Abstract
The phytohormone ethylene plays a crucial role in the production and accumulation of reactive oxygen species (ROS) in plants under stress conditions. Ethylene response factors (ERFs) are important ethylene-signaling regulators functioning in plant defense responses against biotic and abiotic stresses. However, the roles of ERFs during plant adapting to ROS stress have not yet been well documented. Our studies previously reported that a tomato ERF transcription factor TERF1 functions in the regulation of plant ethylene responses and stress tolerance. Here, we report our findings regarding the roles of TERF1 in ROS scavenging. In this study, we revealed that the transcription of TERF1 is regulated by upstream EIN3-like (EIN3, ethylene-insensitive 3) regulators LeEIL3 and LeEIL4 in tomato (Solanum lycopersicum), and is also inducible by exogenous applied ROS-generating reagents. Ectopic expression of TERF1 in tobacco promoted the expression of genes involved in oxidative stress responses, including carbonic anhydrase functioning in hypersensitive defense, catalase and glutathione peroxidase catalyzing oxidative reactions, and GDP-D-mannose pyrophosphorylase functioning in ascorbic acid biosynthesis, reduced the ROS content induced by ethylene treatment, and enhanced stress tolerance of tobacco seedlings to hydrogen peroxide (H2O2). Cumulatively, these findings suggest that TERF1 is an ethylene inducible factor regulating ROS scavenging during stress responses.
ROS production in plants occurs during normal cell metabolism or response to stress conditions through activation of NADPH oxidases1,2,3,4,5,6,7. Virtually all biotic and abiotic stresses could induce a rapid ROS increase in plants, which provokes the oxidation of cellular components to trigger specific plant responses, such as stomatal closure and programmed cell death8,9,10,11. To maintain the ROS levels under control, plants have developed various protective mechanisms for ROS scavenging8,11,12. On the other hand, ROS could act as signaling molecules to transmit physiological signals to the nucleus by oxidizing upstream components of the signaling pathway to ultimately tune up the tolerance responses against stresses7,11,13,14.
The gaseous phytohormone ethylene, which regulates plant growth, development and stress responses, is associated with ROS production under stress conditions15,16,17,18,19,20,21,22. The ethylene-overproducing mutants eto1 and eto3 are more sensitive to ROS stress and exhibit an enhanced ROS-induced spread of cell death23, while the ethylene-insensitive mutants etr1 and ein3 are highly tolerant to ROS stress24,25. Accordingly, ethylene synthesis was shown to be required for “ROS burst” induced cell death24,25,26,27. These facts indicate that ethylene is a positive regulator of ROS production and ROS-induced spread of cell death. On the other hand, the ethylene-induced ROS production in plants was mediated by the NADPH oxidases, which are regulated by multiple stress factors28,29. And, stress-induced ROS production could rapidly promote ethylene production30,31,32,33. These evidences implied a reciprocal regulation between ethylene and ROS production.
The ethylene-signaling pathway has been well studied21,34. Gaseous ethylene is perceived by a family of receptors that negatively regulates ethylene signaling22,34,35,36,37. Then, the negative regulator CTR1 transmits the signal onto the integral membrane protein EIN2 through modulating a cascade of mitogen-activated protein kinases (MAPKs) to activate the transcription factor EIN3, the direct regulator of ethylene response factors (ERFs) that bind the GCC-box in the promoters of ethylene inducible defense-related genes20,35,38. A number of components of ethylene-signaling pathway have been found to be involved in the ROS responses28,33,39,40. The ethylene receptor ETR1 was shown to be a critical node in mediating the cross-talk between ethylene and ROS signaling in stomatal guard cells28. The MAPK cascade is also suggested to be shared by both ethylene and ROS signaling32,41,42. And, mutation in ethylene receptor ETR1 and transcription factor EIN3 could greatly increase the ROS tolerance of Arabidopsis13,25,28,33. The plant specific ERF transcription factors are crucial regulators in plant responses to both biotic and abiotic stresses and regulate a number of stress responsive genes through binding the cis-elements in their promoters, including GCC-box, DRE/CRT, CE1, JERE and CT-rich etc.38,43,44,45,46,47. Recent studies also showed that ERFs are correlated with ROS production39,48,49,50. For instance, Arabidopsis AtERF15 and AtERF71, pepper CaPF1, and tomato JERF3, which function in plant biotic and/or abiotic stress responses, were all reported to be involved in the regulation of ROS production or accumulation39,48,49,51. However, the regulatory relationship between ERFs and ROS production during stress responses is still unclear.
Our studies previously revealed that the tomato ERF transcription factor TERF1 is inducible by ethylene, salinity, drought, cold, and ABA, and that TERF1 functions in plant ethylene responses and diverse stress responses52,53,54,55. In this study, we analyzed the transcriptional regulation of TERF1 by ethylene signaling regulators and ROS-generating reagents, and investigated the roles of TERF1 in regulating plant ROS tolerances, which have provided valuable information to unravel the underlying mechanism by which ERF transcription factors coordinating plant stress tolerance and ROS responses.
Results
Regulation of TERF1 transcription by LeEILs
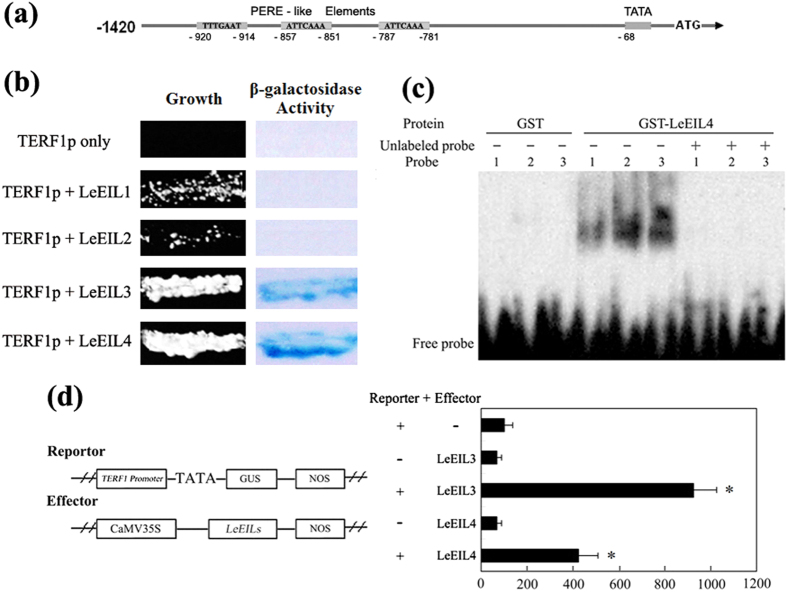
Previous studies on the promoters of ethylene responsive genes identified an element of PERE (primary-ethylene-response element) that is specifically recognized by the ethylene signaling component EIN3 (ETHYLENE-INSENSITIVE3) or EIL1/2 (EIN3-like1/2)35,56. As shown in Fig. 1a, the promoter of TERF1 was found to possess three PERE-like elements (ATTCAAA, - 781 to -787; ATTCAAA, -851 to -857; TTTGAAT, -914 to -920) besides other stress responsive elements53. The PERE-like fragment of TERF1 promoter shows high similarity to those identified in other ethylene responsive genes, such as Arabidopsis PERE element (GGATTCAAG)56 and the PERE element (TTCAAAT) in Os-EBP89 promoter57,58. This evidence indicates that the transcription of TERF1 should be regulated by EIN3 or EIL1/2 through these PERE-like elements. To investigate this hypothesis, Agrobacterium-mediated transient in vivo transcription activation assay and yeast-one-hybrid were employed.
Figure 1. Molecular interaction between LeEILs and TERF1 promoter.
(a) Schematic diagram of TERF1 promoter, showing the position of PERE-like elements. TATA, TATA-box; ATG, initiation codon. (b) Binding of LeEILs to the PERE-like-element-containing fragment from TERF1 promoter in yeast. TERF1p, PERE-like-element-containing fragment. Transformants could grow on the selective medium (Growth, lacking histidine but containing 30 mM 3-AT) were subjected to filter-lifted β-galactosidase activity (β-galactosidase Activity). (c) In vitro interaction between LeEIL4 and the PERE-like elements in TERF1 promoter in gel-shift assay. GST-LeEIL4 indicates GST-tagged LeEIL4 protein, GST is control. Number 1, 2 and 3 indicate probes containing the PERE-like element at position -920–920, -857–851 and -787–781, respectively, and the corresponding unlabeled probes (Unlabeled probe) were used as competitive probes. Free probe indicates unbound probes. (d) Binding of LeEIL3/4 to TERF1 promoter in tobacco leaves. Left panel shows the structures of reporter and effector vectors, the effector vectors are driven by 35S promoter (CaMV35S). NOS, NOS terminator. The relative GUS activity values are the average of three independent triplicates. The GUS activity of non-effector control was set as 100. Error bars indicate ±SD. Asterisks indicate significant differences from the control (P < 0.005, Student’s t test).
In tomato, four homologues of Arabidopsis EIN3 have been identified. They are LeEIL1, LeEIL2, LeEIL3 and LeEIL459,60, which share a similarity about 70% to Arabidopsis EIN3 in amino acid sequence (Fig. S1). In the yeast-one-hybrid assay, LeEIL3 and LeEIL4 were able to activate the expression of histidine and lacZ reporter genes controlled by the PERE-like-element-containing fragment from TERF1 promoter, and allowed the yeast to grow on selective medium lacking histidine, but containing 30 mM 3-AT (3-amino-1,2,4-triazole, a competitive inhibitor of histidine) and to display high β-galactosidase activity, while LeEIL1 and LeEIL2 exhibited a poor capability in growth on the selective medium or in the activation of β-galactosidase activity (Fig. 1b). Further gel-shift assay indicated an in vitro interaction between LeEIL4 and the PERE-like elements in TERF1 promoter (Fig. 1c). In the Agrobacterium-mediated transient in vivo transcription activation assay with tobacco leaves, the effector vectors expressing LeEIL3 and LeEIL4 could strongly activate the expression of TERF1-promoter-controlled GUS gene, which resulted in an increase in the GUS activity as high as 9 times of that by the control vector (Fig. 1d). These data suggested that the transcription of TERF1 is under the regulation of LeEIL3 and LeEIL4.
Transcription of TERF1 is ROS inducible
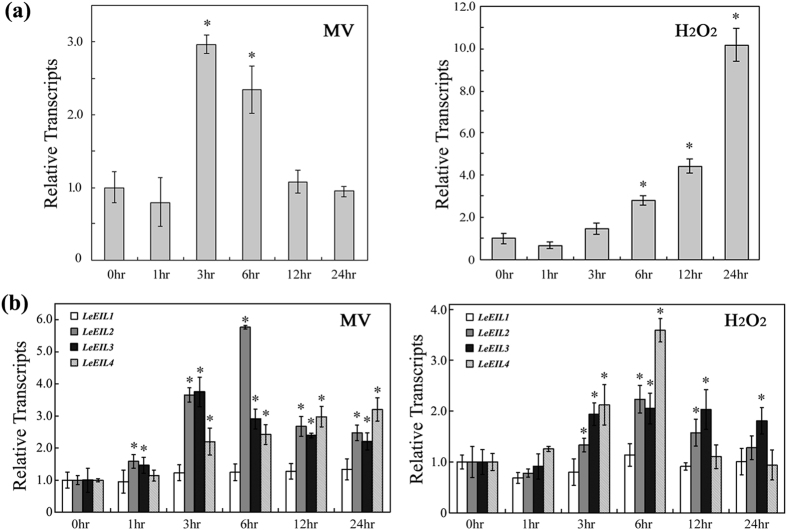
We previously demonstrated that transcription of TERF1 was induced by multiple environmental factors, and that ectopic expression of TERF1 could increase plant tolerance to abiotic stresses52,53,54,55. Abiotic stress responses are highly correlated with ROS production9,10,17,61, and this fact promoted us to determine the involvement of TERF1 in ROS responses. We first analyzed the transcription response of TERF1 in response to methyl viologen (MV, ROS-generating reagent) in tomato leaves, and the results showed that MV treatment caused an increase in TERF1 transcription by 3 folds after 3–6 hr of treatment (Fig. 2a). Further studies revealed that hydrogen peroxide (H2O2) could also induce the transcription of TERF1, which showed a steady increase during the H2O2 treatment and could be induced by over 10 fold after 24 hr of treatment (Fig. 2a). These data showed that TERF1 is responsive to ROS induction. Interestingly, our study suggested that the transcription of LeEILs is also responsible to treatments with methyl viologen or H2O2 (Fig. 2b), supporting an involvement in the ROS stress responses.
Figure 2.
Induction of TERF1 (a) and LeEILs (b) expression by oxidative reagents MV and H2O2. The transcript values are the average of three independent triplicates. The transcription level of each gene in untreated samples (0 hr) was set as “1”. Actin gene was used as internal control. Error bars indicate ±SD. Asterisks indicate significant differences from the data of untreated sample (P < 0.005, Student’s t test).
Expression of TERF1 enhanced H2O2 tolerance of tobacco seedling
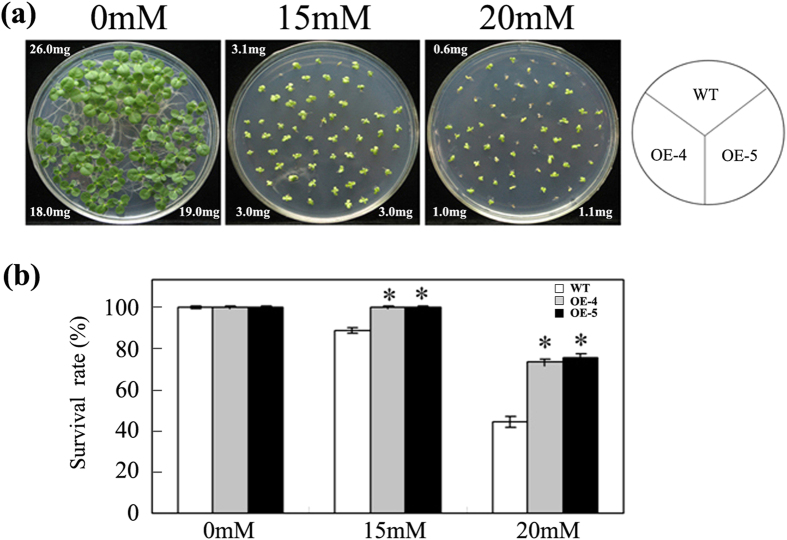
To determine the roles of TERF1 in ROS responses, the TERF1-expressing tobacco plants, which were developed previously52,53, were applied to study the effects of ROS treatment on seedling development, and H2O2 was used as ROS treatment reagent. After 10 days of culture on medium with 15 mM H2O2, the development of both wild type and transgenic tobacco seedlings were arrested, resulting in smaller and yellow leaves, but no significant difference between them could be observed except that a few wild type seedlings start dying. After 10 days of culture on medium with 20 mM H2O2, only a few wild type seedling were alive and the rest were died or dying. In the representative plates shown in Fig. 3a, only 4 wild type seedlings were alive while most seedlings of the transgenic lines were alive except for 3 of them. A greater reduction in the average seedling weight was also observed in wild type plants under the treatment with 20 mM H2O2 (Fig. 3a). Statistic data showed that the survival rate of wild type seedlings was less than 50% upon treatment with 20 mM H2O2 for 14 d, while that of the transgenic tobacco seedling could reach ~80% (Fig. 3b). This indicated that overexpression of TERF1 could significantly increase the tolerance of tobacco seedlings to H2O2.
Figure 3. Expression of TERF1 enhances tobacco tolerance to H2O2 during seedling development.
(a) Seedlings cultured on MS medium (0.6% agar) with indicated concentration of H2O2 after germination. The average fresh weight per seedling (mg) was indicated at the corners of each plant set. (b) Survival rate of tobacco seedlings surveyed after culture on H2O2-containing MS medium for 14 days. Each value represents the average of at least 50 plants of three replicates. WT, wild type tobacco; OE-4/5, TERF1-expressing tobacco lines. Error bars indicate ±SD. Asterisks indicate significant differences from the data of wild type plants (P < 0.005, Student’s t test).
Overexpression of TERF1 decreases ethylene-induced ROS accumulation in tobacco leaves
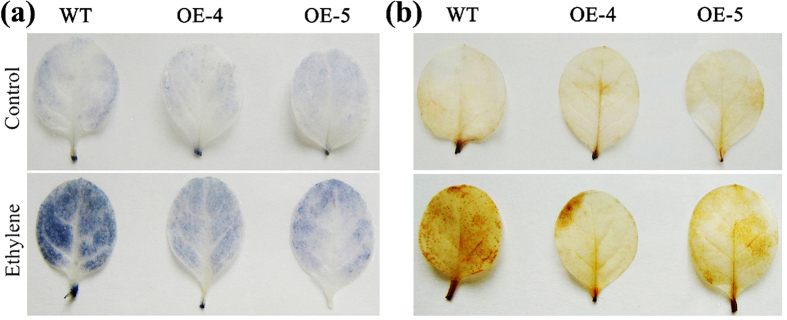
Our previous and present studies have shown that TERF1 is inducible by multiple environmental factors including ROS and ethylene52,54, indicating that TERF1 may be involved in the regulation of ROS production by ethylene. Therefore, the roles of TERF1 in regulating ethylene-induced ROS responses were investigated with the TERF1-expressing tobacco plants52,53. Plant produced ROS includes H2O2, singlet oxygen, superoxide, and the hydroxyl radical12,62, and the contents of ethylene-induced superoxide and H2O2 in TERF1-expressing tobacco seedlings were examined by chemical staining with nitroblue tetrazolium (NBT) and diaminobenzidine tetrahydrochloride (DAB) respectively. As shown in Fig. 4a, NBT staining of superoxide showed that superoxide accumulation kept at low levels under normal conditions and no obvious difference could be observed between wild type and transgenic seedlings. After exposure to ethylene gas (200 ppm) for 3 hr, the superoxide accumulation increased to a much higher level in wild type plants, but remained at a much lower level in the TERF1-expressing seedlings. Similarly, DAB staining of H2O2 showed low H2O2 levels in both wild type and TERF1-expressing plants under normal growth condition. And, the H2O2 content accentuated to a much higher level in wild type plants upon ethylene treatment, but remained at a much lower level in the TERF1-expressing plants (Fig. 4b). These results suggested that TERF1 could decrease ethylene-induced ROS accumulation.
Figure 4. Roles of TERF1 in ROS scavenging.
(a) NBT staining for superoxide in tobacco leaves. (b) DAB staining for H2O2 in tobacco leaves. Control indicates untreated seedlings. Ethylene indicate ethylene-treated seedling. Approximately 15 seedlings were used for each line, and the representative pictures of three replicates are shown.
Expression of TERF1 activated the expression of oxidative-related genes in tobacco
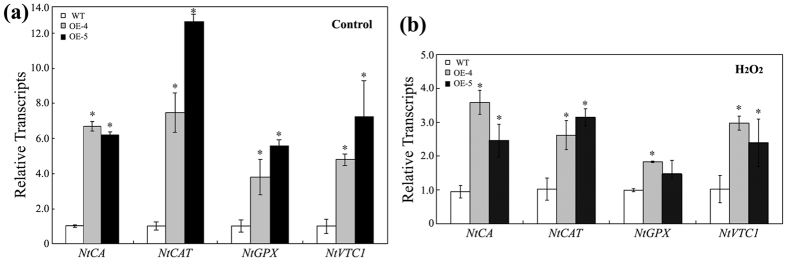
In order to reveal the mechanism by which TERF1 regulates plant responses to ethylene-induced ROS production, we analyzed the roles of TERF1 in regulating oxidative-related genes, including NtCA encoding a carbonic anhydrase functioning in hypersensitive defense63, NtCAT and NtGPX encoding catalase and glutathione peroxidase respectively that use H2O2 as an electron acceptor to catalyze oxidative reactions64,65, and GDP-D-mannose pyrophosphorylase gene (referred to as NtVTC1) functioning in ascorbic acid biosynthesis66,67. The qRT-PCR assays showed that the expression of NtCA and NtCAT was constitutively increased approximately 6 folds in the TERF1-expressing plants compared to that in wild type plants (Fig. 5a). And, the expression levels of NtGPX and NtVTC1 were also increased by over 4 folds in the TERF1-expressing tobacco plants (Fig. 5a). The transcripts of these oxidative-related genes remained higher levels in the TERF1-expressing tobacco plants even after treatment with H2O2 (Fig. 5b). These data support the hypothesis that TERF1 is involved in the regulation of oxidative-related genes, and are helpful to explain the roles of TERF1 in regulating plant responses to ethylene-induced ROS production.
Figure 5.
Expression of TERF1 activates the expression of ROS response related genes in tobacco under normal condition (a) and H2O2 treatment (b). The transcript value for each gene is the average of three independent replicates. NtCA, carbonic anhydrase gene; NtCAT, catalase gene; NtGPX, glutathione peroxidase gene; NtVTC1, GDP-D-mannose pyrophosphorylase gene. The transcription level of each gene in wild type tobacco was set as “1”. NtActin was used as internal control. WT, wild type tobacco; OE-4/5, TERF1-expressing tobacco lines. Error bars indicate ±SD. Asterisks indicate significant differences from the data of wild type plants (P < 0.005, Student’s t test).
Discussion
ROS production is involved in plant stress responses to both biotic and abiotic stresses, and is modulated by signaling pathways of multiple phytohormones, including ABA, salicylic acid (SA) and ethylene etc.6,16,68,69,70,71. Ethylene signaling pathway plays important roles in the regulation of ROS production24,25,26,27,33, and a number of the downstream ERF transcription factors in ethylene pathway function as regulators coordinating both biotic and abiotic stress responses39,48,49,50,51. On the other hand, stress responses in plants are highly related to the production and accumulation of ROS, which involves the regulation by ethylene signaling pathway15,16,26,27,68. Our previous studies showed that an ethylene-inducible tomato ERF transcription factor TERF1 modulates plant tolerance to salt, drought, and cold52,53,54,55. In this study, we further demonstrate that TERF1 is regulated by EILs and plays a role in ROS scavenging in plants, which has extended the understanding to ERF transcription factor in regulating plant stress responses.
Ethylene signal is perceived by a family of membrane receptors, and transducted by the downstream components CTR1, EIN2, EIN3 and ERF transcription factors20,22,26,34,35,36,38. EIN3 as well as its homologues EILs could directly bind the promoters of ERF genes through the PERE elements to regulate their expression35,56,58. In this study, we analyzed the capability of tomato EILs (LeEIL1, LeEIL2, LeEIL3, and LeEIL4) in binding TERF1 promoter and regulating its expression. Yeast-one-hybrid and gel-shift assays indicates that LeEIL3 and LeEIL4 could bind TERF1 promoter through the PERE-like elements. And, the Agrobacterium-mediated transient activation assay also revealed that LeEIL3 and LeEIL4 could activate TERF1-promoter controlled GUS expression in vivo. These findings showed that TERF1 might function as a factor regulating a subset of ethylene signaling pathway downstream of LeEIL3 and LeEIL4. On the other hand, the transcription of TERF1 as well as LeEILs could also be induced by ROS-generating reagents, which indicates their involvement in plant ROS responses. TERF1 was previously evidenced to be an ethylene-inducible factor that acts as a regulator in various plant abiotic stress tolerance52,53,54,55. Whereas, ROS production is always provoked under abiotic stresses, in which ethylene pathway plays important regulatory roles17,25,33,39. These evidences suggest that TERF1 may function as coordinative regulator in responses to abiotic stress- and ethylene-induced ROS stress. Investigating the roles of TERF1 in regulating ROS responses is much helpful to understand the mechanism underlying plant stress responses.
By analyzing the ROS accumulation in TERF1-expressing tobacco plants, we found that the contents of both superoxide and H2O2 were maintained at lower levels upon ethylene treatment, while those in the control plants were remarkably promoted by ethylene. Furthermore, overexpression of TERF1 increased tobacco tolerance to H2O2 during seedling development. These findings indicate that TERF1 may play a role in the regulation of ROS production or scavenging. Plant cells contain multiple enzymes for ROS scavenging, including superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione peroxidase (GPX), and catalase (CAT)1,72,73,74. SODs act as enzymes catalyzing the transformation of highly damaging superoxide to H2O2, and APX, GPX, and CAT function in H2O2 detoxifying1,72,73,74. Previous studies showed that ERF transcription factors could regulate the transcription of ROS metabolic enzymes to change plant ROS tolerance39,48,49,50,51. The transcriptional analyses in this study revealed that overexpression of TERF1 enhanced the transcription of NtCAT and NtGPX, which function in plant ROS scavenging, and activated the expression of NtCA which encodes a carbonic anhydrase that could enhance plant hypersensitive defense to ROS stress63. Ectopic expression of TERF1 also increased the transcription level of NtVCT that encodes GDP-D-mannose pyrophosphorylase, an enzyme catalyzing the biosynthesis of ascorbic acid. And, ascorbic acid, known as vitamin C, is an important substance in plant ROS tolerance66,67,72. Previously, a TERF1 homologous ERF transcription factor JERF3 was found to activate the expression of NtCA via binding the cis-element in NtCA promoter39. TERF1 might regulate the transcription of NtCA in similar manners. The transgenic plants in this study were previously developed lines with high-level ectopic TERF1 expression, and their growth was a bit slower than control plants under normal conditions due to the activation of ethylene responses52. This phenomenon was also observed in this research, however, their growth rate and survival capability were apparently higher than control plants upon H2O2 treatment. These finding imply an involvement of ROS scavenging gene in the growth control. We ever determined if the internal transcription of tobacco ERF1 gene was altered by TERF1-expressing, but found no significant change under normal condition or ethylene induction (data not shown). Cumulatively, these results imply that TERF1 may regulate diverse aspects in plant ROS responses through modulating the transcription of genes related to ROS metabolism.
This work revealed that TERF1 is involved in ROS responses, and expression of TERF1 could decrease ROS accumulation in tobacco plants and increase tobacco tolerance to H2O2 stress. We presume that ethylene signal pathway may play important roles in the regulation of both ROS production and scavenging. That is the reason that some investigation proved the involvement of ethylene in ROS production amplification24,27, while a number of ERF transcription factors have been found functioning in ROS scavenging39,48,49,51. Moreover, ERF transcription factors could regulate plant responses to multiple stresses38,39,48,49, and their functions in ROS responses may be modulated by various signaling pathways. These facts suggest that ERF transcription factors coordinate a complicated regulatory network between ethylene signaling and ROS responses.
Materials and Methods
Plant material and growth conditions
Tomato (Lycopersicon esculentum cv Lichun) seedlings were grown in an indoor growth room at 25 °C under a 16 hr light/8 hr dark photoperiod as previously described52,53. For ROS induction of TERF1 transcription, 4-week-old tomato seedlings were treated by spraying with 50 μm MV or 100 μm H2O2 in 0.1% Tween 20 solution as described by Wu et al.39, and then collected for total RNA preparation at indicated time points.
Tobacco (Nicotiana tabacum cv NC89) plants were grown in the same indoor growth room. The transgenic tobacco plants expressing TERF1 were previously generated by Huang et al.52. T3 transgenic tobacco plants were used for the experiments. Leaves of 6-week-old tobacco seedlings collected for transcriptional assays. For H2O2 induced transcription assay, leaf samples were collected after 6 hr of treatment with H2O2 as described above.
Yeast-one-hybrid assay
The reporter plasmids were constructed as described by Wang et al.75. The PERE-like-element-containing fragment in TERF1 promoter was amplified with primer 5′-TCTTTAATTATAGATATTTTAAAC-3′ and 5′-CGTTAGTACTTATTTGAATGTATC-3′, and cloned as a trimer into the MCS upstream of HIS3 minimal promoter in pHISi-1 vector and that upstream of the lacZ minimal promoter in pLacZi vector (Clontech, USA). Then, these two plasmids were linearized and co-transformed into yeast stain YM4271 to obtain the reporter yeast. The cDNAs of LeEILs were cloned with following primers: 5′-TCATCCTGTGGAAGATGATGATGT-3′ and 5′-ACAACATGTCAACAGACTTCTGGC-3′ for LeEIL1, 5′-TGGCTGCCAAGATGATGATGTTTG-3′ and 5′-CTTGATGTTCATTTGAGTAATCGC-3′ LeEIL2, 5′-TGGTAAATGGGGATATTTGAAGAT-3′ and 5′-CAGTTTAATACTAGTACTAGTTCA-3′ for LeEIL3, and 5′-GAGTTTGTTCTTGTGAAGATGATG-3′ and 5′-ACGTTTCACCAATATCATGGCTAG-3′ for LeEIL4. Then, the coding sequences of LeEILs were fused into downstream of transcriptional activation domain of the yeast vector pB42AD (Clontech, USA), and introduced into the yeast reporter strain respectively. Transformants that could grow on the selective medium lacking histidine but containing 30 mM 3-AT were subjected to filter-lifted β-galactosidase activity according to the manufacturer’s protocol (Clontech, USA).
Gel-shift assay
The coding sequence of LeEIL4 was cloned into the vector pGEX-4T-1 (Amersham, USA), and transformed into Escherichia coli BL21 cells to express the fusion protein GST-LeEIL4. The recombinant protein was purified via GSH-affinity chromatography, and the GST protein was obtained in the same way to act as control. Gel-shift assay was performed using the DIG Gel Shift Kit (Roche, Germany) with the PERE-like elements in TERF1 promoter as probes. Sequences of the probes are 5′-TATAGATATTTTAAACATTTTGAATTATCAATTATTGTGA-3′ for element at position -920–920, 5′-ATAAATATATAAATTTCATTCAAAAAAAATTGAAGATCTC-3′ for element at position -857–851, and 5′-ATAAATTGAACGATACATTCAAATAAGTACTAACGATTAT-3′ for element at position -787–781, in which the core sequences of PERE-like elements are underlined. Probe labeling with DIG-ddUTP, and the preparation and separation of binding reactions were according to the manufacture’s instruction. In the competitive binding assay reactions, a 200-fold excess of the corresponding unlabeled probes were added as specific competitors. After separation in polyacrylamide gel, the probes were transferred onto nylon membrane and crosslinked under UV light, and then detected using anti-Digoxigenin-conjugated alkaline phosphatase included in the kit.
Agrobacterium-mediated transient transcription activation assay
To construct the reporter vector, cauliflower mosaic virus 35S promoter in pCAMBIA1381 was replaced with the TERF1 promoter (-1420–1 bp) cloned previously by Li et al.53 to generate TERF1-promoter-controlled GUS expressing vector. To construct effector vectors, the coding sequences of LeEILs were introduced into pCAMBIA1381 to replace the GUS gene under the control of CaMV 35S promoter. The obtained plasmids were then introduced into Agrobacterium tumefaciens strain LBA4404. Agrobacterium-mediated transient transcription activation assay was performed with tobacco leaves from 6-week-old wild type tobacco seedlings as described previously by Wu et al.39. The GUS activity was measured 48 hr after infiltration as described by Wu et al.39.
Tobacco seedling development under H2O2 stress
For seedling development assay under H2O2 stress, tobacco seeds were surface sterilized, sowed onto plates with MS (Murashige and Skoog) medium, and kept at 4 °C for 3 days to break the dormancy before moving to the indoor growth room. After germination in the growth room, the seeds were transferred onto MS medium plates supplemented with desired concentration of H2O2 and cultured in the same indoor growth room. The survival rate of tobacco seedlings was measured after 14 days of culture on MS medium supplemented with H2O2, and the average fresh weight of seedling was calculated by measuring ten seedlings of each plant set meanwhile.
Detection of ROS accumulation in tobacco leaves
To analyze the ROS accumulation in tobacco leaves, 6-week-old wild type and TERF1-expressing tobacco plants were placed in an 18 L plastic chamber to be treated with ethylene gas (200 ppm), and tobacco leaves was harvested for ROS determination after 3 hr of treatment. Tobacco leaves were stained with nitroblue tetrazolium (NBT) and diaminobenzidine tetrahydrochloride (DAB) to detect the contents of superoxide and H2O2 respectively as described by Lee et al.76.
Transcriptional analyses
Total RNAs were extracted using Trizol reagent (Invitrogen, USA). The first strand cDNA was synthesized with M-MLV reverse transcriptase (Promega, USA) and 1 μg of total RNAs according to the manufacturer’s instructions. Quantitative RT-PCR amplifications were carried out using the following gene-specific primers: 5′-ATGTCAAGCCCACTAGAGAT-3′ and 5′-CTATGATGAAGTCATTAAAAGC-3′ for TERF1 (AY044236); 5′-GCATTGGATACGATACCACACCA-3′ and 5′-GCAGACGAGTACATGGGATCTTT-3′ for LeEIL1 (AF328784); 5′-TGATGACGTGACGAAGCAAGATG-3′ and 5′-TGCACGATCCTCCAACCTCTACA-3′ for LeEIL2 (AF328785); 5′-TCAACTTGGGAGGAAGCGACTAC-3′ and 5′GCACTTACAACCATGGAAACATC-3′ for LeEIL3 (AF328786); 5′-CAGTCCACCAATTACCCTGGAAG-3′ and 5′-GACTGACTATATACGTTTCACCA-3′ for LeEIL4 (AB108840); 5′-CATGCCATTCTCCGTCTTGA-3′ and 5′-GCTAGGAGCCAATGCAGT-3′ for tomato actin gene Tom41 (U60480); 5′-CTGAGAAATATGAGAAGAACC-3′ and 5′-GAAAGACCGAACTCAAGTC-3′ for NtCA (AF454759); 5′-GGAGTCCGCATCAAGAAACA-3′ and 5′-CACATAACTATTTCAGGTTTCA-3′ for NtVTC1 (AB066279); 5′-TGGATCTCATACTGGTCTCA-3′ and 5′-TTCCATTGTTTCAGTCATTCA-3′ for NtCAT (U93244); 5′-GGTTTGCACTCGCTTCAAG-3′ and 5′-AGTAGTGGCAAAACAGGAAG-3′ for NtGPX (AB041518); 5′-CCACACAGGTGTGATGGTTG-3′ and 5′-CACGTCGCACTTCATGATCG-3′ for NtActin (X63603). Actin genes were used as the internal control.
Additional Information
How to cite this article: Zhang, H. et al. Ethylene Response Factor TERF1, Regulated by ETHYLENE-INSENSITIVE3-like Factors, Functions in Reactive Oxygen Species (ROS) Scavenging in Tobacco (Nicotiana tabacum L.). Sci. Rep. 6, 29948; doi: 10.1038/srep29948 (2016).
Supplementary Material
Acknowledgments
This work was supported by the Science and Technology Innovation Program of Chinese Academy of Agricultural Sciences (Elite Youth Program to H.Z.; ASTIP-TRIC05), the Program of Chongqing Tobacco Company (NY20140403030022), the Project from Yunnan Academy of Tobacco Agricultural Sciences (2016YN24), the Key Special Program of China National Tobacco Corporation [110201301009 (JY-09), 110201501015 (JY-02)].
Footnotes
Author Contributions H.Z., A.L. and R.H. designed the research; H.Z., A.L., Z.Z., Z.H., D.Z. and X.L. performed the experiments and analyzed the results; H.Z., A.L., P.L., Z.-F.Z. and R.H. wrote the manuscript.
References
- Vandenabeele S. et al. A comprehensive analysis of hydrogen peroxide-induced gene expression in tobacco. Proc. Natl Acad. Sci. USA 100, 16113–16118 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta D. K. et al. NADPH oxidases differentially regulate ROS metabolism and nutrient uptake under cadmium toxicity. Plant Cell Environ, 10.1111/pce.12711 (2016). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. The plasma membrane NADPH oxidase OsRbohA plays a crucial role in developmental regulation and drought-stress response in rice. Physiol. Plant 156, 421–443 (2016). [DOI] [PubMed] [Google Scholar]
- Ben Rejeb K. et al. NADPH oxidase-dependent H2O2 production is required for salt-induced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 174, 5–15 (2015). [DOI] [PubMed] [Google Scholar]
- Nestler J. et al. Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J. 79, 729–740 (2014). [DOI] [PubMed] [Google Scholar]
- Kwak J. M. et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 22, 2623–2633 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. et al. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 63, 305–317 (2012). [DOI] [PubMed] [Google Scholar]
- Ou X. et al. Stomata prioritize their responses to multiple biotic and abiotic signal inputs. PLoS One 9, e101587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas M. S. & Mano J. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 168, 885–898 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrov V., Hille J., Mueller-Roeber B. & Gechev T. S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6, 69 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio L. A. ROS and RNS in plant physiology: an overview. J. Exp. Bot. 66, 2827–2837 (2015). [DOI] [PubMed] [Google Scholar]
- Gechev T. S., Van Breusegem F., Stone J. M., Denev I. & Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28, 1091–1101 (2006). [DOI] [PubMed] [Google Scholar]
- Overmyer K., Brosche M. & Kangasjarvi J. Reactive oxygen species and hormonal control of cell death. Trends Plant Sci. 8, 335–342 (2003). [DOI] [PubMed] [Google Scholar]
- Considine M. J., Sandalio L. M. & Foyer C. H. Unravelling how plants benefit from ROS and NO reactions, while resisting oxidative stress. Ann. Bot. 116, 469–473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poor P., Kovacs J., Szopko D. & Tari I. Ethylene signaling in salt stress- and salicylic acid-induced programmed cell death in tomato suspension cells. Protoplasma 250, 273–284 (2013). [DOI] [PubMed] [Google Scholar]
- De Cnodder T., Vissenberg K., Van Der Straeten D. & Verbelen J. P. Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane- 1-carboxylic acid: a matter of apoplastic reactions. New Phytol. 168, 541–550 (2005). [DOI] [PubMed] [Google Scholar]
- Wi S. J., Jang S. J. & Park K. Y. Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol. Cells 30, 37–49 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang L. et al. An AP2 domain-containing gene, ESE1, targeted by the ethylene signaling component EIN3 is important for the salt response in Arabidopsis. Plant Physiol. 157, 854–865 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J. L. et al. Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet. 12, e1005760 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M. C., Liao P. M., Kuo W. W. & Lin T. P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol. 162, 1566–1582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbineau F., Xia Q., Bailly C. & El-Maarouf-Bouteau H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 5, 539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Cui X., Sun Y. & Dong C. H. Ethylene signaling and regulation in plant growth and stress responses. Plant Cell Rep. 32, 1099–1109 (2013). [DOI] [PubMed] [Google Scholar]
- Rao M. V., Lee H. I. & Davis K. R. Ozone-induced ethylene production is dependent on salicylic acid, and both salicylic acid and ethylene act in concert to regulate ozone-induced cell death. Plant J. 32, 447–456 (2002). [DOI] [PubMed] [Google Scholar]
- Overmyer K. et al. Ozone-sensitive arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12, 1849–1862 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H., Overmyer K., Keinanen M., Kollist H. & Kangasjarvi J. Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J. 39, 59–69 (2004). [DOI] [PubMed] [Google Scholar]
- Chen G. H., Liu C. P., Chen S. C. & Wang L. C. Role of ARABIDOPSIS A-FIFTEEN in regulating leaf senescence involves response to reactive oxygen species and is dependent on ETHYLENE INSENSITIVE2. J. Exp. Bot. 63, 275–292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann S., Bourdais G., Rietz S. & Robatzek S. Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol. 154, 391–400 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R. et al. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J. 47, 907–916 (2006). [DOI] [PubMed] [Google Scholar]
- Wi S. J., Ji N. R. & Park K. Y. Synergistic biosynthesis of biphasic ethylene and reactive oxygen species in response to hemibiotrophic Phytophthora parasitica in tobacco plants. Plant Physiol. 159, 251–265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlfors R., Brosche M. & Kangasjarvi J. Ozone and nitric oxide interaction in Arabidopsis thaliana: a role for ethylene? Plant Signal. Behav. 4, 878–879 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K. C., Sun P. C. & Lin P. L. Production of reactive oxygen species and induction of signaling pathways for the ACO gene expressions in tomato plants triggered by the volatile organic compound ether. Plant Cell Rep. 30, 599–611 (2011). [DOI] [PubMed] [Google Scholar]
- Ahlfors R. et al. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 40, 512–522 (2004). [DOI] [PubMed] [Google Scholar]
- Poor P. et al. Salt stress-induced production of reactive oxygen- and nitrogen species and cell death in the ethylene receptor mutant Never ripe and wild type tomato roots. Plant Physiol. Biochem. 97, 313–322 (2015). [DOI] [PubMed] [Google Scholar]
- Alonso J. M. & Stepanova A. N. The ethylene signaling pathway. Science 306, 1513–1515 (2004). [DOI] [PubMed] [Google Scholar]
- Guo H. & Ecker J. R. The ethylene signaling pathway: new insights. Curr. Opin. Plant Biol. 7, 40–49 (2004). [DOI] [PubMed] [Google Scholar]
- Schaller G. E. & Bleecker A. B. Ethylene-binding sites generated in yeast expressing the Arabidopsis ETR1 gene. Science 270, 1809–1811 (1995). [DOI] [PubMed] [Google Scholar]
- Rodriguez F. I. et al. A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283, 996–998 (1999). [DOI] [PubMed] [Google Scholar]
- Muller M. & Munne-Bosch S. Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol. 169, 32–41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhang Z., Zhang H., Wang X. C. & Huang R. Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol. 148, 1953–1963 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki M. The role of phytohormone signaling in ozone-induced cell death in plants. Plant Signal. Behav. 3, 166–174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M. A., Walia A., Mansfield S. D. & Ellis B. E. Overexpression of SIPK in tobacco enhances ozone-induced ethylene formation and blocks ozone-induced SA accumulation. J. Exp. Bot. 56, 2195–2201 (2005). [DOI] [PubMed] [Google Scholar]
- Opdenakker K., Remans T., Vangronsveld J. & Cuypers A. Mitogen-Activated Protein (MAP) kinases in plant metal stress: regulation and responses in comparison to other biotic and abiotic stresses. Int. J. Mol. Sci. 13, 7828–7853 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohme-Takagi M. & Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7, 173–182 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q. et al. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10, 1391–1406 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke F. L., Parchmann S., Mueller M. J., Kijne J. W. & Memelink J. Involvement of the octadecanoid pathway and protein phosphorylation in fungal elicitor-induced expression of terpenoid indole alkaloid biosynthetic genes in Catharanthus roseus. Plant Physiol. 119, 1289–1296 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Christiansen K. M. & Innes R. W. Regulation of plant disease resistance, stress responses, cell death, and ethylene signaling in Arabidopsis by the EDR1 protein kinase. Plant Physiol. 138, 1018–1026 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G. P. & Loveridge C. W. HvDRF1 is involved in abscisic acid-mediated gene regulation in barley and produces two forms of AP2 transcriptional activators, interacting preferably with a CT-rich element. Plant J. 37, 326–339 (2004). [DOI] [PubMed] [Google Scholar]
- Park H. Y. et al. AtERF71/HRE2 transcription factor mediates osmotic stress response as well as hypoxia response in Arabidopsis. Biochem. Biophys. Res. Commun. 414, 135–141 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Arabidopsis AtERF15 positively regulates immunity against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea. Front. Plant Sci. 6, 686 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewelam N. et al. Ethylene response factor 6 is a regulator of reactive oxygen species signaling in Arabidopsis. PLoS One 8, e70289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W., Charles T. M. & Newton R. J. Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth. Plant Mol. Biol. 59, 603–617 (2005). [DOI] [PubMed] [Google Scholar]
- Huang Z. et al. Tomato TERF1 modulates ethylene response and enhances osmotic stress tolerance by activating expression of downstream genes. FEBS Lett. 573, 110–116 (2004). [DOI] [PubMed] [Google Scholar]
- Li A., Zhang Z., Wang X. C. & Huang R. Ethylene response factor TERF1 enhances glucose sensitivity in tobacco through activating the expression of sugar-related genes. J. Integr. Plant Biol. 51, 184–193 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Expressing TERF1 in tobacco enhances drought tolerance and abscisic acid sensitivity during seedling development. Planta 222, 494–501 (2005). [DOI] [PubMed] [Google Scholar]
- Gao S. et al. Expression of TERF1 in rice regulates expression of stress-responsive genes and enhances tolerance to drought and high-salinity. Plant Cell Rep. 27, 1787–1795 (2008). [DOI] [PubMed] [Google Scholar]
- Solano R., Stepanova A., Chao Q. & Ecker J. R. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev. 12, 3703–3714 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. J. et al. The OsEBP-89 gene of rice encodes a putative EREBP transcription factor and is temporally expressed in developing endosperm and intercalary meristem. Plant Mol. Biol. 50, 379–391 (2002). [DOI] [PubMed] [Google Scholar]
- Mao C., Wang S., Jia Q. & Wu P. OsEIL1, a rice homolog of the Arabidopsis EIN3 regulates the ethylene response as a positive component. Plant Mol. Biol. 61, 141–152 (2006). [DOI] [PubMed] [Google Scholar]
- Tieman D. M., Ciardi J. A., Taylor M. G. & Klee H. J. Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J. 26, 47–58 (2001). [DOI] [PubMed] [Google Scholar]
- Yokotani N., Tamura S., Nakano R., Inaba A. & Kubo Y. Characterization of a novel tomato EIN3-like gene (LeEIL4). J. Exp. Bot. 54, 2775–2776 (2003). [DOI] [PubMed] [Google Scholar]
- Xue T. et al. Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J. Exp. Bot. 60, 339–349 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli C. G., Gomez F., Martinez D. E. & Guiamet J. J. Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J. Exp. Bot. 55, 1663–1669 (2004). [DOI] [PubMed] [Google Scholar]
- Slaymaker D. H. et al. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl Acad. Sci. USA 99, 11640–11645 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvar B. L. & Ellis B. E. Isolation of a cDNA encoding cytosolic ascorbate peroxidase in tobacco. Plant Physiol. 108, 839–840 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini S., Paolocci F., Borgogni A., Morettini R. & Ederli L. The overexpression of an alternative oxidase gene triggers ozone sensitivity in tobacco plants. Plant Cell Environ. 30, 1545–1556 (2007). [DOI] [PubMed] [Google Scholar]
- Tabata K., Takaoka T. & Esaka M. Gene expression of ascorbic acid-related enzymes in tobacco. Phytochemistry 61, 631–635 (2002). [DOI] [PubMed] [Google Scholar]
- Gao Q. & Zhang L. Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J. Plant Physiol. 165, 138–148 (2008). [DOI] [PubMed] [Google Scholar]
- Pitzschke A., Forzani C. & Hirt H. Reactive oxygen species signaling in plants. Antioxid. Redox Signal 8, 1757–1764 (2006). [DOI] [PubMed] [Google Scholar]
- Straus M. R., Rietz S., Ver Loren van Themaat E., Bartsch M. & Parker & Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J. 62, 628–640 (2010). [DOI] [PubMed] [Google Scholar]
- Kimura M. & Kawano T. Salicylic acid-induced superoxide generation catalyzed by plant peroxidase in hydrogen peroxide-independent manner. Plant Signal. Behav. 10, e1000145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y. et al. AtrbohD and AtrbohF positively regulate abscisic acid-inhibited primary root growth by affecting Ca2+ signaling and auxin response of roots in Arabidopsis. J. Exp. Bot. 64, 4183–4192 (2013). [DOI] [PubMed] [Google Scholar]
- Qin Y. M., Hu C. Y. & Zhu Y. X. The ascorbate peroxidase regulated by H2O2 and ethylene is involved in cotton fiber cell elongation by modulating ROS homeostasis. Plant Signal. Behav. 3, 194–196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar R. K. Plant responses to water stress: role of reactive oxygen species. Plant Signal. Behav. 6, 1741–1745 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum S. A. et al. Osmoregulation and antioxidant production in maize under combined cadmium and arsenic stress. Environ. Sci. Pollut. Res. Int. 10.1007/s11356-016-6382-1 (2016). [DOI] [PubMed] [Google Scholar]
- Wang H. et al. Ectopic overexpression of tomato JERF3 in tobacco activates downstream gene expression and enhances salt tolerance. Plant Mol. Biol. 55, 183–192 (2004). [DOI] [PubMed] [Google Scholar]
- Lee B. H., Lee H., Xiong L. & Zhu J. K. A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14, 1235–1251 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.