Abstract
Objective(s):
Bone marrow-derived mesenchymal stem cells (BM-MSCs) potentials make them appropriate for cell therapy including ability of differentiation and release of anti-inflammatory cytokines and growth factors secreta. For treatment of azoospermia to induce proliferation and differentiation of germ cells, MSCs transplantation has been introduced. The aim of the present experimental case-control study was to histomorphometric evaluation of the germinal cells in seminiferous tubules of azoospermic rats before and after BM-MSCs allotransplantation.
Materials and Methods:
In the present study, BM-MSCs were isolated from six male rats and confirmed. Their testes also served as intact negative controls. The recipient rats (n=6) were received two doses of 10 mg/kg of busulfan with 21 days interval to induce azoospermia. After cessation of spermatogenesis, the rats were allotransplanted with the BM-MSCs into efferent duct of right testes. Thirty-five days later, the right cell-treated testes were compared to left azoospermic ones.
Results:
Histomorphometric analyses showed that the seminiferous tubules treated with BM-MSCs had normal morphology in comparison with azoospermic testes, which were without germinal layer. In most BM-MSCs-treated seminiferous tubules, spermatogenesis was observed.
Conclusion:
The allotransplanted BM-MSCs could induce spermatogenesis in seminiferous tubules of azoospermic rats.
Keywords: Azoospermia, Bone marrow mesenchymal-stem cell, Busulfan, Cell therapy, Rat
Introduction
About 10-15% of all infertile men and 1% of all men are suffering from obstructive or non-obstructive forms of azoospermia (1). Congenital defects, genetic abnormalities, infectious diseases, endocrine disorders, exposure to gonadotoxins, traumas, varicocele, and medications such as chemotherapy drugs are different causes of non-obstructive azoospermia (2). Azoospermia is treated by hormonal or surgical methods with unsatisfactory outcome (2). On the other hand, by increase of the rate of cancers and negative effects of cancer therapy cytotoxic drugs on spermatogenesis, more efforts were focused to compensate their harmful effects (3). For instance, spermatogonial stem cells in different species can be removed by busulfan, a chemotherapeutic agent (4, 5), through disturbance in DNA replication, but it has no effects on DNA synthesis. It is clear that cytotoxic chemotherapy temporarily or even permanently induces azoospermia. On the other hand, busulfan-treated rats are well-established and excellent recipient animal model for evaluating stem cell activity in testis cell populations (5). Therefore, they were used as recipients in this study.
Nowadays, for treatment of male infertility such as proliferation and differentiation malfunction of germ cells, cell transplantation has been introduced (6). Between various methods of cell therapy, stem cell transplantation for restoring organ or tissue function and structure has become a new therapeutic strategy (7-9). However, various types of stem cells including embryonic stem cells or induced pluripotent stem cells are candidates for stem cell therapy, but mesenchymal stem cells (MSCs) are the safest and easily available. MSCs have been isolated from bone marrow (10), adipose tissue (11, 12), endometrium (13, 14), menstrual blood (15) and dental pulp (16) which all have the potential of being a selected source for MSCs therapy of azoospermia. Accumulated evidences suggest that bone marrow harbors bone marrow MSCs (BM-MSCs), hematopoietic stem cells, endothelial stem cells, and multipotential adult progenitor cells (17, 18). In the current decade, BM-MSCs have been shown to have a greater potential for tissue repair, because of their ability to differentiate into various types of tissues in the appropriate in vivo conditions (9). The second ability of BM-MSCs is growth factor secretion that stimulate function restoration of the resident spermatogonia (7). The last mechanism is merging of BM-MSCs with endogenous seminiferous tubule cells to recover the function by injured tissue (19).
After successful transplantation of spermatogonial stem cells in different species, more investigations are developed to evaluate approach of stem cell therapy for treatment of azoospermia (20). Some species animal models of azoospermia including mice and rats were treated by injection of MSCs into seminiferous or testicular tissue (21-23), by the way, without paying attention to the mechanisms of treatment and MSCs sources, all these animal models showed that MSCs therapy can be beneficial to reduce the side effects of chemotherapies on spermatogenesis. Regarding to this therapeutic effects, the structural effect of treatment with BM-MSCs on the histomorphology of male germinal layer were not evaluated in rat azoospermia model. Therefore, the aim of this study was to histomorphometric evaluation of the germinal layer of seminiferous tubules before and after BM-MSCs allotransplantation in busulfan-induced azoospermic rats.
Materials and methods
Animals
The present study was performed according to the animal research instructions of the Ethical Committee of Shiraz University to minimize suffering during the experimental period. Twelve male Sprague-Dawley rats weighing 250-300 g were kept in polypropylene cages and housed in the Laboratory Animal Center, Shiraz University of Medical Sciences, Shiraz, Iran in temperature-controlled room (20-22 °C) under 12 hr light/dark cycle (7.00-19.00 lightning). The rats were fed with standard commercial chow diet ad libitum and had free access to water. They were divided into two groups of azoospermia and control (n=6). The control groups were applied as cell donors and their left testes were used as negative control group. In the azoospermic group, the left testes of azoospermia-induced rats were treated with BM-MSC and their right testes were served as positive control group.
Isolation of BM-MSCs
Rats of negative control group were euthanized by cervical dislocation after intraperitoneal injection of 100 mg/kg ketamine (Woerden, Netherlands) and 7 mg/kg xylazine (Alfazyne, Woerden, Netherlands) for anesthetizing. Incision was made on the skin and both femurs and their muscular tissues were completely removed. BM-MSCs were isolated from the femurs of rats. Under sterile conditions, both ends of the bone were cut and the bone marrow was flushed out using an insulin syringe filled with Dulbecco’s modified eagle medium (DMEM; Biovet, Bulgaria) supplemented with 1% penicillin streptomycin (Sigma, USA). After bone marrow extraction, cells were cultured and BM-MSCs were isolated by modification of the previous reported method (10). In details, bone marrow was diluted with DMEM, and at 1500 rpm for 5 min was centrifuged. The precipitate was cultured in a 75 cm2 flask containing DMEM supplemented with 10% fetal bovine serum (FBS; Biovet, Bulgaria), 1% L-glutamine (Sigma, USA) and 1% penicillin and streptomycin (Sigma, USA) and transferred into CO2 incubator at 37 °C with 5% CO2 and saturated humidity. The medium was changed after 24 hr and then every 72 hr, to remove the non-adherent cells. Cells were sub-cultured two times to obtain a sufficient number of cells using standard methods of trypsinization. Adherent cells were subcultured when they were 80% confluent after phosphate buffer saline (PBS, Gibco, USA) washing and 5 min treatment of the cells with 0.25% trypsin (Gibco, USA). To inactivate enzyme activity, the same volume of supplemented DMEM media was added. Cell passage was continued until passage 2.
Freezing and thawing of BM-MSCs
To coordinate between azoospermia model preparation and cell isolation and characterization, it was necessary to cryopreserve the isolated cells for further steps of the research. For this purpose, the confluent flasks of BM-MSCs in passage 2 were treated with 0.25% trypsin (Gibco, USA) for 3-4 min and then the enzyme was inactivated by equal amount of supplemented DMEM media. After centrifuging the cell suspension at 1500 rpm for 5 min, the supernatant was removed and the precipitate was suspended in mixture of 50% DMEM media, 40% FBS, and 10% dimethyl sulfoxide (DMSO; MP Bio) at a density of 2×106 viable cells/ml and was aliquoted into sterile plastic labeled cryovials. They were freezed in -20 °C for 1 hr and then in -70 °C for 24 hr, and finally transferred to liquid nitrogen for long-term storage. Whenever BM-MSCs were needed, they were taken out for thawing and culturing. The freezed cryovials were partially thawed in a 37 °C water bath and equal volume of DMEM medium was added. Then, cell suspension was centrifuged for 5 min at 1500 rpm. Removing the supernatant, the precipitated BM-MSCs were cultured in a culture flask as mentioned before. Then, using a hemocytometer, the collected cells after 80% confluency were counted.
Detection of mesenchymal marker on BM-MSCs
Reverse transcription polymerase chain reaction (RT-PCR) was applied to detect the presence of BM-MSCs’ specific marker (CD73) expression and absence of expression of hematopoietic stem cells’ specific marker (CD45). Using the column RNA isolation kit (Denazist-Asia, Iran), total RNA of the BM-MSCs was extracted and by a spectrophotometer its concentration was evaluated. Then, synthesis of cDNA (complementary DNA) from RNA samples was performed using AccuPower Cycle Script RT PreMix Kit (Bioneer, Korea). Briefly, for each reaction 15 μl of total RNA was used and with the DEPC water, the volume had been reached up to 20 μl. Twelve thermal cycles were performed with the following way: primer annealing at 20 °C for 30 sec, cDNA synthesis at 42 °C for 4 min, melting secondary structure and cDNA synthesis at 55 °C for 30 sec and heat inactivation at 95 °C for 5 min. In the next step.1 μl of cDNA template was mixed with forward and reverse primers (CD73 and CD45), PCR buffer, Taq DNA polymerase, dNTPs, MgCl2, and H2O. Then, 20 μl of the mixing in microfuge tubes were transferred to thermocycler (Eppendorf Mastercycler Gradient, Eppendorf, Hamburg, Germany) to be run in 30 amplification cycles; denaturation at 95 °C for 30 sec, annealing at 64 °C for 30 sec, and extension at 72 °C for 30 sec with primary denaturation at 95 °C for 2 min and final extension at 72 °C for 5 min. By gel electrophoresis of PCR products and in 1.5% agarose gel medium, the presence of considered bands was evaluated with the aid of DNA safe stain. Under UV radiation, produced bands were visualized by Gel documentation system (UVtec, Cambridge, UK).
Osteogenic and adipogenic differentiations of BM-MSCs
The BM-MSCs at 90% confluence were cultivated for 3 weeks in DMEM, 15% FBS, 10 mM glycerolphosphate, 200 μM L-ascorbic acid, and 100 nM dexamethasone and the osteogenic differentiation medium was changed biweekly. Then, using alizarin red staining, osteogenic differentiation was evaluated. Briefly, BM-MSCs were fixed for 10 min in 4% paraformaldehyde. After that, cells were incubated in 1% alizarin red and 1% ammonium hydroxide for 20 min at room temperature. Cell cultures were washed with 1 ml dH2O 4 times and 5 min each time replacing the water at each 5 min interval and air-dried. A brilliant red staining was resulted from alizarin red dye binding to calcium ions of mineralized deposits secreted by osteoblasts in cultures (all reagents from Sigma-Aldrich, USA).
The cells at 90% confluence were cultivated for 3 weeks in DMEM, 15% FBS, 100 μM L-ascorbic acid, 0.2 mM L-glutamine, 200 μM indomethacin, and 100 nM dexamethasone and the adipogenic differentiation medium was changed biweekly. Then, using Oil Red O staining, adipogenic differentiation was evaluated based on the generation of oil droplets. Briefly, the BM-MSCs were fixed for 10 min with 10% formalin at room temperature and washed twice with water. Then, Oil Red O stain (6 ml of 0.5% Oil Red O in 4 ml distilled water) was added and incubated for 1 hr at room temperature and the cells were rinsed several times with water (all reagents from Sigma-Aldrich, USA).
Busulfan treatment of rats and BM-MSCs transplantation
Six male rats were induced azoospermia by 10 mg/kg intraperitoneally injection of two doses of busulfan (Busilvex®, Pierre Fabre Medicament, Boulogne, France) with 21 days interval. BM-MSCs allotransplantation was performed 35 days after the last busulfan injection. Suspension of BM-MSCs was mixed with equal volume of sterile trypan blue and was loaded into a 1 ml syringe was attached into the polyethylene tube which was connected to a pulled pipette (Figures 1A and B).
Figure 1.
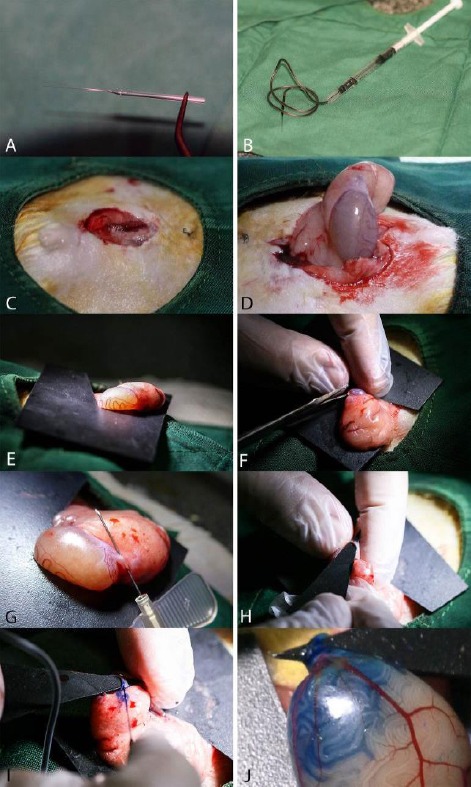
Details of the allotransplantation procedure of bone marrow-derived mesenchymal stem cells (BM-MSCs) in seminiferous tubules of azoospermic rat. A, A pulled glass pipette on the flame with fine tips which diameter is less than efferent duct of rat testis. B, Designed micro-injector consistent of a 1 ml syringe, tube of a 24 gage butterfly needle, and the pre-pulled glass pipette which charged with 106 BM-MSCs were mixed with trypan blue. C, A 2-cm abdominal midline incision is performed. D, The right testis is pulled out by grasping the testicle fat pad. E, A hard black plastic square with v-shape triangular cuts is used for complete fixation of testis. F, Cutting the inferior ligament of the epididymis to facilitate achieving the efferent duct of rat testis. G, Dissecting the efferent duct by a butter fly needle from the covered adipose tissue. H, To make a good contrast between the translucent efferent duct and the fat tissue and the membrane around it, a hard black plastic triangle was inserted underneath the efferent duct. I, Injection of BM-MSCs suspension into efferent duct of rat testis. J, Seminiferous tubules were partially filled with mixture of BM-MSCs suspension
After anaesthetizing of the rats by ketamine and xylazine, their abdominal area was surgically prepared in dorsal recumbency and a 2-cm midline abdominal incision was made to expose the peritoneal cavity (Figure 1C). Using an iris forceps, the fat pad attached to the right testis and epididymis was gently pulled to exteriorize the testis under a stereomicroscope (Model SZN, Optika, Italy; Figure 1D). A thin sterile black plastic card with a v-like cut as a testis holder was put underneath the testis (Figure 1E). Cutting the inferior ligament of the epididymis (Figure 1F), the efferent duct that connects the epididymis to the testis was gently dissected out from surrounding fat tissue using a sharp needle (Figure 1G). By insertion of a triangular black plastic card underneath the duct, a good contrast between the fat tissue and membrane around translucent efferent duct was formed (Figure 1H). The pipette was carefully inserted into the efferent duct and a few mm gently threaded toward the testis and was avoided moving the injection pipette (Figure 1I). Then, the blue suspension of BM-MSCs (106 cells in 100 μL) flowed into the rete testis by gently depressing the syringe plunger to fill almost all seminiferous tubules (Figure 1J). The abdominal wall and the skin were sutured after returning of the testis into abdominal cavity. The untreated left testis with BM-MSCs was served as positive control azoospermia.
Histomorphometric analysis of testes
Thirty-five days after BM-MSCs injection, animals were euthanized with ether and their testes were removed and fixed in a 10% formalin buffer solution. After fixation, ethanol and xylene were used for dehydration step and testes were implanted in paraffin. Testes were embedded in paraffin wax were sectioned at thicknesses of 5 μm. For each testis five vertical sections from the polar and the equatorial regions were sampled. Then hematoxylin-eosin staining was performed on sections and they were examined for any spermatogenic activity by light microscope. For this purpose, all tubules were evaluated in one section per animal for presence of spermatogonia, spermatocytes and spermatids.
Then for evaluation of histomorphometric indices, in 10 circular transverse sections of tubules each from a different region of the testis, outer, inner, and total diameters were measured according to Panahi et al (5). The mean seminiferous tubule diameter (D) was derived by taking the average of two diameters, D1 and D2 at right angles. The cellular and luminal areas and cross sectional area of the tubules were also determined. Cross-sectional area and luminal area (A) of the seminiferous tubules were calculated using the equation A=πD2/4, where π is equivalent to 3.14 and D is the mean total diameter or luminal diameter of seminiferous tubules, respectively. The cellular area was determined by subtraction of luminal area from cross-sectional area.
The testes were also rated for its spermatogenic potential according to the modified spermatogenic index on a modified scale of 0 to 7 (5). The index was based on the presence of the spermatogenic cells throughout the tubules and included number of types of cells, cell layers, and the appearance of late spermatids in the tubules. The index criteria were as follows: 0, no germinal layer cells; 1, presence of only spermatogonia; 2, appearance of spermatogonia and spermatocytes; 3, appearance of spermatogonia, spermatocytes and early round spermatids and up to <25 late spermatids per tubule; 4, appearance of spermatogonia, spermatocytes, and spermatids with 25-50 late spermatids per tubule; 5, appearance of spermatogonia, spermatocytes, and spermatids with 50-75 late spermatids per tubule; 6, appearance of spermatogonia, spermatocytes, and spermatids and up to 75-100 late spermatids per tubule; and 7, all cell types present and >100 late spermatids per tubule.
Statistical analysis
Normality of all data of histomorphometric indices of seminiferous tubules were tested by Kolmogorov-Smirnov test. Means and standard error (SE) of the data were analyzed by one-way ANOVA (SPSS for Windows, version 11.5, SPSS Inc, Chicago, Illinois) and Tukey post hoc test. Mann-Whitney U test was applied to compare the spermatogenesis index of seminiferous tubules between groups. The P-value ≤0.05 was considered to be statistically significant. Group means and their SEM were reported in the graphs which are designed by GraphPad Prism (version 5.01 for Windows, GraphPad software Inc., San Diego, CA, USA).
Table 1.
Sequences of RT-PCR primers used to quantify the expression of bone marrow-derived mesenchymal stem cells' specific marker (CD73) and hematopoietic stem cells' specific marker (CD45) in rat
| Primer | Primer sequence | Amplicon length (bp) |
|---|---|---|
| CD73-F | TGCATCGATATGGCCAGTCC | 208 |
| CD73-R | AATCCATCCCCACCGTTGAC | |
| CD45-F | CCAAGAGTGGCTCAGAAGGG | 450 |
| CD45-R | CTGGGCTCATGGGACCATTT |
Results
Confirmation of BM-MSCs’ characters
After BM-MSCs attached to the culture flasks, they showed a fibroblast-like, spindle-shaped morphology. BM-MSCs started to proliferate 3-4 days after incubation until reaching an 80% confluence (Figure 2A). MSC marker of rats’ BM-MSCs analyzed using a RT-PCR assay. Figure 2B displays presence of MSC marker (CD73) and absence of cell surface marker of hematopoietic stem cell (CD45). To further confirmation of MSC’s characters of isolated cells, the osteogenic and adipogenic differentiation capacity of BM-MSCs of rats were performed. The BM-MSCs were differentiated toward osteoblasts after culture in osteogenic differentiation medium and were verified by positive staining with alizarin red staining (Figure 2C). The presence of intracellular lipid droplets in BM-MSCs were treated by the adipogenic differentiation medium, were confirmed by Oil Red O staining (Figure 2D).
Figure 2.
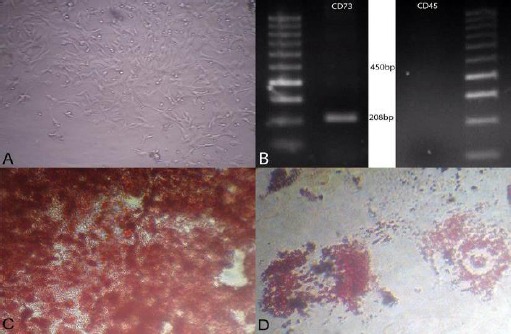
A, Morphological and phenotypic characteristics of rat bone marrow-derived mesenchymal stem cells (BM-MSCs). Stem cells exhibited large, flattened and fibroblast-like morphology (×40). B, Agarose gel electrophoresis of products of reverse transcriptase polymerase chain reaction (RT-PCR) revealing the presence of specific mesenchymal marker (CD73) compared with absence of specific hematopoietic marker (CD45) of rat BM-MSCs. C, BM-MSCs cultivated in osteogenic medium and stained with alizarin red (×100) and D, in adipogenic medium and were stained with Oil Red O at day 21 after induction (×100)
Histological assessment of spermatogenesis
Normal intact rats had more condensed germinal epithelium (Figure 3A) and all of seminiferous tubules were filled by spermatozoa (Figure 3B). After the treatment with busulfan, the seminiferous tubules of the testes were not treated with BM-MSCs were empty and their spermatogenesis process was disrupted (Figure 3C). Sections in positive control group showed only presence of Sertoli cells in the seminiferous tubules. Histological examinations of). testes in busulfan treatment group revealed some degenerative changes such as seminiferous tubular atrophy and degeneration of germinal epithelium in most of the seminiferous tubules. The large vacuolated lumen occupied seminiferous tubules and the atrophic germinal epithelium covered peripheral zone of seminiferous tubules as thin bands (Figure 3D). In the seminiferous tubules with BM-MSCs transplantation, most of the tubules appeared to be filled up with germinal cells (spermatogonia, primary spermatocytes, spermatids and sperms) (Figure 3F). There were some tubes with lack or weak germinal layer regeneration in cell therapy group.
Figure 3.
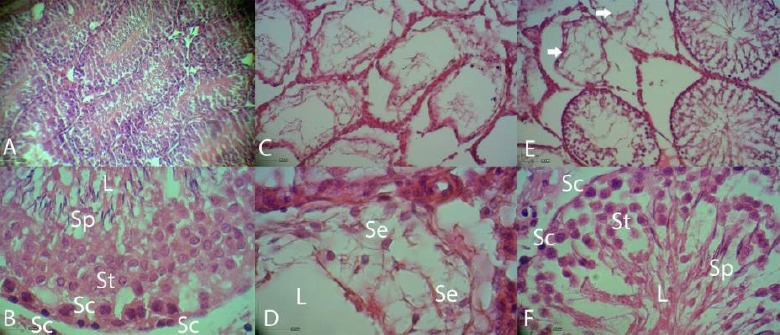
Histopathologic evaluation of busulfan treated rats before and after treatment with bone marrow-derived mesenchymal stem cells (BM-MSCs). A and B, Sections of seminiferous tubules of normal control rat. Tubules have condensed spermatogenic epithelium. C and D, Busulfan treated azoospermic control seminiferous tubules. The seminiferous tubules were partially empty and vacuolated spaces without germinal layer cells indicating the absence of spermatogenesis. E, Sections of seminiferous tubules of treated rat with BM-MSCs. Most of tubules appeared to have spermatogenic cells. Arrows show the untreated seminiferous tubules. F, Presence of different kind of germinal epithelial cells in stem cell-treated seminiferous tubules. Larger spaces between germinal layer cells can be compared with normal tubules. Abbreviations: L, lumen; Se, Sertoli cell; Sc, spermatogonia; S1, spermatocytes; St, spermatid and Sp, spermatozoa. Hematoxylin and eosin staining.
Histomorphometric findings
Histomorphometric evaluations showed the lumen diameter and area of the tubules in rats with BM-MSCs transplantation were less than the azoospermic rats and more than normal ones (P<0.05; Figures 4A and B). The cellular diameter and cellular area of germinal epithelium in the seminiferous tubules in rats with BM-MSCs transplantation were more than azoospermic group (P<0.05; Figures 4B and 4C). However, cellular area in rats with BM-MSCs transplantation was more than normal control rats (P<0.05), but cellular diameters of these two groups were not statistically different (P>0.05). The total diameter and cross sectional area of seminiferous tubules in rats with stem cell transplantation were more than azoospermic rats and also in both groups were more than the normal control group (P<0.05; Figures 4D and 4E). In rats with stem cell transplantation, spermatogenesis index of seminiferous tubules was more than azoospermic rats, and in both groups were less than the normal control rats (P<0.05; Figures 5).
Figure 4.
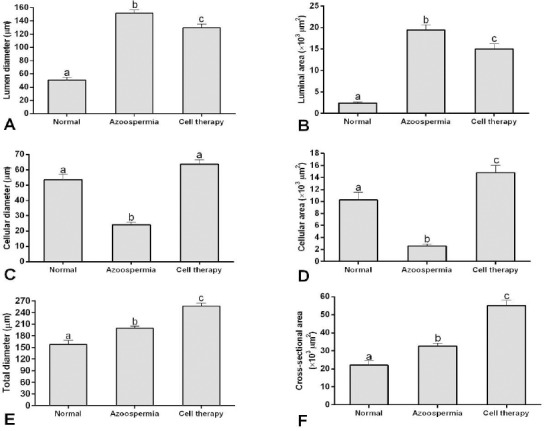
Mean and SEM of histomorphometric indices of seminiferous tubules in busulfan-induced azoospermic testis treated with bone marrow-derived mesenchymal stem cells (cell therapy) in comparison with busulfan treated testes (azoospermia) and intact testis (normal) in rat. A, Lumen diameter (μm), B, Luminal area (μm2), C, Cellular diameter (μm), D, Cellular area (μm2), E, Total diameters (μm), F, Cross sectional area of the tubules (μm2). a, b, c different superscript letters show significant differences between groups (P<0.05)
Figure 5.
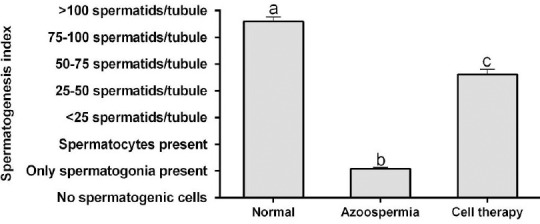
Mean and SEM of spermatogenesis index of seminiferous tubules in busulfan-induced azoospermic testis treated with bone marrow-derived mesenchymal stem cells (cell therapy) in comparison with busulfan treated testes (azoospermia) and intact testis (normal) in rat. a, b, c different superscript letters show significant differences between groups (P<0.05)
Discussion
In azoospermic treatment, damaged somatic environment of the testis is one of limiting step and the most problem for testicular function recovery. Azoospermic patients with damaged testis can not be treated with spermatogonial stem cell transplantation (24). In the present study, the damaged azoospermic seminiferous tubules with busulfan in the rat were allotransplanted by BM-MSCs, which induced spermatogenesis. To obtain advanced differentiated spermatozoa, combination of in vitro differentiation and transplantation was performed (25). Several in vivo studies have been performed to evaluate the differentiation potential of MSCs into spermatozoa in rats and mice animal models. Furthermore, intra-seminiferous tubule injection (9, 26) and intra-testicular injection of BM-MSCs (27) were also found to induce spermatogenesis in seminiferous tubules of azoospermic rats. In addition, Mehrabani et al (28) and Cakici et al (21) demonstrated that adipose tissue-derived MSCs (AT-MSCs) could lead to recovery of fertility in the busulfan-treated azoospermia rat model. Recently, Chen et al (29) showed that sperm differentiation potential of transplantation of human umbilical cord MSCs into seminiferous tubule of immunodeficient mice. In the other rodent species, hamster, intra-seminiferous tubule injection of BM-MSCs activated spermatogenesis in busulfan-induced azoospermic model (30). It seems BM-MSCs were found effective in treating animal model of azoospermia.
Furthermore, although donor and recipient animals were not syngeneic and despite of immunocompetitive character of donor rats, allotransplantation of BM-MSCs in seminiferous tubules of azoospermic rats had therapeutic effects. Consistent with our findings, allogeneic BM-MSCs trans-differentiated into spermatogenic-like-cells and enhanced endogenous fertility recovery in rat by injection into seminiferous tubules of recipient with busulfan induced (9, 26, 27) and testicular torsion (31) azoospermia rats. It is shown that BM-MSCs are not only hypo-immunogenic but also produce immunosurveillance or immunosuppression upon transplantation, therefore they are suitable for allogeneic transplantation (32). In mice after testis rupture, intravenous allogeneic transfusion of BM-MSCs showed immunomodulatory effects on antisperm antibody production (33). Moreover, immunological tolerance of Sertoli cells (34) can cause survival and protection of the allotransplanted BM-MSCs from post-transplantation inflammatory or immune reaction. Except the mentioned items, commenting on this topic, three other points can be noticed in allotransplantation of BM-MSCs in seminiferous tubules. The first one is the immune privilege character of seminiferous tubules. Certain sites of some tissues in immunocompetent wild type mammalians are immune privilege including the eye, the brain, the pregnant uterus and the testicles (35). The second point is the pharmacologic character of busulfan. Busulfan is a chemotherapeutic agent that is mostly used in low doses and for a long time in treatment of chronic myeloid leukemia (36) and before any allotransplantation of hematopoietic cells (37). Therefore, allotransplantation of MSCs can be considered as a method of choice for azoospermia cell therapy.
On the other hand, morphometric findings of the present study showed a new feature of azoospermia cell therapy. Total diameter and cross sectional area of the tubules in rats of the normal control group was less than before and after transplantation. Moreover, the lumen diameter and area of the tubules in rats with BM-MSCs transplantation were more than the normal rats and less than the azoospermic rats. These morphometric changes can be explained that some tubes collapsed after induction of azoospermia by the intratubular hydrostatic pressure and even after cell therapy, the tubular structures of seminiferous which were treated in BM-MSCs-treated rats and untreated ones in azoospermia groups had more space to grow. The intratubular hydrostatic pressure of rats seminiferous tubules are 3.0±0.2 mmHg (38). The contraction ability of myofibroblast cells in peritubular layer may be reduced by increase of total diameter which may cause observed decrease of spermatozoa in epididymis of BM-MSCs treated rats. This phenomenon needs to be investigated in future. In addition, cellular area of the tubules in rats with BM-MSCs transplantation was more than azoospermic and normal control groups. These were caused by increasing total diameter of seminiferous tubules in treated animals and increase of Sertoli cells interval spaces caused more places for cell proliferation. Interestingly, however, cellular diameter of azoospermic rats was less than normal control group, but cellular area of those two groups were not different. In addition, increase of cross sectional area of tubules in azoospermic rats and moreover, greater space for Sertoli cells which were not affected by busulfan leads the germinal layer cells to cover a more two-dimensional area than normal rats.
Furthermore, in the present study, in rats with BM-MSCs transplantation, spermatogenesis index of seminiferous tubules was statistically less than normal control rats. Seminiferous tubules provide cyclic and dynamic regulation of spermatogenesis, and Sertoli cells prepare a microenvironment that induces spermatogonial proliferation and differentiation (39). Our findings also demonstrated that BM-MSCs may ameliorate reconstitution of the tubular microenvironment which helps remained inactivated spermatogonia to proliferate in the host seminiferous tubules but during the recovery period function of the germinal layer could not reach to normal condition.
Conclusion
In the present study, BM-MSCs interactions in the rat testis were evaluated functionally by allotrans-plantation of these cells into seminiferous tubules of azoospermic rats. Injected BM-MSCs induced new formation of testicular germinal cells and this confirms that BM-MSCs therapy could help the partial repair of pathological changes in seminiferous tubules of azoospermic rats. This finding raises the possibility of using BM-MSCs to treat azoospermia in human.
Conflict of Interest
There is no conflict of interest.
Acknowledgment
The authors would like to appreciate the kind of Stem Cell and Transgenic Technology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran for laboratory cooperation. This study was financially supported by Vice Chancellor of Research, School of Veterinary Medicine, Shiraz University, Shiraz, Iran as a DVM thesis with grant# thesis1443, February 2015.
References
- 1.Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics. 2013;68:27–34. doi: 10.6061/clinics/2013(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berookhim BM, Schlegel PN. Azoospermia due to spermatogenic failure. Urol Clin North Am. 2014;41:97–113. doi: 10.1016/j.ucl.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Dohle GR. Male infertility in cancer patients: review of the literature. Int J Urol. 2010;17:327–331. doi: 10.1111/j.1442-2042.2010.02484.x. [DOI] [PubMed] [Google Scholar]
- 4.Panahi M, Karimaghai N, Rahmanifar F, Tamadon A, Vahdati A, Mehrabani D, et al. Stereological evaluation of testes in busulfan-induced infertility of hamster. Comp Clin Pathol. 2014;24:1051–1056. [Google Scholar]
- 5.Panahi M, Keshavarz S, Rahmanifar F, Tamadon A, Mehrabani D, Karimaghai N, et al. Busulfan induced azoospermia: Stereological evaluation of testes in rat. Vet Res Forum. 2015;6:273–278. [PMC free article] [PubMed] [Google Scholar]
- 6.O’Brien KLF, Varghese AC, Agarwal A. The genetic causes of male factor infertility: a review. Fertil Steril. 2010;931:1–12. doi: 10.1016/j.fertnstert.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Leatherman J. Stem cells supporting other stem cells. Front Genet. 2013;4:257–262. doi: 10.3389/fgene.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfister O, della Verde G, Liao R, Kuster GM. Regenerative therapy for cardiovascular disease. Transl Res. 2014;163:307–320. doi: 10.1016/j.trsl.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Liu X, Peng J, He D, Lin T, Zhu J, et al. Potential spermatogenesis recovery with bone marrow mesenchymal stem cells in an azoospermic rat model. Int J Mol Sci. 2014;15:13151–13165. doi: 10.3390/ijms150813151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asadi-Yousefabad S-L, Khodakaram-Tafti A, Dianatpour M, Mehrabani D, Zare S, Tamadon A, et al. Genetic evaluation of bone marrow-derived mesenchymal stem cells by a modified karyotyping method. Comp Clin Pathol. 2015;24:1361–1366. [Google Scholar]
- 11.Shaterzadeh-Yazdi H, Mehrabani D, Khodakaram-Tafti A, Dianatpour M, Zare SH, Tamadon A, et al. Osteogenic potential of subcutaneous adipose-derived stem cells in a rabbit model. Online J Vet Res. 2015;19:436–445. [Google Scholar]
- 12.Lin F. Adipose tissue-derived mesenchymal stem cells: a fat chance of curing kidney disease? Kidney Int. 2012;82:731–733. doi: 10.1038/ki.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrabani D, Rahmanifar F, Mellinejad M, Tamadon A, Dianatpour M, Zare S, et al. Isolation, culture, characterization, and adipogenic differentia-tion of heifer endometrial mesenchymal stem cells. Comp Clin Pathol. 2014;24:1159–1164. [Google Scholar]
- 14.Tamadon A, Mehrabani D, Zarezadeh Y, Rahmanifar F, Dianatpour M, Zare S. Caprine endometrial mesenchymal stromal stem cell: multi-lineage potential, characterization and growth kinetics in breeding and anestrous stages. Vet Med Int. 2016 doi: 10.1155/2017/5052801. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrabani D, Bahrami Nazarabadi R, Dianatpour M, Vahdati A, Tamadon A, Kasraeian M, et al. Growth kinetics, characterization and plasticity of human menstrual blood stem cells. Iran J Med Sci. 2016;41:132–139. [PMC free article] [PubMed] [Google Scholar]
- 16.Mahdiyar P, Zare S, Robati R, Dianatpour M, Torabi K, Tamadon A, et al. Isolation, culture, and characterization of human dental pulp mesenchymal stem cells. Int J Pediatr. 2014;2:44. [Google Scholar]
- 17.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208:421–428. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansour A, Abou-Ezzi G, Sitnicka E, Jacobsen SE, Wakkach A, Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goossens E, Van Saen D, Tournaye H. Spermato-gonial stem cell preservation and transplantation: from research to clinic. Hum Reprod. 2013;28:897–907. doi: 10.1093/humrep/det039. [DOI] [PubMed] [Google Scholar]
- 21.Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, Isık A, et al. Recovery of fertility in azoospermia rats after injection of adipose-tissue-derived mesenchymal stem cells: the sperm generation. Bio Med Res Int. 2013;2013:529589. doi: 10.1155/2013/529589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013;11:537–544. [PMC free article] [PubMed] [Google Scholar]
- 23.Moreno I, Míguez-Forjan JM, Simón C. Artificial gametes from stem cells. Clin Exp Reprod Med. 2015;42:33–44. doi: 10.5653/cerm.2015.42.2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easley IV CA, Simerly CR, Schatten G. Stem cell therapeutic possibilities: future therapeutic options for male-factor and female-factor infertility? Reprod Biomed Online. 2013;27:75–80. doi: 10.1016/j.rbmo.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Hu H-L, Li P, Yang S, Zhang W, Ding H, et al. Generation of male germ cells from induced pluripotent stem cells (iPS cells): an in vitro and in vivo study. Asian J Androl. 2012;14:574–579. doi: 10.1038/aja.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahkook SA, Atwa A, Shahat M, Mansour AM, Bakry S. Mesenchymal stem cells restore fertility in induced azoospermic rats following chemotherapy administration. J Reprod Infertil. 2014;5:50–57. [Google Scholar]
- 27.Monsefi M, Fereydouni B, Rohani L, Talaei T. Mesenchymal stem cells repair germinal cells of seminiferous tubules of sterile rats. Iran J Reprod Med. 2013;11:537. [PMC free article] [PubMed] [Google Scholar]
- 28.Mehrabani D, Hassanshahi MA, Tamadon A, Zare S, Keshavarz S, Rahmanifar F, et al. Adipose tissue-derived mesenchymal stem cells repair germinal cells of seminiferous tubules of busulfan-induced azoospermic rats. J Hum Reprod Sci. 2015;8:103–110. doi: 10.4103/0974-1208.158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Tang QL, Wu XY, Xie LC, Lin LM, Ho GY, et al. Differentiation of human umbilical cord mesenchymal stem cells into germ-like cells in mouse seminiferous tubules. Mol Med Rep. 2015;12:819–828. doi: 10.3892/mmr.2015.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamadon A, Mehrabani D, Rahmanifar F, Raayat Jahromi A, Panahi M, Zare S, et al. Induction of spermatogenesis by bone marrow-derived mesenchy-mal stem cells in busulfan-induced azoospermia in hamster. Int J Stem Cells. 2015;8:134–145. doi: 10.15283/ijsc.2015.8.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabbaghi MA, Bahrami AR, Feizzade B, Kalantar SM, Matin MM, Kalantari M, et al. Trial evaluation of bone marrow derived mesenchymal stem cells (MSCs) transplantation in revival of spermatogenesis in testicular torsion. Middle East Fertil Soc J. 2012;17:243–249. [Google Scholar]
- 32.Bibber B, Sinha G, Lobba ARM, Greco SJ, Rameshwar P. A review of stem cell translation and potential confounds by cancer stem cells. Stem Cells Int. 2013;2013:241048. doi: 10.1155/2013/241048. pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aghamir SMK, Salavati A, Yousefie R, Tootian Z, Ghazaleh N, Jamali M, et al. Does bone marrow-derived mesenchymal stem cell transfusion prevent antisperm antibody production after traumatic testis rupture? Urology. 2014;84:82–86. doi: 10.1016/j.urology.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139:495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]
- 35.Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol Cell Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 36.Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:368–376. doi: 10.1182/asheducation-2010.1.368. [DOI] [PubMed] [Google Scholar]
- 37.Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophospha-mide-based regimens in pediatric hematopoietic cell transplantation: Maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014;20:345–353. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 38.Pholpramool C, Triphrom N, Din-Udom A. Intraluminal pressures in the seminiferous tubules and in different regions of the epididymis in the rat. J Reprod Fertil. 1984;71:173–179. doi: 10.1530/jrf.0.0710173. [DOI] [PubMed] [Google Scholar]
- 39.Garcia T, Hofmann M. Regulation of germ line stem cell homeostasis. Anim Reprod. 2015;12:35–45. [PMC free article] [PubMed] [Google Scholar]


