Abstract
Objective:
The aim of this study was to investigate the effect of pre-treatment with curcumin on metabolic changes induced by two different pathophysiological mechanisms in rats (fructose diet and streptozotocin (STZ)-induced diabetes mellitus).
Materials and Methods:
Five groups with 10 rats per group were investigated: control group (healthy rats), fructose diet groups without any pre-treatment (FD), fructose diet groups with curcumin pre-treatment (FDC), STZ-induced diabetes mellitus without any pre-treatment (SID) and STZ-induced diabetes mellitus with curcumin pre-treatment (SIDC). Systolic blood pressure, and several metabolic and oxidative stress parameters were assessed.
Results:
Systolic blood pressure significantly increased in all groups compared with control group (P<0.001), with significantly lower values on groups with curcumin pre-treatment compared with the group without any pre-treatment and same inducement (FDS vs. FD P<0.0001, SIDC vs. SID P<0.0001). High-density lipoprotein (HDL)-cholesterol was significantly lower in all groups compared with control group (P<0.05) while triglycerides (P<0.05), aspartate aminotransferase (AST, P<0.0001) and alanine aminotransferase (ALT, P<0.0001) were significantly higher. Within the group with same induction, curcumin pre-treatment significantly improved metabolic (total cholesterol, glycaemia, triglycerides, AST, ALT; P<0.05) and oxidative stress parameters (total oxidative status (NOx), Thiol, and malondialdehyde (MDA), P<0.02) compared to untreated groups.
Conclusion:
The pre-treatment with curcumin in our experimental models significantly improved metabolic (total cholesterol, triglycerides, AST and ALT) as well as oxidative stress parameters (MDA, NOx, and Thiol) in both fructose diet and in STZ-induced diabetes in rats. These properties of curcumin may serve to improve the metabolic and oxidative stress conditions in patients with these pathological features.
Keywords: Curcumin, Diabetes mellitus, Fructose, Metabolic syndrome, Oxidative stress, Streptozotocin–induced diabetes
Introduction
The modern lifestyle increased the consumption of refined sugar, particularly fructose, which leads to an increased risk of the development of metabolic disease (1). Experimental studies demonstrated that the chronic rich fructose diet in rats leads to metabolic changes such as non-alcoholic fatty liver diseases (NAFLD), hyperglycemia, and dyslipidemia (2, 3). Several studies put forward the hypothesis that these pathologies are mediated by increased oxidative stress through nitric oxide synthesis induction (4). In high fructose diet rats, hypertension was associated with early renal damage mechanism produced by nitro-oxidative stress (5). Protein inhibitor of neuronal NO synthase (nNOS) proved toinduce sympathoexcitation and hypertension in subjects with metabolic syndrome (6). Metabolic changes similar with those above-described in regard of association with hypertension have been also observed in streptozotocin (STZ)-induced diabetic rats (7). The mechanism of underlying hypertension is not completely understood, but an activation of sympathetic nervous system and elevation of angiotensin-converting enzyme (ACE) have been identified (8). The excessive production of nitric oxide is suggested as one of the pathophysiological mechanisms associated with STZ-induced diabetes (9).
Researches demonstrate that the curcumin (derived from turmeric plant) had anti-bacterial (10), anti-protozoal or anti-fungal (11, 12), anti-oxidant (13), anti-diabetic (14), and anti-tumor effects (15). The constituent of turmeric plant, curcumin is derived from the rhizome of plant Curcuma longa and plays a significant role in regulation of gene activity, which is supposed to be the mechanism of action of curcumin (16). The Turmeric plant belongs to the Zingiberaceae Family, Genus Curcuma (most common species C. longa). The rhizome is the part of the plant that is used as spicy condiment, to preserve food or as coloring material (mainly in India) (17). C. longa has its origins in South Asia, especially China and India. Turmeric has two types of compound: phenolic compounds and terpenoids (a subclass of prenylipids). Curcuminoids, which include curcumin, demethoxy curcumin and bisdemethoxy curcumin is a phenolic compound of turmeric plant (18).
The hypothesis of our study was that curcumin has similar effects on metabolic changes induced by two different pathophysiological mechanisms, named fructose diet (fructose is a compound that is found in food) and by STZ (a naturally occurring compound known to be toxic to the insulin-producing beta cells of the pancreas and used to induce diabetes in animal models (19)). The aim of our study was to investigate the effect of curcumin administration on systolic blood pressure, metabolic changes, and oxidative parameter modifications in models of fructose-induced metabolic syndrome and STZ-induced diabetes rats.
Materials and Methods
Ethics statement
Experimental design and animal handling procedures were approved by Ethics Committee of the Iuliu Haţieganu University of Medicine and Pharmacy, Cluj-Napoca, Romania. The study was carried out according to the Declaration of Helsinki and by respecting the guidelines for the care and use of laboratory animals. A special attention was paid to minimize the number of animals and their suffering.
Animals and diets
Adult male Wistar rats were obtained from the Animal Department of Faculty of Medicine (weighing 200-250 g) and subsequently maintained in poly-propylene cages in the Pathophysiology Department at the “Iuliu Haţieganu” University of Medicine and Pharmacy Cluj-Napoca). The light and dark regime was kept constant and the temperature and humidity were maintained at 24±2 °C and 60±5%, respectively. All animals used in this study had free access to standard pellet (Cantacuzino Institute, Bucharest, Romania) basal diet and water ad libitum.
Rats were randomly divided into five groups, each group with 10 rats:
-Control group (normal) with neither fructose diet nor experimental diabetes mellitus and without any treatment.
-Two groups with fructose diet, one without curcumin pre-treatment (abbreviated as FD, fructose diet control group) and one with curcumin pre-treatment (abbreviated as FDC).
-Two groups with STZ-induced diabetes mellitus, one without curcumin pre-treatment (abbreviated as SID) and one with curcumin pre-treatment (SIDC).
Metabolic syndrome inducement by fructose diet
The metabolic syndrome was induced by high fructose diet. Fructose drinking water was freshly prepared using 10 g of fructose diluted in 100/ml of tap water (20). The fructose drinking water was administered every day for twelve weeks ad libitum.
Diabetes mellitus inducement by streptozotocin
The diabetes mellitus inducement with STZ was made by the injection of a single intraperitoneal dose of STZ in the dose of 60 mg/kg body weight. The drug was dissolved in freshly prepared 0.01 M citrate buffer (pH=4.5) according with Alimohammadi et al (21).
Curcumin pre-treatment
Curcumin was administrated daily by gavages as 1 g/kg body weight, 16 days previous to the inducement of metabolic syndrome in both FDC and SIDC groups. This method was chosen because it was previously demonstrated to reduce the inflammation in diabetic rats (22).
Fructose, STZ, and curcumin procured by Sigma-Aldrich was used in this study. Diagnostic kits were procured from AMP Diagnostic and Sigma – Aldrich (Bio-Zyme, Romania).
Measurements
Measurements were made at baseline (the beginning of the experiment) and at follow-up (in the end of the experiment).
Blood pressure measurement
The systolic arterial blood pressure in conscious, non-anesthetized rats was measured by tail-cuff plethysmography (BIOPAC system 3.7.7). The pressure was monitored before (30 min previous to the experiment) and at the end of the experiment. Three measurements were made for each moment of the experiment and the average was calculated and recorded. group, SIDC: streptozotocin-induced diabetes mellitus with curcumin pre-treatment
Blood sample collection
At the end of the experiment, the blood samples were collected from the retro-orbital plexuses of each rat under light ketamine anesthesia (50 mg/kg body weight by IP route) (23).
Metabolic parameters measurements
Serum was separated and was analyzed for metabolic changes by assessment of glucose (glycemia), total cholesterol (T-C), triglycerides (TG), low density lipoprotein-cholesterol (LDL-C), and high density lipoprotein-cholesterol (HDL-C) by spectrophotometry. Serum levels of aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were measured using an automated technique (Vita Lab Flexor E, Austria).
Determination of oxidative parameters
The oxidative parameters were estimated as follows: malondialdehyde (MDA) according to Yagl method (24), Thiol compound according to Ellman method (25), the indirect assessment of NO synthesis (NOx), total oxidative status (TOS) and total antioxidative capacity (TAC) as in Pârvu et al (26). Jasco V-350 UV-VIS spectrophotometer (Jasco International Co, Ltd, Tokyo, Japan) was used for spectroscopic measurements.
Statistical analysis
Quantitative data were summarized as mean and standard deviation whenever proved normally distributed; otherwise, median, first, and third quartiles were used. Shapiro-Wilks test was used to assess the normal distribution of experimental measurements. The comparisons between groups were performed with student t-test for normally distributed data and Mann-Whitney test or Wilcoxon paired test for data that proved not normally distributed. The relation between NOx and systolic blood pressure was assessed with Pearson correlation coefficient. Statistical analysis was conducted with Statistica software (v. 8) at a significance level of 5% since the comparisons relative to control group was of interest.
Results
All animals included in the study were analyzed (10 rats per group).
Systolic blood pressure proved significantly (P<0.001) higher in all investigated groups compared to controls at the end of the experiment (Figure 1).
Figure 1.
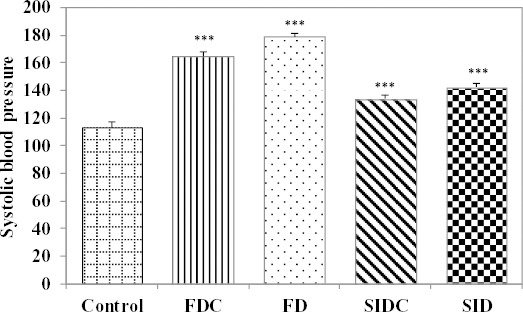
Systolic blood pressure on inducement groups compared to control group (***P<0.001 vs. control group); FD: fructose diet group, FDC: fructose diet group with curcumin pre-treatment, SID: streptozotocin-induced diabetes mellitus group, SIDC: streptozotocin-induced diabetes mellitus with curcumin pre-treatment
The mean of systolic blood pressure in the group with fructose diet proved significantly lower in the group with curcumin pre-treatment compared with the group without curcumin pre-treatment (FDC:FD= 164.90±3.14:178.90±2.60; P<0.0001). Furthermore, the mean of systolic blood pressure in the SIDC proved significantly lower compared with the SID (SIDC: SID=133.70±2.50:142.40±2.72; P<0.0001).
The animals with fructose diet proved significantly higher values of systolic blood pressure (164.90±3.14) compared to animals with STZ-induced diabetes mellitus (133.70±2.50; P<0.0001, see Figure 1).
In regards of metabolism, the characteristics of the investigated variables measured at the end of the experiment proved significantly different in all investigated groups compared to control group (Figure 2), with one exception. The value of total cholesterol proved not significantly different in the group with fructose diet and curcumin pre-treatment compared with controls (ns, Figure 2).
Figure 2.
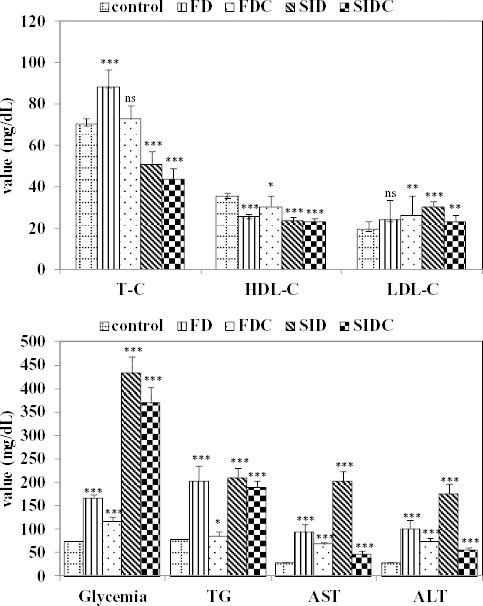
Metabolic measurements according with the group and comparisons with control group (ns= not statistically significant; * P<0.05 vs. control group; ** P<0.01 vs. control group; *** P<0.001 vs. control group); T-C: total cholesterol, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, TG: triglycerides, AST: aspartate aminotransferase, ALT: alanine aminotransferase, FD: fructose diet group, FDC: fructose diet groups with curcumin pre-treatment, SID: streptozotocin-induced diabetes mellitus group, SIDC: streptozotocin-induced diabetes mellitus with curcumin pre-treatment group
The efficacy of the curcumin pre-treatment was investigated by comparing the groups with the same induction and with two exceptions significantly lower values were observed in the curcumin pre-treatment groups (Figure 3). In the group with high fructose diet, no significant difference was observed in regard of LDL-C, while in the SIDC group, no significant difference was observed in regard of HDL-C (see Figure 3).
Figure 3.
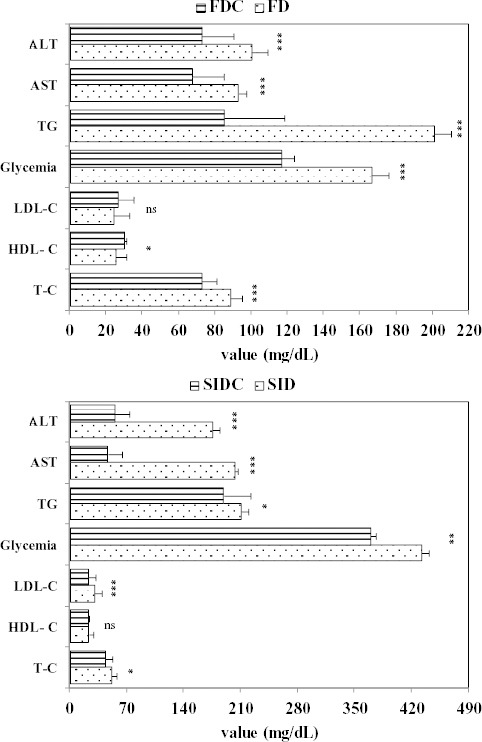
Pre-treatment efficacy in regards of metabolic measurements (ns = not statistically significant; * P<0.05; ** P<0.01; *** P<0.001 for comparisons of the same inducement group with and without curcumin pre-treatment; T-C: total cholesterol, HDL-C: high density lipoprotein-cholesterol, LDL-C: low density lipoprotein-cholesterol, TG: triglycerides, AST: aspartate aminotransferase, ALT: alanine aminotransferase, FD: fructose diet group, FDC: fructose diet groups with curcumin pre-treatment, SID: streptozotocin-induced diabetes mellitus group, SIDC: streptozotocin-induced diabetes mellitus with curcumin pre-treatment
With one exception, represented by LDL-choles-terol, significant differences in terms of meta-bolic biochemical parameters were observed when the group with fructose-induced metabolic syndrome with curcumin pre-treatment (FDC) was compared with STZ-induced diabetes mellitus with curcumin pre-treatment (SIDC) (Figure 4).
Figure 4.
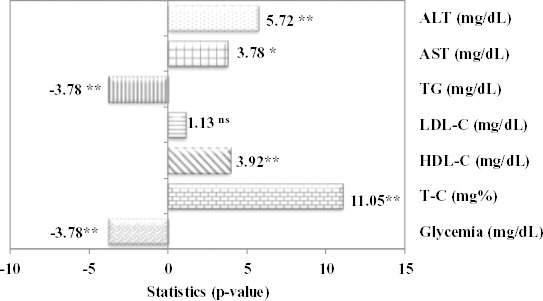
Statistical parameter and associated P-value for comparison between fructose diet groups with curcumin pre-treatment (FDC) and streptozotocin-induced diabetes mellitus with curcumin pre-treatment group (SIDC) (ns = not statistically significant; ** P<0.01, comparison between FDC and SIDC; Mann-Whitney test for glycemia, TG and AST; Student t-test for T-C, HDL-C, LDL-C and ALT
The comparisons of investigated oxidative stress parameters determined at the end of the experiment showed significant differences compared to the controls for most parameters (Figure 5).
Figure 5.
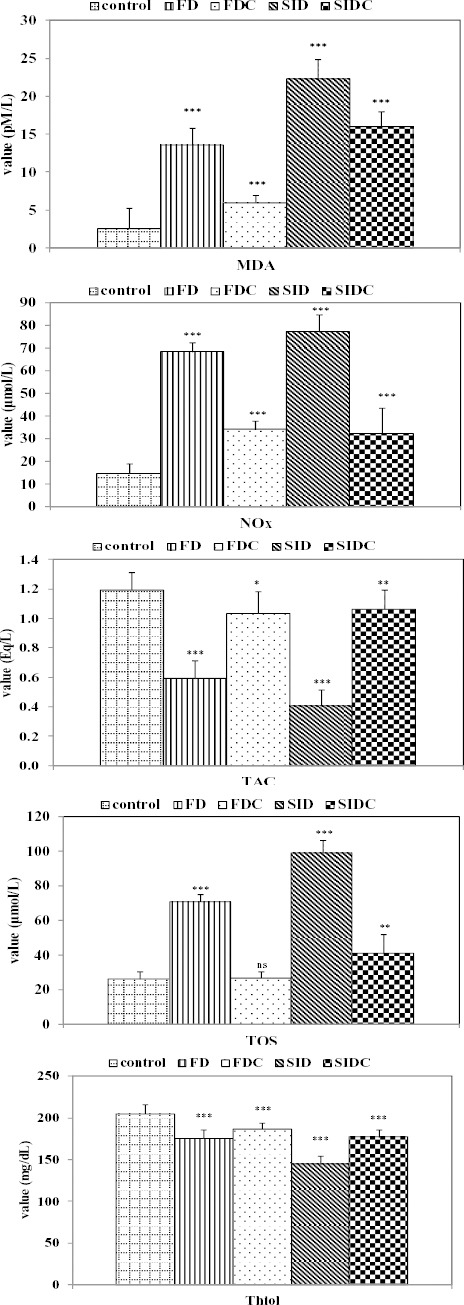
Characteristics of oxidative stress parameters by groups and comparisons with the control group (ns = not statistically significant; * P<0.05 vs. control group; ** P<0.01 vs. control group; *** P<0.001 vs. control group); MDA: malondialdehyde, Thiol: Thiol compound, NOx: indirect assessment of NO synthesis, TOS: total oxidative status, TAC: total antioxidative capacity, FD: fructose diet group, FDC: fructose diet groups with curcumin pre-treatment, SID: streptozotocin-induced diabetes mellitus group, SIDC: streptozotocin-induced diabetes mellitus group with curcumin pre-treatment
MDA (P<0.0001), NOx (P<0.0001) and TOS (P=0.0002) proved significantly higher in FD group compared with FDC, while Thiol (P=0.0175) and TAC (P=0.0006) proved significantly higher in FDC group compared with FD group. Similar differences were observed when SID group was compared with SIDC group, with MDA (P<0.0001), NOx (P<0.0001) and TOS (P=0.0025) significantly higher in SID group compared with SIDC, while Thiol (P<0.0001) and TAC (P=0.0002) with significantly higher in SIDC group compared with SID group.
Even the oxidative stress parameter did not reach the normal values for none of the investigated groups. In the most of the cases, the closest values to the normal were found in FDC. The values of TOS and MAD proved significantly lower in FDC group compared with SIDC group, while the values of Thiol proved significantly higher in FDC group compared with SIDC (Figure 6).
Figure 6.
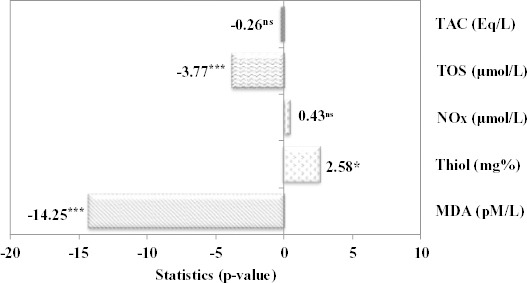
Statistical parameter and associated value for comparison between fructose diet groups with curcumin pre-treatment (FDC) and streptozotocin induced metabolic syndrome with curcumin pre-treatment group (SIDC) (ns = not statistically significant; * P<0.05; *** P<0.001); Mann-Whitney Test for TOS, and TAC, Student t-test for independent sample for MDA, Thiol, and NOx
The relation between systolic blood pressure and NOx proved with one exception positive; the exception was observed in control group where the relation was negative (Figure 7).
Figure 7.
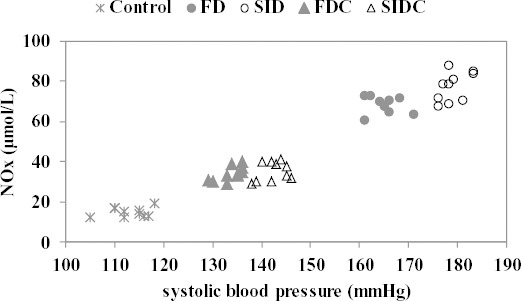
Relationship between systolic blood pressure and NOx by groups; FD: fructose diet group, FDC: fructose diet groups with curcumin pre-treatment, SID: streptozotocin-induced diabetes mellitus group, SIDC: streptozotocin-induced diabetes mellitus with curcumin pre-treatment
With one exception, observed in SID group (r=0.6780, P=0.0310), no significant linearity was observed between systolic blood pressure and NOx (Control: r= 0.2453, P=0.494; FD: r= -0.1772, P=0.624; SID: r=0.4873, P= 0.153; FDC: r=0.6780, P=0.031; SIDC: r=0.2984, P=0.645).
Discussion
The effect of curcumin administration on systolic blood pressure, metabolic changes, and oxidative parameters was successfully investigated on two different inducements, fructose diet and STZ-induced diabetes mellitus. Despite the fact that the investigated inducements have different mechanisms, similar beneficial effect of curcumin was observed in both investigated experimental models (fructose diet, and STZ administration). The evaluation of systolic blood pressure showed a significantly increase in all investigated groups compared to controls. Nevertheless, the curcumin pre-treatment assure a significantly lower mean of systolic blood pressure in fructose-induced metabolic syndrome group (FDC: FD=164.90±3.14:178.90±2.60; P<0.0001) as well as in STZ-induced diabetes mellitus group (SIDC: SID=133.70±2.50:142.40±2.72; P<0.0001).
The presence of hypertension associated with fructose diet rats and STZ induced diabetic rats have already been demonstrated (27, 8). It has also been demonstrated that the increase of ACE activity leads to sympathetic dysfunction in both experimental rats groups (FD and SID) (27). There is evidence that the aldosterone administration aggravates metabolic disturbances induced by fructose diet in rats (28). Fructose diet can also produce hypertension in experimental studies in rats (29). There is evidence that oxidative stress can produce neurogenic hypertension by the influence of central nervous system. Oxidative stress in rostral ventrolateral medulla (RVLM) augments the sympathetic activity, which is involved in the neural mechanism of hypertension (30). In our experiment, there is a significant decrease of systolic arterial blood pressure in curcumin pre-treatment groups (FDC and SIDC groups). Since ACE catalyzes the conversion of angiotensin I to angiotensin II, and angiotensin II may trigger the inflammatory effect and oxidative stress (31), the connection between a sympathetic activation and oxidative stress may exist in our experimental groups. The reduction of systolic blood pressure due to curcumin pre-treatment in our experimental groups can be explained by the reduction of oxidative stress. In our study, the metabolic parameters had different pattern according with both inducement and curcumin pre-treatment. Total cholesterol proved significantly higher in fructose-induced metabolic syndrome compared with controls, but not different when curcumin pre-treatment fructose-induced metabolic syndrome group was compared with controls. With one exceptions, significant increased values are observed in LDL-C, glycemia, TG, AST and ALT in all investigated groups. The exception is represented by LDL-C in FD group that proved not significantly different with control. The curcumin pre-treatment proved with one exception represented by LDL-C to reduce the values of all investigated metabolic parameters. The same effect of reducing the increase of metabolic parameters was also observed on STZ-induced diabetes mellitus, but this time the exception was represented by HDL-C and the highest decrease was observed in regard of ALT.
The comparisons of groups with curcumin pre-treatment showed significant differences in regards of all metabolic parameters when FDC was compared with SIDC, with higher values on SIDC group compared with FDC for glycemia and TG and higher values on FDC group compared with SIDC in regard of T-C, HDL-C, AST and ALT. The curcumin pre-treatment in our experimental groups is based on the potential of curcumin to improve metabolic parameters demonstrated in previous experimental metabolic syndrome (32, 33) and on its anti-oxidant effect (34). The effect of curcumin is also based on the increase of postprandial serum insulin levels without affecting the plasma glucose in healthy human subjects (35). The potency of curcumin in improvement of lipid profile in experimental high-fat-diet rats has been previously demonstrated (35). Furthermore, it has also been demonstrated that curcumin supplementation of diet can improve lipid profile and prevent accumulation of fat in the liver by enhancing triglyceride transport out of the liver by its hypotriglyceridemic effect (36). A significant hepatic injury induced by high fructose diet or by STZ has already demonstrated by the high level of ALT and AST (37). The STZ induced diabetic rats are also associated with increased oxidative stress and hepatic injury (38). Hepato-protective effect of curcumin was previously demonstrated in an experimental model of oxidative stress on rats (39).
Oxidative stress is often an imbalance between pro-oxidants and anti-oxidants. Evaluation of oxidative stress parameters (pro-oxidative stress markers such as MDA, NOx, and TOS, and antioxidative stress markers such as THIOL and TAC) showed significantly increased values of MDA, NOx, and TOS (except TOS in FDC group) when each inducement group was compared to control group, and significantly decreased values of Thiol and TAC. Curcumin treatment reduced MDA and TOS values significantly more in FDC group compared with SIDC group, and increased the values of Thiol significantly more in FDC group compared with SIDC group. MDA, generated by decomposition of polyunsaturated fatty acid peroxides is largely used as an indicative of oxidative stress in cells and tissues (40). Measurements of MDA levels, as a biomarker of oxidative stress, demonstrate that a significant lipid peroxidation exists in our both experimental groups (FD and SID). These results demonstrate that the fructose diet and STZ are able to start lipid peroxidation either directly or by exhausting antioxidative defense substances. Other authors demonstrate that a fructose diet in rats can induce the increase the MDA level, which can facilitate oxidative damage (41). It has also been demonstrated that one of the possible mechanisms of diabetes-related tissue damage in experimental diabetes induced by STZ is the modification of various proteins via secondary degradation products resulting from lipid peroxidation such as MDA (42). Because our study groups (FD and SID) were found to have a significantly increased MDA, there is an increased susceptibility to cells and tissue damage due to the increase of this metabolite in the blood. Based on the obtained results, it can be suggested that the curcumin treatment can reduce the lipid peroxidation, demonstrated by the decreasing of MDA in both curcumin pre-treatment groups (FDC and SIDC).
The increase of NO production as a biomarker of nitro-oxidative stress was demonstrated by measurement of inorganic nitrites and nitrates (NOx), the stable end metabolites of NO. The high concentration of NOx in all groups (FD and SID) compared with control group demonstrates the increase of NO synthesis. The NO molecule behaves as a potent pro-oxidant producing peroxynitrite in the presence of super oxide anion (43). Enhanced production of NO resulting from oxygen-derived free radical production has been proven to induce the increase of lipid peroxidation and MDA production (44). The production of NO is enhanced by hyperglycemia and hyperlipidemia, which are associated with increased oxidative stress. The correlation of increased NO production with pro-inflammatory cytokines as is IL-6 and TNF-alpha has been reported in human (45). In inflammatory status, activated macrophages and neutrophils produce large amounts of NO through iNOS activity (46). In our experimental groups, the presence of hyperglycemia and hyperlipidemia, and increased oxidative stress can lead to increase NO synthesis. The significant decrease of NOx and TOS (markers of the degree of oxidative stress) after pre-treatment with curcumin demonstrates its potency to reduce the oxidative stress and implicitly the inflammatory reaction. Anti-oxidant and anti-inflammatory properties of curcumin were already demonstrated even though the exact molecular mechanisms are not entirely known (47).
Thiol groups (-SH) play an important role in anti-oxidant reaction and also in reaction of catalysis, regulation, electron transport, and thus preserving the correct structure of proteins (48). For this reason, Thiol production compounds have a significant effect on the redox state of plasma because they are an important anti-oxidant system. Cells have a natural reservoir of Thiol compounds such as glutathione (GSH), cysteine (CSH), and homocysteine (HCSH). They play an in vivo role of redox buffer. The reduction of GSH is associated with pathological oxidative stress induced by high fat diet in rats (49). Similar influence on total anti-oxidant capacity and Thiol of other plants (such as Aronia melanocarpa) on high fructose diet rat experimental model has also been demonstrated (50).
In this study, no significant differences were observed in regard of TAC and NOx when FDC group was compared with SIDC group. However, the values of MAD and respectively TOC proved significantly higher in SIDC group compared with FDC, while the values of Thiol proved significantly higher in FDC group compared with SIDC.
Association analysis showed a significant linear relation between NOx and systolic blood pressure for SID group. The regulation of sympathetic vasomotor tone, as a mechanism of hypertension, can be induced by NO action in rostral ventrolateral medulla (51).
Conclusion
The curcumin pre-treatment proved to improve metabolic changes (hyperglycemia, hypercholes-terolemia, hypertriglyceridemia) and oxidative stress induced by both fructose-induced metabolic syndrome and STZ-induced diabetes in rats, even these two experimental models have different pathophysiological mechanism of metabolic and oxidative stress changes. The systolic blood pressure is also significantly reduced by curcumin compared to untreated groups. These ameliorating effects on metabolic parameter and systolic blood pressure of curcumin are exerted through its potency to reduce oxidative stress. Future studies are needed for pathophysiological mechanisms elucidation due to curcumin pre-treatment.
Conflict of interests
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article. The authors declared no conflict of interest.
Acknowledgement
No financial source to declare.
References
- 1.Tappy L, Lê KA, Tran C, Paquot N. Fructose and metabolic diseases: new findings, new questions. Nutrition. 2010;26:1044–1049. doi: 10.1016/j.nut.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, et al. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009;139:2067–2071. doi: 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- 3.Mamikutty N, Thent ZC, Sapri SR, Sahruddin NN, Mohd Yusof MR, Haji Suhaimi F. The establishment of metabolic syndrome model by induction of fructose drinking water in male Wistar rats. Biomed Res Int. 2014;2014:263897. doi: 10.1155/2014/263897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spruss A, Kanuri G, Uebel K, Bischoff SC, Bergheim I. Role of the inducible nitric oxide synthase in the onset of fructose-induced steatosis in mice. Antioxid Redox Signal. 2011;14:2121–2135. doi: 10.1089/ars.2010.3263. [DOI] [PubMed] [Google Scholar]
- 5.Cosenzi A, Bernobich E, Bonavita M, Gris F, Odoni G, Bellini G. Role of nitric oxide in the early renal changes induced by high fructose diet in rats. Kidney Blood Press Res. 2002;25:363–369. doi: 10.1159/000068694. [DOI] [PubMed] [Google Scholar]
- 6.Wu KL, Chao YM, Tsay SJ, Chen CH, Chan SH, Dovinova I, et al. Role of nitric oxide synthase uncoupling at rostral ventrolateral medulla in redox-sensitive hypertension associated with metabolic syndrome. Hypertension. 2014;64:815–824. doi: 10.1161/HYPERTENSIONAHA.114.03777. [DOI] [PubMed] [Google Scholar]
- 7.Mohamad Shahi M, Haidari F, Shiri MR. Com-parison of effect of resveratrol and vanadium on diabetes related dyslipidemia and hyperglycemia in streptozotocin induced diabetic rats. Adv Pharm Bull. 2011;1:81–86. doi: 10.5681/apb.2011.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musial DC, da Silva Júnior ED, da Silva RM, Miranda-Ferreira R, Lima-Landman MT, Jurkiewicz A, et al. Increase of angiotensin-converting enzyme activity and peripheral sympathetic dysfunction could contribute to hypertension development in streptozotocin-induced diabetic rats. Diab Vasc Dis Res. 2013;10:498–504. doi: 10.1177/1479164113496441. [DOI] [PubMed] [Google Scholar]
- 9.Haluzík M, Nedvídková J, Skrha J. The influence of NO synthase inhibitor and free oxygen radicals scavenger-methylene blue-on streptozotocin-induced diabetes in rats. Physiol Res. 1998;47:337–341. [PubMed] [Google Scholar]
- 10.Negi PS, Jaypraskasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: a by product from curcumin manufacture. J Agric Food Chem. 1999;47:4297–4300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 11.Nagajyothi F, Zhao D, Weiss LM, Tanowitz HB. Curcumin treatment provides protection against Trypanosoma cruzi infection. Parasitol Res. 2012;110:2491–2499. doi: 10.1007/s00436-011-2790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias Ferreira F, Mossini SA, Dias Ferreira FM, Arrotéia CC, da Costa CL, Nakamura CV, et al. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Scientific World Journal. 2013;2013:343804. doi: 10.1155/2013/343804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaprakasha GK, Rao LJ, Sakarian KK. Antioxidant activities of curcumin, demethoxi curcumin and bisdemethoxy curcumin. Food Chem. 2006;98:720–724. [Google Scholar]
- 14.Minaiyan M, Zolfaghari B, Kamal A. Effect of hydroalcoholic and buthanolic extract of cucumis sativus seeds on blood glucose level of normal and streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2011;14:436–442. [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 16.Ashan H, Perveen N, Khan NU, Hadi SM. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivetes demethoxy curcumin and bisdemethoxi curcumin. Chem Biol Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 17.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- 18.Bengmark S1, Mesa MD, Gil A. Plant-derived health: the effects of turmeric and curcuminoids. Nutr Hosp. 2009;24:273–281. [PubMed] [Google Scholar]
- 19.Rossini AA, Like AAA, Chick WL, Appel MC, Cahill GF., Jr Studies of streptozotocin-induced insulitis and diabetes. Proc Natl Acad Sci USA. 1977;74:2485–2489. doi: 10.1073/pnas.74.6.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amr AA, Elshazly SM. Ursodeoxycholic acid ameliorates fructose-induced metabolic syndrome in rats. PLoS One. 2014;9:e106993. doi: 10.1371/journal.pone.0106993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alimohammadi S, Hobbenaghi R, Javanbakht J, Kheradmand D, Mortezaee R, Tavakoli M, et al. Protective and antidiabetic effects of extract from Nigella sativa on blood glucose concentrations against streptozotocin (STZ)-induced diabetic in rats: an experimental study with histopathological evaluation. Diagn Pathol. 2013;8:137. doi: 10.1186/1746-1596-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, et al. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 23.Amani M, Noroozzadeh A, Badalzadeh R, Khosh-baten A. Effect of ascorbic acid supplementation on nitric oxide metabolites and systolic blood pressure in rats exposed to lead. Indian J Pharmacol. 2010;42:78–81. doi: 10.4103/0253-7613.64501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yagl K. Assay for blood plasma and serum peroxides. Methods Enzymol. 1984;105:28–31. doi: 10.1016/s0076-6879(84)05042-4. [DOI] [PubMed] [Google Scholar]
- 25.Hu ML. Measurement of protein thiol groups and glutathuine in plasma. Methods Enzymol. 1994;233:380–384. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 26.Pârvu AE, Pârvu M, Vlase L, Miclea P, Mot AC, Silaghi-Dumitrescu R. Anti-inflammatory effects of Allium Schonoprasum L leaves. J Physiol Pharmacol. 2014;65:309–315. [PubMed] [Google Scholar]
- 27.Kawamura T, Yoshida K, Sugawara A, Nagasaka M, Mori N, Takeuchi K, et al. Regulation of skeletal muscle peroxisome proliferator-activated receptor gamma expression by exercise and angiotensin-converting enzyme inhibition in fructose-fed hypertensive rats. Hypertens Res. 2004;27:61–70. doi: 10.1291/hypres.27.61. [DOI] [PubMed] [Google Scholar]
- 28.Sherajee SJ, Rafiq K, Nakano D, Mori H, Kobara H, Hitomi H, et al. Aldosterone aggravates glucose intolerance induced by high fructose. Eur J Pharmacol. 2013;720:63–68. doi: 10.1016/j.ejphar.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]
- 30.Wu KL, Chan SH, Chan JY. Neuroinflammation and oxidative stress in rostral ventrolateral medulla contribute to neurogenic hypertension induced by systemic inflammation. J Neuroinflammation. 2012;9:212. doi: 10.1186/1742-2094-9-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soucek M, Kára T. [Stress-induced hypertension and diabetes mellitus] Vnitr Lek. 2001;4:315–319. [PubMed] [Google Scholar]
- 32.Amin F, Mehmood MH, Siddiqui BS, Khatoon N, Gilani AH. Co-administration of Black seeds and Turmeric shows enhanced efficacy in preventing metabolic syndrome in fructose-fed rats. J Cardiovasc Pharmacol. 2015;65:176–183. doi: 10.1097/FJC.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 33.Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med Sci Monit. 2007;13:BR286–292. [PubMed] [Google Scholar]
- 34.Hu GX, Lin H, Lian QQ, Zhou SH, Guo J, Zhou HY, et al. Curcumin as a potent and selective inhibitor of 11β-hydroxysteroid dehydrogenase 1:improving lipid profiles in high-fat-diet-treated rats. PLoS One. 2013;8:e49976. doi: 10.1371/journal.pone.0049976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickenberg J, Ingemansson SL, Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr J. 2010;9:43. doi: 10.1186/1475-2891-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan K. Dietary spices as beneficial modulators of lipid profile in conditions of metabolic disorders and diseases. Food Funct. 2013;4:503–521. doi: 10.1039/c2fo30249g. [DOI] [PubMed] [Google Scholar]
- 37.Hassanin A, Malek HA, Saleh D. Heparin modulation on hepatic nitric oxide synthase in experimental steatohepatitis. Exp Ther Med. 2014;8:1551–1558. doi: 10.3892/etm.2014.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abolfathi AA, Mohajeri D, Rezaie A, Nazeri M. Protective effects of green tea extract against hepatic tissue injury in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2012;2012:740671. doi: 10.1155/2012/740671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Rubaei ZM, Mohammad TU, Ali LK. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak J Biol Sci. 2014;17:1237–1241. doi: 10.3923/pjbs.2014.1237.1241. [DOI] [PubMed] [Google Scholar]
- 40.Janero DR. Malondialdehide and thiogbarbituric acid-reactivity as adiagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 41.Suganthi R, Rajamani S, Ravichandran MK, Anuradha CV. Effect of food seasoning spices mixture on biomarkers of oxidative stress in tissues of fructose-fed insulin-resistant rats. J Med Food. 2007;10:149–153. doi: 10.1089/jmf.2005.058. [DOI] [PubMed] [Google Scholar]
- 42.Lung CC, Pinnas JL, Yahya MD, Meinke GC, Mooradian AD. Malondialdehyde modified proteins and their antibodies in the plasma of control and streptozotocin induced diabetic rats. Life Sci. 1993;52:329–337. doi: 10.1016/0024-3205(93)90225-r. [DOI] [PubMed] [Google Scholar]
- 43.Goss SP, Singh RJ, Hogg N, Kalyanaraman B. Reactions of *NO, *NO2 and peroxynitrite in membranes: physiological implications. Free Radic Res. 1999;31:597–606. doi: 10.1080/10715769900301171. [DOI] [PubMed] [Google Scholar]
- 44.Husain K, Hazelrigg SR. Oxidative in jury due to chronic nitric synthase inhibition in rat: effect of regular exercise on the heart. Biochim Biophys Acta. 2002;1587:75–82. doi: 10.1016/s0925-4439(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 45.Volpe CM, Abreu LF, Gomes PS, Gonzaga RM, Veloso CA, Nogueira-Machado JA. The production of nitric oxide, IL-6, and TNF-alpha in palmitate-stimulated PBMNCs is enhanced through hyperglycemia in diabetes. Oxid Med Cell Longev. 2014;2014:479587. doi: 10.1155/2014/479587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nathan C, Xie Q-W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 47.Aggarwal BB, Harikumar KB. Potential thera-peutic effects of curcumin, the antiinflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rokutan K, Thomas JA, Sies H. Specific S-thiolation of a 30-kDa cytosolic protein from rat liver under oxidative stress. Eur J Biochem. 1989;179:233–239. doi: 10.1111/j.1432-1033.1989.tb14546.x. [DOI] [PubMed] [Google Scholar]
- 49.Fisher-Wellman KH, Gilliam LA, Lin CT, Cathey BL, Lark DS, Neufer PD. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med. 2013;65:1201–1208. doi: 10.1016/j.freeradbiomed.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Francik R, Krośniak M, Sanocka I, Bartoń H, Hebda T, Francik S. Aronia melanocarpa treatment and antioxidant status in selected tissues in wistar rats. Biomed Res Int. 2014;2014:457085. doi: 10.1155/2014/457085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kishi T. Regulation of the sympathetic nervous system by nitric oxide and oxidative stress in the rostral ventrolateral medulla: 2012 Academic Conference Award from the Japanese Society of Hypertension. Hypertens Res. 2013;36:845–851. doi: 10.1038/hr.2013.73. [DOI] [PubMed] [Google Scholar]


