Abstract
The transcription factor SKN7 is a highly conserved protein among fungi and was initially recognized as a response regulator that protects cells from oxidative stress and maintains cell wall integrity in yeast. Orthologs of SKN7 are extensively present in biocontrol agents of plant pathogens, but they had not been functionally characterized. Here, we identified and characterized the transcription factor SKN7 in the nematode endoparasitic fungus Hirsutella minnesotensis. Null mutant lacking HIM-SKN7 (HIM_03620), which was generated by a gene disruption strategy, demonstrated reduced conidiation, increased sensitivity to high temperature, hydrogen peroxide, mannitol and ethanol, and reduced fungal resistance to farnesol. However, over-expression mutant showed increased conidial production, thermotolerance and resistance to farnesol, suggesting that HIM-SKN7 regulates antiapoptotic-like cell death in H. minnesotensis. Moreover, the results showed that in null mutant, H. minnesotensis had decreased endoparasitic ability as compared to wild type and over-expression strain. During the infection process, the relative expression of the HIM-SKN7 gene was significantly induced in the wild type and over-expression strain. The results of the present study advance our understanding of the functions of the SKN7 gene in biocontrol agents, in particular, nematode endoparasitic fungi.
Hirsutella minnesotensis (Ophiocordycipitaceae, Hypocreales, Ascomycota) is the dominant biocontrol agent of Heterodera glycines (soybean cyst nematode, SCN) and is a representative nematode endoparasitic fungi in nature. The species produces globose conidia that adhere to the cuticle during the movement of the secondary stage juvenile (J2) of SCN and subsequently penetrate, digest and eventually kill the nematode1,2,3. Field and greenhouse experiments have shown that H. minnesotensis is a potent factor that regulates the SCN population in the soil and contributes to soil suppressiveness in a soybean monoculture system4,5. Adequate conidial production in soil is important for H. minnesotensis to parasitize SCN. Environmental factors, such as temperature, soil type and water content, which are required for the efficient activity of H. minnesotensis, have been studied under diverse conditions6. Conidia activity may be perturbed by temperature, nutrition, abiotic stresses and secondary metabolites7,8, but conidial cells are capable of combating high temperature, ultraviolet radiation and the generation of reactive oxygen species intermediates during respiration. Fungi respond to these unfavorable conditions by inducing the expression of stress related proteins, among that the role of heat shock transcription factor and its homolog SKN7 plays central part.
Heat shock proteins are a conserved family of proteins that are encoded by several heat shock genes. The expression of these genes is regulated by heat shock transcription factor proteins (HSPs). Heat shock transcription factors (HSFs) recognize conserved DNA binding motifs (heat shock elements, HSEs) that are found in the promoter region of heat shock genes, and activate their transcription to promote survival and respond to abiotic stress, temperature and pathogen infection9,10. There is a high degree of homology between the sequence of SKN7 and the DNA binding domain of heat shock transcription factors (HSFs). A Skn7 gene contains a potential receiver domain, which is found in the two-component signal transduction family of proteins in prokaryotes and is extensively present across the kingdoms11,12,13. Skn7 is a stress-responsive transcription factor with a uniform architecture that is composed of a N-terminal DNA binding domain that is homologous to a heat shock transcription factor (HSF) and a C-terminal receiver domain14. These domains are highly conserved among fungi. The receiver domain regulates Skn7 transcriptional activity by His-Asp phosphorelay signaling via the phosphorylation of a conserved aspartate15. As such, Skn7 is an important regulatory factor that regulates multi-functional responses in fungi and other organisms.
In yeast, it has been revealed that SKN7 and HSF1 collaborate to achieve the maximal induction of heat shock genes in response to oxidative stress16, and the disruption of SKN7 in Saccharomyces cerevisiae resulted in sensitivity to oxidizing agents and the induction of TRX2 genes17. The deletion of SKN7 gene, which is also known as POS918 and BRY119, resulted in the sensitivity of yeast cells to hydrogen peroxide. The over-expression of SKN7 inhibited cell wall defects that have been linked to the mutation of KRE9 gene14, as well as the lethality associated with the loss of the G1 transcription factors SBF and MBF, indicating the role of SKN7 in the regulation of the cell cycle19. Moreover, the disruption of SKN7 was shown to be lethal in pkc1∆ background19, and the PKC1 MAP kinase pathway has been reported to be involved in cell wall biosynthesis20. In the human fungal pathogen Cryptococcus neoformans, SKN7 mutants were less virulent and sensitive to reactive oxygen species, as compared to those with wild-type21. These results suggest that SKN7 plays functional roles in the response to different stresses and might be involved in the expression of genes that regulate the cell wall.
H. minnesotensis diverged from Ophiocordyceps sinensis around 23.9–33.9 Mya and from Tolypocladium inflatum around 29.7–39.7 Mya. Phylogenetic studies suggest that H. minnesotensis clusters with entomopathogens and is closely linked to the caterpillar fungus O. sinensis2. When analyzing the function of Skn7 in other fungi, it was of immense interest to investigate the role of Skn7 in H. minnesotensis, a dominant endoparasite of H. glycines. In the present study, we have functionally characterized a gene within the H. minnesotensis wild type strain, WT-3608, encoding a HSF-type DNA-binding domain along with REC (signal receiver domain), and designated it as transcription factor, HIM-SKN7. We developed HIM-SKN7 knockout and over-expression mutant of H. minnesotensis WT-3608 and found that HIM-SKN7 regulates conidiation, thermotolerance, abiotic stress resistance, antiapoptotic like cell death and nematode endoparasitic efficiency.
Results
Phylogeny, gene structure and motifs analyses
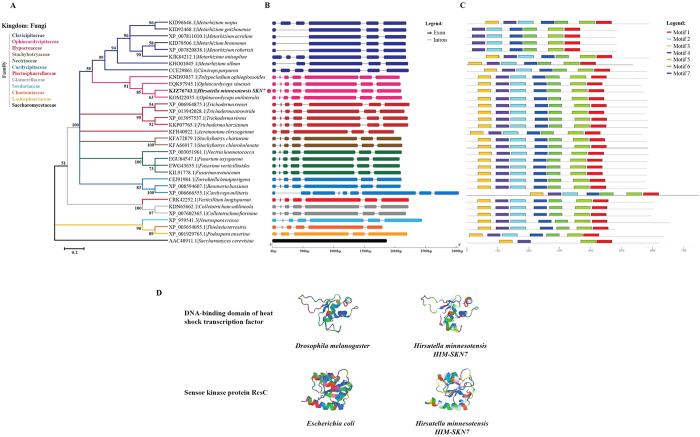
The HIM-SKN7 ORF sequence was 2105 bp and encoded the HSF-type DNA-binding domain and REC (signal receiver domain). The coding sequence of HIM-SKN7 was 1737 bp and encoded a protein sequence of 578 amino acids, with an estimated molecular mass of 63 kDa and an isoelectric point of 6.29. The phylogenetic analysis revealed that HIM-SKN7 (KJZ76743.1) is closely related to the homolog Ophiocordyceps unilateralis (KOM22035.1), an entomopathogenic fungus, and therefore is clustered with different families of hypocrealean fungi (Fig. 1A). Gene structure analysis demonstrated that HIM-SKN7 and its orthologous genes contained 3 to 8 introns. However, most commonly, the genes had 6 introns of different length (Fig. 1B). Analysis of the conserved motifs in HIM-SKN7 and its orthologs revealed 6–7 motifs, with an average width ranging from 42–50. Motif 5 was not present in the protein sequences of KID92468.1, XP_007811010.1, KID78506.1 and XP_007820838.1 (Fig. 1C). The prediction of the tertiary structure showed 40% similarity between the HSF DNA-binding domain of HIM-SKN7 and Drosophila melanogaster, and 34% similarity with the sensor kinase protein ResC from Escherichia coli (Fig. 1D).
Figure 1. Phylogenetic analysis, gene structure and motifs comparison.
(A) Phylogenetic analysis of the different orthologs of HIM-SKN7 (KJZ76743.1|Hirsutella minnesotensis SKN7). The protein sequences were aligned with ClustalW, and a Maximum likelihood (ML) tree was generated using a WAG model. (B) Genomic and coding sequences were used for gene structure analysis to identify the number of introns and exons. (C) The motifs analysis comparison showed various conserved regions in the protein sequences of HIM-SKN7 and its different orthologs. (D) Prediction of the tertiary structure of the conserved domain in HIM-SKN7 and comparison with that of Drosophila melanogaster and Escherichia coli.
Targeted gene disruption, over-expression and phenotypic characterization
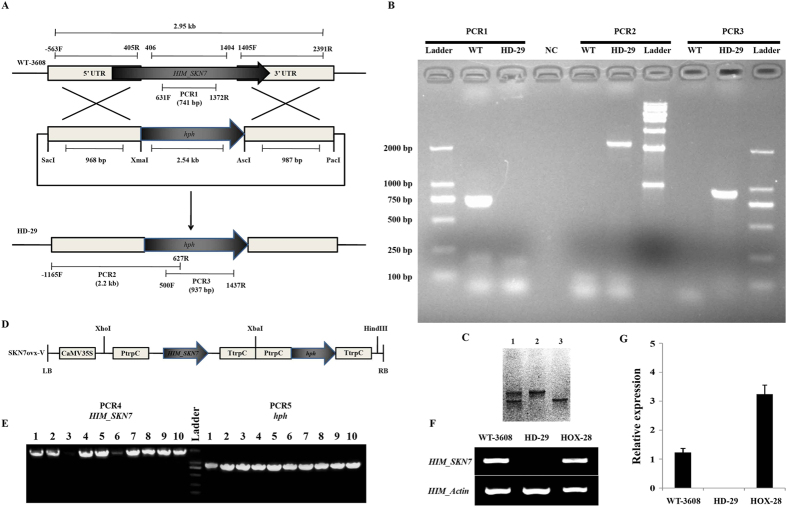
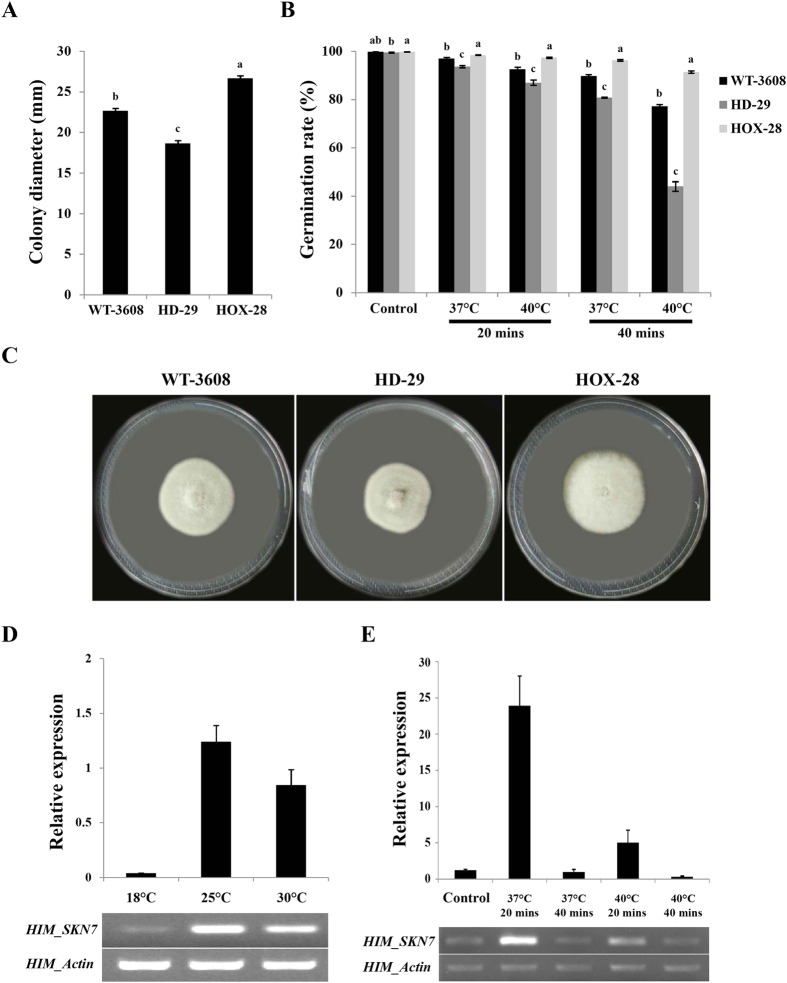
The functional capabilities of HIM-SKN7 were investigated in the wild type strain of H. minnesotensis (WT-3608) by performing targeted gene disruption and an over-expression strategy (Fig. 2A,D). The gene-disrupted and over-expression transformants were screened phenotypically on potato dextrose agar (PDA) supplemented with hygromycin (hph) for revealing the function of HIM-SKN7, and the results were confirmed by PCR (Fig. 2B,C,E), RT-PCR (Fig. 2F) and real-time RT-PCR (Fig. 2G). The relative expression analysis showed that the knockout (HD-29) strain showed no transcription activity and the over-expression (HOX-28) strain showed an increase in transcript levels, as compared to WT-3608. The knockout HD-29 showed no significant difference, while over-expression HOX-28 showed a significant increase in mycelial growth, as compared to WT-3608. Moreover, HD-29 produced fewer conidia and fresh biomass, as compared to HOX-28 and WT-3608 (Table 1). These results revealed the prominent role of HIM-SKN7 in the phenotypic characteristics of the nematode endoparasitic fungus H. minnesotensis.
Figure 2. Generation of HIM-SKN7 disruption and over-expression mutant.
(A) A schematic illustration for the disruption of the HIM-SKN7 gene. The hph gene was used as a selection marker after transformation. (B) PCR verification. PCR1 was performed to amplify the disrupted region of the HIM-SKN7 gene. The expected product size was 741 kb for WT, and no amplification was expected for knockout strain. PCR2 was performed to verify the replacement of the HIM-SKN7 gene by the hph gene. The forward primer was designed to bind 0.6 kb upstream of the 5′UTR (used in vector for replacement), and the reverse primer designed to bind near the center of the hph gene. The expected product size of mutant was 2.2 kb, and no amplification was expected for the WT strain. PCR3 was performed to amplify the hph gene from the mutant. The expected product size was 937 bp for the mutant and no amplification was expected for the WT. (C) Amplification of HIM-SKN7 ORF. lane 1, negative transformants; lane 2, positive transformants; lane 3, wild-type 3608. (D) A schematic illustration for the over-expression of the HIM-SKN7 gene. The hph gene was used as a selection marker after transformation. (E) PCR verification. PCR4 was performed to ensure the correct orientation of the HIM-SKN7 gene in the vector, pCAMBIA 3300. The forward primer was designed to bind to the promoter region, and reverse primer was designed to bind to the gene. The expected product size was ~1.9 kb. PCR5 was performed to confirm the presence of the hph gene in mutants. The expected product size was 937 bp. (F) RT-PCR verification to confirm the loss of HIM-SKN7 transcripts from knockout HD-29. (G) Real-time RT-PCR verification to confirm the over-expression of the HIM-SKN7 gene. The actin gene was used as an internal control.
Table 1. Comparison of phenotypic characteristics between the mutants and wild type 3608 of H. minnesotensis.
| Strains | Colony diameterA(mm) | BiomassB(mg/plate) | ConidiationC (106conidia/plate) |
|---|---|---|---|
| WT-3608 | 14.5 ± 0.34b | 22.50 ± 0.95b | 2.41 ± 0.14b |
| HD-29 | 14.83 ± 0.31b | 21.83 ± 0.91b | 1.29 ± 0.11c |
| HOX-28 | 16.33 ± 0.21a | 59.67 ± 2.21a | 3.28 ± 0.23a |
(A) Colony diameter was recorded on PDA plates following incubation at 25 °C for 14 days. (B) Biomass (fresh-weight) was calculated from 14-day-old cultures. (C) Conidia production in14-day-old cultures were counted using a haemocytometer. The mean values and standard error were calculated from 6 replicates. The letters within each column indicate statistically significant differences of P ≤ 0.05.
Deletion of HIM-SKN7 increased the sensitivity of H. minnesotensis to heat shock
To reveal the function of HIM-SKN7 in response to high temperatures, we first cultured WT-3608 on PDA, and incubated at temperature ranging from 18 °C to 40 °C for 14 days. We found no significant difference in mycelial growth in WT-3608 grown at temperature ranging from 18 °C to 30 °C. Fungal growth was completely suppressed at temperatures ≥37 °C. When we compared the transcript level of WT-3608 grown at 18 °C and 30 °C, the relative expressions were significantly changed, but did not observe any increases with that of control at 25 °C (Fig. 3D). To test the thermotolerance of H. minnesotensis against high temperature and time, 5 mm mycelium agar plug from WT-3608, HD-29 and HOX-28 were heat shocked for 2 h at 37 °C and inoculated on fresh PDA plates. Mycelial growth was triggered after 7 days and significantly inhibited in the knock out strain, HD-29, as compared to WT-3608 and the over-expression strain, HOX-28 (Fig. 3A,C). Subsequently, H. minnesotensis WT-3608 was challenged with heat shock at 37 °C and 40 °C for 20 and 40 minutes, and the transcript levels were measured following these two high temperature treatments for two different time periods. The expression levels were increased to 20 fold at 37 °C for 20 minutes and started reduces with increased temperature and time (Fig. 3E). To examine the effect of high temperature on conidia germination and survival, the spores of all strains were heat shocked at 37 °C and 40 °C for 20 and 40 minutes along with control. We found a significantly lower germination rate for HD-29, as compared to WT-3608 and HOX-28 (Fig. 3B). These observations indicate that the HIM-SKN7 gene also regulates thermotolerance, and protects H. minnesotensis from adverse temperature conditions.
Figure 3. H. minnesotensis survival assays under heat stress challenge.
(A) The colony diameter of H. minnesotensis strains that were heat shocked at 37 °C for 2 h. (B) The conidial germination percentage of WT-3608, HD-29 and HOX-28 after being exposing to heat shock at 37 °C and 40 °C for 20 and 40 minutes. (C) The colony morphology of H. minnesotensis WT-3608, HD-29 and HOX-28 at 25 °C after heat shock for 2 h at 37 °C. (D) The relative expression of HIM-SKN7 in WT-3608 at 18 °C, 25 °C and 30 °C. (E) The relative expression of HIM-SKN7 in WT-3608 after heat shock at 37 °C and 40 °C for 20 and 40 minutes along with control. The actin gene was used as an internal control.
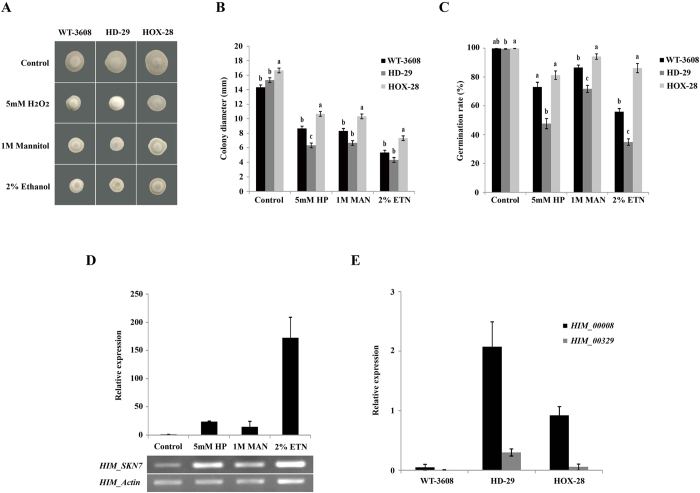
HIM-SKN7 is required to combat abiotic stresses
Abiotic stresses, such as 5 mM H2O2, 1M mannitol and 2% ethanol, were added to PDA along with untreated control and all strains were cultured for 14 days at 25 °C to compare mycelia growth rate. The knock out HD-29, was sensitive to all stress treatments, as compared to WT-3608, while the over-expression strain, HOX-28, showed rapid mycelial growth under all stresses (Fig. 4A,B). To determine the conidial germination percentage, the conidial suspensions were treated with 5 mM H2O2, 1 M mannitol and 2% ethanol. Significant differences in the germination rates of all strains were observed, and HOX-28 showed a higher germination percentage than WT-3608 and HD-29. The conidial germination capability of the knockout strain, HD-29, was reduced to 15–25% under different stresses (Fig. 4C). In WT-3608, an increase in the relative expressions levels of HIM-SKN7 was also observed under these stress conditions (Fig. 4D). The relative expressions levels of heat and stress regulating heat shock transcription factors (HSFs), designated as HIM_00008 (KJZ80158.1) and HIM_00329 (KJZ80479.1), were also increased in the knockout strain, HD-29 and over-expression strain, HOX-28 (Fig. 4E). These results showed that HIM-SKN7 stabilizes cell activity by increasing its expression levels under different stress conditions, and cooperates with heat shock transcription factors in H. minnesotensis in order to combat abiotic stresses.
Figure 4. Growth assay and conidia survival assay following abiotic stress challenge.
(A) The phenotypes of WT-3608, HD-29 and HOX-28 grown on PDA amended with 5 mM H2O2 (HP), 1 M mannitol (MAN) and 2% ethanol (ETN) for 14 days along with untreated control. (B) The colony diameter (mm) of all strains in the presence of 5mM H2O2 (HP), 1 M mannitol (MAN) and 2% ethanol (ETN) along with untreated control. The data were measured at 14 dpi. (C) The spore germination percentage after abiotic stress treatments. All strains were sensitive to 5 mM H2O2 (HP), 1 M mannitol (MAN) and 2% ethanol (ETN). (D) Transcription and relative expression of the HIM-SKN7 gene in response to 5 mM H2O2 (HP), 1M mannitol (MAN) and 2% ethanol (ETN) along with control. The WT-3608 were grown in PDB for 4 d and used for RT-PCR and qRT-PCR. The actin gene was used as an internal control. (E) The relative expression of heat shock transcription factors, HIM_00008 and HIM_00329, in WT-3608, knockout HD-29 and over-expressed HOX-28.
HIM-SKN7 is involved in anti-apoptotic like cell death in H. minnesotensis
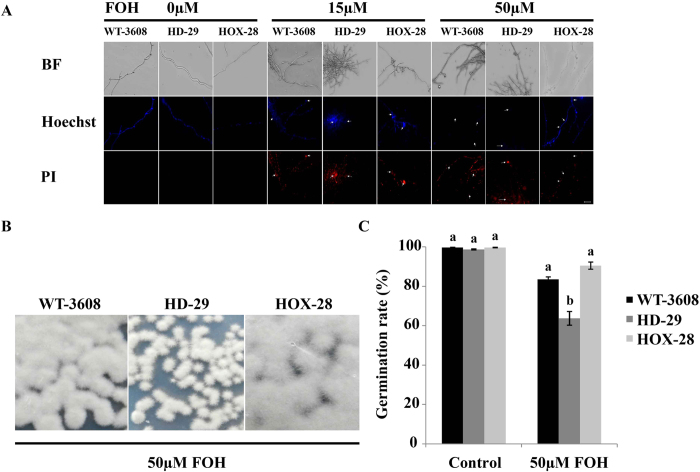
To investigate the contribution of HIM-SKN7 in apoptotic like cell death, we exposed germlings of WT-3608, HD-29 and HOX-28 to the various concentrations (0 μM, 15 μM and 50 μM) of the apoptosis-inducing compound, farnesol (FOH). The FOH-treated mycelium and conidia were co-stained with Hoechst and propidium iodide (PI) dyes and observed under a fluorescence microscope. The microscopy results showed high levels of apoptosis and necrosis-like features, such as intense chromatin condensation and marginalization, in all strains. A dose of 50 μM was observed to be highly toxic and caused severe necrosis in HD-29 and WT-3608 (Fig. 5A). For the conidial survival assay, the conidia of all strains were treated with 50 μM FOH along with blank control. The treatments had significant effect and resulted in decreased conidial germination in the knock out HD-29, as compared to WT-3608 and HOX-28 (Fig. 5B,C). These results indicate that HIM-SKN7 regulates apoptotic-like cell death and provides functional cell stability in H. minnesotensis.
Figure 5. Induction of apoptosis in H. minnesotensis.
(A) 10 days old germlings from WT-3608, HD-29 and HOX-28 were treated with farnesol (FOH) for 4 h at 25 °C, and the samples were double stained with Hoechst 33342/PI dyes. Fluorescence microscopy showed dose dependent apoptosis and necrosis of hyphae at 15 μM and 50 μM FOH. Bar = 50 μm. (B) The conidia survival assay on PDA treated with 50 μM FOH. (C) The conidia germination percentage of all strains exposed to FOH. HD-29 was more sensitive than WT-3608 and HOX-28 to 50 μM FOH.
Disruption of HIM-SKN7 reduces the nematode endoparasitic ability of H. minnesotensis
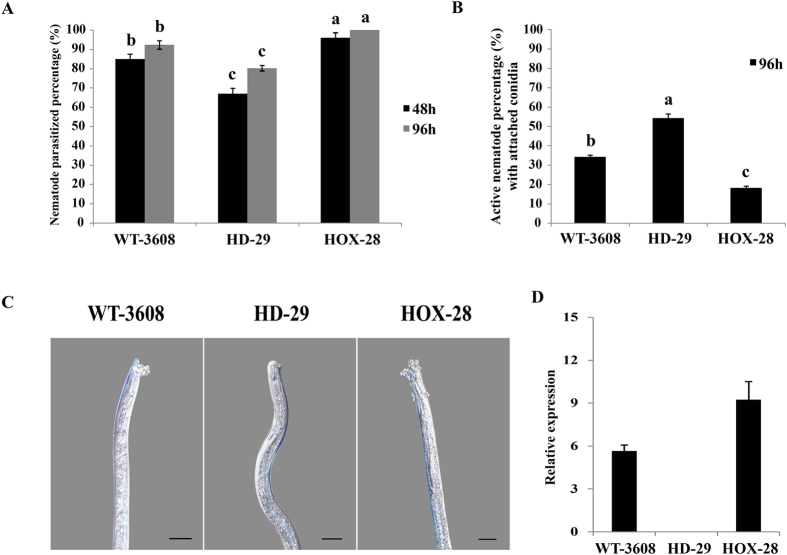
The ability of all strains to parasitize secondary stage juveniles (J2) of H. glycines was assayed on CMA in 12 wells tissue culture plates. First, a 10 μl conidia suspension (2 × 105 conidia ml−1) of all strains was allowed to germinate for 14 days, and after which 100 J2 of SCN were inoculated into each well. The parasitized and alive nematodes were monitored at 48 h and 96 h under an inverted microscope. The ability of the knock out HD-29, to parasitize J2 was decreased by 20% as compared to WT-3608. For HOX-28, the parasitized J2 percentage was 100% at 96 h (Fig. 6A). However, when active and alive J2 with adhered conidia were calculated at 96 h, the percentage of alive nematodes was higher in the knockout strain, HD-29, while there was also a significant response between WT-3608 and the over-expressed HOX-28 to completely parasitize the nematodes (Fig. 6B,C). During the infection process, the relative expression of HIM-SKN7 was increased 4- to 7-fold at 24 h of J2 inoculation in WT-3608 and HOX-28, respectively (Fig. 6D). The nematode parasitism assays provide an understanding into the role of HIM-SKN7 in H. minnesotensis virulence and its efficiency in parasitizing the second stage juveniles of H. glycines.
Figure 6. Parasitism of Heterodera glycines J2 (SCN) by Hirsutella minnesotensis.
(A) The percentage of nematodes parasitized by WT-3608, HD-29 and HOX-28 at 48 h and 96 h of SCN inoculation. HD-29 parasitized fewer J2, as compared to WT-3608 and HOX-28. (B) The percentage of active J2 with adhered conidia. The highest mortality rate was recorded in HOX-28, followed by WT-3608 and HD-29. The data were recorded at 96 h. (C) Conidia of H. minnesotensis WT-3608, HD-29 and HOX-28 adhered to the cuticle of H. glycines. Bar = 10 μm (D) An increased in the relative expression of the HIM-SKN7 gene was observed during the infection process. RNA was extracted from WT-3608, HD-29 and HOX-28 after 24 h of nematode inoculation. The actin gene was used as an internal control.
Discussion
Nematophagous fungi are natural enemies of plant pathogenic nematodes, and provide promising strategies for managing crops against nematodes in order to achieve high yields. The soybean cyst nematode is a ubiquitous plant parasitic nematode that is capable of causing huge losses in the soybean producing regions of the world, especially those that lack available management practices, including crop rotation and resistant cultivars22. Hirsutella minnesotensis is a competent bio-control agent of H. glycines that produces globose conidia that adhere to cuticle of secondary stage juvenile (J2) of SCN and eventually penetrate and digest the nematode. While the whole genome analysis of H. minnesotensis was previously published2, the specific functional genes that are expressed during stress, conidiation, parasitism and other cellular functions remain to be investigated. For this purpose, we characterized a highly conserved member of stress regulatory gene, HIM-SKN7, which shows high homology with the DNA binding domain of heat shock transcription factors.
Heat shock transcription factors (HSFs) play critical roles in regulating survival and normal metabolic processes under diverse stresses and acute conditions. The involvement of HSFs has been documented in conidiation, thermotolerance, virulence and the refolding of damaged proteins, and in particular, in maintaining cell homeostasis8,23,24. The SKN7 stress regulatory transcription factor was observed to interact with HSF1, and together, they resulted in the high induction of heat shock genes in response to oxidative stress in yeast and increased the sensitivity of yeast cells to oxidative agents in null mutants, which was associated with the regulation of different regulatory genes14,16,17. In the present study, HIM-SKN7 was found to regulate various cellular functions, including conidiation, thermotolerance and resistance against abiotic stresses, apoptotic-like cell death and nematode parasitism in H. minnesotensis. Null mutant lacking HIM-SKN7 showed reduced biomass formation and conidiation production, as compared to the wild type strain and HIM-SKN7 over-expression mutant (Table 1). The regulation of some conidiation pathways by such conserved families of proteins in the bio-control fungus, Coniothyrium minitans, have been investigated and provide insights into genetically enhancing conidiation8,25,26. This indicates the regulation of conidiation in H. minnesotensis by HIM-SKN7, which is significant because plentiful conidia production by bio-control agents could lead to the manufacturing of commercial products for controlling plant pathogenic nematodes.
Thermotolerance and resistance against abiotic stresses are required by all organisms, including fungi, in order to survive in adverse environmental conditions. The heat shock response occurs via the induction of heat shock genes, and the disruption of such genes in different organisms has been shown to inhibit thermotolerance and increase the sensitivity of the organisms to abiotic stresses, including oxidative stress16,27,28,29. The over-expression of HIM-SKN7 in H. minnesotensis increased its thermotolerance against heat and abiotic stresses, as compared to the wild type strain and knock out HD-29. This revealed that in this biocontrol agent fungus, SKN7 probably interacts with heat shock genes to induce thermotolerance. In fungi, Drosophila melanogaster and mammals, heat shock factor proteins have the fundamental capability for stress sensing and are converted from monomers to homotrimers in response to heat and oxidative stresses8,28,30,31. When the wild type strain was heat treated to 30 °C, no significant increases in the transcript level of HIM-SKN7 was observed, as compared to control (25 °C), but an increase was observed at high temperatures. The heat treatments at 37 °C and 40 °C for 20 and 40 mins disturbed the normal growth of the wild type strain and knock out mutant, but the over-expression mutant successfully survived, and their mycelia and conidia were observed to be tolerant against heat and abiotic stresses. The increased expression of two HSFs (HIM_00008 and HIM_00329) that play important roles in cellular functioning and thermotolerance was also observed (Fig. 4E), and the over-expression of these genes enhanced the regulation of tolerance to diverse environmental conditions and stresses8,32,33,34. These results emphasize the contribution of HIM-SKN7 to stabilizing H. minnesotensis against heat and abiotic stresses by interacting with heat shock factor proteins.
The induction of apoptotic-like cell death by farnesol (FOH), a 15-carbon isoprenoid alcoholic natural compound and a main precursor of the isoprenoid or sterol biosynthesis pathway35, has been reported in many fungi, including Metarhizium robertisii, Candida albicans, S. cerevisiae, and Aspergillus nidulans36,37. In this study, we observed that FOH triggered cellular apoptosis, as well as necrosis like features, including intense chromatin condensation and marginalization, in the nematode endoparasitic fungus H. minnesotensis. The plate assay and microscopic observations showed low conidial germination at high FOH concentration (50 μM) in HIM-SKN7 knock out strain, as compared to the wild type and over-expression strain. A recent study revealed that the deletion of Bax inhibitor 1 (Bl-1) perturbed fungal development, virulence and thermotolerance and also reduced fungal resistance to FOH, which suggests that cell death was mediated by the endoplasmic reticulum stress signaling pathway in entomopathogenic fungus M. robertsii36. These results revealed the role of HIM-SKN7 in regulating cellular homeostasis against apoptosis-like cell death and providing tolerance against such alcoholic compounds.
Nematophagous fungi are a promising control strategy for plant pathogenic nematodes in the natural ecosystem. In the present study, we tested the ability of transformed strains to parasitize the secondary stage juveniles of H. glycines. H. minnesotensis is a cold-adapted fungus that preferentially parasitizes the cyst nematodes in nature6, and recently, a transformation system involving GFP-labeling was established in H. minnesotensis to investigate the infection process in H. glycines3. H. minnesotensis is abundant in soils with soybean monocultures, suggesting that it is an indicator of natural SCN suppressive soils in different locations in China and the USA4,6,38. The over-expression of HIM-SKN7 enhanced conidial adherence and the parasitic capability of H. minnesotensis, as compared to the wild type strain (Fig. 6C). Moreover, the ability of the knock out HD-29 to parasitize the nematodes was reduced with that of WT-3608. At present, functional genetic studies in nematophagous fungi, especially in Hirsutella spp. are limited. Stress-related genes have been described to be involved in the process of virulence in insect pathogenic fungi36 and the nematode egg parasitic fungus P. chlamydosporia, and the induction of serine proteases and chitinase-encoding genes was previously investigated in the nematode-trapping fungus Arthrobotrys oligospora, during parasitism39,40. Our results showed the involvement of HIM-SKN7 in nematode parasitism of H. minnesotensis.
In conclusion, this is the first study to report multi-functions of HIM-SKN7 in nematode endoparasitic fungus H. minnesotensis. Our findings provide a comprehensive understanding of the role of this stress-responsive transcription factor in conidiation, thermotolerance, abiotic stresses, apoptotic-like cell death and virulence. The deletion of HIM-SKN7 decreased the tolerance of the fungus towards acute temperature and abiotic stresses and reduced the endoparasitic ability of H. minnesotensis against H. glycines. Our results also indicate that HIM_SKN7 regulates apoptosis-like cell death and that its over-expression increased conidiation, which would facilitate the commercial production of this nematode endoparasitic fungus for controlling plant pathogenic nematodes.
Materials and Methods
Strains, media and cultural conditions
The Hirsutella minnesotensis wild type strain 3608 was maintained on PDA and used throughout this study. All the mutant strains were also maintained on potato dextrose agar (PDA) at 25 °C with appropriate antibiotics. The Escherichia coli strain DH5α, and the Agrobacterium tumefaciens strain EHA105 were prepared and stored at −80 °C. For cloning and plasmid DNA, bacterial strains were propagated in Luria Bertani (LB) medium supplemented with the appropriate antibiotic when required.
Phylogenetic, gene structure and conserved motifs analyses
To establish the phylogenetic relationship between HIM-SKN7 (KJZ76743.1) and its orthologs, protein sequences from representative fungal species belonging to different families in Phylum Ascomycota were aligned using the ClustalW program of the Molecular Evolutionary Genetics Analysis (MEGA version 6.0) software suite41. A maximum likelihood tree was generated with the WAG Model using the following parameters: a Nearest-Neighbor-Interchange heuristic method for tree inference, 1,000 bootstrap replications for phylogeny test, and a complete deletion for gaps/missing data. Gene structure, including introns and exons, was investigated using the online Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/), which is based on genomic and coding sequences42. The conserved motifs were determined by the MEME online server43. The following parameters were used: maximum numbers of different motifs, 07; minimum motif width, 30; maximum motif width, 50; the other parameters were kept at their default settings. The tertiary structure of HIM-SKN7 was constructed using SWISS-TOOL workshop44.
Construction strategy of the HIM-SKN7 disruption and over-expression vectors
To elucidate the function of HIM-SKN7 gene, a gene disruption vector was constructed by following the homologous recombination strategy. The 968 bp and 987 bp DNA fragment from the upstream and downstream regions of HIM-SKN7 were amplified from the wild type strain using the primers, 5Sac-FP/5Xma-RP and 3Asc-FP/3Pac-RP, respectively. Both PCR amplified fragments were sequenced to verify the true gene sequence. The purified fragments were cloned into the pMD18-T vector and digested with Sac1-Xma1 (upstream) and Asc1-Pac1 (downstream), before being ligated into the binary vector, pAg1-H3. Additionally, the disruption vector pAg1-H3 was digested with the same restriction enzymes to facilitate the ligation of the upstream and downstream fragments to the corresponding sites containing the hygromycin phosphotransferase (hph) gene cassette, resulting the formation of SKN7pAg1-H3.
To construct the over-expression vector, an over-expression cassette was created using promoter (PtrpC) and terminator (TtrpC) from Aspergillus nidulans with a 1.8 kb fragment of the HIM-SKN7 ORF that was amplified from WT-3608 with the primer pair SKN7OX-F and SKN7OX-R. The hygromycin resistant gene cassette was constructed using the 1.4 kb hph gene and was ligated into PtrpC and TtrpC using the preliminary cloning vector, pSKH. The HIM-SKN7 gene cassette and hph resistance gene cassette were ligated into the backbone of the pCAMBIA3300 binary vector by digesting it with Xcm1 and Xba1, respectively, resulting in the formation of SKN7ovx-V. To confirm the orientation of the HIM-SKN7 gene in the over-expression vector, a forward primer from PtrpC and a gene specific reverse primer PromSP3/SKN7OX-R were used to amplified ~1.9 kb DNA fragment. The ligation of the hph gene cassette was confirmed by amplifying the 937 bp fragment with the primer pair Hyg-NF and Hyg-NR. All the primers are listed in Table 2.
Table 2. The primers used in this study.
| Primer | Sequence (5’-3’) | Expected Size | Roles | Source |
|---|---|---|---|---|
| Primer used for clone DNA from H. minnesotensis | ||||
| 5Sac-FP | CGAGCTCCTGGTAAAATCTGTCCCG | 968 bp | Amplified 5′ UTR and partial sequence of HIM-SKN7 | This study |
| 5Xma-RP | CCCCGGGCTGTCGGATAAAACTGGA | This study | ||
| 3Asc-FP | GGCGCGCCAATAAGGTGATGAAGCAA | 987 bp | Amplified 3′ UTR and partial sequence of HIM-SKN7 | This study |
| 3Pac-RP | CTTAATTAAACCCAATGCGGCACAAGC | This study | ||
| SKN7OX-F | TCTCAAGGTAGCGGCAACAAT | 1.8 kb | Amplified HIM-SKN7 ORF for over-expression and to confirm replacement with hph cassete | This study |
| SKN7OX-R | CCCGCACTGTCTCCATCGTGT | This study | ||
| Primer used for identification of null mutants | ||||
| 5-HD-FP | GCCGAGGACTTCACCACTA | 741 bp | Amplified HIM-SKN7 gene (PCR 1) | This study |
| 3-HD-RP | GCAGCCCATTGACTTGAGA | This study | ||
| HD-F5N | GGCAGTTCGTGGTTTAGTCG | 2.2 kb | Amplified sequence of UTR and partial sequence of hph gene (PCR 2) | This study |
| Hyg-R3 | CTTTGTAGAAACCATCGGCG | This study | ||
| Hyg-NF | AAGTTCGACAGCGTCTCC | 937 bp | Amplified the gene hph (PCR 3) | This study |
| Hyg-NR | TTCCACTATCGGCGAGTA | This study | ||
| Primer used for identification of over-expression mutants | ||||
| PromSP3 | AGGTAAGTGAACGACCCGGTC | 1891 bp | Amplifying ORF and partial sequence of PtrpC (PCR4) | This study |
| SKN7OX-R | CCCGCACTGTCTCCATCGTGT | This study | ||
| Hyg-NF | AAGTTCGACAGCGTCTCC | 937 bp | Amplified the gene hph (PCR 5) | This study |
| Hyg-NR | TTCCACTATCGGCGAGTA | This study | ||
| Primer used for RT-PCR and RT-qPCT | ||||
| HRT-F | ACCTAAGCCCATACAACG | 254 bp | mRNA transcription of HIM-SKN7 gene | This study |
| HRT-R | TAAATCATTCATCGGGTCAT | |||
| HIM-08F | GGAAACCCAGAAACGACCCT | 149 bp | mRNA transcription of HIM_00008 gene | This study |
| HIM-08R | AGTCGGGTTCGGCTTGTTGT | |||
| HIM-29F | TTCTGTATGGGCTGCGTCTC | 181 bp | mRNA transcription of HIM_00329 gene | This study |
| HIM-29R | TCTGGCTCATCAACAAACCC | |||
| Actin-F | CCCCATCTACGAGGGTTT | 554 bp | Used as internal control | This study |
| Actin-R | GGGAAGCGAGAATGGAAC | |||
Transformation and screening of HIM-SKN7 disruption and over-expression mutants
The disruption vector SKN7pAg1-H3 and the over-expression vector SKN7ovx-V were transformed into A. tumefaciens EHA105 using the heat shock method. Fresh conidia (10 days old) of H. minnesotensis were transformed with the A. tumefaciens-mediated transformation method, as previously described8. To screen for HIM-SKN7 disrupted and over-expression transformants, candidates were grown on PDA supplemented with 200 μg/ml hygromycin B and 500 μg/ml cefotaxime. The positive transformants harboring the homologous recombination event were confirmed by PCR, RT-PCR and real-time RT-PCR.
Expression profiling of HIM-SKN7 in Hirsutella minnesotensis
All strains were cultured for 4 days in PDB at 25 °C (150 rpm), and the collected mycelium was processed immediately for further RNA extraction. TRIzol reagent (Ambion) was used to extract RNA manually and treated with DNaseI (Invitrogen) by following the kit protocol. First strand cDNA synthesis was performed using FastQuant RT Kit (with gDNase) (TIANGEN) according to the manufacturer instructions.
Expression analysis was carried out by reverse transcriptase-PCR (RT-PCR) and SYBR green-based real-time RT-PCR on a CFX96 Real-Time System (Bio-Rad). A gene specific primer pair, HRT-F/HRT-R was used to amplify a 254 bp region of HIM-SKN7 ORF by RT-PCR. The reaction condition were 3 min at 95 °C and, 30 cycles of 40 s at 94 °C, 30 s at 51 °C and 30 s at 72 °C, followed by 10 min at 72 °C. The real-time RT-PCR conditions were 2 min at 95 °C and, 40 cycles of 20 s at 95 °C, 15 s at 51 °C and 20 s at 72 °C. The HIM-Actin gene was amplified with the primer pair, Actin-F/Actin-R, and was used as an internal control for all reactions.
Heat shock and abiotic stress assay
To test the thermotolerance of all strains under different temperatures, temperature ranges for heat shock assay were initially determined by culturing the WT-3608 on PDA plates, and incubating the plates at 18 °C, 25 °C, 30 °C, 37 °C and 40 °C for 2 weeks. After the initial screening, mycelium from H. minnesotensis WT-3608, knock out HD-29 and over-expressed HOX-28 were subjected to heat shock treatment at 37 °C for 2 h to determine the incubation time that was required. Finally, the heat shock treatment for conidia of all strains was performed at 37 °C and 40 °C for 20 and 40 minutes in a water bath. The heat-treated conidial suspensions (1 × 105 conidia ml−1; 10 μl) were inoculated in PDB and incubated for 3 days at 25 °C (150 rpm) for the examination of conidia germination and survival. To test the transcriptional activity of the gene, HIM-SKN7, during the heat stress, its relative expression levels were measured by real-time RT-PCR.
All strains were cultured on PDA supplemented with 5 mM H2O2, 1 M mannitol and 2% ethanol for 14 days. Radial hyphal growth was measured to observe the tolerance of all strains to stress conditions. For the conidia survival assay, conidia were collected in 0.05% Tween-80, and spore suspensions (1 × 105 conidia ml−1; 10 μl) from all strains were inoculated in PDB amended with 5 mM H2O2, 1 M mannitol and 2% ethanol. The samples were incubated for 3 days at 25 °C (150 rpm) to record germination and survival percentages. The relative expression of HIM-SKN7 was measured for each treatment. All strains were generated in triplicate, and the experiment was repeated twice.
Apoptosis assay
The 10-day old germlings of WT-3608, knock out HD-29 and over-expressed HOX-28 were treated with the apoptosis-inducing compound, farnesol (FOH, TCI), at concentrations of 15 μM and 50 μM along with control for 4 h at 25 °C. The dyes Hoechst 33342 and PI (Shanghai Yeasen Biotechnology Co. Ltd.) were used to stain nuclei and necrotic cells, respectively. Observations were made using fluorescence microscopy with a ZEISS microscope (Axio Imager.A2). The spore germination percentage of all strains was determined by conidia treatment with 50 μM FOH for 24 h, and conidial suspensions (1 × 105 conidia ml−1; 10 μl) were transferred to PDB for 3 days and on PDA for 14 days at 25 °C. All strains were generated in triplicate, and the experiment was repeated three times.
Nematode parasitism bioassays
To reveal the role of the HIM-SKN7 in the parasitism of H. glycines, a 10 μl conidial suspension (2 × 105 conidia ml−1) of all strains were thoroughly spread onto 1 ml corn meal agar in 12 well tissue culture plates (Nest Biotechnology Co. Ltd.). The plates were incubated at 20 °C for 14 days and then, 100 second-stage juveniles (J2) of H. glycines were placed in 20 μl of sterilized water suspension and inoculated into each well. The plates were placed in an illuminated flow hood to allow the evaporation of excess water from each well in order to enhance the adhesion of conidia with the J2 of H. glycines. Nematodes were washed out from the wells of the plate with 1 ml of 0.05% Tween-80 at 48 h and 96 h. Using an inverted microscope (CK40-F200, Olympus), the J2 with conidia attached to cuticle and parasitized nematodes were calculated based on the total number of nematodes collected using an inverted microscope (CK40-F200, Olympus). The mycelia of WT-3608, HD-29 and HOX-28 were collected at 24 h after nematode inoculation and the relative expressions of HIM-SKN7 were measured. All strains were generated in triplicate, and the experiment was repeated three times.
Statistical analysis
Analysis of variance (ANOVA) was used for evaluating the results. Duncan’s Multiple Range (DMR) test was used to compare the treatment means values. Treatments were considered significant when P ≤ 0.05.
Additional Information
How to cite this article: Hussain, M. et al. The transcription factor SKN7 regulates conidiation, thermotolerance, apoptotic-like cell death and parasitism in the nematode endoparasitic fungus Hirsutella minnesotensis. Sci. Rep. 6, 30047; doi: 10.1038/srep30047 (2016).
Acknowledgments
This research was supported by the National Key Basic Research Program of China (973 Program, Grant No. 2013CB127506), the National Natural Science Foundation of China (Grant No. 30800732). MH is thankful to CAS-TWAS for the award of a PhD Fellowship.
Footnotes
Author Contributions M.H., M.I.H., N.W. and L.B. performed the experiments. M.H. and M.I.H. wrote the main manuscript text. M.H., M.I.H., M.X. and X.L. designed the experiments. X.L. revised the manuscript. All authors reviewed the manuscript.
References
- Liu X. & Chen S. Parasitism of Heterodera glycines by Hirsutella spp. in Minnesota soybean fields. Biol. Control 19, 161–166 (2000). [Google Scholar]
- Lai Y. et al. Comparative genomics and transcriptomics analyses reveal divergent lifestyle features of nematode endoparasitic fungus Hirsutella minnesotensis. Genome Biol. Evol. 6, 3077–3093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J. et al. Development of a transformation system for Hirsutella spp. and visualization of the mode of nematode infection by GFP-labeled H. minnesotensis. Sci. Rep. 5, 10477, doi: 10.1038/srep10477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. & Chen S. Screening isolates of Hirsutella species for biocontrol of Heterodera glycines. Biocontrol Sci. Techn. 11, 151–160 (2001). [Google Scholar]
- Liu S. & Chen S. Effectiveness of Hirsutella minnesotensis and H. rhossiliensis in control of the soybean cyst nematode in four soils with various pH, texture, and organic matter. Biocontrol Sci. Techn. 19, 595–612 (2009). [Google Scholar]
- Xiang M., Xiang P. a., Liu X. & Zhang L. Effect of environment on the abundance and activity of the nematophagous fungus Hirsutella minnesotensis in soil. FEMS Microbiol. Ecol. 71, 413–417 (2010). [DOI] [PubMed] [Google Scholar]
- Calvo A. M., Wilson R. A., Bok J. W. & Keller N. P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66, 447–459 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid M. I., Zeng F., Cheng J., Jiang D. & Fu Y. Disruption of heat shock factor 1 reduces the formation of conidia and thermotolerance in the mycoparasitic fungus Coniothyrium minitans. Fungal Genet. Biol. 53, 42–49 (2013). [DOI] [PubMed] [Google Scholar]
- Morimoto R., Sarge K. & Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J. Biol. Chem. 267, 21987–21990 (1992). [PubMed] [Google Scholar]
- Lindquist S. & Craig E. The heat-shock proteins. Annu. Rev. Genet. 22, 631–677 (1988). [DOI] [PubMed] [Google Scholar]
- Chang C. & Meyerowitz E. Eukaryotes have “two-component” signal tranducers. Res. Microbiol. 145, 481–486 (1994). [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Shaulsky G. & Wang N. Histidine kinases in signal transduction pathways of eukaryotes. J. Cell Sci. 110, 1141–1145 (1997). [DOI] [PubMed] [Google Scholar]
- Wurgler-Murphy S. M. & Saito H. Two-component signal transducers and MAPK cascades. Trends Biochem. Sci. 22, 172–176 (1997). [DOI] [PubMed] [Google Scholar]
- Brown J., North S. & Bussey H. SKN7, a yeast multicopy suppressor of a mutation affecting cell wall beta-glucan assembly, encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J. Bacteriol. 175, 6908–6915 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler J. S. & West A. H. Fungal Skn7 stress responses and their relationship to virulence. Eukaryot. Cell 10, 156–167 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitt D. C. et al. The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol. Biol. Cell 11, 2335–2347 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan B. A. et al. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 16, 1035–1044 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krems B., Charizanis C. & Entian K.-D. The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr. Genet. 29, 327–334 (1996). [DOI] [PubMed] [Google Scholar]
- Morgan B., Bouquin N., Merrill G. & Johnston L. A yeast transcription factor bypassing the requirement for SBF and DSC1/MBF in budding yeast has homology to bacterial signal transduction proteins. EMBO J. 14, 5679 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igual J., Johnson A. & Johnston L. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15, 5001 (1996). [PMC free article] [PubMed] [Google Scholar]
- Wormley F., Heinrich G., Miller J., Perfect J. & Cox G. Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect. Immun. 73, 5022–5030 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Li J. & Zhang D. History and status of soybean cyst nematode in China. Int. J. Nematol. 7, 18–25 (1997). [Google Scholar]
- Nicholls S. et al. Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genet. Biol. 48, 297–305 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åkerfelt M., Morimoto R. I. & Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Bio. 11, 545–555 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F. et al. A fungal cell wall integrity-associated MAP kinase cascade in Coniothyrium minitans is required for conidiation and mycoparasitism. Fungal Genet. Biol. 49, 347–357 (2012). [DOI] [PubMed] [Google Scholar]
- Gong X. et al. L-arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet. Biol. 44, 1368–1379 (2007). [DOI] [PubMed] [Google Scholar]
- Montero-Barrientos M. et al. Overexpression of a Trichoderma HSP70 gene increases fungal resistance to heat and other abiotic stresses. Fungal Genet. Biol. 45, 1506–1513 (2008). [DOI] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J. & Kingston R. E. In vitro activation of purified human heat shock factor by heat. Biochemistry 34, 1902–1911 (1995). [DOI] [PubMed] [Google Scholar]
- Gurley W. B. HSP101: a key component for the acquisition of thermotolerance in plants. Plant Cell 12, 457–460 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M., Orosz A. & Wu C. Direct sensing of heat and oxidation by Drosophila heat shock transcription factor. Mol. Cell 2, 101–108 (1998). [DOI] [PubMed] [Google Scholar]
- Goodson M. L. & Sarge K. D. Heat-inducible DNA binding of purified heat shock transcription factor 1. J. Biol. Chem. 270, 2447–2450 (1995). [DOI] [PubMed] [Google Scholar]
- Todgham A. E., Schulte P. M. & Iwama G. K. Cross‐Tolerance in the Tidepool sculpin: The role of heat shock proteins. Physiol. Biochem. Zool. 78, 133–144 (2005). [DOI] [PubMed] [Google Scholar]
- Echave P. et al. DnaK dependence of mutant ethanol oxidoreductases evolved for aerobic function and protective role of the chaperone against protein oxidative damage in Escherichia coli. Proc. Nat. Acad. Sci. USA 99, 4626–4631 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino M., Hibino T., Tanaka Y., Nii N. & Takabe T. Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytica acquires resistance to salt stress in transgenic tobacco plants. Plant Sci. 146, 81–88 (1999). [Google Scholar]
- Edwards P. A. & Ericsson J. Sterols and isoprenoids: signaling molecules derived from the cholesterol biosynthetic pathway. Annu. Rev. Biochem. 68, 157–185 (1999). [DOI] [PubMed] [Google Scholar]
- Xu Q. & Reed J. C. Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol. Cell 1, 337–346 (1998). [DOI] [PubMed] [Google Scholar]
- Chen Y., Duan Z., Chen P., Shang Y. & Wang C. The Bax inhibitor MrBI-1 regulates heat tolerance, apoptotic-like cell death, and virulence in Metarhizium robertsii. Sci. Rep. 5, 10625, doi: 10.1038/srep10625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R., Liu X., Jian H. & Li S. Detection of Hirsutella spp. and Pasteuria sp. parasitizing second-stage juveniles of Heterodera glycines in soybean fields in China. Biol. Control 33, 223–229 (2005). [Google Scholar]
- Lopez-Llorca L. V. et al. Expression of serine proteases in egg-parasitic nematophagous fungi during barley root colonization. Fungal Genet. Biol. 47, 342–351 (2010). [DOI] [PubMed] [Google Scholar]
- Yang J. et al. Characterization and functional analyses of the chitinase-encoding genes in the nematode-trapping fungus Arthrobotrys oligospora. Arch. Microbiol. 195, 453–462 (2013). [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A. & Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B. et al. GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31, 1296–1297 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T. L., Johnson J., Grant C. E. & Noble W. S. The MEME Suite. Nucleic Acids Res. 43, gkv416, doi: 10.1093/nar/gkv416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J. & Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 (2006). [DOI] [PubMed] [Google Scholar]