Abstract
Objective(s):
Nepeta binaludensis Jamzad (Lamiaceae) has been used in folk medicine of Iran to cure various diseases. The plant is an endemic species to the country that has recently been identified in Razavi Khorasan province. To evaluate the antioxidant and anti-melanogenesis of N. binaludensis, in this study the inhibitory activity of different extracts of N. binaludensis in murine melanoma B16F10 cells is investigated.
Materials and Methods:
The effects of petroleum ether, dichloromethane, ethyl acetate, and methanol extracts isolated from the plant on melanogenesis in B16 melanoma cells were investigated. To assess the inhibitory effects of this plant on melanogenesis, various assays were used including cytotoxicity, inhibition of mushroom tyrosinase and cellular tyrosinase, determination of melanin content, the effect of extracts on reactive oxygen species and western blot analysis of proteins involved in melanogenesis process.
Results:
The content of melanin and the activity of tyrosinase were significantly reduced with different extracts of N. binaludensis in cells. Reactive oxygen species was also significantly decreased following the treatment of cell with the mentioned extracts, while a resazurin assay showed no cytotoxicity. Furthermore, we have found that the plant decreased the amount of tyrosinase and microphthalmia-associated transcription factor proteins, which verify the role of suppression of microphthalmia-associated transcription factor protein in melanogenesis inhibition.
Conclusion:
Taken together the data indicate that N. binaludensis has inhibitory activity on melanin synthesis with no cytotoxic effects in B16 melanoma cells. Therefore, it merits future investigations to apply as whitening agent in hyperpigmentation.
Keywords: Lamiaceae, Melanogenesis, Melanoma B16F10, Microphthalmia-associated, Nepeta binaludensis, Tyrosinase, Transcription factor
Introduction
The synthesis and distribution of melanin play important roles in mammalian skin pigmentation. Melanin is synthesized in melanin-containing granules, namely the melanosomes of melanocytes. Enzymes such as tyrosine, tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP-2) regulate melanogenesis (1-3). The two initial steps of melanogenesis process, the hydroxylation of tyrosine to 3, 4-dihydroxyphenylalanine (DOPA) and hydroxylation of DOPA to DOPAquinone are catalyzed by tyrosinase. TRP-2 catalyzes the rearrangement of DOPAchrome to 5, 6-dihydroxyindole- 2-carboxylic acid (DHICA) and the oxidation of DHICA to indole-5,6-quinone-2-carboxylic acid is catalyzed by TRP-1. Melanin production plays an important role in preventing UV irradiation and is mediated by various signaling pathways (4). The major pathway in un-dependent stimulation of melanin production is supposed to be a cAMP-dependent pathway (5). UV exposure of keratinocytes in the skin causes the production and release of α-melanocyte-stimulating hormone (α-MSH), a peptide hormone which binds to the melanocortin 1 receptor, activating adenylate cyclase via G proteins increase the levels of intracellular cAMP (6). Then, protein kinase A, which phosphorylates the transcription factor cAMP response element-binding protein at Ser133 and increases the expression of microphthalmia-associated transcription factor (MITF), is activated by cAMP. It is believed that MITF, which is characterized by an essential basic helix-loop helix leucine zipper structure, regulates melanocyte pigmentation, proliferation, and survival (2). After binding with the M-box in the promoter regions of the genes, MITF mediates the transcriptional activation of pigmentation genes, such as tyrosinase, TRP-1, and TRP-2 (Figure 1) (2, 3). Excessive melanin biosynthesis causes several skin disorders, including melanoma, melasma, lentigines, freckles, nevus, and age spots. Increased numbers of melanocytes or activity of melanogenic enzymes cause epidermal and dermal hyperpigmentation (7). Chronic inflammation, ultraviolet light, and abnormal α-melanocyte stimulating hormone (α-MSH) release are among the most important factors causing these disorders (8, 9). Inhibition of tyrosinase activity is related to anti-melanogenesis, and many tyrosinase inhibitors such as kojic acid, arbutin (10), and linoleic acid (11) are used in the treatment of hyperpigmentation. However, many of the skin-whitening agents exhibit toxicity toward melanocytes and produce adverse side effects.
Figure 1.
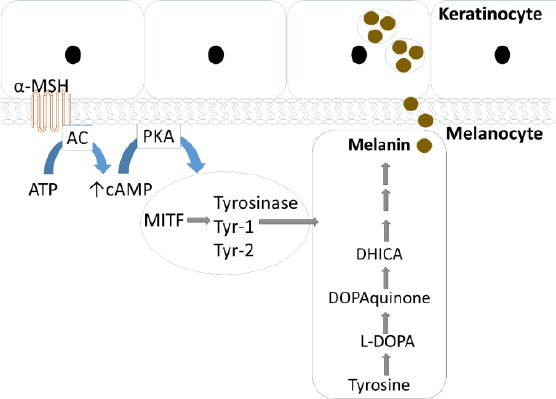
Schematic representation of melanin synthesis. Melanin is synthetized in melanocytes in response to up regulation of cAMP after α-melanocyte-stimulating hormone (α-MSH) secretion from keratinocytes and activation of melanocortin 1 receptor (MC1R). Increase in expression of microphthalmia-associated transcription factor (MITF) activates expression of enzymatic machinery including tyrosinase and tyrosinase-related protein 1 and 2 (Tyrp1 &2), which regulate the synthesis of melanin within melanosomes. After conversion of tyrosine to melanin it is then translocate to melanosomes in epidermal keratinocytes
Nepeta is a genus comprising about 250 species of annual or perennial shrubs of under the flowering plant family Lamiaceae, to 1.2 m high, found mostly in Europe and Asia as well as North Africa (12, 13). There are about 70 species of this genus found in Iran which 38 species of them are endemic to the country with diverse pharmacological activities (14-16). In this report, we focused on Nepeta binaludensis which is endemic to Iran (17). In Iran, different species of Nepeta have been used traditionally as anti-convulsant, anti-septic, anti-bacterial, diuretic, expectorant, anti-tussive, anti-asthmatic and febrifuge activities. In spite of widespread medicinal properties of Nepeta plant genus, their traditional medicinal uses are not documented on scientific research or investigations, and other therapeutic effects have not been identified. N. binaludensis is distributed in limited area in Binalud Mountains, Razavi Khorasan Province in Northeast of Iran. N. binaludensis grows in habitats at altitude of 2300-2700 m with an annual rainfall ranging 350-370 mm (17-19). In traditional medicine, the plant is used for treatment of different diseases such as respiratory disorders, asthma, common cold, headache, migraine, colic, digestive disorders, rheumatism, and cardio vascular disorders (18).
Recently, studies have focused on discovering new drugs from natural resources to cure skin diseases such as melanoma and introduce whitening products duo to their minimal side effects and more potency. Many of these studies focused on tyrosinase activity and expression levels of factors involving in the melanogenesis process, and there are few articles investigated the intracellular signal transduction cascade.
Many skin disorders are caused by oxidants such as reactive oxygen species, thus discovering drugs with antioxidant activities as well as anti-tyrosinas effects seems necessary. Antioxidants by scavenging free radicals improve health of the body. In recent years, although plenty of synthetic drugs are proposed for the treatment of skin diseases, the observed side effects have stopped their progression (20). Thus, scientists are searching for bioactive compounds in natural products. In several studies, the antioxidant activity of the plants belonging to Nepeta genus have been reported. In some investigations polyphenolic compounds are reported as antioxidant (21, 22), while in others the essential oils extracted from this plant are proposed as bioactive components (23, 24). In addition, the antioxidant activity of the phenolic is well accepted (25) (26, 27). In this study, we have studied the anti-melanogenesis effect of methanol extract of N. binaludensis and different fractions of it on cultured murine B16F10 melanoma cells.
Materials and Methods
Chemicals and Reagents
Resazurin from Sigma (Saint Louis, MO, USA); RPMI-1640 and FCS from PAA; β-actin and anti-rabbit IgG and HRP linked antibody from Cell Signaling technology (Boston, USA); Tyrosinase (H-109) and MITF (H-50) rabbit polyclonal antibody from Santa Cruz Biotechnology, Inc. (Dallas, Texas 75220 USA) and Western blotting detection reagent from Bio-RaD (USA); α-melanocyte stimulating hormone, 3,4-dihydroxy-L-phenylalanine, mushroom tyrosinase, protease inhibitor cocktail, phosphatase inhibitor cocktail, kojic acid, phenylmethylsulfonyl fluoride and QuantiPro BCA Assay Kit from Sigma (Steinheim, Germany); all solvents as analytical grade were purchased from Dr Mojallali Lab (Tehran, Iran).
Plant materials
The aerial parts of N. binaludensis were collected in June 2013 at attitude 2000-3000 m from north of Binalud mountains, Razavi Khorassan province, Iran. Voucher specimens have been identified in the Herbarium of Faculty of Pharmacy, Mashhad Medical University, and deposited under Accession No. 13060. The air dried aerial parts were powdered and then applied in the following process.
Extraction of plant material
Plant materials were extracted with pure methanol for 24 h by percolation method at room temperature. The whole methanol extract was filtered and the solvent was evaporated under reduced pressure at 40-45 °C to afford a crude methanol extract. This extract was then suspended in 95% methanol and successively partitioned between petroleum ether, dichloromethane, ethyl acetate, normal n-butanol, and water. The extracts were evaporated under reduced pressure (Rotary evaporator) until they were dried and then stored at -20°C until use. To prepare different concentrations, a stock solution (50 mg/ml) of each extract was made at first. For this purpose, the extracts were dissolved in dimethylsulfoxide (DMSO) and then was subjected to cytotoxic and apoptosis assays. The concentration of DMSO in sample test was lower than 0.01%.
Cells and cell culture
B16F10 melanoma cells, purchased from Pasteur Institute of Iran (Tehran), were maintained as a monolayer culture in Roswell Park Memorial Institute medium (RPMI 1640; PAA, Austria) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, USA), 100 units/ml of penicillin, 0.1 mg/ml of streptomycin (antibiotic-antimycotic; PAA, Austria) in a humidified atmosphere containing 5% CO2 in air at 37°C. The culture medium was changed every 2 d. Kojic acid was used as positive control and cell containing medium as blank.
Cell viability assay
Resazurin (Sigma, MO, USA), is a cell health indicator by using the reducing power of live cells and convert to resorufin. The reduction occurs in live cytosol of the cell. Resazurin is a non-toxic cell permeable compound, blue in color and virtually non-fluorescent but resorufin is red in color and highly fluorescent. Resazurin in viable cells continuously convert to resorufin, this change increases the total fluorescence and color of the media surrounding cells (28).
B16F10 murine melanoma cells were seeded onto 96-well dishes at a density of 104 cells per well. Then resazurin (14 mg/dl; 20 μl) was added to each well after 48 h incubation writh 50 μg/ml of each extracts. The absorbance was measured at 570 and 600 nm in a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek, Winooski, USA) after 4 hr incubation at 37 °C. Cells incubated only with 0.05% of DMSO were used as a negative control and culture medium was used as background. All experiments were performed in triplicate.
Measurement of melanin content
The melanin content was measured by a modification of the method described by Hosoi et al (29). B16F10 melanoma cells were seeded at a density of 5×104 cells per dish in 96-well culture plates and cultivated by the method described above. The concentration of 50 μg/ml of each extract was added to the medium and incubated further for 24 h. Then, the medium was removed, and the cells were washed twice with phosphate-bufferd saline (PBS) and harvested by trypsinization. The harvested cells were pelleted and the cell membrane was dissolved in Triton X-100. The purified melanin was dissolved in 2 M NaOH for 30 min at 100°C. The absorbance was measured at 405 nm and melanin content was compared with control untreated cells.
Mushroom tyrosinase activity assay
Murine B16F10 melanoma cells were seeded onto 96-well dishes at a density of 104 cells per dish and incubated as described above for 24 h. After incubation, the extracts were added at 50 μg/ml, kojic acid was used as positive control and cell containing medium as negative control, and further incubated for 24 hr. 160 μl of 5 mM L-DOPA (in 100 mM sodium phosphate buffer pH 6.8) was added to the wells plus 20 μl of mushroom tyrosinase and shacked for 5 min. After 30 min; the absorbance was measured at 475 nm by ELISA Reader.
Cellular tyrosinase activity assay
Tyrosinase activity was analyzed by spectrophotometry following the oxidation of DOPA to DOPAchrome. B16F10 melanoma cells (5×104 cells/well) were plated in 96-well dishes and incubated for 24 hr. They were then treated with concentration of 50 μg/ml of the extract. After trypsinization, the harvested cells were pelleted and washed with PBS. Then pelleted cells were lysed with 100 μl sodium phosphate buffer 100 mM (pH 6.8). After 30 min, the lysates were centrifuged at 10,000 rpm for 20 min at 4 °C. The supernatant was transferred to 1 ml sterile microtube and kept in -80 °C. 100 μl of this protein suspension as well as 100 μl of DOPA 5 mM was added to each well of a 96-well plate. After 2 hr incubation, the absorbance was measured at 475 nm.
Western blotting
B16F10 melanoma cells were cultured in 25 cm2 flasks as described above. Cells were treated with all extracts at the concentration of 50 μg/ml for 24 hr. The cells were then lysed in a buffer containing 50 mM Tris-HCl with pH 7.4, 2 mM EGTA, 1 mM phenylmethyl-sulfonyl fluoride, 10 mM β-Glycerophosphate, 10 mM β-mercaptoethanol, 1 mM sodium orthovanadate, 0.1% deoxycholic acid sodium salt.
Proteins (50 μg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and transferred electrophoretically to polyvinylidene difluoride membranes and then were blocked overnight in 5% skim milk in TBST (20 mM Tris-HCl pH 7.4, 100 mM NaCl, and 0.1% Tween 20) buffer at 4°C. After washing in TBST buffer, they were then incubated for 3 hr with a primary antibody: rabbit anti-tyrosinase antibody (1:300), anti-MITF antibody (1:300). After incubation with an anti-rabbit IgG (1:2000) as secondary antibody the bands were detected using the ECL Prime Western Blotting Detection System (BioRaD, USA). Bands were scanned by GS-800, and band intensities were quantified by measuring optical densities with Quantity One software (Bio-Rad).
Cellular ROS level determination
About 5×104 B16F10 melanoma cells were cultured in 96-well plates and treated with 50 μg/ml of each extract and positive control for 24 hr. After cell exposure to 24 mM H2O2 at 37 °C for 30 min, 2’, 7’-dichlorofluorescein diacetate (DCFH-DA) was added to each well and incubated for the next 30 min. The amount of ROS was measured according to fluorescence intensities of DCF at excitation wavelength 504 nm and emission wavelength 524 nm using a Synergy H4 Hybrid Multi-Mode fluorescent Microplate Reader (BioTek, Winooski, USA). Control cells received 24 mM H2O2 and Kojic acid was used as a positive control.
Statistics
Values were expressed as mean±standard deviation (SD) of three different experiments. To check for quantitative differences between the groups, analysis of variance (ANOVA) and the Tukey-Kramer Multiple Comparison tests were performed. P<0.05 was considered as statistically significant.
Results
Effect of N. binaludensis extracts on melanin synthesis in B16F10 cells without cytotoxicity
To study the effect of different extracts of N. binaludensis on melanin synthesis, the melanin content of extract-treated B16F10 melanoma cells was quantified. All extracts were significantly decreased the melanin content in cells and there was no significant difference between kojic acid and methanol extract (Figure 2). In addition, investigating the effect of N. binaludensis extracts on B16F10 melanoma cell proliferation showed that the extracts had no significant cytotoxic effect on B16F10 cells at the concentration used (Figure 3). These results indicate that N. binaludensis extract exerts anti-melanogenic effects on B16F10 melanoma cells without inducing cytotoxicity.
Figure 2.
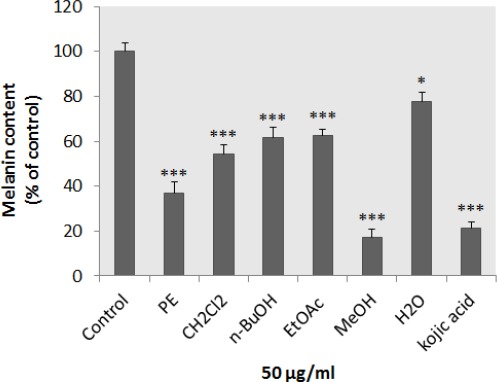
Effect of Nepeta binaludensis on melanin content in B16F10 murine melanoma cells. B16F10 cells were incubated without (control) and with, 50 μg/ml of different N. binaludensis extracts for 48 hr. Melanin content was measured as described in ‘‘Materials and Methods.’’ As shown in the figure all extracts significantly decreased the melanin content in B16F10 cells. Results were expressed as percentages relative to control, and are presented as mean±SD of triplicate samples. Statistically significant difference between extract-treated cells and control ***(P<0.001)
Figure 3.
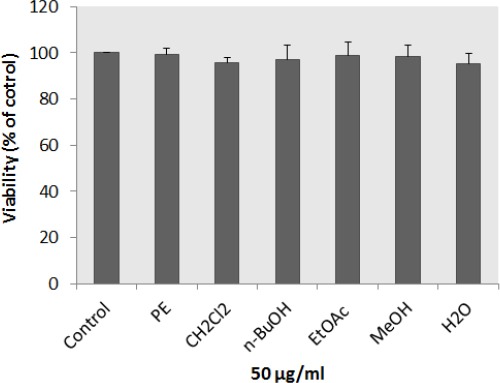
Cytotoxic effects of Nepeta binaludensis extracts on murine melanoma cells. After incubation of B16F10 melanoma cells with concentration of 50 μg/ml of different extracts of N. binaludensis in a 96-well plate for 48 hr, cell viability was determined by Resazurin assay. Percentage values in the treated cells were compared with respect to that in the control cells. Data are expressed as mean±SD for triplicate samples
Effect of N. binaludensis extracts on mushroom tyrosinase activity
To determine whether N. binaludensis extracts affect tyrosinase activity directly, we performed a mushroom tyrosinase assay using L-DOPA as substrate and mushroom tyrosinase as enzyme source. Tyrosinase is a key enzyme catalyzing the rate-limiting step in melanin biosynthesis. As shown in Figure 4, petroleum ether, dichloromethane, and methanol extracts of N. binaludensis exerted a significant inhibitory effect on L-DOPA oxidation by mushroom tyrosinase and significantly reduced the activity of mushroom tyrosinase. These results suggest that petroleum ether extract of N. binaludensis displayed the most inhibitory effects on mushroom tyrosinase activity and there was no significant difference between petroleum ether extract and kojic acid.
Figure 4.
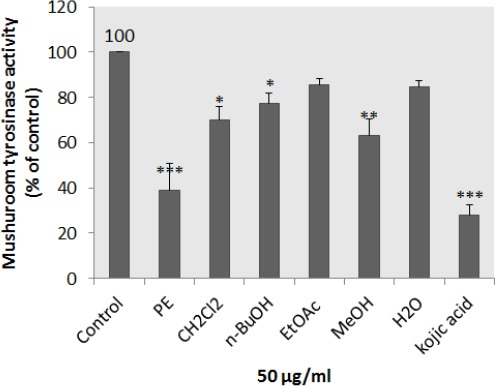
Effect of Nepeta binaludensis extracts on mushroom tyrosinase in B16F10 murine cells. Concentration 50 μg/ml of different N. binaludensis extracts and 2 mM of kojic acid were incubated with mushroom tyrosinase and L-DOPA at 37 °C. Mushroom tyrosinase activity was measured by the change in absorption at 475 nm. Results were expressed as percentages relative to control, and are presented as mean±SD of triplicate samples. Statistically significant difference between extract-treated cells and control *(P < 0.05), **(P < 0.01) and ***(P <0.001)
Inhibitory effect of N. binaludensis extracts on B16F10 melanoma cellular tyrosinase activity
The anti-melanogenesis effect of different extracts of N. binaludensis on cellular tyrosinase activity was assessed. As shown in Figure 5, suppression of tyrosinase activity in the cultured B16F10 melanoma cells was occurred after treatment of the cells with 50 μg/ml of each extract except aqueous extract.
Figure 5.
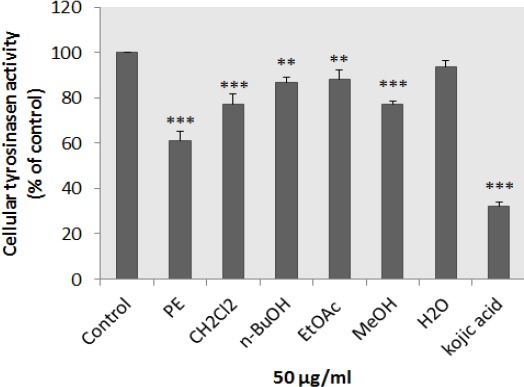
Effect of Nepeta binaludensis extracts on cellular tyrosinase in B16F10 murine melanoma cells. After incubation of B16F10 melanoma cells with concentration 50 μg/ml of different N. binaludensis extracts for 48 hr, cellular tyrosinase activity was assessed as described in ‘‘Materials and Methods.’’ Results were expressed as percentages relative to control, and are presented as mean±SD of triplicate samples. Statistically significant difference between extract-treated cells and control **(P<0.01) and ***(P<0.001)
Effect of N. binaludensis extracts on Cellular ROS level
The antioxidant capacity of different extracts was measured in respect to intracellular ROS levels. 24 mM H2O2 was exposed to cells which were pretreated with plant extracts. As shown in Figure 6, all extracts could significantly suppress the oxidative stress induced by H2O2 in the cells.
Figure 6.
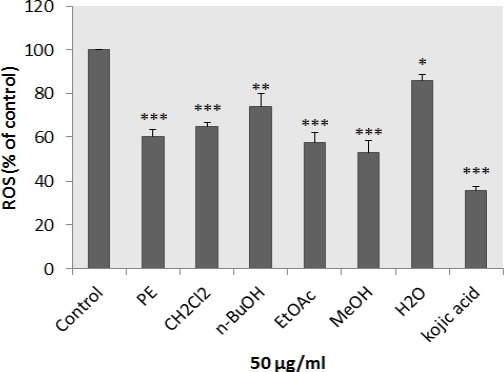
Antioxidant effects of Nepeta binaludensis extracts and cellular reactive oxygen species (ROS) level in B16F10 murine melanoma cells. The cells were treated with concentration 50 μg/ml of different N. binaludensis extracts or kojic acid (2.0 mM) for 24 hr and then the ROS content was measured by the DCF-DA assay. Results were expressed as percentages relative to control, and are presented as mean±SD of triplicate samples. Statistically significant difference between extract-treated cells and control ** (P<0.01) and *** (P<0.001)
The level of tyrosinase and MITF protein by N. binaludensis extracts
To determine the tyrosinase protein level in the cells treated with N. binaludensis extracts, we performed western blot analysis (Figure 7) and the intracellular effect of different extracts on melanogenic related proteins such as tyrosinase and MITF as an indicator of melanogenesis was evaluated. As shown in Figure 7, tyrosinase protein levels were significantly decreased by methanol extract of N. binaludensis. Beta-Actin was used as internal control. These results suggest that the anti-melanogenesis effect of the mentioned extracts on B16F10 cells is associated with the down regulation of tyrosinase, the most important enzyme in melanogenesis.
Figure 7.
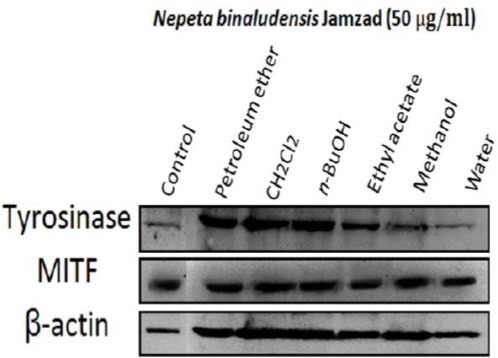
Effect of Nepeta binaludensis on tyrosinase and MITF protein level in B16F10 murine melanoma cells. B16F10 melanoma cells were treated with 50 μg/ml of different N. binaludensis extracts for 48 hr. The loading control was β-actin antibody. The relative intensities of tyrosinase and MITF protein compared with the β-actin using Quantity One software
Discussion
Melanoma, the fifth and sixth most common cancer among men and women respectively, is the deadliest form of skin cancer and one of the most challenging human cancers. Recently, the demand for anti-melanogenetic agents has increased all over the world, not only for treatment of cancers but also for cosmetics as well. As the age increases, melanin-rich spots occur on the skin and various methods are employed to remove these spots. Thus, regulating melanogenesis is an interesting field of research and we are aiming to find new agents for this purpose. 3,4-dihydroxyacetophenone (30), 4,4´-dihydroxybiphenyl (31), phospholipase D1 (32), phospholipase D2 (33), 4-n-butylresorcinol (34), miconazole (35), glycolic acid and lactic acid (36) are reported as anti-melanogenesis agents for the treatment of different skin diseases such as vitiligo, porphyritic amiroidosis and hemochromatosis. Duo to side effects of synthetic drugs, our main objective is to find new agents that can regulate melanogenesis from natural resources.
In this study, we have investigated whether different extracts of N. binaludensis extracts had a hypopigmentation effect in B16F10 melanoma cells, and found that all extracts significantly down-regulated melanin synthesis without showing significant cytotoxicity. Generally, petroleum ether extract of N. binaludensis was the most active extract in all tests. This extract considerably inhibited melanogenesis without cytotoxicity (Figures 2 & 3). After 48 hr of treatment with the extracts, B16F10 cells were still completely viable, which is important point in using plant for medical or cosmetic purposes. The anti-melanogenesis effect of potent extracts without any cytotoxicity is an encouraging factor for developing natural anti-melanogenesis agent.
Melanin synthesis occurs predominantly in the melanosomes, which are lysosome-like structures of melanocytes. Melanin is packaged and delivered to the keratinocytes by melanosomes. The two types of melanin, pheomelanin and eumelanin, are different not only in color but also in the size, shape, and the packing of their granules (37). Both pheomelanin and eumelanin are produced from the same precursor, tyrosine, in a common tyrosinase-dependent pathway. Melanogenesis is known to be regulated by the tyrosinase gene family, which includes tyrosinase, TRP-1, and TRP-2 (2). Tyrosinase controls two key steps in the melanin synthesis pathway by catalyzing the hydroxylation of tyrosine to DOPA, followed by the oxidation of DOPA to DOPAquinone (1, 3), and thus melanin production is correlated with the expression level and the catalytic activity of tyrosinase.
To investigate the mechanism of N. binaludensis extract in inhibiting melanogenesis, first we determined whether N. binaludensis extract could inhibit tyrosinase activity directly in cell-free assay systems. We used mushroom tyrosinase as enzyme source. In this assay, kojic acid was used as a positive control. It has been reported that kojic acid can affect melanin synthesis in melanocytes and melanoma cells. Although all extracts almost had some inhibitory effect on tyrosinase activity, the petroleum ether, dichlomethan and methanol extracts of N. binaludensis significantly decreased the mushroom tyrosinase activity after 24 hr of treatment and all extracts inhibited the cellular tyrosinase activity (Figures 4 & 5). More inhibition of melanogenesis and decrease of tyrosinase activity with petroleum ether and methanol extracts suggested the presence of similar compound responsible for anti-melanogesis in these extracts. Moreover, ROS was also significantly decreased after treatment of the cells with all extracts showing antioxidant capacity of the extracts.
Microphthalmia-associated transcription factor (MITF), one of the most important nuclear transcription factors, regulates melanogenesis by activation of tyrosinase, TRP1 and TRP2 transcription (38). Studies have shown that in addition to regulating melanogenesis, MITF also plays a central role in regulation of melanocyte development and survival (39, 40). The importance and complex role of MITF in tumorigenesis and progression of melanoma have been revealed by different studies (41, 42). MITF has recently been investigated as a potential target for melanoma therapy (43, 44). The petroleum ether and methanol extracts of N. binaludensis considerably decreased the amount of tyrosinase and microphthalmia-associated transcription factor proteins. Many signal transduction pathways have been found to balance melanin production. Mitogen-activated protein kinase pathways, particularly the extracellular signal-regulated kinase (ERK) 1/2 pathway, are perhaps involved in MITF regulation (45, 46). Significant MITF degradation occurs after phosphorylation of MITF at serine 73 by ERK, leading to ubiquitin-dependent proteasomal degradation. In the present study, we found that while N. binaludensis reduced tyrosinase level in cells, it did not have a significant effect on the level of MITF protein.
Since several studies have revealed many potent bioactive plant derived compounds for regulating melanogenesis and treatment of skin problems, as well as for cosmetic purposes (47) (48), We suggest further investigation to determine the exact bioactive compounds of N. binaludensis. In addition, according to investigations and literature survey we have done, there is no similar study on the genus Nepeta which emphasizes more investigations on other species.
Conclusion
In summary, the present study revealed that the N. binaludensis extracts inhibited cellular melanin biosynthesis and tyrosinase activity in B16F10 murine melanoma cells, leading to decrease in tyrosinase level. These results indicate that the N. binaludensis extracts are effective inhibitors of melanogenesis and can be useful as a therapeutic treatment for skin hyperpigmentation disorders.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
This work was supported by grants from Research Affairs of Mashhad University of Medical Sciences, Mashhad, Iran, the Specialized Research Fund (No. 910875) for the Pharmacy doctorate Program. The results described in this paper were part of student thesis. We are also grateful for the editorial assistance of Mrs. Z. Gonzalez.
References
- 1.Fang D, Kute T, Setaluri V. Regulation of Tyrosinase-related Protein-2 (TYRP2) in Human Melanocytes: Relationship to Growth and Morphology. Pigment Cell Res. 2001;14:132–139. doi: 10.1034/j.1600-0749.2001.140209.x. [DOI] [PubMed] [Google Scholar]
- 2.Fang D, Setaluri V. Role of microphthalmia transcription factor in regulation of melanocyte differentiation marker TRP-1. Biochem Biophys Res Commun. 1999;256:657–663. doi: 10.1006/bbrc.1999.0400. [DOI] [PubMed] [Google Scholar]
- 3.Kameyama K, Sakai C, Kuge S, Nishiyama S, Tomita Y, Ito S, et al. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell Res. 1995;8:97–104. doi: 10.1111/j.1600-0749.1995.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 4.Michaela Brenner M, Hearing VJ. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Busca R, Ballotti R. Cyclic AMP a key messenger in the regulation of skin pigmentation. Pigment Cell Res. 2000;13:60–69. doi: 10.1034/j.1600-0749.2000.130203.x. [DOI] [PubMed] [Google Scholar]
- 6.Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–994. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 7.Ortonne JP, Nordlund JJ. Mechanisms That Cause Abnormal Skin Color, The Pigmentary System. Blackwell Publishing Ltd; 2007:521–537. [Google Scholar]
- 8.Im S, Kim J, On WY, Kang WH. Increased expression of alpha-melanocyte-stimulating hormone in the lesional skin of melasma. Br J Dermatol. 2002;146:165–167. doi: 10.1046/j.1365-2133.2002.4513_3.x. [DOI] [PubMed] [Google Scholar]
- 9.Kang WH, Yoon KH, Lee ES, Kim J, Lee KB, Yim H, et al. Melasma: histopathological characteristics in 56 Korean patients. Br J Dermatol. 2002;146:228–237. doi: 10.1046/j.0007-0963.2001.04556.x. [DOI] [PubMed] [Google Scholar]
- 10.Maeda K, Fukuda M. Arbutin: mechanism of its depigmenting action in human melanocyte culture. J Pharmacol Exp Ther. 1996;276:765–769. [PubMed] [Google Scholar]
- 11.Ando H, Watabe H, Valencia JC, Yasumoto K, Furumura M, Funasaka Y, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J Biol Chem. 2004;279:15427–15433. doi: 10.1074/jbc.M313701200. [DOI] [PubMed] [Google Scholar]
- 12.Mabberley DJ. The Plant-Book. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 13.Rechinger KH. In: Nepeta. Flora Iranica, Labiatae. 30. Rechinger KH, editor. Graz: Akademische Druc-u Verlagsanstalt; 1982. pp. 108–216. [Google Scholar]
- 14.Taskina A, Javan M, Sonboli A, Semnanian S. Evaluation of the anti-nociceptive and anti-inflammatory effects of essential oil of Nepeta pogonosperma Jamzad et Assadi in rats. Daru. 2012;20:48. doi: 10.1186/2008-2231-20-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emami SA, Aghazari F. Les Phanerogames endemiques de la flore d’Iran. Teheran: Publications de I’Universitéde’Iran des Sciences Médicales; 2011. [Google Scholar]
- 16.Ghahreman A, Attar F. Biodiversity of plant species in Iran. Tehran: Tehran University Publication; 1999. [Google Scholar]
- 17.Jamzad Z. Nepeta menthoides Boiss. & Buhse and species allied to it in Iran. Iran J Bot. 1991;5:17–27. [Google Scholar]
- 18.Nadjafi F, Koocheki A, Honermeier B, Asili J. Autecology, ethnomedicinal and phytochemical studies of Nepeta binaludensis Jamzad a highly endangered medicinal plant of Iran. J Essent Oil Bear Pl. 2009;12:97–110. [Google Scholar]
- 19.Phillips R, Rix M. The Botanical Garden. London: Mac Milan; 2002. [Google Scholar]
- 20.García-Gavín J, González-Vilas D, Fernández-Redondo V, Toribio J. Pigmented contact dermatitis due to kojic acid. A paradoxical side effect of a skin lightener. Contact Dermat. 2010;62:63–64. doi: 10.1111/j.1600-0536.2009.01673.x. [DOI] [PubMed] [Google Scholar]
- 21.Ali L, Ali S, Rizvi TS, Khan AL, Hassan Z, Al-Harrasi A, et al. Antioxidant flavonoids from Nepeta floccosa benth. Rec Nat Prod. 2015;9:567–571. [Google Scholar]
- 22.Kraujalis P, Venskutonis PR, Ragazinskiene O. Antioxidant activities and phenolic composition of extracts from Nepeta plant species. in 6th Baltic Conference on Food Science and Technology: Innovations for Food Science and Production, FOODBALT-2011. Jelgava. 2011 [Google Scholar]
- 23.Mothana RA. Chemical composition, anti-microbial and antioxidant activities of the essential oil of Nepeta deflersiana growing in Yemen. Rec Nat Prod. 2012;6:189–193. [Google Scholar]
- 24.Pandey AK, Mohan M, Singh P, Tripathi NN. Chemical Composition, Antioxidant and Anti-microbial Activities of the Essential Oil of Nepeta hindostana (Roth) Haines from India. Rec Nat Prod. 2015;9:224–233. [Google Scholar]
- 25.Kurek-Górecka A, Rzepecka-Stojko A, Górecki M, Stojko J, Sosada M, Swierczek-Zieba G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules. 2014;19:78–101. doi: 10.3390/molecules19010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raederstorff D. Antioxidant activity of olive polyphenols in humans: A review. Int J Vit Nutr Res. 2009;79:152–165. doi: 10.1024/0300-9831.79.3.152. [DOI] [PubMed] [Google Scholar]
- 28.O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- 29.Hosoi J, Abe E, Suda T, Kuroki T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1 alpha, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985;45:1474–1478. [PubMed] [Google Scholar]
- 30.Kim YJ, No JK, Lee JS, Kim MS, Chung HY. Anti-melanogenic activity of 3,4-dihydroxyacetophenone: inhibition of tyrosinase and MITF. Biosci Biotechnol Biochem. 2006;70:532–534. doi: 10.1271/bbb.70.532. [DOI] [PubMed] [Google Scholar]
- 31.No JK, Kim YJ, Lee JS, Chung HY. Inhibition of melanogenic activity by 4,4’-dihydroxybiphenyl in melanoma cells. Biol Pharm Bull. 2006;29:14–16. doi: 10.1248/bpb.29.14. [DOI] [PubMed] [Google Scholar]
- 32.Ohguchi K, Banno Y, Nakagawa Y, Akao Y, Nozawa Y. Negative regulation of melanogenesis by phospholipase D1 through mTOR/p70 S6 kinase 1 signaling in mouse B16 melanoma cells. J Cell Physiol. 2005;205:444–451. doi: 10.1002/jcp.20421. [DOI] [PubMed] [Google Scholar]
- 33.Kageyama A, Oka M, Okada T, Nakamura S, Ueyama T, Saito N, et al. Down-regulation of melano-genesis by phospholipase D2 through ubiquitin proteasome-mediated degradation of tyrosinase. J Biol Chem. 2004;279:27774–27780. doi: 10.1074/jbc.M401786200. [DOI] [PubMed] [Google Scholar]
- 34.Kim DS, Kim SY, Park SH, Choi YG, Kwon SB, Kim MK, et al. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol Pharm Bull. 2005;28:2216–2219. doi: 10.1248/bpb.28.2216. [DOI] [PubMed] [Google Scholar]
- 35.Mun YJ, Lee SW, Jeong HW, Lee KG, Kim JH, Woo WH. Inhibitory effect of miconazole on melanogenesis. Biol Pharm Bull. 2004;27:806–809. doi: 10.1248/bpb.27.806. [DOI] [PubMed] [Google Scholar]
- 36.Usuki A, Ohashi A, Sato H, Ochiai Y, Ichihashi M, Funasaka Y. The inhibitory effect of glycolic acid and lactic acid on melanin synthesis in melanoma cells. Exp Dermatol. 2003;2:43–50. doi: 10.1034/j.1600-0625.12.s2.7.x. [DOI] [PubMed] [Google Scholar]
- 37.Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- 38.Otreba M, Rok J, Buszman E, Wrzesniok D. Regulation of melanogenesis: the role of cAMP and MITF. Postepy Hig Med Dosw. 2012;66:33–40. [PubMed] [Google Scholar]
- 39.Li J, Song JS, Bell RJA, Tran TNT, Haq R, Liu H, et al. YY1 regulates melanocyte development and function by cooperating with MITF. PLoS Genetics. 2012;8:e1002688. doi: 10.1371/journal.pgen.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12:406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Paillerets BBD, Lesueur F, Bertolotto C. A germline oncogenic MITF mutation and tumor susceptibility. Eur J Cell Biol. 2014;93:71–75. doi: 10.1016/j.ejcb.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Bertolotto C, Lesueur F, Giuliano S, Strub T, De Lichy M, Bille K, et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature. 2011;480:94–98. doi: 10.1038/nature10539. [DOI] [PubMed] [Google Scholar]
- 43.Hartman ML, Czyz M. Pro-survival role of MITF in melanoma. J Invest Dermatol. 2015;135:352–358. doi: 10.1038/jid.2014.319. [DOI] [PubMed] [Google Scholar]
- 44.Rudloff U, Samuels Y. TYRO3-mediated regulation of MITF: A novel target in melanoma? Pigment Cell Melanoma Res. 2010;23:9–11. doi: 10.1111/j.1755-148X.2009.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawano M, Matsuyama K, Miyamae Y, Shinmoto H, Kchouk ME, Morio T, et al. Anti-melanogenesis effect of Tunisian herb Thymelaea hirsuta extract on B16 murine melanoma cells. Exp. Dermatol. 2007;16:977–984. doi: 10.1111/j.1600-0625.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu M, Hemesath TJ, Takemoto CM, Horstmann MA, Wells AG, Price ER, et al. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes Dev. 2000;14:301–312. [PMC free article] [PubMed] [Google Scholar]
- 47.Yamahara M, Sugimura K, Kumagai A, Fuchino H, Kuroi A, Kagawa M, et al. Callicarpa longissima extract, carnosol-rich, potently inhibits melanogenesis in B16F10 melanoma cells. J Nat Med. 2016;70:28–35. doi: 10.1007/s11418-015-0933-5. [DOI] [PubMed] [Google Scholar]
- 48.Huang HC, Ho YC, Lim JM, Chang TY, Ho CL, Chang TM. Investigation of the Anti-Melanogenic and Antioxidant Characteristics of Eucalyptus camaldule-nsis Flower Essential Oil and Determination of Its Chemical Composition. Int J Mol Sci. 2015;16:10470–10490. doi: 10.3390/ijms160510470. [DOI] [PMC free article] [PubMed] [Google Scholar]


