Abstract
Objective(s):
Three-dimensional biomimetic scaffolds have widespread applications in biomedical tissue engineering due to similarity of their nanofibrous architecture to native extracellular matrix. Co-culture system has stimulatory effect on chondrogenesis of adult mesenchymal stem cells. This work presents a co-culture strategy using human articular chondrons and adipose-derived stem cells (ASCs) from infrapatellar fat pad (IPFP) for cartilage tissue production.
Materials and Methods:
Isolated stem cells were characterized by flowcytometry. Electrospun and polycaprolactone (PCL) scaffolds (900 nm fiber diameter) was obtained from Bon Yakhteh (Tehran-Iran) and human infrapatellar fat pad-derived stem cells (IPFP-ASCs) were seeded on them. IPFP-ASCs on scaffolds were co-cultured with articular chondrons using transwell. After 21 day, chondrogenic differentiation of stem cell was evaluated by determining the genes expression of collagen2, aggrecan and Indian hedgehog using real-time RT-PCR.
Results:
Genes expression of collagen2, aggrecan by IPFP-ASCs did not alter significantly in comparison with control group. Howevers, expression of Indian hedgehog decreased significantly compared to control group (P< 0.05).
Conclusion:
These findings indicate that chondrons obtained from osteoarthritic articular cartilage did not stimulate chondrogenic differentiation of IPFP-ASCs in co-culture.
Keywords: Chondron, Co-culture, Nanofiber, Poly-e-caprolactone scaffold
Introduction
Articular cartilage (AC) is an aneural, avascular tissue that covers the diarthrodial joints and due to its avascularity it has limited capacity for regeneration. Therefore, injuries to the AC in adults, do not heal spontaneously and progress towards osteoarthritis (OA) (1, 2).
Tissue engineering represents aviable choice to repair cartilage and restore joint function (3, 4). This approach requires cell sources, matrix or scaffolds, and appropriate growth factors to promote chondrogenesis (5). The optimum cell source for cartilage tissue engineering is still not being established. Adult mesenchymal stem cells (MSCs) have been investigated as one of the common cell source for cartilage repair (6, 7). MSCs could be obtained from bone marrow and different other tissues such as adipose tissue, and many other organs (8). Adipose tissue offers an easy available and abundant source for adult stem cells, with minimal donor-site morbidity. Some have used adipose stem cells derived from the infrapatellar fat pad (IPFP), because it has been demonstrated that adipose-derived stem cells (ASCs) from the IPFP encapsulated in fibrin, express collagen type 2 and aggrecan. Additionally, its matrix sulphated glycosaminoglycan (sGAG) content reaches 50% of native cartilage (9).
Scaffolds as a second element in the tissue engineering, provide a 3D environment that are desired for the production of cartilaginous tissue. Polycaprolactone (PCL) is a synthetic polymer that has intriguing properties appropriate for cartilage tissue engineering applications, such as good degradability, compatibility and mechanical strength (10). Nevertheless, its low surface wettability due to its rather hydrophobicity affects cell attachment and proliferation. So several surface treatments of polyesters have been attempted to increase their surface hydrophilicity in order to improve the adhesion of cells to PCL (11, 12). Such methods as alkaline hydrolysis and plasma treatment of polymeric scaffolds were proved to improve cell adhesion and enhance cell proliferation and functions (13-15).
The third components of the tissue engineering triad, stimulating factors, have been used to induce, accelerate, and/or enhance cartilage formation. In vitro co-culture systems provide a strong tool for cartilage tissue engineering (16) and is used to replace growth factors. The extracellular matrix (ECM) of tissues is a complex reservoir of mediators and growth factors. Therefore, whole tissue chips or ECM components have been used to improve neo-cartilage formation by cultured chondrocytes and chondrocyte-like cells (17, 18).
The chondrocyte and its associated narrow pericellular matrix (PCM) are termed as chondron (19). Previous studies have demonstrated that the PCM of chondron, is primarily defined by the presence of type 6 collagen, but also contains high concentration of proteoglycans including aggrecan (20), hyaluronan, laminins and nidogen-2 (21), decorin and fibronectin (22), and as well as collagen types 2, 9 (23), and collagen type 11 (24), relative to the ECM. In general, the small proteoglycans are thought to have inhibitory functions such as restricting collagen fibrillogenesis, limiting fibronectin adhesion, and binding TGF-β modulate matrix synthesis or mitogenic activity (25). The differentiation of chondroprogenitors or MSCs to chondrocyte are characterized by the deposition of cartilage matrix containing collagen 2 and aggrecan. Furthermore, Indian hedgehog (IHH) is one of three hedgehog that specifically expressed by flattened prehypertrophic chondrocytes during development of embryo (26, 27). In the present study the impact PCM of chondrons, in an indirect co-culture model, on chondrogenic potential of IPFP-derived stem cells has been examined. The stem cells were seeded on PCL scaffolds and chondrons were situated on transwell. Chondrogenisity was evaluated by gene expression using Real-time RT-PCR and data were analyzed.
Materials and Methods
Cell isolation, culture and doubling time
IPFP was obtained from patients (aged 24, 25 and 46 years; n= 3) undergoing anterior cruciate ligament (ACL) surgery. Before surgery the purpose of study was explained to the patients and a written consent was obtained from each patient. Briefly the tissue was washed 3 times with phosphate buffered saline (PBS, Sigma, USA), and diced finely and then digested with 0.1% collagenase 1 (Gibco, USA) for 50-55 min at 37 °C. Enzymatic activity was neutralized by Dulbecco’s modified Eagle’s medium (DMEM-low glucose, Gibco, UK), containing 10% etal bovine serum (FBS, Gibco, E.U. Approved (South American)) and centrifuged at 1400 rpm for 10 min. Then, the pellet was resuspended, washed 2 times with medium, and seeded on culture flask. Medium of culture flask containing DMEM, 10% FBS, 1% penicillin-streptomycin (Sigma-Aldrich, USA) and maintained in incubator at 37°c, 5% CO2 and 97% humidity (28). At the time of passage, cell viability was determined by trypan blue staining. For freezing, after culturing through passage 1 or 2, the cells were suspended in a cryopreservation medium containing 90% FBS and 10% dimethylsulfoxide (DMSO, Sigma-Aldrich, USA).
Doubling time from passage 0 to 1 was calculated using the algorithm:
Doubling Time = T [log 2 / log (N2 / N1)]
T= days of expansion; N1= number of plating cells;
N2 = number of harvested cells at the end
Enzymatic isolations of chondrons
Articular cartilage was obtained from patients (aged 46, 55, and 62 years; n = 3) who underwent total hip or knee arthroplasty due to osteoarthritis. Only macroscopically normal-looking cartilage was diced for chondrons isolation. Chondron isolation was accorded on a previously published protocol (29) with slight modification. The cartilage pieces were treated with 0.3% dispase (Gibco, USA) and 0.2% collagenase 2 (Gibco, USA) in PBS for 5 hr. Enzymes activity were neutralized with DMEM, containing 10% FBS and centrifuged at 1400 rpm for 10 min. The cells were washed and seeded on culture flasks in medium containing DMEM, 10% FBS, 1% penicillin– streptomycin, and 25 μg/ml ascorbic acid (Sigma-Aldrich, USA). For freezing, after 24 hr, floating cells were washed and suspended in a cryopreservation medium containing 90% FBS and 10% DMSO.
Scaffold characterization
PCL scaffold was obtained from a Stem Cell Technology Company (Bon Yakhteh-Tehran, Iran). According to companiʼs instruction the nanofibrous PCL sheet was plasma treated. Before using, scanning electron microscope (MIRA3 FEG-SEM) was used for the observation of the structural morphology of scaffold. The samples of scaffold were cut from the nanofiberous sheet using a 7 mm dermal punch and coated with gold by a sputter-coater. Diameter of fibers in the electrospun scaffold is mean of 10 nanofibers which were measured on scanning electron micrographs. The average fiber diameter was determined from measurements taken perpendicular to the long axis of the fibers within representative microscopic fields (30).
Cell surface epitope characterization and flowcyto-metry
IPFP-ASCs from passage 2 were characterized for mesenchymal stem cell surface protein expression by flowcytometry. The monoclonal antibodies used were PE labeled anti-CD90 and FITS labeled anti-CD44 (BD Bioscience, USA), PerCP labeled anti-CD31 (R&D systems) and CD45 (Abcam, UK). The cells (3×105 cells) were washed and incubated with antibodies for 25 min at 4 °C temperature in dark environment. At least 10,000 events were acquired on BD caliber (BD ebioscience), and the data were analyzed using flowing software (PerttuTerho, Version: 2.5.1).
Seeding of IPFP-ASCs into PCL scaffolds and co-culture
The scaffolds were sterilized under ultraviolet (UV) light for 2 hr on each side. The sterilized 7 mm scaffold discs were placed into 24-well culture plate. Each scaffold was seeded with IPFP-ASCs at concentration of 5×105 cells/specimen and then incubated for 2 hr to allow the cells to attach. Then 1 ml medium [DMEM, 1% FBS, 1% penicillin–streptomycin, and 25 μg/ml ascorbic acid] was added to each well. For co-culturing transwells (0.4 μm pore size, polyester membrane, Greiner, Germany) were placed into the 24-wells plates that had IPFP-ASCs /scaffold. The chondrons were suspended in chondrogenic medium [DMEM, 1% FBS, 1% penicillin–streptomycin, and 25 μg/ml ascorbic acid] at a concentration of 5 ×105 cell/300 μl. Cell suspension was added to the transwell. The 24-well culture plate was incubated at 37°c in 5% CO2 for 21 days. Hundred μl medium were removed from the lower chambers and equal volume fresh medium were added to upper chambers every 2 days.
Real-Time RT-PCR
After 21 days, each IPFP-ASCs seeded scaffold was homogenized under liquid nitrogen using a mortar. RNA was isolated from the samples by using RNX-Plus (Sinaclon, IRAN) according to the manufacturer’s instructions. The concentration of RNA was estimated spectrophotometrically with NanoDrop 1000 Spectrophotometer (Wilmington, DE, USA) at A260/280. The RNA samples were reverse transcribed into first-strand cDNA using the AccuPower® RT PreMix (Bioneer). Real Time-PCR reactions were performed using the SYBRGreen PCR Mastermix (Applied Biosystems, USA), according to the manufacturer’s instructions. These gene primers were purchased from (Bioneer). The cartilage-specific oligonucleotide primers which were used, were aggrecan (forward 5’-AGGGCGAGTGGAAT-GATGTT-3’; reverse 5’-GGTGGCTGTGCCCTTTTTAC-3’), and collagen type 2a1 (forward 5’-ATGCCACA-CTCAAGTCCCTCAA-3’; reverse 5’-CGCAAGTCTCG-CCAGTCTCC-3’), (IHH) (forward 5’-CACTTCTGCC-TGGTCCTGTT-3’; reverse 5’-GGGCTGAACTGCTTG-TAGG-3’), housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward, 5’-CAAGATCATCAGCAATGCCTCC-3’; reverse, 5’-GC-CA-TCACGCCACAGTTTCC-3’). All experiments were performed in triplicate for each sample. Interpreta-tion of the results was performed using the Pfaffle method and the CT values were normalized with respect to GAPDH expression.
Statistical analysis
Results are presented as mean±standard deviation. T-test method was used for statistical analysis. P<0.05 were considered statistically significant.
Results
Cell morphology and doubling time
IPFP-ASCs were able to adhere to tissue culture flasks where as non-adherent cells such as red blood cells, were removed by media change. Cells proliferated rapidly and doubling time was 1.99 ± 0.36 days. The initial adherent cells grew into spindle, or triangular-shaped cells (Figure 1A). At passage 1 (P1), the shape of cells shifted towards a fibroblast-like form (Figure 1B). At the second passage, IPFP-ASCs appeared to adopt a more uniform fibroblast-like shape (Figure 1C).
Figure 1.
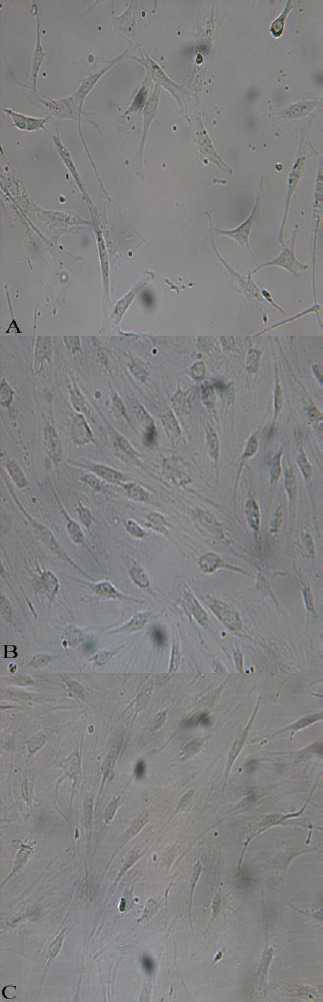
Morphology of IPFP-ASCs in culture observed with an inverted phase-contrast microscope at different passages. The shape of cells changed during passages: p0 (A), p1 (B) and passage2 (C), 20X
Scaffold characterization
SEM imaging was first performed to observe the structural morphology of fibers in the electrospun PCL scaffold. The surface of plasma treated scaffold was smooth. The average diameter of nanofibers was approximately 900 nm. The orientation of the fibers were random (Figure 2).
Figure 2.

Architecture of electrospun PCL nanofiber scaffold as seen with a scanning electron microscope at 4.00KX
Cell surface epitope characterization and flowcytometry
IPFP-ASCs expressed the mesenchymal stem cell markers such as CD44 (94.32%), CD90 (97.82%). However, few cells expressed hematopoietic marker such as CD45 (3.18%), or the endothelial marker CD31 (4.88%). Figure 3 shows positive and negative mesenchymal cell surface markers at passage 2.
Figure 3.
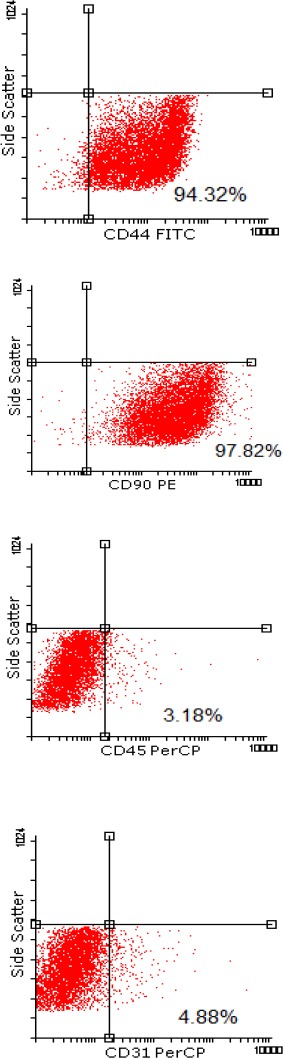
Cells were tested against human antigens CD31, CD44, CD45, CD90. All experiments were conducted at passage 2
Cell culture and co-culture
Before using the chondrons for co-culturing, they were cultured in DMEM, containing 10% FBS, 1% penicillin–streptomycin, and 25 μg/ml ascorbic acid. After 15 days of culture in flask, PCM of the chondrocytes disappeared and chondrocytes attached on floor of flask. These events happened gradually. In the 1st day, all the chondrons had PCM and were floating. Figure 4A shows the cells at the 1st day of culture, the white arrows indicate a group of chondronsconsisting of a few cells which are inconnection with adjacent group by a tail-like segment that ensure linear continuity between adjacent groups. But on the 8th day, some of them lost PCM and transformed to flat form cells (Figure 4B). On the 15th day all of chondrons lost their PCM and the cells adhered to culture flasks (Figure 4C).
Figure 4.
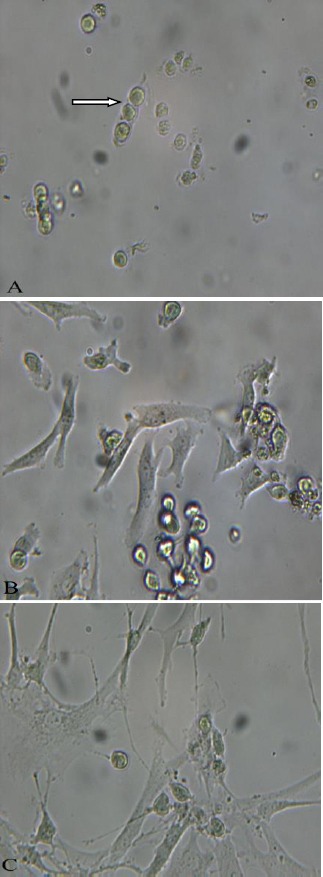
Morphology of cultured chondrons as seen with an inverted phase-contrast microscope during 15 day culture, 1st day (A), 8th day (B), 15th day (C), 10X
Figure 5 shows the IPFP-ASCs seeded on PCL scaffold. As the Figure 5 shows, the cultured cells on PCL, have roundish shape and have a few rocesses after 21 days.
Figure 5.
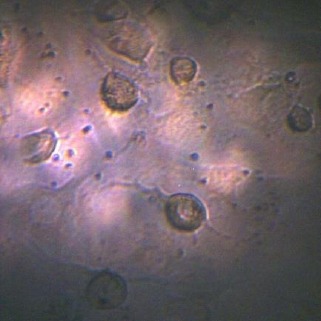
IPFP-ASCs on PCL scaffold after 21 days, observed with inverted phase contrast microscope, 20X
Real-Time RT-PCR
To determine the effects of chondron co-culture on IPFP-ASCs chondrogenesis, we examined the mRNA levels of collagen type 2 (a1), aggrecan, and IHH in IPFP-ASCs/scaffold by quantitative RT-PCR. For the co-culture group, IPFP-ASCs/scaffold showed slight decrease in collagen type 2 (a1) and aggrecan (Figure 6A, 6B) but the changes were not significant. However the mRNA levels of IHH had severe reduction (Figure 6C) and it was statistically significant (P< 0.05).
Figure 6.
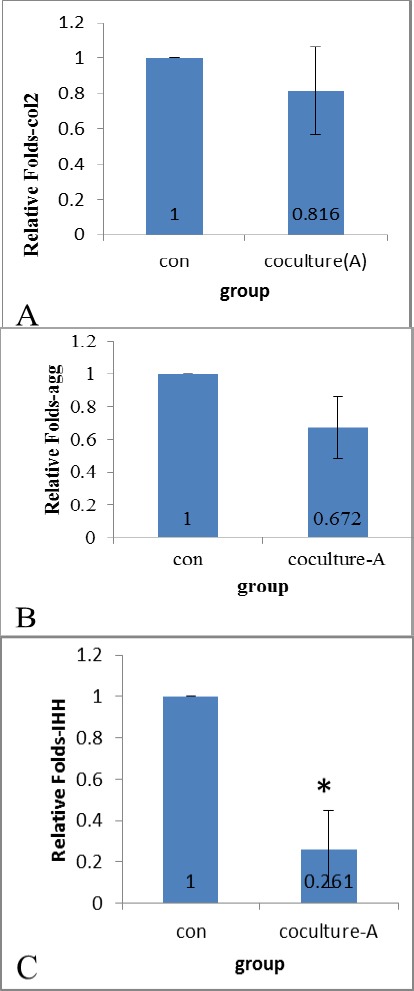
Relative genes expression of chondrogenic cell/PCL and cell/PCL co-culture. Co-culture conditions decreased expression collagen2a1 (A), aggrecan (B) and IHH (C * = P< 0.05
Discussion
In this study we investigated whether chondrons are able for chondrogenic induction of ASCs from the IPFP on the nanofibrous PCL scaffold.
The study showed plastic-adherent property of ASCs in culture, 94.32% of ASCs expressed the MSCs positive CD markers (CD44) and 97.82% of ASCs expressed the CD90. While about 2% of ASCs expressed negative cell surface marker (CD31: platelet endothelial cell adhesion molecule and CD45: leukocyte common antigen), at second passage. So the cell surface epitope characterization and flowcytometry of IPFP population showed a similar staining pattern to that of bone marrow-derived stem cells. Our result showed the percentage of negative markers is high, but it seems to decrease with increasing passage number because cells became more homogeneous. Taken together, the results suggest that the ASCs from the IPFP is a relatively homogenous population of mesenchymal cells with low contamination by endothelial and leukocyte cells.
Two properties have been established in the present study. First it is shown that ASCs isolated from the IPFP were more homogenous fibroblast-like shape in passage 2. Second, we found that the contents of pericellular matrix of chondrons released into the culture medium by 15 days. Based on these findings, we used chondrons for co-culture in transwell to evaluate the effect of PCM on ASCs. In an study it is demonstrated that chondrocytes on nanofibrous scaffolds had higher rates of proliferation and maintained a rounded morphology, which is characteristic of the chondrocyte phenotype (31). Consistent with this result, the seeded IPFP-ASCs on scaffold, in our study, had a round morphology. We also found that 21 day after culture, the attached IPFP-ASCs on nanofibrous PCL scaffold still had roundish shape and grew a few branched processes.
Previous study showed IPFP-ASCs can successfully undergo chondrogenesis using TGFβ3 and BMP6 and the cartilage-like tissue (32). Release of growth factors such as transforming growth factor beta 1 (TGFβ1), insulin-like growth factor 1 (IGF-1), bone morphogenetic proteins 2 (BMP2) were demonstrated in the supernatants of co-cultured bone marrow stem cells (BMSC) and articular chondrocytes (33). In our study, probably releasing of similar factors were involved in cell differentiation.
Some studies showed that presence of the PCM in chondrons had a profound effect on chondrocyte gene expression (34-36). Up-regulation of the heat shock protein 70, may contribute to the robustness and active matrix production of chondrons (34). In this study, we expected that the release of factors from PCM of chondrocytes into the microenviron-ment could be absorbed by the nanofibrous PCL and stimulate IPFP-ASCs. The nanofibrous constructs were found to selectively enhance the adsorption of specific proteins, such as fibronectin and vitronectin (37). Since previous studies have reported that fibronectin binds to growth factors (38), so we assumed that growth factors may also attach to factors released from chondrons. However, the results of real-time RT-PCR in this study suggest that co-culture conditions had deleterious effect on chondrogenesis. From this point, our findings are consistent with a previous study showing that conditioned medium from primary osteoarthritic (P0) chondrocytes could not induce chondrogenic differentiation of MSCs (39).
Previous studies have also demonstrated that injured and osteoarthritic joints show significantly higher levels of pro-inflammatory cytokines. For example interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α), as well as procatabolic enzymes and mediators such as metalloproteinases (MMPs), aggrecanases, prostaglandins, and nitric oxide are overexpressed in these joints which could reason tissue degradation, pain, and inflammation (40-42). Other studies showed the deleterious effects of IL-1 on the chondrogenesis of ASCs (43) and MSCs (44-47). Based on these studies, it is probable that in the present study, the presence of similar substances in the microenvironment during the initial 21-day culture period inhibited gene expression. In addition to these substances in the microenvironment, there may be other factors overshadowed.
On the other side, it is known that IHH is secreted from mature cells, it seems that co-culture condition in our study, inhibited cell maturation and therefore secretion of IHH was reduced.
There are limitations related to the design of the current study. First, it was not possible to use chondrons from healthy normal cartilage of young adults, so we used sample of OA patients which would have yielded different, perhaps more dramatic, results. Second, the conditioned medium is expected to contain various growth factors and cytokines secreted from chondrons but it was not checked in the present study.
Conclusion
In summary, our findings show that nanofibrous scaffold is ideal for IPFP-ASCs attachment. The differential effects of co-culture with chondrons from OA patients did not improve chondrogenesis of IPFP-ASCs.
Acknowledgment
This research was approved and financially supported by Research Deputy, Tabriz University of Medical Sciences, Tabriz, Iran.
References
- 1.Buckwalter J, Mankin H. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course lect. 1997;47:487–504. [PubMed] [Google Scholar]
- 2.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465. [PubMed] [Google Scholar]
- 3.Caplan AI. Tissue engineering designs for the future: new logics, old molecules. Tissue Eng. 2000;6:1–8. doi: 10.1089/107632700320838. [DOI] [PubMed] [Google Scholar]
- 4.Tuli R, Li W-J, Tuan RS. Current state of cartilage tissue engineering. Arthritis Res Ther. 2003:235–238. doi: 10.1186/ar991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magazine E. From UMassWiki. Tissue Eng. 1993;60:920. [Google Scholar]
- 6.Tuan RS. Stemming cartilage degeneration: adult mesenchymal stem cells as a cell source for articular cartilage tissue engineering. Arthritis Rheum. 2006;54:3075–3078. doi: 10.1002/art.22148. [DOI] [PubMed] [Google Scholar]
- 7.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dragoo J, Samimi B, Zhu M, Hame S, Thomas B, Lieberman J, et al. Tissue-engineered cartilage and bone using stem cells from human infrapatellar fat pads. J Bone Joint Surg Br. 2003;85:740–747. [PubMed] [Google Scholar]
- 10.Woodruff MA, Hutmacher DW. The return of a forgotten polymer—polycaprolactone in the 21st century. Prog Polym Sci. 2010;35:1217–1256. [Google Scholar]
- 11.Lee HU, Jeong YS, Jeong SY, Park SY, Bae JS, Kim HG, et al. Role of reactive gas in atmospheric plasma for cell attachment and proliferation on biocompatible poly ɛ-caprolactone film. Appl Surf Sci. 2008;254:5700–5705. [Google Scholar]
- 12.Yildirim ED, Pappas D, Güçeri S, Sun W. Enhanced cellular functions on polycaprolactone tissue scaffolds by O2 plasma surface modification. Plasma Proc Polym. 2011;8:256–267. [Google Scholar]
- 13.Wang YQ, Cai JY. Enhanced cell affinity of poly (L-lactic acid) modified by base hydrolysis: Wettability and surface roughness at nanometer scale. Curr Appl Phys. 2007;7:108–111. [Google Scholar]
- 14.Ng R, Zhang X, Liu N, Yang ST. Modifications of nonwoven polyethylene terephthalate fibrous matrices via NaOH hydrolysis: Effects on pore size, fiber diameter, cell seeding and proliferation. Proc Biochem. 2009;44:992–998. [Google Scholar]
- 15.Lee HU, Kang YH, Jeong SY, Koh K, Kim JP, Bae JS, et al. Long-term aging characteristics of atmospheric-plasma-treated poly (ɛ-caprolactone) films and fibres. Polym Degrad Stab. 2011;96:1204–1209. [Google Scholar]
- 16.Hendriks J, Riesle J, van Blitterswijk CA. Co-culture in cartilage tissue engineering. J Tissue Eng Regen Med. 2007;1:170–178. doi: 10.1002/term.19. [DOI] [PubMed] [Google Scholar]
- 17.Arana CJ, Diamandis EP, Kandel RA. Cartilage tissue enhances proteoglycan retention by nucleus pulposus cells in vitro. Arthritis Rheum. 2010;62:3395–3403. doi: 10.1002/art.27651. [DOI] [PubMed] [Google Scholar]
- 18.Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, et al. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments in vitro matrix production. Am J Sports Med. 2011;39:2355–2361. doi: 10.1177/0363546511417172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szirmai J. Biopolymere und Biomechanik von Bindegewebssystemen. Springer; 1974. The concept of the chondron as a biomechanical unit; pp. 87–91. [Google Scholar]
- 20.Poole CA, Matsuoka A, Schofield JR. Chondrons from articular cartilage. III. Morphologic changes in the cellular microenvironment of chondrons isolated from osteoarthritic cartilage. Arthritis Rheum. 1991;34:22–35. doi: 10.1002/art.1780340105. [DOI] [PubMed] [Google Scholar]
- 21.Salmivirta K, Talts JF, Olsson M, Sasaki T, Timpl R, Ekblom P. Binding of mouse nidogen-2 to basement membrane components and cells and its expression in embryonic and adult tissues suggest complementary functions of the two nidogens. Exp Cell Res. 2002;279:188–201. doi: 10.1006/excr.2002.5611. [DOI] [PubMed] [Google Scholar]
- 22.Poole CA, Honda T, Skinner SJ, Schofield JR, Hyde KF, Shinkai H. Chondrons from articular cartilage (II): analysis of the glycosaminoglycans in the cellular microenvironment of isolated canine chondrons. Connect Tissue Res. 1990;24:319–330. doi: 10.3109/03008209009152158. [DOI] [PubMed] [Google Scholar]
- 23.Poole CA, Flint MH, Beaumont BW. Chondrons extracted from canine tibial cartilage: preliminary report on their isolation and structure. J Orthop Res. 1988;6:408–419. doi: 10.1002/jor.1100060312. [DOI] [PubMed] [Google Scholar]
- 24.Smith GN, Jr, Hasty KA, Brandt KD. Type XI collagen is associated with the chondrocyte surface in suspension culture. Matrix. 1989;9:186–192. doi: 10.1016/s0934-8832(89)80049-6. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg LC. Structure and function of dermatan sulfate proteoglycans in articular cartilage. Articular Cartilage and Osteoarthritis. New York: Raven Press; 1992. pp. 45–63. [Google Scholar]
- 26.Van der Kraan P, Van den Berg W. Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage. 2012;20:223–232. doi: 10.1016/j.joca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Wei F, Zhou J, Wei X, Zhang J, Fleming BC, Terek R, et al. Activation of Indian hedgehog promotes chondrocyte hypertrophy and upregulation of MMP-13 in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2012;20:755–763. doi: 10.1016/j.joca.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabiri A, Esfandiari E, Hashemibeni B, Kazemi M, Mardani M, Esmaeili A. Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochem Biophys Res Commun. 2012;424:234–238. doi: 10.1016/j.bbrc.2012.06.082. [DOI] [PubMed] [Google Scholar]
- 29.Lee GM, Poole CA, Kelley SS, Chang J, Caterson B. Isolated chondrons: a viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthritis Cartilage. 1997;5:261–274. doi: 10.1016/s1063-4584(97)80022-2. [DOI] [PubMed] [Google Scholar]
- 30.Heydarkhan-Hagvall S, Schenke-Layland K, Dhanasopon AP, Rofail F, Smith H, Wu BM, et al. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials. 2008;29:2907–2914. doi: 10.1016/j.biomaterials.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li WJ, Jiang YJ, Tuan RS. Chondrocyte phenotype in engineered fibrous matrix is regulated by fiber size. Tissue Eng. 2006;12:1775–1785. doi: 10.1089/ten.2006.12.1775. [DOI] [PubMed] [Google Scholar]
- 32.Ye K, Felimban R, Traianedes K, Moulton SE, Wallace GG, Chung J, et al. Chondrogenesis of infrapatellar fat pad derived adipose stem cells in 3D printed chitosan scaffold. PloS One. 2014;9:e99410. doi: 10.1371/journal.pone.0099410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, et al. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406–9414. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Fan J, Becker K, Graff R, Lee G, Francomano C. Comparison of gene expression profile between human chondrons and chondrocytes: a cDNA microarray study. Osteoarthritis Cartilage. 2006;14:449–459. doi: 10.1016/j.joca.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Wang QG, Magnay JL, Nguyen B, Thomas CR, Zhang Z, El Haj AJ, et al. Gene expression profiles of dynamically compressed single chondrocytes and chondrons. Biochem Biophys Res Commun. 2009;379:738–742. doi: 10.1016/j.bbrc.2008.12.111. [DOI] [PubMed] [Google Scholar]
- 36.Shieh A, Athanasiou K. Dynamic compression of single cells. Osteoarthritis Cartilage. 2007;15:328–334. doi: 10.1016/j.joca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Woo KM, Jun JH, Chen VJ, Seo J, Baek JH, Ryoo HM, et al. Nano-fibrous scaffolding promotes osteoblast differentiation and biomineralization. Biomaterials. 2007;28:335–343. doi: 10.1016/j.biomaterials.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Adv Mater. 2009;21:3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 39.Aung A, Gupta G, Majid G, Varghese S. Osteoarthritic chondrocyte–secreted morphogens induce chondrogenic differentiation of human mesenchymal stem cells. Arthritis Rheum. 2011;63:148–158. doi: 10.1002/art.30086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 41.Goldring MB, Otero M. Inflammation in osteo-arthritis. Curr Opin Rheumatol. 2011;23:471. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fermor B, Weinberg J, Pisetsky D, Misukonis M, Fink C, Guilak F. Induction of cyclooxygenase-2 by mechanical stress through a nitric oxide-regulated pathway. Osteoarthritis Cartilage. 2002;10:792–798. doi: 10.1053/joca.2002.0832. [DOI] [PubMed] [Google Scholar]
- 43.Estes B, Fermor B, Guilak F, editors. The influence of interleukin-1 and mechanical stimulation on human adipose derived adult stem cells undergoing chondrogenesis. Transactions of the Orthopaedic Research Society Annual Meeting. 2004 [Google Scholar]
- 44.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 45.Heldens GT, Blaney Davidson EN, Vitters EL, Schreurs BW, Piek E, van den Berg WB, et al. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2011;18:45–54. doi: 10.1089/ten.TEA.2011.0083. [DOI] [PubMed] [Google Scholar]
- 46.Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Research article Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment 2010. doi: 10.1186/ar3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Felka T, Schafer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17:1368–1376. doi: 10.1016/j.joca.2009.04.023. [DOI] [PubMed] [Google Scholar]


