunol. 2005;162:28–42. doi: 10.1016/j.jneuroim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 157.Deshane J., Garner C.C., Sontheimer H. Chlorotoxin inhibits glioma cell invasion via matrix metalloproteinase-2. J Biol Chem. 2003;278:4135–4144. doi: 10.1074/jbc.M205662200. [DOI] [PubMed] [Google Scholar]
- 158.Wang C.W., Ning L.P., Wang H.W., Lu Z.J., Li X.G., Fan X.Y. A peptide-mediated targeting gene delivery system for malignant glioma cells. Int J Nanomedicine. 2013;8:3631–3640. doi: 10.2147/IJN.S44990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Y.Y., Yan Z.Q., Wei D.X., Zhong J., Liu L., Zhang L. Tumor-penetrating peptide functionalization enhances the anti-glioblastoma effect of doxorubicin liposomes. Nanotechnology. 2013;24:405101. doi: 10.1088/0957-4484/24/40/405101. [DOI] [PubMed] [Google Scholar]
- 160.Shevtsov M.A., Yakovleva L.Y., Nikolaev B.P., Marchenko Y.Y., Dobrodumov A.V., Onokhin K.V. Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma. Neuro Oncol. 2014;16:38–49. doi: 10.1093/neuonc/not141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Locatelli E., Naddaka M., Uboldi C., Loudos G., Fragogeorgi E., Molinari V. Targeted delivery of silver nanoparticles and alisertib: in vitro and in vivo synergistic effect against glioblastoma. Nanomedicine (London) 2014;9:839–849. doi: 10.2217/nnm.14.1. [DOI] [PubMed] [Google Scholar]
- 162.Fang C., Wang K., Stephen Z.R., Mu Q.X., Kievit F.M., Chiu D.T. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl Mater Interfaces. 2015;7:6674–6682. doi: 10.1021/am5092165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang J., Lei Y., Xie C., Lu W.Y., Yan Z.Q., Gao J. Targeted gene delivery to glioblastoma using a C-end rule RGERPPR peptide-functionalised polyethylenimine complex. Int J Pharm. 2013;458:48–56. doi: 10.1016/j.ijpharm.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 164.Chen S.Q., Ahmadiantehrani M., Publicover N.G., Hunter K.W., Jr, Zhu X.S. Thermal decomposition based synthesis of Ag-In-S/ZnS quantum dots and their chlorotoxin-modified micelles for brain tumor cell targeting. RSC Adv. 2015;74:60612–60620. doi: 10.1039/C5RA11250H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Costa P.M., Cardoso A.L., Custódia C., Cunha P., de Almeida L.P., de Lima M.C.P. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: a new multimodal gene therapy approach for glioblastoma. J Control Release. 2015;207:31–39. doi: 10.1016/j.jconrel.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 166.Gao H.L., Yang Z., Cao S.J., Xiong Y., Zhang S., Pang Z.Q. Tumor cells and neovasculature dual targeting delivery for glioblastoma treatment. Biomaterials. 2014;35:2374–2382. doi: 10.1016/j.biomaterials.2013.11.076. [DOI] [PubMed] [Google Scholar]
- 167.Gao H.L., Xiong Y., Zhang S., Yang Z., Cao S.J., Jiang X.G. RGD and interleukin-13 peptide functionalized nanoparticles for enhanced glioblastoma cells and neovasculature dual targeting delivery and elevated tumor penetration. Mol Pharm. 2014;11:1042–1052. doi: 10.1021/mp400751g. [DOI] [PubMed] [Google Scholar]
- 168.Gao H.L., Hu Y., Xiong Y., Zhang S., Yang J.R., Yu L. Glioma targeting and anti-glioma effect of interleukin 13 peptide and RGD peptide dual functionalized nanoparticles. Curr Pharm Biotechnol. 2013;14:1118–1126. doi: 10.2174/1389201015666140425102937. [DOI] [PubMed] [Google Scholar]
- 169.Vinters H.V., Gilbert J.J. Cerebral amyloid angiopathy: incidence and complications in the aging brain. II. The distribution of amyloid vascular changes. Stroke. 1983;14:924–928. doi: 10.1161/01.str.14.6.924. [DOI] [PubMed] [Google Scholar]
- 170.Agyare E.K., Jaruszewski K.M., Curran G.L., Rosenberg J.T., Grant S.C., Lowe V.J. Engineering theranostic nanovehicles capable of targeting cerebrovascular amyloid deposits. J Control Release. 2014;185:121–129. doi: 10.1016/j.jconrel.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luther T., Flössel C., Mackman N., Bierhaus A., Kasper M., Albrecht S. Tissue factor expression during human and mouse development. Am J Pathol. 1996;149:101–113. [PMC free article] [PubMed] [Google Scholar]
- 172.Gao H.L., Pan S.Q., Yang Z., Cao S.J., Chen C., Jiang X.G. A cascade targeting strategy for brain neuroglial cells employing nanoparticles modified with angiopep-2 peptide and EGFP-EGF1 protein. Biomaterials. 2011;32:8669–8675. doi: 10.1016/j.biomaterials.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 173.Chung N.S., Wasan K.M. Potential role of the low-density lipoprotein receptor family as mediators of cellular drug uptake. Adv Drug Deliv Rev. 2004;56:1315–1334. doi: 10.1016/j.addr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 174.Li J.W., Feng L., Fan L., Zha Y., Guo L.R., Zhang Q.Z. Targeting the brain with PEG-PLGA nanoparticles modified with phage-displayed peptides. Biomaterials. 2011;32:4943–4950. doi: 10.1016/j.biomaterials.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gao H.L., Qian J., Cao S.J., Yang Z., Pang Z.Q., Pan S.Q. Precise glioma targeting of and penetration by aptamer and peptide dual-functioned nanoparticles. Biomaterials. 2012;33:5115–5123. doi: 10.1016/j.biomaterials.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 176.Gao H.L., Yang Z., Zhang S., Pang Z.Q., Liu Q.F., Jiang X.G. Study and evaluation of mechanisms of dual targeting drug delivery system with tumor microenvironment assays. Acta Biomater. 2014;10:858–867. doi: 10.1016/j.actbio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 177.Townsend M., Mehta T., Selkoe D.J. Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 178.Allaman I., Gavillet M., Bélanger M., Laroche T., Viertl D., Lashuel H.A. Amyloid-β aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bartnik D., Funke S.A., Andrei-Selmer L.C., Bacher M., Dodel R., Willbold D. Differently selected d-enantiomeric peptides act on different Aβ species. Rejuvenation Res. 2010;13:202–205. doi: 10.1089/rej.2009.0924. [DOI] [PubMed] [Google Scholar]
- 180.van Groen T., Kadish I., Wiesehan K., Funke S.A., Willbold D. In vitro and in vivo staining characteristics of small, fluorescent, Aβ42-binding d-enantiomeric peptides in transgenic AD mouse models. ChemMedChem. 2009;4:276–282. doi: 10.1002/cmdc.200800289. [DOI] [PubMed] [Google Scholar]
- 181.Zhang C., Wan X., Zheng X.Y., Shao X.Y., Liu Q.F., Zhang Q.Z. Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer׳s disease mice. Biomaterials. 2014;35:456–465. doi: 10.1016/j.biomaterials.2013.09.063. [DOI] [PubMed] [Google Scholar]
- 182.Zhang C., Zheng X.Y., Wan X., Shao X.Y., Liu Q.F., Zhang Z.M. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer׳s disease. J Control Release. 2014;192:317–324. doi: 10.1016/j.jconrel.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 183.Nie Y., Schaffert D., Rödl W., Ogris M., Wagner E., Günther M. Dual-targeted polyplexes: one step towards a synthetic virus for cancer gene therapy. J Control Release. 2011;152:127–134. doi: 10.1016/j.jconrel.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 184.Xu Q., Liu Y.X., Su S.S., Li W., Chen C.Y., Wu Y. Anti-tumor activity of paclitaxel through dual-targeting carrier of cyclic RGD and transferrin conjugated hyperbranched copolymer nanoparticles. Biomaterials. 2012;33:1627–1639. doi: 10.1016/j.biomaterials.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 185.Zhang P.C., Hu L.J., Yin Q., Feng L.Y., Li Y.P. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood–brain barrier penetration and glioma targeting therapy. Mol Pharm. 2012;9:1590–1598. doi: 10.1021/mp200600t. [DOI] [PubMed] [Google Scholar]
- 186.Li Y., He H., Jia X.R., Lu W.L., Lou J.N., Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33:3899–3908. doi: 10.1016/j.biomaterials.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 187.He H., Li Y., Jia X.R., Du J., Ying X., Lu W.L. PEGylated poly(amidoamine) dendrimer–based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32:478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 188.Ying X., Wen H., Lu W.L., Du J., Guo J., Tian W. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J Control Release. 2010;141:183–192. doi: 10.1016/j.jconrel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 189.Byeon H.J., Thao le Q., Lee S., Min S.Y., Lee E.S., Shin B.S. Doxorubicin-loaded nanoparticles consisted of cationic- and mannose-modified-albumins for dual-targeting in brain tumors. J Control Release. 2016;225:301–313. doi: 10.1016/j.jconrel.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 190.Yan H.H., Wang J.Y., Yi P.W., Lei H., Zhan C.Y., Xie C. Imaging brain tumor by dendrimer-based optical/paramagnetic nanoprobe across the blood–brain barrier. Chem Commun (Camb) 2011;47:8130–8132. doi: 10.1039/c1cc12007g. [DOI] [PubMed] [Google Scholar]
- 191.Yang Z.Z., Li J.Q., Wang Z.Z., Dong D.W., Qi X.R. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35:5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 192.Chen Y.C., Chiang C.F., Chen L.F., Liang P.C., Hsieh W.Y., Lin W.L. Polymersomes conjugated with des-octanoyl ghrelin and folate as a BBB-penetrating cancer cell–targeting delivery system. Biomaterials. 2014;35:4066–4081. doi: 10.1016/j.biomaterials.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 193.Gao J.Q., Lv Q., Li L.M., Tang X.J., Li F.Z., Hu Y.L. Glioma targeting and blood–brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34:5628–5639. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 194.Li X.Y., Zhao Y., Sun M.G., Shi J.F., Ju R.J., Zhang C.X. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials. 2014;35:5591–5604. doi: 10.1016/j.biomaterials.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 195.Yue P.J., He L., Qiu S.W., Li Y., Liao Y.J., Li X.P. OX26/CTX-conjugated PEGylated liposome as a dual-targeting gene delivery system for brain glioma. Mol Cancer. 2014;13:191. doi: 10.1186/1476-4598-13-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kuo Y.C., Chen Y.C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int J Pharm. 2015;479:138–149. doi: 10.1016/j.ijpharm.2014.12.070. [DOI] [PubMed] [Google Scholar]
- 197.Zhang J., Chen N., Wang H., Gu W., Liu K., Ai P.H. Dual-targeting superparamagnetic iron oxide nanoprobes with high and low target density for brain glioma imaging. J Colloid Interface Sci. 2016;469:86–92. doi: 10.1016/j.jcis.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 198.Bana L., Minniti S., Salvati E., Sesana S., Zambelli V., Cagnotto A. Liposomes bi-functionalized with phosphatidic acid and an ApoE-derived peptide affect Aβ aggregation features and cross the blood–brain barrier: implications for therapy of Alzheimer disease. Nanomedicine. 2014;10:1583–1590. doi: 10.1016/j.nano.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 199.Sonali Agrawal P., Singh R.P., Rajesh C.V., Singh S., Vijayakumar M.S. Transferrin receptor–targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23:1788–1798. doi: 10.3109/10717544.2015.1094681. [DOI] [PubMed] [Google Scholar]
- 200.Gao X.H., Qian J., Zheng S.Y., Xiong Y., Man J.H., Cao B.X. Up-regulating blood brain barrier permeability of nanoparticles via multivalent effect. Pharm Res. 2013;30:2538–2548. doi: 10.1007/s11095-013-1004-9. [DOI] [PubMed] [Google Scholar]
- 201.Pardridge W.M. Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv. 2015;12:207–222. doi: 10.1517/17425247.2014.952627. [DOI] [PubMed] [Google Scholar]
- 202.Maletínská L., Blakely E.A., Bjornstad K.A., Deen D.F., Knoff L.J., Forte T.M. Human glioblastoma cell lines: levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000;60:2300–2303. [PubMed] [Google Scholar]
- 203.Ito S., Ohtsuki S., Terasaki T. Functional characterization of the brain-to-blood efflux clearance of human amyloid-β peptide (1–40) across the rat blood–brain barrier. Neurosci Res. 2006;56:246–252. doi: 10.1016/j.neures.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 204.Li H.Y., Qian Z.M. Transferrin/transferrin receptor-mediated drug delivery. Med Res Rev. 2002;22:225–250. doi: 10.1002/med.10008. [DOI] [PubMed] [Google Scholar]
- 205.Demeule M., Régina A., Ché C., Poirier J., Nguyen T., Gabathuler R. Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther. 2008;324:1064–1072. doi: 10.1124/jpet.107.131318. [DOI] [PubMed] [Google Scholar]
- 206.Xin H.L., Sha X.Y., Jiang X.Y., Chen L.C., Law K., Gu J.J. The brain targeting mechanism of angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles. Biomaterials. 2012;33:1673–1681. doi: 10.1016/j.biomaterials.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 207.Xin H.L., Jiang X.Y., Gu J.J., Sha X.Y., Chen L.C., Law K. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 208.Sun X.Y., Pang Z.Q., Ye H.X., Qiu B., Guo L.R., Li J.W. Co-delivery of pEGFP-hTRAIL and paclitaxel to brain glioma mediated by an angiopep-conjugated liposome. Biomaterials. 2012;33:916–924. doi: 10.1016/j.biomaterials.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 209.Ni D.L., Zhang J.W., Bu W.B., Xing H.Y., Han F., Xiao Q.F. Dual-targeting upconversion nanoprobes across the blood brain barrier for magnetic resonance/fluorescence imaging of intracranial glioblastoma. ACS Nano. 2014;8:1231–1242. doi: 10.1021/nn406197c. [DOI] [PubMed] [Google Scholar]
- 210.Ruan S.B., Yuan M.Q., Zhang L., Hu G.L., Chen J.T., Cun X.L. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–435. doi: 10.1016/j.biomaterials.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 211.Su Z.G., Xing L., Chen Y.N., Xu Y.R., Yang F.F., Zhang C. Lactoferrin-modified poly(ethylene glycol)–grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol Pharm. 2014;11:1823–1834. doi: 10.1021/mp500238m. [DOI] [PubMed] [Google Scholar]
- 212.Wiley D.T., Webster P., Gale A., Davis M.E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci U S A. 2013;110:8662–8667. doi: 10.1073/pnas.1307152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Du D., Chang N.D., Sun S.L., Li M.H., Yu H., Liu M.F. The role of glucose transporters in the distribution of p-aminophenyl-α-d-mannopyranoside modified liposomes within mice brain. J Control Release. 2014;182:99–110. doi: 10.1016/j.jconrel.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 214.Hao Z.F., Cui Y.X., Li M.H., Du D., Liu M.F., Tao H.Q. Liposomes modified with P-aminophenyl-α-d-mannopyranoside: a carrier for targeting cerebral functional regions in mice. Eur J Pharm Biopharm. 2013;84:505–516. doi: 10.1016/j.ejpb.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 215.Jiang X.Y., Xin H.L., Ren Q.Y., Gu J.J., Zhu L.J., Du F.Y. Nanoparticles of 2-deoxy-d-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35:518–529. doi: 10.1016/j.biomaterials.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 216.Kharya P., Jain A., Gulbake A., Shilpi S., Jain A., Hurkat P. Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting. J Nanopart Res. 2013;15:2022–2026. [Google Scholar]
- 217.Elfinger M., Maucksch C., Rudolph C. Characterization of lactoferrin as a targeting ligand for nonviral gene delivery to airway epithelial cells. Biomaterials. 2007;28:3448–3455. doi: 10.1016/j.biomaterials.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 218.Legrand D., Pierce A., Elass E., Carpentier M., Mariller C., Mazurier J. Lactoferrin structure and functions. Adv Exp Med Biol. 2008;606:163–194. doi: 10.1007/978-0-387-74087-4_6. [DOI] [PubMed] [Google Scholar]
- 219.Liu Z.Y., Jiang M.Y., Kang T., Miao D.Y., Gu G.Z., Song Q.X. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials. 2013;34:3870–3881. doi: 10.1016/j.biomaterials.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 220.Plate K.H., Breier G., Millauer B., Ullrich A., Risau W. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53:5822–5827. [PubMed] [Google Scholar]
- 221.Fan C.H., Ting C.Y., Liu H.L., Huang C.Y., Hsieh H.Y., Yen T.C. Antiangiogenic-targeting drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34:2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 222.Wang F., Wang Y.C., Dou S., Xiong M.H., Sun T.M., Wang J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano. 2011;5:3679–3692. doi: 10.1021/nn200007z. [DOI] [PubMed] [Google Scholar]
- 223.Shao K., Ding N., Huang S.X., Ren S.M., Zhang Y., Kuang Y.Y. Smart nanodevice combined tumor-specific vector with cellular microenvironment-triggered property for highly effective antiglioma therapy. ACS Nano. 2014;8:1191–1203. doi: 10.1021/nn406285x. [DOI] [PubMed] [Google Scholar]
- 224.Wu G.Y., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 225.Shilo M., Sharon A., Baranes K., Motiei M., Lellouche J.P., Popovtzer R. The effect of nanoparticle size on the probability to cross the blood–brain barrier: an in-vitro endothelial cell model. J Nanobiotechnology. 2015;13:19. doi: 10.1186/s12951-015-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kibria G., Hatakeyama H., Ohga N., Hida K., Harashima H. The effect of liposomal size on the targeted delivery of doxorubicin to Integrin αvβ3-expressing tumor endothelial cells. Biomaterials. 2013;34:5617–5627. doi: 10.1016/j.biomaterials.2013.03.094. [DOI] [PubMed] [Google Scholar]
- 227.Hirsjärvi S., Sancey L., Dufort S., Belloche C., Vanpouille-Box C., Garcion E. Effect of particle size on the biodistribution of lipid nanocapsules: comparison between nuclear and fluorescence imaging and counting. Int J Pharm. 2013;453:594–600. doi: 10.1016/j.ijpharm.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 228.Popović Z., Liu W.H., Chauhan V.P., Lee J., Wong C., Greytak A.B. A nanoparticle size series for in vivo fluorescence imaging. Angew Chem Int Ed Engl. 2010;49:8649–8652. doi: 10.1002/anie.201003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zan M.H., Li J.J., Luo S.Z., Ge Z.S. Dual pH-triggered multistage drug delivery systems based on host-guest interaction-associated polymeric nanogels. Chem Commun (Camb) 2014;50:7824–7827. doi: 10.1039/c4cc03120b. [DOI] [PubMed] [Google Scholar]
- 230.Ruan S.B., Cao X., Cun X.L., Hu G.L., Zhou Y., Zhang Y.J. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials. 2015;60:100–110. doi: 10.1016/j.biomaterials.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 231.Ruan S.B., He Q., Gao H.L. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale. 2015;7:9487–9496. doi: 10.1039/c5nr01408e. [DOI] [PubMed] [Google Scholar]
- 232.Rolland J.P., Maynor B.W., Euliss L.E., Exner A.E., Denison G.M. Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc. 2005;127:10096–10100. doi: 10.1021/ja051977c. [DOI] [PubMed] [Google Scholar]
- 233.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M.V., Somasundaran P. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 234.Aggarwal P., Hall J.B., McLeland C.B., Dobrovolskaia M.A., McNeil S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61:428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 236.Mirshafiee V., Mahmoudi M., Lou K.Y., Cheng J.J., Kraft M.L. Protein corona significantly reduces active targeting yield. Chem Commun (Camb) 2013;49:2557–2559. doi: 10.1039/c3cc37307j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ponka P., Lok C.N. The transferrin receptor: role in health and disease. Int J Biochem Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 238.Xie R., Dong L., Du Y.F., Zhu Y.T., Hua R., Zhang C. In vivo metabolic labeling of sialoglycans in the mouse brain by using a liposome-assisted bioorthogonal reporter strategy. Proc Natl Acad Sci U S A. 2016;113:5173–5178. doi: 10.1073/pnas.1516524113. [DOI] [PMC free article] [PubMed] [Google Scholar]