Abstract
AIM
To compare the effect of anti-vascular endothelial growth factor (VEGF) monotherapy versus photodynamic therapy (PDT) and anti-VEGF combination treatment in age-related macular degeneration (AMD).
METHODS
A computerized online search was performed using PubMed, Web of Science and the Cochrane Library. Studies that compared anti-VEGF monotherapy with PDT and anti-VEGF combination treatment of AMD and were designed as randomized controlled trials were included. The means and standard deviations of the best-corrected visual acuity (BCVA), central retinal thickness (CRT), number of treatments and proportions of patients who gained BCVA ≥15, 10, 5, or 0 letters at 12th month were extracted. A systematic review and Meta-analysis of the comparison of the two approaches was conducted using Review Manager 5.2. Subgroup. A sensitivity analysis was also performed.
RESULTS
Eight studies were included. When the subgroup and sensitivity analysis was conducted, the results indicated that in the findings that included the monotherapy group and PDT (standard fluence, SF) group of Kaiser's study, the patients in the monotherapy group had a better BCVA compared with the combination group at 12th month in the PDT (SF) subgroup [weighted mean difference (WMD): 3.54; 95%CI: 0.36 to 6.73; P=0.03], and there were more patients who gained ≥15 letters of BCVA in the monotherapy group compared with the combination group in the total result [odds ratio (OR): 1.41; 95%CI: 1.02 to 1.95; P=0.04]. The same conclusion was obtained in the total result that included the monotherapy group and PDT (reduced fluence, RF) group of Kaiser's study (OR: 1.56; 95%CI: 1.13 to 2.15; P=0.007). However, there were no significant differences in the other indexes between the two therapies.
CONCLUSION
We found that anti-VEGF monotherapy is more effective on the recovery of visual acuity than combination therapy and more researches with lager sample size should be performed to study on the effect of the two therapy approaches on CRT and number of injections.
Keywords: age-related macular degeneration, anti-vascular endothelial growth factor, photodynamic therapy, Meta-analysis
INTRODUCTION
Age-related macular degeneration (AMD) is one of the leading causes of blindness in elderly individuals in developed countries[1]–[2]. AMD affects more than 1.75 million individuals in the United States. As a result of the rapid aging of the US population, this number will increase to approximately 3 million individuals by 2020[3]. AMD is also a regular ophthalmic disease in elderly individuals in Asia[4].
The pathophysiology of AMD is complex. Oxidative stress, inflammation and angiogenesis mainly contribute to the disease progression at the molecular level. The neovascular form of AMD (nAMD) is linked to choroidal neovascularization (CNV), and it causes severe vision loss because of an abnormal growth of blood vessels in the retina[5]. Vascular endothelial growth factor (VEGF), a potent angiogenic molecule, plays an important role in the development of CNV in nAMD[6] in the tissue microenvironment. Treatments using anti-VEGF monotherapy have been established as the standard therapy for CNV and AMD. In these cases, patients require multiple treatments[7] to slow down the growth of new abnormal blood vessels. Photodynamic therapy (PDT) is another treatment approach for AMD patients. It includes intravenous injection of verteporfin, a photosensitizing drug, which injures newly formed CNV and thus reduces the risk of vision loss and retards disease progression in patients with AMD. Its efficacy and safety in nAMD have been demonstrated by several studies[8]–[10].
It was hypothesized that the combination of these two treatments may have a synergistic effect on improving visual acuity (VA) and reducing the center retinal thickness (CRT), the CNV and the number of anti-VEGF treatments[5],[11]. Currently, there is no consensus regarding whether combination therapy is more effective compared with anti-VEGF monotherapy. We performed a comprehensive, computerized, online search of the randomized controlled trials that have compared anti-VEGF monotherapy versus PDT and anti-VEGF combination treatment in AMD. Using all available data, a systematic review and Meta-analysis of the comparison of the two therapies was performed to estimate the efficacy of anti-VEGF monotherapy and combination therapy.
METHODS
Literature Search
We searched PubMed, Web of Science and the Cochrane Library using the following search terms: (“age related macular degeneration” OR “AMD” OR “macular degeneration”) and (“PDT” OR “photodynamic therapy” OR “visudyne”) and (“anti-VEGF” OR “vascular endothelial growth factors” OR “endothelial growth factors” OR “angiogenesis inhibitors” OR “angiogenesis inducing agents”) and other alternative names (“macugen” OR “pegaptanib” OR “lucentis” OR “rhufab” OR “ranibizumab” OR “bevacizumab” OR “avastin”). All related articles that were published prior to January 31, 2015 without language or geographic limitations were considered.
Selection Criteria
Studies were included only if they fulfilled all of the following six criteria: 1) all patients had a professional ophthalmic examination and were diagnosed as AMD; 2) the study design was limited to randomized controlled trials, and the full-text was available; 3) interventions included anti-VEGF monotherapy (inner ocular injection with ranibizumab or bevacizumab) and combined PDT and anti-VEGF therapy, and the time of follow-up was at least 12mo; 4) endpoints included at least one of the following: the best-corrected visual acuity (BCVA), CRT, number of treatments and proportion of patients who gained ≥15, 10, 5, or 0 letters of BCVA at 12th month; 5) raw data were available; and 6) for studies published by the same group regarding the same population, only the most recent report or the report with the largest sample size was included for the analysis.
Data Extraction
Two reviewers (Tong Y and Zhao KK) independently extracted the data and evaluated the quality. The following variables were extracted from each study: 1) the characteristics of the included studies, i.e., the name of the first author, year of publication, location, follow-up time, mean age and sex ratio of the study participants; 2) the means and standard deviations (SDs) of the BCVA at the endpoint; 3) the means and SDs of the CRT at the endpoint; 4) the means and SDs of the number of treatments at the endpoint; and 5) the proportion of patients who gained BCVA ≥15; 10; 5; 0 letters at the endpoint. An independent review and resolution by a third reviewer (Feng D) was sought if the two reviewers disagreed.
Statistical Analysis
Data were collected and analyzed using Review Manager 5.2 software. We calculated the pooled odds ratios (ORs) and 95% confidence intervals (CIs) for the dichotomous outcomes, as well as the weighted mean differences (WMDs) and 95% CIs for the continuous outcomes. The differences between the monotherapy and combination groups were displayed via forest plot. Q-statistic and I2 statistic were used to measure the difference in the between-study heterogeneity. If the heterogeneity was statistically significant (P<0.1 and I2>50%), we chose a random-effects model. Otherwise, a fixed-effects model was used. Furthermore, a subgroup analysis was conducted to determine the effect of the fluence used in the PDT therapy, and a sensitivity analysis was performed because of the different design of Kaiser et al's[17] study.
RESULTS AND DISCUSSION
Literature Search
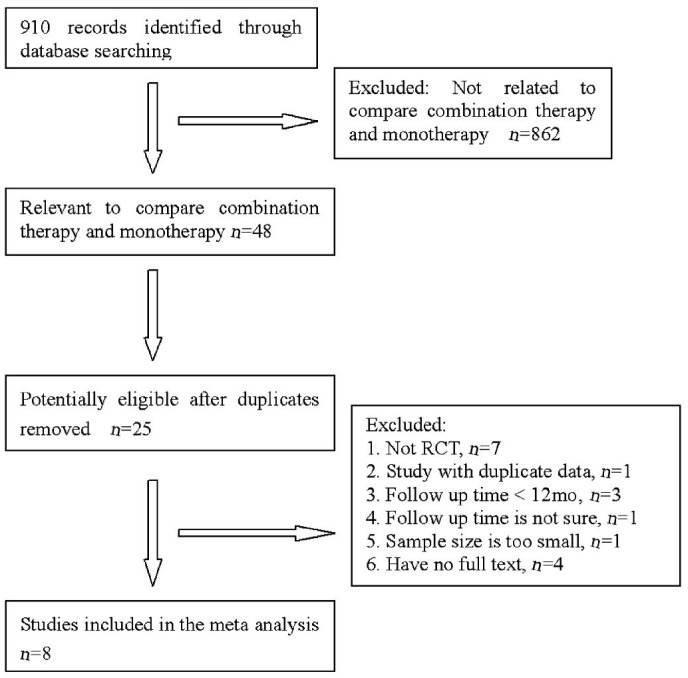
Forty-eight relevant studies were identified by our initial search, which included 8 studies eligible for inclusion in the review[12]–[19]. The follow-up times in all studies comprised 12mo. In some cases, raw data were kindly provided by the author[13], and some were procured from Novartis' data on file[15],[17]. Figure 1 is a flow diagram of the selection of eligible studies. The characteristics of the included studies are summarized in Table 1. The combined sample size for this Meta-analysis was 800, which included 409 individuals in the monotherapy group and 391 individuals in the combination group. The average ages ranged from 65.3 to 79.1y in the monotherapy group and 63.2 to 80.3y in the combination group. The gender ratios (male/female) of the two groups varied from 0.53 (16/30) to 1.6 (8/5) in the monotherapy group and 0.36 (13/36) to 2.0 (12/6) in the combination group. All included studies comprised randomized controlled trials; 3 studies were conducted in America, 4 studies were conducted in Europe, and 1 study was conducted in Korea. Table 1 indicates the features of the included studies. Table 2 presents the means and SDs of the BCVA, the CRT, the numbers of treatments of the patients at 12th month and the proportion of the patients who gained BCVA ≥15, 10, 5, 0 letters at 12th month in the included studies.
Figure 1. The literature search process.
Table 1. The feature of included studies.
| First author | Public year | Study type | Location | Follow-up (mo) | Groups | Patients (n) | Mean age (a) | Gender radio (M/F) | Therapy |
| Larsen[15] | 2012 | RCT | Europe | 12 | Monotherapy | 133 | 75.5 | 59/74 | IVR (3+PRN) |
| Combination | 122 | 76.8 | 44/78 | PDT (SF1+PRN); IVR (3+PRN) | |||||
| Kaiser[17] | 2012 | RCT | America | 12 | Monotherapy | 112 | NR | NR | IVR (11) |
| PDT (SF) combination | 104 | NR | NR | PDT (SF1+PRN); IVR (3+PRN) | |||||
| PDT (RF) combination | 105 | NR | NR | PDT (RF1+PRN); IVR (3+PRN) | |||||
| Krebs[16] | 2013 | RCT | Austria | 12 | Monotherapy | 24 | 77.71 | NR | IVR (3+PRN) |
| Combination | 20 | 80.25 | NR | PDT (SF1+PRN); IVR (3+PRN) | |||||
| Vallance[13] | 2010 | RCT | UK | 12 | Montherapy | 9 | NR | NR | IVR (3+PRN) |
| Combination | 9 | NR | NR | PDT (SF1+PRN); IVR (3+PRN) | |||||
| Lim[14] | 2012 | RCT | Korea | 12 | Monotherapy | 13 | 66.7 | 8/5 | IVB (3+PRN) |
| Combination | 23 | 68.9 | 12/6 | PDT (SF1+PRN); IVB (3+PRN) | |||||
| Williams[12] | 2012 | RCT | American | 12 | Monotherapy | 27 | 79.1 | NR | IVB (1+PRN) |
| PDT (RF) combination | 29 | 79.3 | NR | PDT (RF1+PRN); IVB (1+PRN) | |||||
| Costagliola[19] | 2010 | RCT | Italy | 12 | Monotherapy | 45 | 65.3 | 20/25 | IVB (1+PRN) |
| PDT (RF) combination | 40 | 63.2 | 18/22 | PDT (RF1+PRN); IVB (1+PRN) | |||||
| Datseris[18] | 2015 | RCT | America | 12 | Monotherapy | 46 | 74 | 16/30 | IVB (1+PRN) |
| PDT (RF) combination | 49 | 73 | 13/36 | PDT (RF1+PRN); IVB (1+PRN) |
RCT: Randomized control trials; Monotherapy: Group which accept anti-VEGF treatment only; PDT (SF): PDT with standard fluence; PDT (RF): PDT with reduced fluence; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; IVR: Intravitreal ranibizumab; IVB: Intravitreal bevacizumab; PRN: As needed; NR: No record.
Table 2. Means and SDs of examination results of patients in each included study at 12th month.
| Study (first author) | Groups | Patients (n) | Means of BCVA (SD, letter) | CRT (SD, µm) | Treatments n (SD) | Patients gained BCVA [letter, n (%)] |
|||
| ≥15 | ≥10 | ≥5 | ≥0 | ||||||
| Larsen[15] | Monotherapy | 132 | 59.4 (18.8) | 232 (54.54) | 5.1 (2.01) | 34 (25.8) | 51 (38.6) | 69 (52.3) | 87 (65.9) |
| PDT(SF) combination | 121 | 57.1 (18.3) | 219.9 (61.05) | 4.8 (2.03) | 22 (18.2) | 45 (37.2) | 61 (50.4) | 86 (71.1) | |
| Kaiser[17] | Monotherapy | 110 | 63 (18.88) | 284.59 (75.49) | NR | 45 (41.1) | 65 (58.9) | 72 (65.3) | 87 (78.9) |
| PDT(SF) combination | 103 | 59 (17.47) | 292.92 (79.27) | NR | 32 (31.3) | 50 (48.2) | 57 (55.4) | 77 (74.7) | |
| PDT(RF) combination | 105 | 59 (18.03) | 305.72 (80.45) | NR | 26 (24.7) | 45 (42.4 | 62 (58.8) | 74 (70.6) | |
| Krebs[16] | Monotherapy | 22 | 57.09 (24.61) | 291.87 (70.00) | 7.17 (2.44, n=24) | NR | NR | NR | NR |
| PDT(SF) combination | 19 | 46.89 (28.30) | 268.83 (90.81) | 5.8(2.31, n=20) | NR | NR | NR | NR | |
| Vallance[13] | Monotherapy | 9 | 59.44 (11.05) | 233.11 (47.93) | 4.6 (0.96) | 1 (11.1) | 3 (33.3) | NR | NR |
| PDT(SF) combination | 9 | 52.78 (18.74) | 193.67 (37.52) | 4.3 (0.82) | 1 (11.1) | 1 (11.1) | NR | NR | |
| Lim[14] | Monotherapy | 13 | NR | 239.3 (129.37) | 3.3 (0.46) | NR | NR | NR | NR |
| PDT(SF) combination | 23 | NR | 211.89 (128.89) | 3.22 (0.42) | NR | NR | NR | NR | |
| Williams[12] | Monotherapy | 27 | NR | NR | NR | 9 (33) | NR | NR | NR |
| PDT(RF) combination | 29 | NR | NR | NR | 9 (31) | NR | NR | NR | |
| Costagliola[19] | Monotherapy | 45 | 57 (18) | 223 (72) | NR | 21 (47) | NR | NR | NR |
| PDT(RF) combination | 40 | 61 (16) | 244 (60) | NR | 14 (35) | NR | NR | NR | |
| Datseris[18] | Monotherapy | 46 | NR | 286.00 (57.99) | NR | 20 (43.5) | NR | NR | NR |
| PDT(RF) combination | 49 | NR | 290.84 (96.26) | NR | 21 (42.8) | NR | NR | NR | |
Monotherapy: Group which accept anti-VEGF treatment only; Combination: PDT and anti-VEGF combination treatment; PDT (SF): PDT with standard fluence; PDT (RF): PDT with reduced fluence; BCVA: Best-corrected visual acuity; CRT: Central retinal thickness; NR: No record or record is incomplete.
Best-corrected Visual Acuity
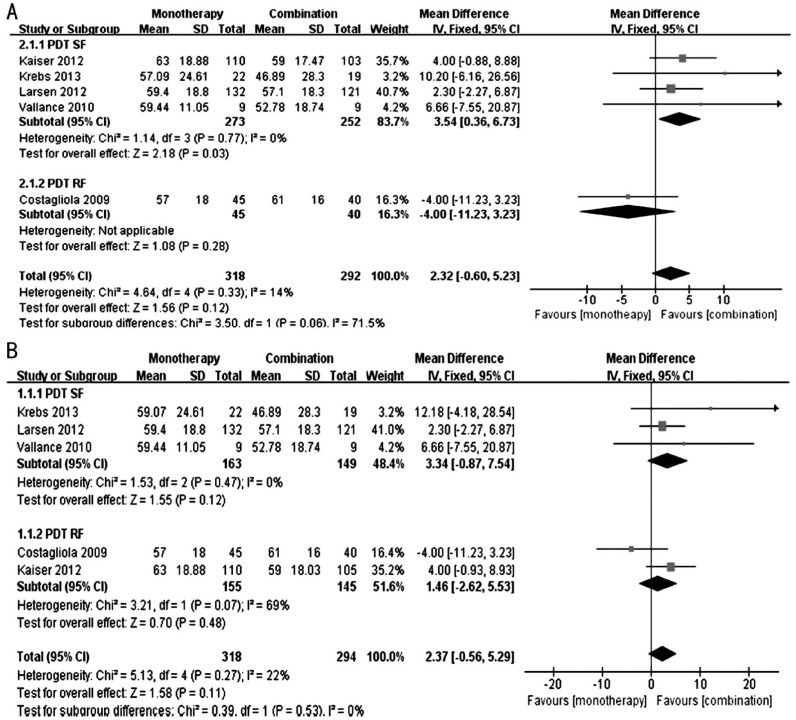
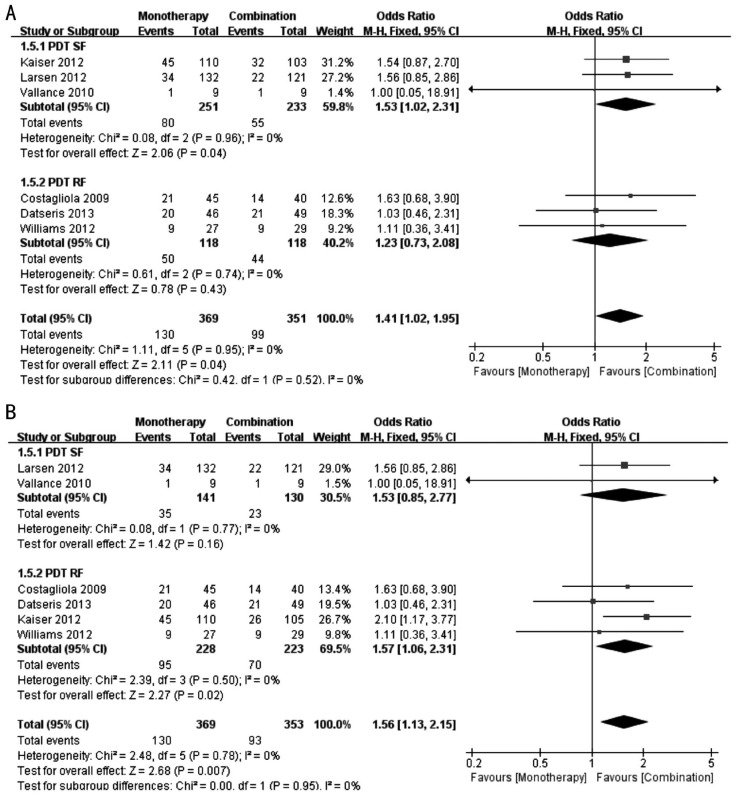
Figure 2 shows the forest plots of the effect on the BCVA. We performed a subgroup analysis to determine the effect of the fluence used in the PDT therapy, and a sensitivity analysis was performed because Kaiser et al's[17] study has three groups: monotherapy group, PDT (standard fluence, SF) group and PDT (reduced fluence, RF) group; we discussed the results that included the monotherapy group and PDT (SF) group of Kaiser's study, as well as the results that included the monotherapy group and PDT (RF) group of Kaiser et al's[17] study.
Figure 2. Forest plots of the effect on the BCVA.
A: Comparison of BCVA at 12th month between monotherapy group and combination group [including monotherapy group and PDT (SF) group of Kaiser et al's[17] study]; B: Comparison of BCVA at 12th month between monotherapy group and combination group [including monotherapy group and PDT (RF) group of Kaiser et al's[17] study].
Figure 2A shows the results that included the monotherapy group and PDT (SF) group of Kaiser et al's[17] study. In the PDT (SF) subgroup, the patients in the monotherapy group exhibited a better BCVA compared with the combination group at 12th month (WMD: 3.54; 95%CI: 0.36 to 6.73; P=0.03), with no evidence of heterogeneity (I2=0%, P=0.77). The PDT (RF) subgroup included only one study[19] (WMD: -4.00; 95%CI: -11.23 to 3.23, P=0.28). In the total result, the mean difference in the BCVA was not significant between the monotherapy and combination groups (WMD: 2.32; 95%CI: -0.60 to 5.23; P=0.12), with no significant heterogeneity (I2=14%, P=0.33).
Figure 2B shows the results that included the monotherapy group and PDT (RF) group of Kaiser's study. In the PDT (SF) subgroup, the mean difference in the BCVA was not significant between the monotherapy and combination groups (WMD: 3.34; 95%CI: -0.87 to 7.54; P=0.12), with no evidence of heterogeneity (I2=0%, P=0.47). In the PDT (RF) subgroup, the mean difference in the BCVA was not significant between the monotherapy and combination groups (WMD: 1.46; 95%CI: -2.62 to 5.53; P=0.48), with significant heterogeneity (I2=69%, P=0.07). Overall, the mean difference in the BCVA was not significant between the monotherapy and combination groups (WMD: 2.37; 95%CI: -0.56 to 5.29; P=0.11), with no significant heterogeneity (I2=22%, P=0.27).
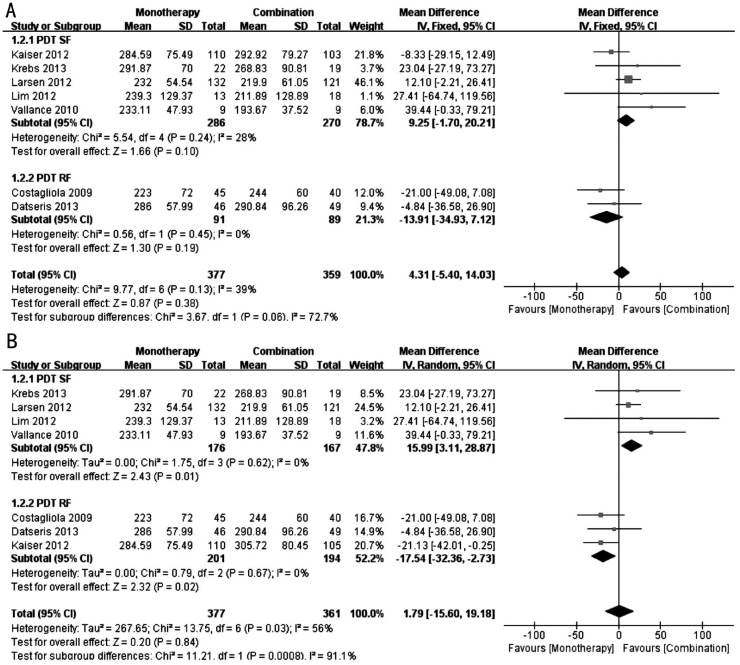
Central Retinal Thickness
Figure 3 shows the forest plots of the effect on the CRT. We also performed subgroup and sensitivity analyses. Figure 3A shows the results that included the monotherapy group and PDT (SF) group of Kaiser et al's[17] study. In the PDT (SF) subgroup, the mean difference in the CRT was not significant between the monotherapy and combination groups (WMD: 9.25; 95%CI: -1.70 to 20.21; P=0.10), with no significant heterogeneity (I2=28%, P=0.24). The PDT (RF) subgroup was also not significantly different between the two groups (WMD: -13.91; 95%CI: -34.93 to 7.12; P=0.19), with no significant heterogeneity (I2=0%, P=0.45). In the total result, the mean difference in the CRT was not significant between the monotherapy and combination groups (WMD: 4.31; 95%CI: -5.40 to 14.03; P=0.38), with no significant heterogeneity (I2=39%, P=0.13).
Figure 3. Forest plots of the effect on the CRT.
A: Comparison of CRT at 12th month between monotherapy group and combination group(including monotherapy group and PDT (SF) group of Kaiser et al's[17] study); B: Comparison of CRT at 12th month between monotherapy group and combination group [including monotherapy group and PDT (RF) group of Kaiser et al's[17] study].
Figure 3B shows the results that included the monotherapy group and PDT (RF) group of Kaiser's study. In the PDT (SF) subgroup, the CRT was thinner in the combination group compared with the monotherapy group (WMD: 15.99; 95%CI: 3.11 to 28.87; P=0.01), with no evidence of heterogeneity (I2=0%, P=0.62). In the PDT (RF) subgroup, the CRT was thinner in the monotherapy group compared with the combination group (WMD: -17.54; 95%CI: -32.36 to -2.73; P=0.02), with no significant heterogeneity (I2=0%, P=0.67). In the total result, the mean difference in the CRT was not significant between the monotherapy and combination groups (WMD: 1.79; 95%CI: -15.60 to 19.18; P=0.84), with significant heterogeneity (I2=56%, P=0.03).
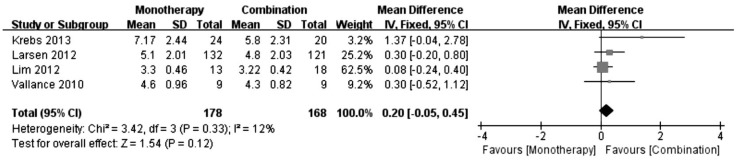
Number of Treatments
Figure 4 shows the forest plots of the effect on the number of treatments. The mean difference in the number of treatments was not significant between the monotherapy and combination groups (WMD: 0.20; 95%CI: -0.05 to 0.45; P=0.12), with no significant heterogeneity (I2=12%, P=0.33).
Figure 4. Comparison of number of treatments between monotherapy group and combination group.
Best-corrected Visual Acuity More Than 15, 10, 5, or 0 Letters
Figure 5 shows the forest plots of the proportion of patients who gained ≥15 letters of BCVA at 12th month. Subgroup and sensitivity analyses were performed.
Figure 5. Forest plots of the proportion of patients who gained ≥15 letters of BCVA at 12th month.
A: Comparison between monotherapy group and combination group [including monotherapy group and PDT (SF) group of Kaiser et al's[17] study]; B: Comparison between monotherapy group and combination group [including monotherapy group and PDT (RF) group of Kaiser et al's[17] study].
Figure 5A shows the results that included the monotherapy group and PDT (SF) group of Kaiser et al's[17] study. In the PDT (SF) subgroup, the proportion of patients who gained ≥15 letters of BCVA in the monotherapy group was increased compared with the combination group (OR: 1.53; 95%CI: 1.02 to 2.31; P=0.04), with no significant heterogeneity (I2=0%, P=0.96). The PDT (RF) subgroup was not significantly different between the two groups (OR: 1.23; 95%CI: 0.73 to 2.08; P=0.43), with no significant heterogeneity (I2=0%, P=0.74). In the total result, the proportion of patients who gained ≥15 letters of BCVA in the monotherapy group was increased compared with the combination group (OR: 1.41; 95%CI: 1.02 to 1.95; P=0.04), with no significant heterogeneity (I2=0%, P=0.95).
Figure 5B shows the results that included the monotherapy group and PDT (RF) group of Kaiser et al's[17] study. The PDT (SF) subgroup was not significantly different between the two groups (OR: 1.53; 95%CI: 0.85 to 2.77; P=0.16), with no evidence of heterogeneity (I2=0%, P=0.77). In the PDT (RF) subgroup, the proportion of patients who gained ≥15 letters of BCVA in the monotherapy group was increased compared with the combination group (OR: 1.57; 95%CI: 1.06 to 2.31; P=0.02), with no significant heterogeneity (I2=0%, P=0.50). In the total results, the proportion of patients who gained ≥15 letters of BCVA in the monotherapy group was increased compared with the combination group (OR: 1.56; 95%CI: 1.13 to 2.15; P=0.007), with no significant heterogeneity (I2=0%, P=0.78).
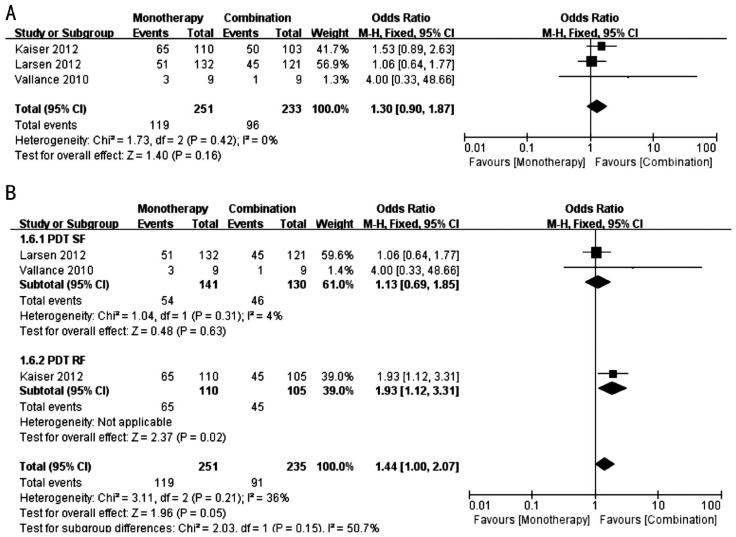
A subgroup analysis, sensitivity analysis and discussion were also conducted in the analysis of the effect of the proportion of patients who gained ≥10, 5, 0 letters of BCVA at 12th month. With the exception of the proportion of patients who gained ≥10 letters in the monotherapy group, which was increased compared with the PDT (RF) combination group in Kaiser's study (Figure 6B), all other comparisons were not significantly different (Figures 6–8).
Figure 6. Forest plots of proportion of patients who gained ≥10 letters of BCVA at 12th month.
A: Comparison between monotherapy group and combination group [including monotherapy group and PDT (SF) group of Kaiser et al's[17] study]; B: Comparison between monotherapy group and combination group [including monotherapy group and PDT (RF) group of Kaiser et al's[17] study].
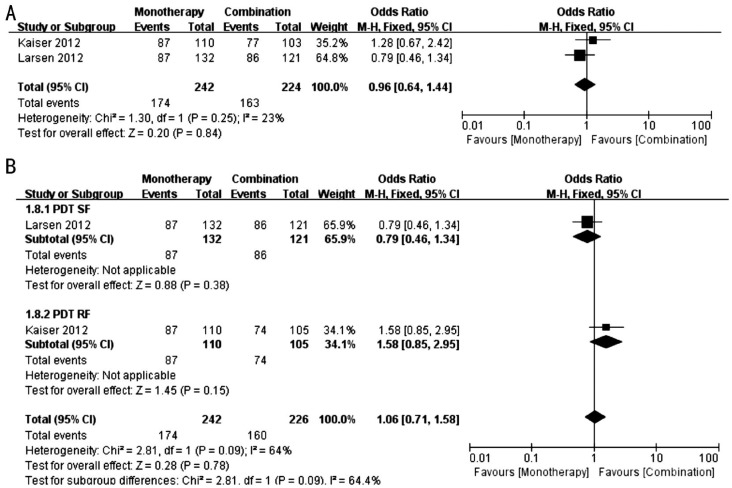
Figure 8. Forest Plots of proportion of patients who gained ≥0 letters of BCVA at 12th month.
A: Comparison between monotherapy group and combination group [including monotherapy group and PDT (SF) group of Kaiser et al's[17] study]; B: Comparison between monotherapy group and combination group [including monotherapy group and PDT (RF) group of Kaiser et al's[17] study].
Figure 7. Forest plots of proportion of patients who gained ≥5 letters of BCVA at 12th month.
A: Comparison between monotherapy group and combination group [including monotherapy group and PDT (SF) group of Kaiser et al's[17] study]; B: Comparison between monotherapy group and combination group [including monotherapy group and PDT (RF) group of Kaiser et al's[17] study].
DISCUSSION
The aim of our study is to compare the efficacy of PDT and intravitreal anti-VEGF monotherapy versus PDT and anti-VEGF combination treatment in AMD. Three of the eight included studies compared reduced fluence PDT and anti-VEGF combination therapy with anti-VEGF monotherapy, whereas the other three included studies compared standard fluence PDT and anti-VEGF combination therapy with anti-VEGF monotherapy; Kaiser et al's[17] study compared both the standard and reduced fluence PDT and anti-VEGF combination therapy with anti-VEGF monotherapy. Thus, we performed subgroup and sensitivity analyses and discussed the results that included the monotherapy group and the PDT (SF) group of Kaiser's study and the results that included the monotherapy group and the PDT (RF) group of Kaiser's study. When we included the different groups of Kaiser's study in the sensitivity analysis, the results were different in the different subgroups.
Kaiser et al's[17] study demonstrated that monotherapy may lead to a better BCVA compared with combination therapy. In Krebs et al's study[16], the patients in the monotherapy group gained a mean of 5.1 letters, whereas the patients in the combination group lost a mean of 7.1 letters at 12th month after the accepted different treatments. Larsen et al's[15] study also indicated that monotherapy is superior in VA recovery. Vallance et al's[13] study exhibited similar results, but the difference was not significant. However, in Costagiliola et al's[19] study, the improvement in VA was greater in the combination group compared with the monotherapy group, but the difference was not statistically significant either. When a Meta-analysis was performed for these studies, we determined that both the combination therapy and the anti-VEGF monotherapy improved the BCVA at 12th month. In the sensitivity analysis, in the PDT (SF) subgroup of the results that included the monotherapy group and PDT (SF) group of Kaiser et al's[17] study, the patients in the anti-VEGF monotherapy group exhibited a better BCVA compared with the combination group at 12th month. Furthermore, the Meta analysis of the proportions of patients who gained ≥15, 10, 5, 0 letters in the BCVA indicated that more patients gained ≥15 letters in the BCVA in the monotherapy group compared with the combination group. This finding may indicate that anti-VEGF monotherapy may improve BCVA better than the combination treatment. However, this finding may also be affected by the design of Kaiser et al's[17] study: ranibizumab monotherapy was administered monthly and 12 times in total in the monotherapy group, which may lead to a better therapeutic effect compared with other studies while patients in other studies accepted less treatments. However, in the results that included the monotherapy group and PDT (SF) group of Kaiser's study, there was no significant difference in the BCVA between the two groups in the PDT (RF) subgroup and the total result. In the results that included the monotherapy group and PDT (RF) group of Kaiser's study, both subgroups were not significantly different in the BCVA between the two groups. There were no differences in the ratios of the patients who gained more BCVA ≥10, 5, 0 letters between the two groups.
In all included studies, the CRT was reduced at 12th month using both approaches. However, it remains unclear which approach is better. For example, in Kaiser et al's[17] study, a decrease in the mean CRT was identified for the ranibizumab monotherapy (172.2 µm), PDT (SF) combination (151.7 µm), and PDT (RF) combination (140.9 µm) groups from the baseline at 12th month (P=0.400 and 0.050 for the SF and RF combination groups, respectively). In Costagliola et al's[19] study, the mean change from baseline in the center point thickness was approximately 107 µm in the monotherapy group and 77 µm in the combination group through 12mo (P=0.002 and 0.003 for the monotherapy and combination groups, respectively). However, in Krebs et al's[16] study, the retinal thickness decreased 81.49 µm in the monotherapy group and 138.2 µm in the combination group (P value was not provided). In Larsen et al's[15] study, the mean change in the CRT at 12th month was reduced 115.3 µm in the combination group and 107.7 µm in the monotherapy group, which was not significantly different between the groups. In Vallance et al's[13] study, the mean CRT was reduced by 138 µm in the combination group and 103 µm in the monotherapy group (P=0.57). In the sensitivity analysis, in the results that included the monotherapy group and PDT (SF) group of Kaiser's study, there was no significant difference between the two groups. In the results that included the monotherapy group and PDT (RF) group of Kaiser's study, the CRT was thinner in the combination group compared with the monotherapy group in the PDT (SF) subgroup, and the result was opposite in the PDT (RF) subgroup. This finding is likely a result of the fluence of the PDT or was affected by the design of Kaiser's study as previously discussed. In the total result, there was no significant difference in these two groups. Overall, the findings were opposite and were not significantly different in several included studies; thus, additional studies with larger sample sizes should be conducted to determine which approach has a better effect on the CRT.
The treatment approaches are different in several included studies; thus, we performed a Meta-analysis for four studies that used the same approach and had complete data to compare the number of treatments between the two groups. Krebs et al's[16] study considered that a significant reduction in the number of required intravitreal injections may be achieved by additional PDT treatment; however, we did not identify a significant difference in the number of treatments between the two groups in the total result. In Larsen et al's[15] study, the patients received 4.8 ranibizumab injections, on average, in the combination group versus 5.1 injections, on average, in the monotherapy group in 12mo; the mean number of ranibizumab retreatments was 1.9 in the combination group and 2.2 in the monotherapy group (P=0.14). In Vallance et al's[13] study, after the initial injection, both groups required a mean of 1.3 retreatments with ranibizumab over the 12mo of the trial. Datseris et al[18] and Costagliola et al's [19] studies indicated that low fluence PDT and anti-VEGF combination therapy significantly reduced the reinjection rate compared with monotherapy. Williams et al's[12] study also considered that low fluence PDT and anti-VEGF combination therapy may lead to fewer reinjections; however, the difference was not significant based on a Chi-square test. Additional studies with larger sample sizes should be performed to determine whether low fluence PDT and anti-VEGF combination therapy may reduce the number of injections.
In Si et al's[20] study, they compared a combination of ranibizumab and photodynamic therapy with ranibizumab monotherapy in the treatment of AMD and obtained similar results compared with the current study. The differences between our studies are that we included ranibizumab and bevacizumab as the anti-VEGF therapy and eight studies were included in our analysis.
In conclusion, we determined that anti-VEGF monotherapy is better for visual recovery compared with combination therapy. As some included studies suggested, low fluence PDT combined with anti-VEGF therapy may reduce the frequency of reinjection. Fewer injections may be useful to reduce the risk of side effects and the financial burden to patients; however, it may not improve VA similar to anti-VEGF monotherapy. To determine the best therapeutic schedule, it is advisable to consider the patient's demand and the doctor's proper judgments based on the practical situation of the patient. We did not identify significant differences in the other indexes between the two therapeutic approaches. More researches with lager sample size should be performed to study on the effect of the two therapy approaches on CRT and number of injections.
Acknowledgments
Yao Tong conceived of the study and participated in its design; analysis and interpretation of data and drafted the manuscript. Zhao-Yang Wang participated in design the study. Ke-Ke Zhao and Dong Feng participated in performed the statistical analysis. Manas Biswal helped to amend the mistakes of grammar. Pei-Quan Zhao and Yun Zhang helped to revise the manuscript.
Foundations: Supported by the National Natural Science Funds of China (No.81371040); Shanghai Pujiang Program (No.15PJD028).
Conflicts of Interest: Tong Y, None; Zhao KK, None; Feng D, None; Biswal M, None; Zhao PQ, None; Wang ZY, None; Zhang Y, None.
REFERENCES
- 1.VanNewkirk MR, Weih LM, McCarty CA, Taylor HR. Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the visual impairment project. Ophthalmology. 2001;108(5):960–967. doi: 10.1016/s0161-6420(01)00554-1. [DOI] [PubMed] [Google Scholar]
- 2.Harvey PT. Common eye diseases of elderly people: identifying and treating causes of vision loss. Gerontology. 2003;49(1):1–11. doi: 10.1159/000066507. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DS, O'Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J, Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki R, Yasuda M, Song SJ, Chen SJ, Jonas JB, Wang JJ, Mitchell P, Wong TY. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser PK. Verteporfin photodynamic therapy and antiangiogenic drugs: potential for combination therapy in exudative age-related macular degeneration. Curr Med Res Opin. 2007;23(3):477–487. doi: 10.1185/030079907X167624. [DOI] [PubMed] [Google Scholar]
- 6.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157(1):135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaide RF. Rationale for combination therapy in age-related macular degeneration. Retina (Philadelphia, Pa) 2009;29(6 Suppl):S5–S7. doi: 10.1097/IAE.0b013e3181ad237a. [DOI] [PubMed] [Google Scholar]
- 8.Verteporfin in Photodynamic Therapy Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 9.Bressler NM, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol. 2001;119(2):198–207. [PubMed] [Google Scholar]
- 10.Kaiser PK, Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Verteporfin therapy of subfoveal choroidal neovascularization in age related macular degeneration: 5-year results of two randomized clinical trials with an open-label extension: TAP report no 8. Graefes Arch Clin Exp Ophthalmol. 2006;244(9):1132–1142. doi: 10.1007/s00417-005-0199-9. [DOI] [PubMed] [Google Scholar]
- 11.Wolf-Schnurrbusch UE, Brinkmann CK, Berger L, Wolf S. Effects of combination therapy with verteporfin photodynamic therapy and ranibizumab in patients with age-related macular degeneration. Acta Ophthalmol. 2011;89(6):585–590. doi: 10.1111/j.1755-3768.2009.01747.x. [DOI] [PubMed] [Google Scholar]
- 12.Williams PD, Callanan D, Solley W, Avery RL, Pieramici DJ, Aaberg T. A prospective pilot study comparing combined intravitreal ranibizumab and half-fluence photodynamic therapy with ranibizumab monotherapy in the treatment of neovascular age-related macular degeneration. Clin Ophthalmol. 2012;6:1519–1525. doi: 10.2147/OPTH.S31010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallance JH, Johnson B, Majid MA, Banerjee S, Mandal K, Bailey CC. A randomised prospective doublemasked exploratory study comparing combination photodynamic treatment and intravitreal ranibizumab vs intravitreal ranibizumab monotherapy in the treatment of neovascular age-related macular degeneration. Eye (Lond) 2010;24(10):1561–1567. doi: 10.1038/eye.2010.84. [DOI] [PubMed] [Google Scholar]
- 14.Lim JY, Lee SY, Kim JG, Lee JY, Chung H, Yoon YH. Intravitreal bevacizumab alone versus in combination with photodynamic therapy for the treatment of neovascular maculopathy in patients aged 50 years or older: 1-year results of a prospective clinical study. Acta Ophthalmol. 2012;90(1):61–67. doi: 10.1111/j.1755-3768.2009.01841.x. [DOI] [PubMed] [Google Scholar]
- 15.Larsen M, Schmidt-Erfurth U, Lanzetta P, Wolf S, Simader C, Tokaji E, Pilz S, Weisberger A, MONT BLANC Study Group Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results. Ophthalmology. 2012;119(5):992–1000. doi: 10.1016/j.ophtha.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Krebs I, Vécsei Marlovits V, Bodenstorfer J, Glittenberg C, Ansari Shahrezaei S, Ristl R, Binder S. Comparison of Ranibizumab monotherapy versus combination of Ranibizumab with photodynamic therapy with neovascular age-related macular degeneration. Acta Ophthalmol. 2013;91(3):e178–e183. doi: 10.1111/aos.12018. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser PK, Boyer DS, Cruess AF, Slakter JS, Pilz S, Weisberger A, DENALI Study Group Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month results of the DENALI study. Ophthalmology. 2012;119(5):1001–1010. doi: 10.1016/j.ophtha.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Datseris I, Kontadakis GA, Diamanti R, Datseris I, Pallikaris IG, Theodossiadis P, Tsilimbaris MK. Prospective comparison of low-fluence photodynamic therapy combined with intravitreal bevacizumab versus bevacizumab monotherapy for choroidal neovascularization in age-related macular degeneration. Semin Ophthalmol. 2015;30(2):112–117. doi: 10.3109/08820538.2013.833268. [DOI] [PubMed] [Google Scholar]
- 19.Costagliola C, Romano MR, Rinaldi M, dell'Omo R, Chiosi F, Menzione M, Semeraro F. Low fluence rate photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Br J Ophthalmol. 2010;94(2):180–184. doi: 10.1136/bjo.2009.159343. [DOI] [PubMed] [Google Scholar]
- 20.Si JK, Tang K, Bi HS, Guo DD, Guo JG, Du YX, Cui Y, Pan XM, Wen Y, Wang XR. Combination of ranibizumab with photodynamic therapy vs ranibizumab monotherapy in the treatment of age-related macular degeneration: a systematic review and meta-analysis of randomized controlled trials. Int J Ophthalmol. 2014;7(3):541–549. doi: 10.3980/j.issn.2222-3959.2014.03.28. [DOI] [PMC free article] [PubMed] [Google Scholar]