Abstract
Particulate pollution from anthropogenic and natural sources is a severe problem in China. Sulfur and oxygen isotopes of aerosol sulfate (δ34Ssulfate and δ18Osulfate) and water-soluble ions in aerosols collected from 2012 to 2014 in Beijing are being utilized to identify their sources and assess seasonal trends. The mean δ34S value of aerosol sulfate is similar to that of coal from North China, indicating that coal combustion is a significant contributor to atmospheric sulfate. The δ34Ssulfate and δ18Osulfate values are positively correlated and display an obvious seasonality (high in winter and low in summer). Although an influence of meteorological conditions to this seasonality in isotopic composition cannot be ruled out, the isotopic evidence suggests that the observed seasonality reflects temporal variations in the two main contributions to Beijing aerosol sulfate, notably biogenic sulfur emissions in the summer and the increasing coal consumption in winter. Our results clearly reveal that a reduction in the use of fossil fuels and the application of desulfurization technology will be important for effectively reducing sulfur emissions to the Beijing atmosphere.
In recent years, most parts of northern and eastern China have been strongly affected by haze events, arousing public and official concerns1,2. Haze results from a high concentration of submicron (10–100 nm) particles with high scattering coefficients and high relative humidity in the atmosphere tends to aggravate these effects, which may cause visibility reduction to 3–4 km3. Sulfate serving as cloud condensation nuclei4,5 is an important component of atmospheric aerosols6. Such sulfate aerosols influence the surface temperature of the Earth7, acid rain formation8,9, and human health10, playing a key role in environmental chemistry and climate change6,11,12. Therefore, knowing the source(s) of aerosol sulfate and its transport and transformation in the atmosphere will form the necessary base for improving the air quality in Beijing.
Stable isotopes of sulfur and oxygen are used for identifying potential sources of atmospheric sulfate13,14,15,16. The sulfur isotope ratios (δ34S) of sea salt sulfate, dimethyl sulfide (DMS) and anthropogenic sulfate are +21‰17, +18.9 to +20.3‰18 and +1 to +11‰19,20, respectively. However, the δ34S values of biogenic sulfur released from soils and wetlands are much lighter, ranging from −10 to −2‰21,22,23. The relative contributions for each source of sulfate can be calculated according to their distinct δ34S values16,20. Moreover, if sources of sulfate cannot be distinguished due to their similar sulfur isotope values, the oxygen isotopic composition of sulfate (δ18Osulfate) can provide additional evidence24. δ18Osulfate is influenced by source variation and mixing25. Hence, paired sulfur and oxygen isotopes a powerful tool for constraining the source of sulfate24,26.
Sulfur and oxygen isotopes have also been used to investigate the oxidation processes of SO2 and transport pathways of sulfur in the atmosphere14,27,28. Sulfur isotopes show distinctive isotope fractionations for different oxidation reactions of SO228,29,30,31. Sulfur isotope enrichment in sulfate can be caused by heterogeneous oxidation of SO229,30, while sulfur isotope depletion in sulfate may be generated from homogeneous oxidation31. However, the sulfur isotope fractionation factor α = 1.14 for homogeneous oxidation has been estimated based on RRKM transition-state theory32. Furthermore, a recent study shows that only transition metal-catalysed oxidation of SO2 can lead to the enrichment of lighter sulfur isotopes in sulfate, compared with oxidation by OH, H2O2 and O328. In addition, the oxygen isotope composition of sulfate may reflect oxidation processes27, since oxygen isotopes of sulfate and water are not exchanged under ambient conditions25. Primary sulfate formed in the emission source may be enriched in heavy oxygen isotope (δ18O > +20‰)33,34, while the δ18O values of secondary sulfate range from ~−10‰ to +20‰25. The proportion of primary and secondary sulfate can be estimated based on an oxygen isotope apportionment model14.
Seasonal variations in sulfur and oxygen isotopes of atmospheric sulfate and SO2 have been observed25,35,36,37,38. The sulfur isotope ratios often show low values in summer and high values in winter13,35,37, while it displays an opposite seasonal tendency in Central Europe38. The seasonality of sulfur isotopes is attributed to several possible factors, including seasonal changes of the sources of atmospheric sulfur35,38, seasonal variations in the proportion of oxidation pathways37,39, seasonal changes in the isotope fractionation factors due to temperature-dependence36 and seasonality in reservoir effects39. The δ18O value of sulfate in precipitation also shows seasonal variations, which could be related to changes in the oxygen isotopic composition of local precipitation25.
In this work, the sources of sulfate aerosols in Beijing and their formation, migration and transformation are studied by using stable sulfur and oxygen isotopes, which may provide a theoretical basis for air quality improvement in the city of Beijing. Furthermore, the seasonality in sulfur and oxygen isotopes of sulfate aerosols is discussed in order to better understand isotope fractionation during the oxidation of SO2.
Results
Water-soluble ions
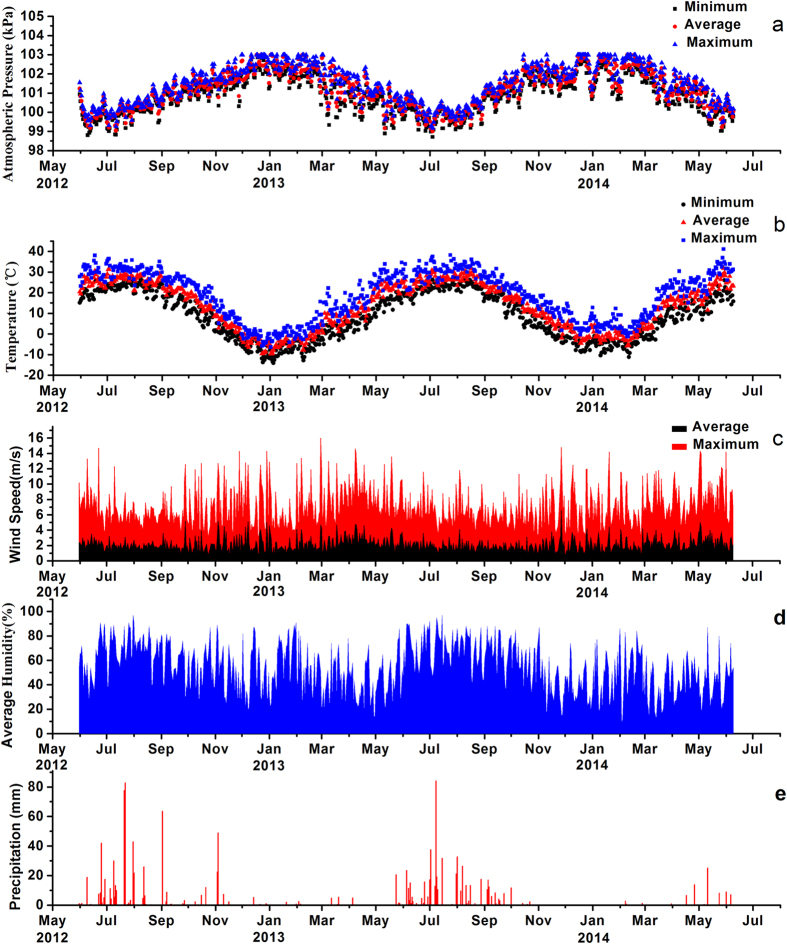
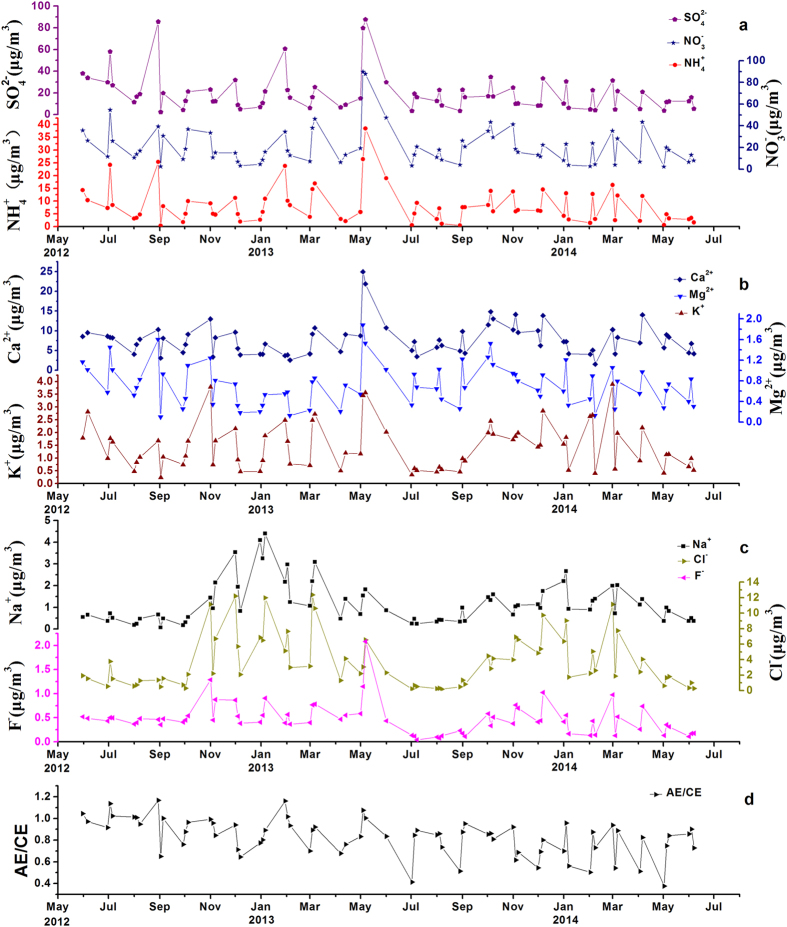
Total suspended particulates (TSP) were sampled on a 3-day basis from May 31, 2012 to June 10, 2014 (n = 73) in Beijing (Fig. 1). Figure 2 shows the meteorological data during the sampling. The concentrations of water-soluble inorganic ions (WSII) in the TSP during the sampling period are shown in Table 1 and Fig. 3. The predominant ions in the TSP were  ,
, 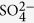 ,
, 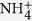 and Ca2+, which together account for ~85% of WSII. Nitrate and sulfate are the dominant anions, varying from 2.3 to 89.8 μg/m3 (mean = 21.1 ± 17.5 μg/m3, n = 70) and from 2.4 to 87.7 μg/m3 (mean = 20.0 ± 18.0 μg/m3, n = 70), respectively; while the concentrations of
and Ca2+, which together account for ~85% of WSII. Nitrate and sulfate are the dominant anions, varying from 2.3 to 89.8 μg/m3 (mean = 21.1 ± 17.5 μg/m3, n = 70) and from 2.4 to 87.7 μg/m3 (mean = 20.0 ± 18.0 μg/m3, n = 70), respectively; while the concentrations of 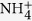 and Ca2+ range from 0.3 to 38.5 μg/m3 (mean = 8.3 ± 7.2 μg/m3, n = 70) and from 1.5 to 25.0 μg/m3 (mean = 7.8 ± 4.1 μg/m3, n = 70), respectively. The average concentrations of F−, Na+, K+ and Mg2+ are lower than 2.0 μg/m3.
and Ca2+ range from 0.3 to 38.5 μg/m3 (mean = 8.3 ± 7.2 μg/m3, n = 70) and from 1.5 to 25.0 μg/m3 (mean = 7.8 ± 4.1 μg/m3, n = 70), respectively. The average concentrations of F−, Na+, K+ and Mg2+ are lower than 2.0 μg/m3.
Figure 1. Sampling location in Beijing, China.
Total suspended particulates (TSP) were sampled on a 3-day basis from May 31, 2012 to Jun 10, 2014 (n = 73) at the roof of a building (around 10 meters above ground level) in the site. Modified after Guo et al. (2013)56. Reprinted from Environmental Pollution, 176(2013), Guo et al., Tracing the source of Beijing soil organic carbon: A carbon isotope approach, 208–214, Copyright (2013), with permission from Elsevier.
Figure 2. Meteorological parameters from May 2012 to June 2014 in Beijing, China (data from China Meteorological Data Network57).
Table 1. Statistical parameters of stable isotopes (δ34Ssulfate and δ18Osulfate) and water-soluble ions in Beijing aerosols (SD represents standard deviation).
| Species | Spring | Summer | Autumn | Winter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Average | SD | Range | Average | SD | Range | Average | SD | Range | Average | SD | |
| Na+(μg/m3) | 0.4–1.8 | 1.1 | 0.5 | 0.1–1.0 | 0.5 | 0.2 | 0.2–2.1 | 1.1 | 0.6 | 0.7–4.4 | 2.0 | 1.1 |
| NH4+(μg/m3) | 0.6–38.5 | 9.9 | 12.6 | 0.3–25.3 | 7.4 | 7.0 | 1.8–14.0 | 7.5 | 3.7 | 1.4–23.8 | 8.8 | 5.9 |
| K+(μg/m3) | 0.4–3.6 | 1.6 | 1.1 | 0.2–2.8 | 1.0 | 0.6 | 0.7–3.8 | 1.8 | 0.8 | 0.4–3.9 | 1.6 | 1.0 |
| Mg2+(μg/m3) | 0.2–1.9 | 0.8 | 0.5 | 0.1–1.6 | 0.8 | 0.4 | 0.3–1.5 | 0.9 | 0.4 | 0.1–1.2 | 0.5 | 0.3 |
| Ca2+(μg/m3) | 4.7–24.9 | 11.3 | 6.9 | 3.1–10.7 | 6.8 | 2.3 | 3.4–14.8 | 9.8 | 3.7 | 1.5–13.8 | 6.2 | 3.1 |
| F−(μg/m3) | 0.1–2.1 | 0.7 | 0.6 | 0.0–0.5 | 0.3 | 0.2 | 0.3–1.3 | 0.6 | 0.3 | 0.1–1.0 | 0.5 | 0.3 |
| Cl−(μg/m3) | 0.6–6.6 | 2.8 | 1.7 | 0.2–3.8 | 1.0 | 0.8 | 0.3–11.1 | 4.3 | 3.1 | 1.7–12.4 | 6.5 | 3.5 |
| NO3−(μg/m3) | 2.3–89.8 | 30.7 | 32.7 | 2.5–54.6 | 19.6 | 13.9 | 9.3–43.4 | 25.7 | 12.2 | 2.7–46.3 | 16.3 | 12.6 |
| SO42−(μg/m3) | 3.7–87.7 | 25.2 | 31.3 | 2.4–85.7 | 22.1 | 18.5 | 4.3–34.7 | 16.6 | 8.2 | 4.0–60.7 | 17.4 | 13.5 |
| δ34Ssulfate | 4.5–9.2 | 6.4 | 1.5 | 3.4–7.0 | 5.0 | 0.9 | 5.0–9.4 | 6.8 | 1.3 | 7.1–11.3 | 8.6 | 0.9 |
| δ18Osulfate | 7.2–12.5 | 10.6 | 1.7 | 3.8–12.3 | 9.3 | 2.1 | 8.2–12.6 | 11.1 | 1.3 | 11.3–16.1 | 13.4 | 1.4 |
Figure 3. Variations in concentrations of water-soluble ions and ratios of the anion equivalents (AE) to the cation equivalents (CE) in atmospheric aerosols from May 2012 to June 2014 in Beijing, China.
The concentrations of WSII show seasonal changes (Fig. 3). 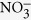 , F− and Ca2+ concentrations are relatively high in spring and autumn compared to summer and winter. The
, F− and Ca2+ concentrations are relatively high in spring and autumn compared to summer and winter. The 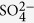 concentration is slightly lower in winter than in summer. The concentrations of Cl− and Na+ are much higher in winter than in summer, spring and autumn.
concentration is slightly lower in winter than in summer. The concentrations of Cl− and Na+ are much higher in winter than in summer, spring and autumn.
The ion balance as an indicator of the acidity of the aerosols was calculated using the ratios of the anion equivalents (AE) to the cation equivalents (CE) in TSP samples2 (Fig. 3d). The ratios of AE/CE during the sampling period range from 0.38 to1.17 with a mean value of 0.83 ± 0.17 (n = 70). Most of ratios of AE/CE are lower than 1.0, which shows no seasonal changes.
Sulfur and oxygen isotopes
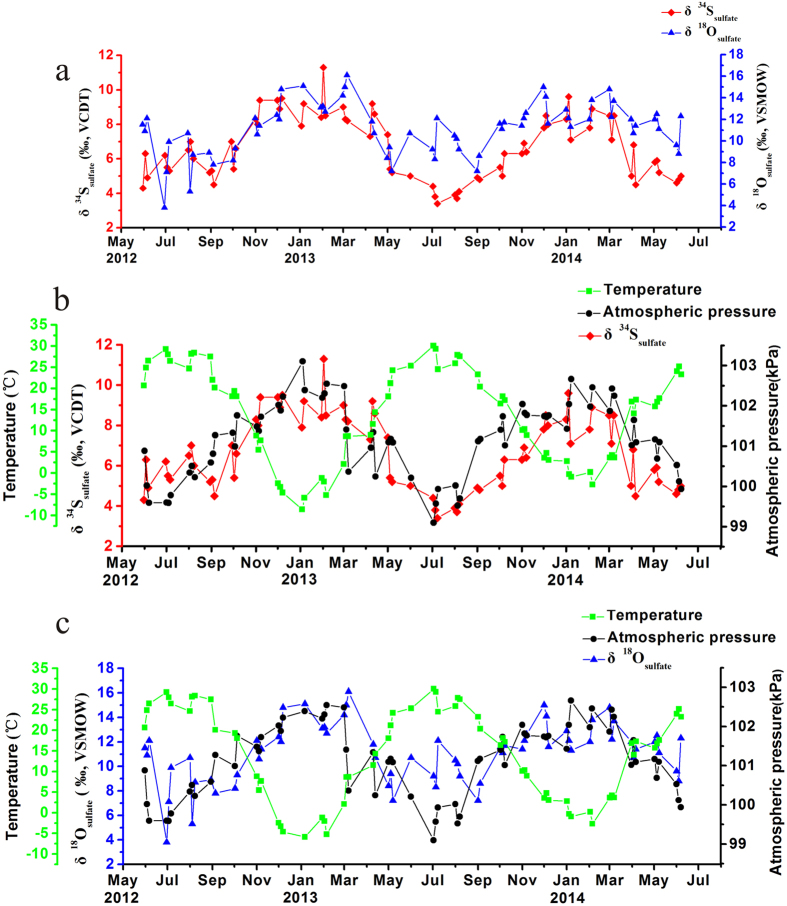
Sulfur and oxygen isotopic compositions of sulfate (δ34Ssulfate and δ18Osulfate) in Beijing aerosols are presented in Fig. 4a. The δ34Ssulfate values range from 3.4 to 11.3‰ with a mean value of 6.6 ± 1.8‰ (n = 70). The δ18Osulfate values vary from 3.8 to 16.1‰ around a mean value of 11.1 ± 2.4‰ (n = 66). Both isotope records reveal seasonal changes, i.e., low values in summer and high values in winter (Fig. 4a). The average values of δ34Ssulfate in spring, summer, autumn and winter are 6.4 ± 1.5‰ (n = 12), 5.0 ± 0.9‰ (n = 24), 6.8 ± 1.3‰ (n = 12) and 8.6 ± 0.9‰ (n = 22), respectively. The mean values of δ18Osulfate are 10.6 ± 1.7‰ (n = 11), 9.3 ± 2.1‰ (n = 23), 11.1 ± 1.3‰ (n = 11) and 13.4 ± 1.4‰ (n = 21) for spring, summer, autumn and winter, respectively.
Figure 4. Variations in δ34Ssulfate and δ18Osulfate values of atmospheric aerosols from May 2012 to June 2014 in Beijing, China and their relationship with the mean temperature (72 h) and mean atmospheric pressure (72 h).
Discussion
In order to determine the relationship between ions in TSP (as well as the sulfur and oxygen isotopic compositions), correlation coefficients are calculated (Table 2). A strong correlation is observable between 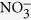 and
and 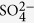 (r = 0.83), implying the similar chemical behavior in cloud processes40.
(r = 0.83), implying the similar chemical behavior in cloud processes40. 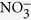 and
and 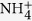 (r = 0.90) as well as
(r = 0.90) as well as 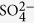 and
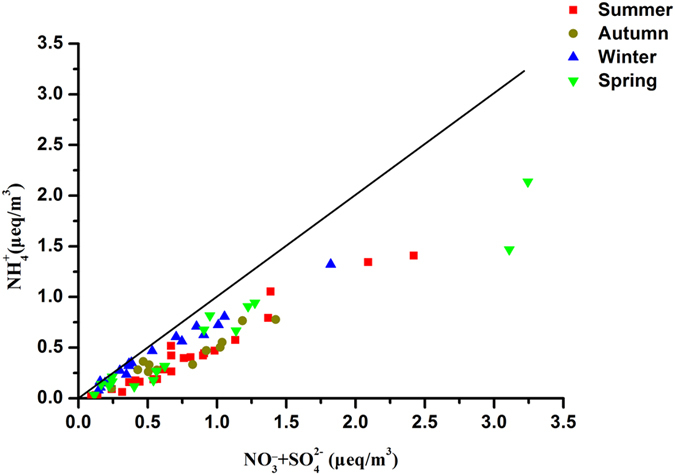
and 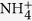 (r = 0.93) show strong correlations, indicating that NH4NO3 and (NH4)2SO4 may be two main species in TSP. At lower concentrations, the ratio of
(r = 0.93) show strong correlations, indicating that NH4NO3 and (NH4)2SO4 may be two main species in TSP. At lower concentrations, the ratio of 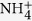 to
to 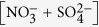 almost equals 1.0 (Fig. 5), which implies an ammonium-rich environment for these samples. However, the ratio of
almost equals 1.0 (Fig. 5), which implies an ammonium-rich environment for these samples. However, the ratio of 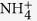 to
to 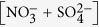 is lower than 1.0 at increasing
is lower than 1.0 at increasing 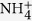 concentrations, suggesting that nitrate may exist in other chemical forms besides NH4NO3. A significant correlation is also found between Ca2+ and Mg2+ (r = 0.81), which may be an indicator of terrestrial sources (e.g. soil and miral dust). In addition, strong correlations between Ca2+ and
concentrations, suggesting that nitrate may exist in other chemical forms besides NH4NO3. A significant correlation is also found between Ca2+ and Mg2+ (r = 0.81), which may be an indicator of terrestrial sources (e.g. soil and miral dust). In addition, strong correlations between Ca2+ and 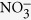 (r = 0.81) as well as Mg2+ and
(r = 0.81) as well as Mg2+ and 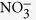 (r = 0.84) indicate heterogeneous chemistry on mineral dust41.
(r = 0.84) indicate heterogeneous chemistry on mineral dust41.
Table 2. Correlation coefficients for water-soluble ions and stable isotopes of atmospheric aerosol in Beijing (*Significant at 0.05 level; **Significant at 0.01 level).
| Na+ | NH4+ | K+ | Ca2+ | Mg2+ | F− | Cl− | NO3− | SO42− | δ34S | δ18O | |
| Na+ | 1.00 | ||||||||||
| NH4+ | 0.29** | 1.00 | |||||||||
| K+ | 0.41** | 0.72** | 1.00 | ||||||||
| Ca2+ | 0.14 | 0.64** | 0.70** | 1.00 | |||||||
| Mg2+ | 0.06 | 0.72** | 0.68** | 0.81** | 1.00 | ||||||
| F− | 0.47** | 0.63** | 0.73** | 0.69** | 0.47** | 1.00 | |||||
| Cl− | 0.83** | 0.39** | 0.66** | 0.31** | 0.21* | 0.66** | 1.00 | ||||
| NO3− | 0.15 | 0.90** | 0.72** | 0.81** | 0.84** | 0.62** | 0.26* | 1.00 | |||
| SO42− | 0.16 | 0.93** | 0.61** | 0.63** | 0.75** | 0.54** | 0.19 | 0.83** | 1.00 | ||
| δ34S | 0.66** | −0.01 | 0.26* | −0.17 | −0.27* | 0.34** | 0.67** | −0.19 | −0.13 | 1.00 | |
| δ18O | 0.55** | −0.04 | 0.27* | −0.15 | −0.24 | 0.10 | 0.57** | −0.14 | −0.26* | 0.56** | 1.00 |
Figure 5. Ammonium equivalent concentration as a function of sum of the sulfate and nitrate equivalent concentrations in TSP from Beijing.
The relative importance of mobile versus stationary sources of nitrogen and sulfur in the atmosphere can be indicated by the mass ratio of 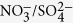 42,43. A high
42,43. A high 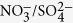 ratio implies that mobile sources of the pollutants are predominant over stationary sources42. However, the majority of the ratios are lower than 0.8 during the heating period, suggesting the predominance of stationary sources (emission from coal combustion) over mobile sources of pollutants.
ratio implies that mobile sources of the pollutants are predominant over stationary sources42. However, the majority of the ratios are lower than 0.8 during the heating period, suggesting the predominance of stationary sources (emission from coal combustion) over mobile sources of pollutants.
The Cl− and Na+ concentrations are positively correlated (r = 0.83) and display an increase in winter (Fig. 3c), suggesting they have a common source. As the prevailing winds in Beijing’s winter are north and northwest, a significant contribution from seawater in Beijing aerosols can be ruled out. In addition, the ratio of Cl− to Na+ in winter is 3.2 ± 1.2, which is different from the ratio in seawater of 1.1743. Studies reported that high Cl− concentration in Beijing aerosols may be related to coal combustion2,43. During combustion, complex changes in coal particles may cause the vaporization of volatile elements, including sodium44. Sodium vaporised from coal during combustion, may be present in the gas phase or bound in particulate aerosols in the flue gases, which can be emitted to the atmosphere44. Significant correlations exist as well for δ34S and Cl− (r = 0.67) and for δ34S and Na+ (r = 0.66), which provides additional evidence for a common origin and the significant contribution of coal combustion to the atmospheric sulfate pool.
Water-soluble sulfate in aerosol is derived from both primary (e.g. sea salt, dust, fly ash) and secondary (e.g. oxidation of SO2 and H2S) sulfates14,37, all characterized by their own distinct isotopic composition. Consequently, the sulfur isotopic composition of sulfate in Beijing aerosols reveals a mixture from different sulfate sources with high and low δ34S values (Fig. 6). Volcanism as a source of sulfate in Beijing aerosols can be excluded with no volcanic activities in North China45. Also, a significant contribution from sea salt is not very likely as suggested by the low concentration of Na+ (mean = 1.2 ± 1.0 μg/m3, n = 70). In addition, the weak correlation between 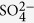 and Na+ (r = 0.16) as well as
and Na+ (r = 0.16) as well as 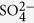 and Cl−(r = 0.19) also suggest that seawater sulfate provides only a very small contribution to the aerosols in Beijing.
and Cl−(r = 0.19) also suggest that seawater sulfate provides only a very small contribution to the aerosols in Beijing.
Figure 6. δ34Ssulfate and sulfate concentration in aerosols of Beijing compared to a ternary mixing model.
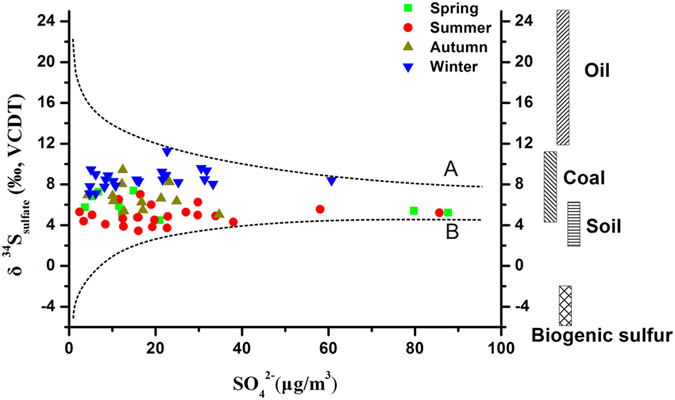
Curve A represents a mixture between sulfur in oil with a δ34S value of 20.5‰ and sulfur in coal with a δ34S value of 6.6‰; Curve B represents a mixture between biogenic sulfur with a δ34S value of −10‰ and sulfur in coal with a δ34S value of 6.6‰.
Some SO2 emissions from industry and transportation ultimately originate from oil combustion. It has been estimated that 15.3 million tons of petroleum products were consumed in Beijing in 2012, including 4.4 million tons by industry and 6.1 million tons from transportation46. The sulfur content in oil from North China ranges from 0.1% to 0.6% and its δ34S value varies between 13.7‰ and 24.2‰ (mean = 20.5‰, n = 4)47. The emission rate of SO2 from oil combustion is relatively constant with almost no seasonal change in the consumption of petroleum. Therefore, the SO2 emissions from oil combustion in the study area are a steady source of sulfate in the aerosol that is characterized by a relatively high δ34S value.
The contribution from coal combustion to the atmospheric sulfur pool of China is very significant15,37. In Beijing, the total consumption of coal was 22.7 million tons in 2012 based on the China Energy Statistical Yearbook (2013)46. With an average sulfur content of 0.77wt.% in coal from North China48, an estimated 174.8 thousand tons of sulfur were released into Beijing’s atmosphere in 2012 assuming that there is no desulfurization implemented into the coal combustion processes. During the winter, the consumption of coal in China is increasing due to heating, which will affect the overall δ34S value of atmospheric sulfate. It has been shown before that the sulfur isotopic composition of atmospheric sulfur in the different regions of China is closely related to the sulfur isotope signature of the coal used in the respective area37. Reported δ34S values for coal from North China (mean = +6.6‰) are higher than for coal from South China (mean = −0.32‰)47,48. The average δ34S value of sulfate in aerosol from Beijing (6.6 ± 1.8‰, n = 70), determined in this study, is similar to that for coal used in North China, indicating that coal combustion is a significant, if not the most important contributor to the atmospheric sulfate pool.
Biogenic sulfur from wetlands and soils is an important source for atmospheric sulfate, especially in the summer22,23, and δ34S values for biogenic sulfur are generally negative, ranging from −10 to −2‰21,22,23. Low δ34S values recorded for Beijing summer aerosols may, thus, reflect a larger contribution from biogenic sulfur, in contrast to the winter season where low temperatures greatly attenuate (or inhibit) microbial activity in wetlands and soils.
Sulfates in aerosols can also originate from terrigenous sources (e.g. soil or mineral dusts). In this study, a significant correlation can be seen between 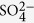 and Ca2+ concentrations (r = 0.63), which suggests a terrigenous contribution to the aerosol sulfate pool. Ca2+ as a reference element for mineral dust is used for calculating the proportion of this contribution (fts) to sulfate in the aerosol by following the equation49:
and Ca2+ concentrations (r = 0.63), which suggests a terrigenous contribution to the aerosol sulfate pool. Ca2+ as a reference element for mineral dust is used for calculating the proportion of this contribution (fts) to sulfate in the aerosol by following the equation49:
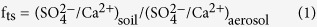 |
where the ratio of 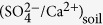 is 0.1850.
is 0.1850.
The δ34S values of sulfate in soils from North China vary from 2.0 to 7.0‰51,52.
Assuming that the seasonal change in the δ34S values of aerosol sulfate reflects temporal variations in the proportional ctributions from different sulfate sources, these proportions can be estimated using the following equations16,20:
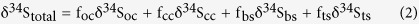 |
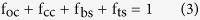 |
where foc, fcc, fbs and fts represent the fractional contributions of oil combustion, coal combustion, biogenic source and terrigenous source, respectively, and δ34Soc, δ34Scc, δ34Sbs and δ34Sts represent the corresponding δ34S value of each sulfur source.
In winter, biogenic sulfur is likely negligible since the soil microbial activity is weak. Hence, we assume that in winter, fbs equals 0. In addition, the contribution of oil combustion is relatively constant throughout the year as there is no seasonal variation in oil consumption. By solving equations (1)–(3), , , the contributions of sulfate sources can be calculated (results listed in Table 3), assuming a δ34Soc value of 20.5 ± 4.8‰47, a δ34Scc value of 6.6 ± 3‰47,48, a δ34Sbs value of −6 ± 4‰21,22,23 and a δ34Sts value of 4.5 ± 3.5‰51,52 as the respective δ34S signature of each sulfur source. The results show that the average contributions of coal combustion, oil combustion, biogenic sulfur and terrigenous sulfate to sulfate in aerosols of Beijing are 49.6 ± 7.5%, 17.6 ± 8.6%, 19.8 ± 9.9% and 10.1 ± 6.2%, respectively, but exhibiting strong seasonal differences (Table 3).
Table 3. Relative contributions of sulfate sources to the sulfate in aerosols of Beijing (nd. represents no data).
| Date | Sample | δ34S (‰) | δ18O (‰) | Contribution of terrigenous sulfate (%) | Contribution of biogenic sulfur (%) | Contribution of coal combustion (%) | Contribution of oil combustion (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Min. | Max. | Min. | Max. | |||||
| 2012/5/31 | Fu30 | 4.3 | 11.5 | 4.0 | 30.5 | 50.7 | 33.7 | 41.8 | 11.5 | 23.7 |
| 2012/6/6 | Fu32 | 4.9 | 12.1 | 5.1 | 26.0 | 46.9 | 36.6 | 45.3 | 11.5 | 23.7 |
| 2012/6/30 | Fu42 | 6.2 | 3.8 | 5.2 | 15.3 | 38.2 | 45.1 | 55.9 | 11.5 | 23.7 |
| 2012/7/3 | Fu43 | 5.5 | 7.1 | 2.6 | 21.3 | 43.5 | 42.4 | 52.5 | 11.5 | 23.7 |
| 2012/7/6 | Fu44 | 5.3 | 9.9 | 5.5 | 22.9 | 44.3 | 38.7 | 47.9 | 11.5 | 23.7 |
| 2012/8/1 | Fu54 | 6.5 | 10.7 | 6.3 | 12.9 | 36.0 | 46.2 | 57.1 | 11.5 | 23.7 |
| 2012/8/4 | Fu55 | 7.0 | 5.3 | 7.1 | 8.8 | 32.5 | 48.8 | 60.5 | 11.5 | 23.7 |
| 2012/8/8 | Fu56 | 6.0 | 8.7 | 7.4 | 16.8 | 38.9 | 42.1 | 52.2 | 11.5 | 23.7 |
| 2012/8/30 | Fu63 | 5.2 | 8.9 | 2.2 | 23.9 | 45.7 | 40.6 | 50.3 | 11.5 | 23.7 |
| 2012/9/2 | Fu64 | 5.3 | nd. | 22.7 | 19.9 | 38.5 | 27.2 | 33.7 | 11.5 | 23.7 |
| 2012/9/5 | Fu66 | 4.5 | 7.8 | 7.4 | 28.7 | 48.6 | 32.5 | 40.3 | 11.5 | 23.7 |
| 2012/9/29 | Fu75 | 7.0 | nd. | 18.7 | 7.4 | 29.2 | 40.6 | 50.3 | 11.5 | 23.7 |
| 2012/10/2 | Fu76 | 5.4 | 8.2 | 9.2 | 21.5 | 42.4 | 36.9 | 45.6 | 11.5 | 23.7 |
| 2012/10/5 | Fu77 | 6.6 | 9.3 | 7.7 | 11.9 | 34.9 | 45.8 | 56.8 | 11.5 | 23.7 |
| 2012/11/1 | Fu86 | 8.3 | 12.1 | 10.1 | 0.0 | 23.7 | 54.7 | 67.7 | 11.5 | 23.7 |
| 2012/11/4 | Fu87 | 8.0 | 10.6 | 5.0 | 1.0 | 26.7 | 56.9 | 70.4 | 11.5 | 23.7 |
| 2012/11/7 | Fu88 | 9.4 | 11.4 | 12.0 | 0.0 | 15.5 | 61.1 | 75.6 | 11.5 | 23.7 |
| 2012/12/1 | Fu96 | 9.4 | 12.4 | 5.4 | 0.0 | 0.0 | 54.9 | 68.0 | 15.3 | 31.5 |
| 2012/12/4 | Fu97 | 8.9 | 12.0 | 11.0 | 0.0 | 0.0 | 53.2 | 65.9 | 13.3 | 27.4 |
| 2012/12/7 | Fu98 | 9.5 | 14.8 | 13.8 | 0.0 | 0.0 | 46.1 | 57.1 | 16.8 | 34.6 |
| 2013/1/3 | Fu107 | 7.9 | nd. | 6.8 | 0.0 | 0.0 | 64.8 | 80.2 | 7.5 | 15.4 |
| 2013/1/6 | Fu108 | 9.2 | 15.1 | 5.6 | 0.0 | 0.0 | 55.8 | 69.1 | 14.7 | 30.1 |
| 2013/1/30 | Fu116 | 8.4 | 13.1 | 1.1 | 0.0 | 0.0 | 66.4 | 82.2 | 9.7 | 19.9 |
| 2013/2/2 | Fu117 | 11.3 | 13.2 | 3.1 | 0.0 | 0.0 | 43.0 | 53.3 | 25.3 | 51.9 |
| 2013/2/5 | Fu118 | 8.5 | 12.7 | 2.9 | 0.0 | 0.0 | 64.1 | 79.4 | 10.2 | 21.0 |
| 2013/3/1 | Fu127 | 9.0 | 14.2 | 12.2 | 0.0 | 0.0 | 51.1 | 63.3 | 14.2 | 29.2 |
| 2013/3/4 | Fu128 | 8.3 | 15.0 | 10.3 | 0.0 | 0.0 | 58.2 | 72.0 | 10.2 | 21.0 |
| 2013/3/7 | Fu129 | 8.2 | 16.1 | 7.6 | 0.0 | 0.0 | 61.5 | 76.1 | 9.4 | 19.4 |
| 2013/4/7 | Fu141 | 7.3 | nd. | 12.6 | 6.0 | 29.3 | 46.6 | 57.8 | 11.5 | 23.7 |
| 2013/4/13 | Fu144 | 8.6 | 10.7 | 17.9 | 0.0 | 18.9 | 51.7 | 64.0 | 11.5 | 23.7 |
| 2013/5/1 | Fu155 | 7.4 | 8.4 | 10.6 | 5.3 | 29.1 | 48.9 | 60.5 | 11.5 | 23.7 |
| 2013/5/4 | Fu156 | 5.4 | 9.4 | 5.6 | 21.9 | 43.5 | 39.4 | 48.8 | 11.5 | 23.7 |
| 2013/5/7 | Fu157 | 5.2 | 7.2 | 4.5 | 23.5 | 44.9 | 39.1 | 48.4 | 11.5 | 23.7 |
| 2013/6/1 | Fu169 | 5.0 | 10.7 | 6.5 | 25.1 | 45.9 | 36.1 | 44.7 | 11.5 | 23.7 |
| 2013/7/2 | Fu331 | 4.4 | 9.2 | 26.8 | 26.4 | 43.0 | 18.7 | 23.1 | 11.5 | 23.7 |
| 2013/7/5 | Fu332 | 3.8 | 8.3 | 6.7 | 34.2 | 53.2 | 28.6 | 35.4 | 11.5 | 23.7 |
| 2013/7/8 | Fu333 | 3.4 | 12.1 | 3.9 | 37.8 | 56.6 | 28.0 | 34.7 | 11.5 | 23.7 |
| 2013/8/1 | Fu341 | 3.9 | 10.5 | 8.3 | 33.5 | 52.3 | 27.9 | 34.5 | 11.5 | 23.7 |
| 2013/8/4 | Fu342 | 3.7 | 10.2 | 6.1 | 35.1 | 54.0 | 28.4 | 35.1 | 11.5 | 23.7 |
| 2013/8/7 | Fu343 | 4.1 | 9.2 | 13.4 | 31.0 | 49.3 | 25.8 | 31.9 | 11.5 | 23.7 |
| 2013/9/1 | Fu352 | 4.9 | 7.2 | 7.8 | 25.9 | 46.2 | 34.5 | 42.7 | 11.5 | 23.7 |
| 2013/9/4 | Fu353 | 4.8 | 8.6 | 4.8 | 27.2 | 47.8 | 35.8 | 44.4 | 11.5 | 23.7 |
| 2013/10/2 | Fu362 | 5.5 | 11.6 | 12.1 | 20.2 | 40.8 | 35.6 | 44.0 | 11.5 | 23.7 |
| 2013/10/5 | Fu363 | 5.0 | 11.1 | 7.7 | 24.4 | 45.0 | 35.8 | 44.3 | 11.5 | 23.7 |
| 2013/10/8 | Fu364 | 6.3 | 11.7 | 14.0 | 13.7 | 35.2 | 39.3 | 48.6 | 11.5 | 23.7 |
| 2013/11/1 | Fu373 | 6.3 | 11.4 | 7.4 | 14.1 | 36.8 | 44.3 | 54.8 | 11.5 | 23.7 |
| 2013/11/4 | Fu374 | 6.9 | 12.1 | 25.3 | 6.8 | 27.4 | 35.7 | 44.3 | 11.5 | 23.7 |
| 2013/11/7 | Fu375 | 6.4 | 12.6 | 16.7 | 12.4 | 33.6 | 38.2 | 47.3 | 11.5 | 23.7 |
| 2013/12/1 | Fu383 | 7.8 | 15.0 | 22.1 | 0.0 | 0.0 | 50.9 | 63.0 | 8.7 | 17.8 |
| 2013/12/4 | Fu384 | 8.5 | 14.1 | 13.3 | 0.0 | 0.0 | 54.1 | 67.0 | 11.4 | 23.5 |
| 2013/12/7 | Fu385 | 8.0 | 11.6 | 7.5 | 0.0 | 0.0 | 62.9 | 77.8 | 8.5 | 17.4 |
| 2014/1/1 | Fu393 | 8.3 | 12.9 | 12.7 | 0.0 | 0.0 | 55.7 | 69.0 | 10.6 | 21.8 |
| 2014/1/4 | Fu394 | 9.6 | 12.1 | 4.3 | 0.0 | 0.0 | 54.4 | 67.3 | 16.5 | 33.8 |
| 2014/1/7 | Fu395 | 7.1 | 11.3 | 12.1 | 0.0 | 0.0 | 65.3 | 80.9 | 4.1 | 8.4 |
| 2014/2/2 | Fu403 | 7.8 | 12.0 | 15.1 | 0.0 | 0.0 | 57.2 | 70.9 | 8.1 | 16.7 |
| 2014/2/5 | Fu404 | 8.9 | 13.8 | 4.1 | 0.0 | 0.0 | 59.6 | 73.8 | 12.8 | 26.3 |
| 2014/3/1 | Fu411 | 8.5 | 14.8 | 5.9 | 0.0 | 0.0 | 61.0 | 75.5 | 10.7 | 22.1 |
| 2014/3/4 | Fu412 | 7.1 | 12.2 | 15.7 | 0.0 | 0.0 | 61.9 | 76.7 | 4.4 | 9.0 |
| 2014/3/7 | Fu413 | 8.5 | 13.7 | 6.9 | 0.0 | 0.0 | 60.2 | 74.5 | 10.7 | 22.0 |
| 2014/4/3 | Fu422 | 6.8 | 10.7 | 23.7 | 7.7 | 28.5 | 36.3 | 44.9 | 11.5 | 23.7 |
| 2014/4/6 | Fu423 | 4.5 | 11.4 | 12.0 | 28.1 | 47.2 | 29.2 | 36.2 | 11.5 | 23.7 |
| 2014/5/2 | Fu432 | 5.8 | 12.0 | 27.9 | 15.4 | 33.9 | 26.7 | 33.1 | 11.5 | 23.7 |
| 2014/5/5 | Fu433 | 5.9 | 12.5 | 14.0 | 16.7 | 37.6 | 36.9 | 45.7 | 11.5 | 23.7 |
| 2014/5/8 | Fu434 | 5.2 | 11.1 | 12.3 | 22.3 | 42.5 | 33.7 | 41.7 | 11.5 | 23.7 |
| 2014/6/1 | Fu441 | 4.6 | 9.6 | 6.4 | 27.9 | 48.1 | 34.0 | 42.1 | 11.5 | 23.7 |
| 2014/6/4 | Fu443 | 4.8 | 8.8 | 7.7 | 26.7 | 46.9 | 33.9 | 42.0 | 11.5 | 23.7 |
| 2014/6/7 | Fu444 | 5.0 | 12.3 | 14.1 | 23.7 | 43.3 | 31.1 | 38.6 | 11.5 | 23.7 |
It has been shown that the seasonal change in the proportion of different oxidation pathways of atmospheric sulfur dioxide to aerosol sulfate may also lead to a seasonality in δ34Ssulfate37,39. The sulfur isotope fractionation factors (α) for different oxidation reactions of SO2 are distinct to each other28,29,30,31. Experimental studies show that the fractionation factor for heterogeneous oxidation is 1.0165 ± 0.001 at 25 °C29,30. The fractionation factor during gas-phase oxidation by the OH radical (homogeneous oxidation) is 0.991, which is determined by using an ab initio quantum mechanical calculation31. In contrast, results from laboratory measurements show that the fractionation factors during homogeneous oxidation and aqueous oxidation by H2O2 and O3 are greater than 1.0, while the fractionation factor for oxidation by transition metal ion catalysis (TMI-catalysis) is αFe = 0.9894 ± 0.0043 at 19 °C28. A recent study shows that the changing proportion among oxidation by TMI-catalysis, OH and H2O2 was the main cause for the seasonality in the δ34S values of sulfate versus SO239. However, sulfate from aqueous SO2 oxidation by TMI-catalysis only accounts for 9–17% of the global sulfate production53. Thus, it cannot resolve the difference in δ34Ssulfate observed between summer and winter in Beijing aerosol sulfate.
A strong negative correlation between mean air temperature and δ34Ssulfate in aerosol (r = −0.83, Fig. 4b) is apparent. This could indicate that the seasonality in δ34S of atmospheric sulfate may result from a seasonal variation in sulfur isotope fractionation factors influenced by temperature36,54. Caron et al. revealed that during the oxidation of SO2 to sulfate, the δ34Ssulfate value increases by 0.08–0.15‰ with a decrease in temperature of 1 °C54. Considering a temperature difference of 30 °C between summer and winter in Beijing, this effect may, thus, cause a seasonal variation in δ34Ssulfate of 2.4 to 4.35‰. The maximum difference between the δ34S values in summer and winter, however, is 7.9‰, which is substantially higher. Consequently, the temperature effect alone cannot explain the seasonal difference in δ34S. In addition, a recent study shows that for the oxidation of SO2 by OH radicals, H2O2 and transition metal ion catalysis (TMI-catalysis), the coefficients of the temperature effect on the fractionation factors are 0.004 ± 0.015‰°C1−, 0.085 ± 0.004‰°C1− and 0.237 ± 0.004‰°C1−, respectively39. This indicates that the temperature effect on the fractionation factor is negligible for the OH radical pathway, but can be more significant for the oxidation by TMI-catalysis and H2O239. A temperature difference of 30 °C between summer and winter could account for a maximum seasonal isotopic difference via TMI-catalysis of 1.2‰, again insufficient for the observed maximal seasonality in δ34Ssulfate.
In addition to temperature, a positive correlation between atmospheric pressure and δ34Ssulfate values can be observed (r = 0.69, Fig. 4b). Leung et al. evaluated the sulfur isotope fractionation factor (α) during the oxidation of SO2 by OH radicals based on the RRKM transition-state theory, and found that the factor is a function of pressure and temperature, i.e., α = 1.1646 + 0.0198(P/Torr)0.1769 −0.3092[(T/K)/1000]32. The maximum difference in atmospheric pressure between summer and winter is around 4 kpa (30 Torr), which may cause a change in the δ34Ssulfate value by 0.45‰ for the OH oxidation of SO2. It suggests that the change in atmospheric pressure may have only a minor effect on the variation in δ34Ssulfate.
Considering that changes in the sulfur isotopic fractionation resulting from meteorological boundary conditions (i.e. air temperature and atmospheric pressure) are insufficient to explain the observed seasonality in δ34Ssulfate, respective variations are more likely reflecting temporal changes in the proportional contributions from different sulfate sources during different times of the year, as has been reported from other areas before38. It is proposed here that during the summer, biogenic sulfur emissions which are characterized by negative δ34S values, are an important source of atmospheric sulfate22,23, leading a decrease in the overall δ34S value of aerosol sulfate in the summer. In contrast, the increase in coal consumption for heating during winter time (and with it an increase in the proportional importance of this contribution to the overall sulfate pool) will cause a shift to a more positive overall δ34S value for aerosol sulfate in the winter.
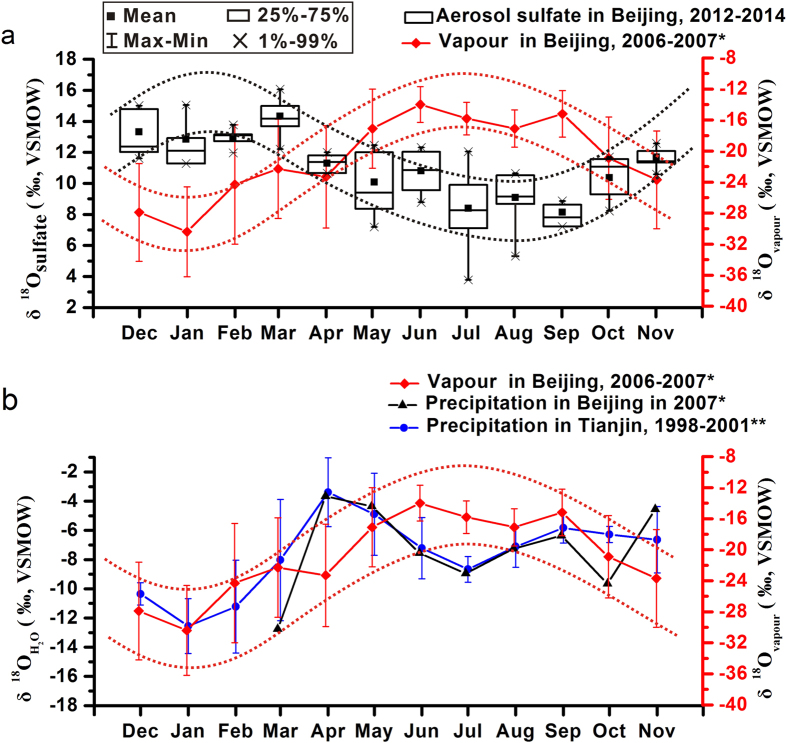
Evidence in particular for the latter, i.e. the increasing coal combustion in the winter, comes from the oxygen isotopic composition of sulfate aerosol (δ18Osulfate). It also exhibits strong seasonal changes, with the highest values in winter and low values in summer (Fig. 4a). Previous studies have shown that high-temperature combustion processes, thereby oxidizing the sulfur dioxide to sulfate, will lead to 18O enriched aerosol sulfate25,33, and δ18Osulfate values of +35 to +40‰ have been reported33. Consequently, a higher contribution from coal combustion in the winter will cause more positive δ18Osulfate values of sulfate aerosols. In addition, the lack of a positive correlation between the δ18Osulfate and the δ18OH2O (Fig. 7) supports the assumption that sulfate formed at high temperatures, rather than heterogeneous, i.e. aqueous oxidation of SO2 is the important process during the winter, because the latter would result in a positive correlation between δ18Osulfate and the δ18OH2O55. This explains the obvious decoupling of both oxygen isotope records (Fig. 7) and argues that the observed increase in δ18Osulfate seen in the winter is reflecting most likely a source effect, i.e. the high-temperature combustion of coal, generating an 18O enriched primary sulfate aerosol.
Figure 7. Relationship between δ18Osulfate in aerosols and δ18O in atmospheric water vapor and precipitation58,59.
The “*” represents the data from Wen et al. (2010)58; the “**” represents the data from GNIP Database59.
Although an influence of the meteorological boundary conditions, i.e. air temperature and atmospheric pressure, on the observed seasonality in the sulfur and oxygen isotope compositions of sulfate in Beijing aerosol cannot be ruled out, the tight coupling of the temporal trend in δ34Ssulfate and δ18Osulfate is best explained as a variation related to the source of aerosol sulfate. Due to their pronounced seasonality, the two strongest variables in this respect are contributions from biogenic sulfur emissions, limited to the summer, and increasing coal consumption in the winter. In particular in the winter, coal combustion is the main contributor to the Beijing aerosol sulfate pool as evidenced by the paired shift to high δ34S and δ18O values for sulfate in aerosol. Moreover, the temporal variation in PM2.5 concentration during the sampling period exhibits an obvious seasonal trend (Fig. 8) that is similar to the temporal records of aerosol sulfate sulfur isotopic composition and the maximum contribution from coal combustion to the aerosol sulfate pool, i.e. high in winter and low in summer. This strongly underlines the conclusion that coal combustion is the major contributor to the Beijing aerosol (sulfate) pool. While the biogenic sulfur emissions are likely more difficult to control, a reduction in the usage of coal, especially the high sulfur coal, and the application of desulfurization measures for coal powered industries will be important in reducing sulfur emissions to the Beijing atmosphere, which will ultimately improve Beijing’s air quality.
Figure 8. Variations in δ34Ssulfate values of sulfate aerosols, maximum contribution of coal combustion and PM2.5 concentration in Beijing, May 2012–June 2014.
The data of PM2.5 concentration are from Beijing US Embassy60.
Methods
Sample Collection
Total suspended particulates (TSP) were sampled on a 3-day basis from May 31, 2012 to June 10, 2014 (n = 73) on top of the roof (around 10 meters above ground level) of the Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing (Fig. 1). The samples were collected using a high volume air sampler (Qingdao Laoshan, KC1000) with a flow rate of 1.0 m3 min−1 and pre-combusted (450 °C for 6 h) quartz fiber filters (20 cm × 25 cm, Pallflex). After sampling, a pre-combusted glass jar (150 ml) with Teflon lined screw cap was used to store each filter in a freezer at −20 °C until geochemical analyses.
The meteorological data during sampling were obtained from China Meteorological Data Sharing Service System (http://cdc.nmic.cn/home.do, Fig. 2). The daily average values of air temperature, air humidity, wind speed and atmospheric pressure were calculated based on the observation data at 2.00 a.m., 8.00 a.m., 14.00 p.m. and 20.00 p.m. The detection limits of air temperature, precipitation, air humidity, wind speed and atmospheric pressure are 0.1 °C, 0.1 mm, 1%, 0.1 m/s and 0.1 hPa, respectively.
Analytical Methods
Using a circular hole-puncher, two circular pieces with a diameter of 47 mm were cut from each filter (20 cm × 25 cm), shredded and soaked in 200 ml of Milli-Q water for 30 minutes added by ultrasonification14. Subsequently, the filters were kept in water overnight in order to quantitatively extract the water-soluble ions. The quartz filter fibers were removed by filtration using 0.45 mm millipore filters. 10 ml of the solution were taken for ion concentration analysis, and the remaining solution was acidified to pH<2 by addition of HCl solution and heated to boiling. The dissolved sulfate in the solution was precipitated as barite by adding 25 ml of 8.5% BaCl2 solution and the glass beaker with the solution was kept at 80 °C for additional 3 hours. The BaSO4 precipitates were collected on 0.22 μm acetate millipore filters and rinsed with 150 mL Milli-Q water to remove Cl−15. The millipore filters with the precipitates were dried in an oven at 45 °C for 48 hours. Then the S and O isotopic compositions of the BaSO4 precipitates were analyzed. The blank samples were also analyzed after the same method.
The concentrations of the water-soluble ions (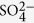 ,
, 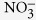 , Cl−, F−,
, Cl−, F−, 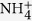 , Na+, K+, Ca2+ and Mg2+) were analyzed by ion chromatography (Dionex ICS900). An IonPacTM AS23 column (4 × 250 mm, Dionex) and an IonPacTM CS12A column (4 × 250 mm, Dionex) were used f the determination of anions and cations, respectively. The 4.5 mM sodium carbonate and 0.8 mM sodium bicarbonate were used as the eluent for anions; the 20 mM methansulfonic acid (MSA) was used as the eluent for cations. The detection limits were below 0.07 μg/m3 for cations and anions.
, Na+, K+, Ca2+ and Mg2+) were analyzed by ion chromatography (Dionex ICS900). An IonPacTM AS23 column (4 × 250 mm, Dionex) and an IonPacTM CS12A column (4 × 250 mm, Dionex) were used f the determination of anions and cations, respectively. The 4.5 mM sodium carbonate and 0.8 mM sodium bicarbonate were used as the eluent for anions; the 20 mM methansulfonic acid (MSA) was used as the eluent for cations. The detection limits were below 0.07 μg/m3 for cations and anions.
The sulfur isotope measurements (δ34S) were performed at the Institute of Geographic Sciences and Natural Resources Research, Chinese Academy of Sciences, using an Elemental Analyzer (EA) coupled to a Delta V Advantage Isotope Ratio Mass Spectrometer (IRMS). Results are expressed in the standard delta notation relative to the Vienna Canyon Diablo Troilite standard (VCDT). The reproducibility of the measurements was better than ±0.2‰. The oxygen isotope values (δ18O) were measured by a Thermal Conversion Elemental Analyzer (TCEA) coupled to an IRMS. The results are reported in the standard delta notation against the Vienna Standard Mean Ocean Water (VSMOW). The reproducibility of the measurements was better than ±0.3‰.
Sulfate Source Apportionment Calculation
Assuming that the seasonal change in δ34Saerosol sulfate reflects temporal variations in the proportional contributions from different sulfate sources, these proportions can be estimated by using the following equations16,20,49:
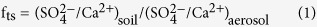 |
where the ratio of 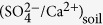 is 0.1850;
is 0.1850;
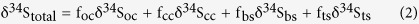 |
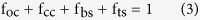 |
where foc, fcc, fbs and fts represent the fractional contributions of oil combustion, coal combustion, biogenic source and terrigenous source, respectively, and δ34Soc, δ34Scc, δ34Sbs and δ34Sts represent the corresponding δ34S value of each sulfur source. We assume a δ34Soc value of 20.5 ± 4.8‰47, a δ34Scc value of 6.6 ± 3‰47,48, a δ34Sbs value of −6 ± 4‰21,22,23 and a δ34Sts value of 4.5 ± 3.5‰51,52 as the respective δ34S signature of each sulfur source.
First, the fractional contribution from a terrigenous source has been calculated based on the equation (1) and the ratio of (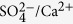 ) measured in aerosol.
) measured in aerosol.
Second, in winter, biogenic sulfur emissions are greatly attenuated to likely negligible since the soil microbial activity is weak. Hence, we assume that in winter, fbs equals 0. Now, the fractional contributions from oil combustion and from coal combustion in winter can be calculated according to equations (2) and (3).
Third, the contribution from oil combustion is relatively constant throughout the year as there is no seasonal variation in oil consumption. Assuming that the average value of fcc in winter equals the fractional contribution from oil combustion in spring, summer and autumn, the contributions of coal combustion and biogenic source in these seasons can also be computed.
Additional Information
How to cite this article: Han, X. et al. Using stable isotopes to trace sources and formation processes of sulfate aerosols from Beijing, China. Sci. Rep. 6, 29958; doi: 10.1038/srep29958 (2016).
Acknowledgments
This work was funded by National High Technology Research and Development Program of China (973 Program: No. 2014CB238906), the Joint NSFC-ISF Research Program (No. 4151101008), Project of Chinese Academy of Sciences (No. XDB15020401), the Sino-German Center (No. GZ1055), NSFC (No. 41250110528), and the Feature Institute Program of the Chinese Academy of Sciences (Comprehensive Technical Scheme and Integrated Demonstration for Remediation of Soil and Groundwater in Typical Area). The authors thank the US Department of State for providing the Beijing air quality data used in this analysis. The observational data are not fully verified or validated.
Footnotes
Author Contributions X.H. performed the data analyses and wrote the manuscript; Q.G. and C.L. contributed to the conception of the study and contributed to the manuscript; Q.G., P.F., H.S., J.H. and M.P. helped to perform the analyses and provided constructive discussions; L.W., J.Y., R.W. and L.T. performed the experiments; H.R. collected the samples.
References
- Huang R.-J. et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 514, 218–222 (2014). [DOI] [PubMed] [Google Scholar]
- Gao J. et al. The variation of chemical characteristics of PM2.5 and PM10 and formation causes during two haze pollution events in urban Beijing, China. Atmos. Environ. 107, 1–8 (2015). [Google Scholar]
- Shen X. J. et al. Characterization of submicron aerosols and effect on visibility during a severe haze-fog episode in Yangtze River Delta, China. Atmos. Environ. 120, 307–316 (2015). [Google Scholar]
- Matsumoto K., Tanaka H., Nagao I. & Ishizaka Y. Contribution of particulate sulfate and organic carbon to cloud condensation nuclei in the marine atmosphere. Geophys. Res. Lett. 24, 655–658 (1997). [Google Scholar]
- Rose D. et al. Calibration and measurement uncertainties of a continuous-flow cloud condensation nuclei counter (DMT-CCNC): CCN activation of ammonium sulfate and sodium chloride aerosol particles in theory and experiment. Atmos. Chem. Phys. 8, 1153–1179 (2008). [Google Scholar]
- IPCC. Climate Change 2007: the Physical Science Basis. In: Solomons S. et al. (Eds), Contribution of Working Group 1 to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge (2007). [Google Scholar]
- Charlson R. J. et al. Climate forcing by anthropogenic aerosols. Science. 255, 423–430 (1992). [DOI] [PubMed] [Google Scholar]
- Singer A., Shamay Y., Fried M. & Ganor E. Acid rain on Mt Carmel, Israel. Atmos. Environ. 27, 2287–2293 (1993). [Google Scholar]
- Zhou G. & Tazaki K. Seasonal variation of gypsum in aerosol and its effect on the acidity of wet precipitation on the Japan Sea side of Japan. Atmos. Environ. 30, 3301–3308 (1996). [Google Scholar]
- Pope C.A. & Dockery D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manage. 56, 709–742 (2006). [DOI] [PubMed] [Google Scholar]
- Andreae M. O. & Rosenfeld D. Aerosol-cloud-precipitation interactions. Part 1. The nature and sources of cloud-active aerosols. Earth-Sci. Rev. 89, 13–41 (2008). [Google Scholar]
- Abbatt J. P. D. et al. Solid ammonium sulfate aerosols as ice nuclei: A pathway for cirrus cloud formation. Science 313, 1770–1773 (2006). [DOI] [PubMed] [Google Scholar]
- Inomata Y., Ohizumi T., Take N., Sato K. & Nishikawa M. Transboundary transport of anthropogenic sulfur in PM2.5 at a coastal site in the Sea of Japan as studied by sulfur isotopic ratio measurement. Sci. Total Environ. 553, 617–625 (2016). [DOI] [PubMed] [Google Scholar]
- Norman A. L. et al. Aerosol sulphate and its oxidation on the Pacific NW coast: S and O isotopes in PM2.5. Atmos. Environ. 40, 2676–2689 (2006). [Google Scholar]
- Guo Z. et al. Identification of sources and formation processes of atmospheric sulfate by sulfur isotope and scanning electron microscope measurements. J. Geophys. Res. 115, D00K07 (2010). [Google Scholar]
- Lin C. T., Baker A. R., Jickells T. D., Kelly S. & Lesworth T. An assessment of the significance of sulphate sources over the Atlantic Ocean based on sulphur isotope data. Atmos. Environ. 62, 615–621 (2012). [Google Scholar]
- Tostevin R. et al. Multiple sulfur isotope constraints on the modern sulfur cycle. Earth Planet. Sci. Lett. 396, 14–21 (2014). [Google Scholar]
- Amrani A., Said-Ahmad W., Shaked Y. & Kiene R. P. Sulfur isotope homogeneity of oceanic DMSP and DMS. Proc. Natl. Acad. Sci. USA 110, 18413–18418 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R. et al. Large sulfur-isotope anomaly in nonvolcanic sulfate aerosol and its implications for the Archean atmosphere. Proc. Natl. Acad. Sci. USA 111, 11979–11983 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman A. L. et al. Sources of aerosol sulphate at Alert: apportionment using stable isotopes. J. Geophys. Res. 104, 11619–11631 (1999). [Google Scholar]
- Liu G. S., Hong Y. T., Piao H. C. & Zeng Y. Q. Study on sources of sulfur in atmospheric particulate matter with stable isotope method. China Environ. Sci. 12(6), 426–429 (in Chinese with English abstract, 1996). [Google Scholar]
- Nriagu J.O., Holdway D.A. & Coker R.D. Biogenic sulfur and the acidity of rainfall in remote areas of Canada. Science 237, 1189–1192 (1987). [DOI] [PubMed] [Google Scholar]
- Mast M. A., Turk J. T., Ingersoll G. P., Clow D. W. & Kester C. L. Use of stable sulfur isotopes to identify sources of sulfate in Rocky Mountain snowpacks. Atmos. Environ. 35, 3303–3313 (2001). [Google Scholar]
- Proemse B. C., Mayer B. & Fenn M. E. Tracing industrial sulfur contributions to atmospheric sulfate deposition in the Athabasca oil sands region, Alberta, Canada. Appl. Geochem. 27, 2425–2434 (2012). [Google Scholar]
- Holt B. D. & Kumar R. Oxygen isotope fractionation for understanding the sulphur cycle. In: Krouse H., Grinenko V. (Eds), Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment. John Wiley & Sons, pp. 27–41(Chapter 2, 1991). [Google Scholar]
- Schiff S. L., Spoelstra J., Semkin R. G. & Jeffries D. S. Drought induced pulses of from a Canadian shield wetland: use of δ34S and δ18O in to determine sources of sulfur. Appl. Geochem. 20, 691–700 (2005). [Google Scholar]
- Shaheen R. et al. Tales of volcanoes and El-Niño southern oscillations with the oxygen isotope anomaly of sulfate aerosol. Proc. Natl. Acad. Sci. USA 110, 17662–17667 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. et al. Sulfur isotope fractionation during oxidation of sulfur dioxide: gas-phase oxidation by OH radicals and aqueous oxidation by H2O2, O3 and iron catalysis. Atmos. Chem. Phys. 12, 407–423 (2012). [Google Scholar]
- Eriksen T. E. Sulfur isotope-effects. I. Isotopic-exchange coefficient for sulfur isotopes 34S-32S in the system SO2g-HSO3−aq at 25, 35, and 45 °C. Acta Chem. Scand. 26, 573–580 (1972a). [Google Scholar]
- Eriksen T. E. Sulfur isotope-effects. III. Enrichment of 34S by chemical exchange between SO2g and aqueous-solutions of SO2. Acta Chem. Scand. 26, 975–979 (1972b). [Google Scholar]
- Tanaka N., Rye D. M., Xiao Y. & Lasaga A. C. Use of Stable Sulfur Isotope Systematics for Evaluating Oxidation Reaction Pathways and in-Cloud Scavenging of Sulfur-Dioxide in the Atmosphere. Geophys. Res. Lett. 21, 1519–1522 (1994). [Google Scholar]
- Leung F. Y., Colussi A. J. & Hoffmann M. R. Sulfur isotopic fractionation in the gas-phase oxidation of sulfur dioxide initiated by hydroxyl radicals. J. Phys. Chem. A 105, 8073–8076 (2001). [Google Scholar]
- Holt B. D., Kumar R. & Cunningham P. T. Primary sulfates in atmospheric sulfates: estimation by oxygen isotope ratio measurements. Science 217, 51–53 (1982). [DOI] [PubMed] [Google Scholar]
- Dominguez G. et al. Discovery and measurement of an isotopically distinct source of sulfate in Earth’s atmosphere. Proc. Natl. Acad. Sci. USA 105, 12769–12773 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohizumi T., Fukuzaki N. & Kusakabe M. 1997. Sulfur isotopic view on the sources of sulfur in atmospheric fallout along the coast of the Sea of Japan. Atmos. Environ. 31, 1339–1348 (1997). [Google Scholar]
- Alewell C., Mitchell M. J., Likens G. E. & Krouse R. Assessing the origin of sulfate deposition at the Hubbard Brook Experimental Forest. J. Environ. Qual. 29, 759–767 (2000). [Google Scholar]
- Mukai H. et al. Regional characteristics of sulfur and lead isotope ratios in the atmosphere at several Chinese urban sites. Environ. Sci. Technol. 35, 1064–1071 (2001). [DOI] [PubMed] [Google Scholar]
- Novák M., Jackova I. & Prechova E. Temporal trends in the isotope signature of air-borne sulfur in central Europe. Environ. Sci. Technol. 35, 255–260 (2001). [DOI] [PubMed] [Google Scholar]
- Harris E., Sinha B., Hoppe P. & Ono S. High-precision measurements of 33S and 34S fractionation during SO2 oxidation reveal causes of seasonality in SO2 and sulfate isotopic composition. Environ. Sci. Technol. 47, 12174–12183 (2013). [DOI] [PubMed] [Google Scholar]
- Li X. et al. Chemical composition and size distribution of airborne particulate matters in Beijing during the 2008 Olympics. Atmos. Environ. 50, 278–286 (2012). [Google Scholar]
- Li W. J. & Shao L. Y. Observation of nitrate coatings on atmospheric mineral dust particles. Atmos. Chem. Phys. 9, 1863–1871 (2009). [Google Scholar]
- Arimoto R. et al. Relationships among aerosol constituents from Asia and the North Pacific during PEM-West A. J. Geophys. Res. 101, 2011–2023 (1996). [Google Scholar]
- Yao X. et al. The water-soluble ionic composition of PM2.5 in Shanghai and Beijing, China. Atmos. Environ. 36, 4223–4234 (2002). [Google Scholar]
- Clarke L.B. The fate of trace elements during coal combustion and gasification: an overview. Fuel 72, 731–736 (1993). [Google Scholar]
- Liu J. Volcanoes in China, Beijing: Science Press, 1–219 (in Chinese, 1999). [Google Scholar]
- Department of Energy Statistics, National Bureau of Statistics, People’s Republic of China. China Energy Statistical Yearbook. China Statistics Press, Beijing (2013). [Google Scholar]
- Maruyama T. et al. Sulfur isotope ratios of coals and oils used in China and Japan. Nippon Kagaku Kaishi 1, 45–51 (in Japanese, 2000). [Google Scholar]
- Hong Y., Zhang H. & Zhu Y. Sulfur isotopic characteristics of coal in China and sulfur isotopic fractionation during coal-burning process. Chinese J. Geochem. 12(1), 51–59 (1993). [Google Scholar]
- Zhang M., Wang S., Wu F., Yuan X. & Zhang Y. Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmos. Res. 84, 311–322 (2007). [Google Scholar]
- Legrand M. et al. Sulfur-containing species (methanesulfonate and SO4) over the last climatic cycle in the Greenland Ice Core Project (central Greenland) ice core. J. Geophys. Res. 102, 26663–26679 (1997). [Google Scholar]
- Guo Q. et al. Tracing the sources of sulfur in Beijing soil with stable sulfur isotopes. J. Geochem. Explor. 161, 112–118 (2016). [Google Scholar]
- Xiao H.Y., Li N. & Liu C.Q. Source identification of sulfur in uncultivated surface soils from four Chinese provinces. Pedosphere 25, 140–149 (2015). [Google Scholar]
- Alexander B., Park R.J., Jacob D.J. & Gong S. Transition metal-catalyzed oxidation of atmospheric sulfur: Global implications for the sulfur budget. J. Geophys. Res. 114, D02309, 10.1029/2008JD010486 (2009). [DOI] [Google Scholar]
- Caron F., Tessier A., Kramer J. R., Schwarcz H. P. & Rees C. E. Sulfur and oxygen isotopes of sulfate in precipitation and lakewater, Quebec, Canada. Appl. Geochem. 1(5), 601–606 (1986). [Google Scholar]
- Van Stempvoort D. R. & Krouse H. R. Controls of δ18O in sulfate: a review of experimental data and application to specific environments. In: Alpers C. N., Blowes P. W. (Eds) Environmental Geochemistry of Sulfide Oxidation, American Chemical Society, Washington D.C., pp. 446–480 (1994). [Google Scholar]
- Guo Q. et al. Tracing the source of Beijing soil organic carbon: A carbon isotope approach. Environ. Pollut. 176, 208–214 (2013). [DOI] [PubMed] [Google Scholar]
- China Meteorological Data Network. Meteorological data from international exchange station of Beijing (2014). Accessible at: http://data.cma.cn/ (Date of access: 20th November 2015).
- Wen X. F., Zhang S. C., Sun X. M., Yu G. R. & Lee X. Water vapor and precipitation isotope ratios in Beijing, China. J. Geophys. Res. 115, D01103, 10.1029/2009JD012408 (2010). [DOI] [Google Scholar]
- IAEA/WMO. Global Network of Isotopes in Precipitation-the GNIP Database. (2016). Accessible at: http://www.iaea.org/water. (Date of access: 19th May 2016)
- Beijing U.S. Embassy. The Mission China air quality monitoring program-Beijing Air Quality Data. (2014). Accessible at: http://www.stateair.net/web/historical/1/1.html. (Date of access: 20th May 2016).