ABSTRACT
Safety checklists in medicine are designed to identify a potential error before it results in harm to a patient. The World Health Organization (WHO) safety checklist was widely implemented in surgical practice in the UK after significant reductions in death, and peri-operative complications were achieved in eight countries worldwide in the ‘Safe Surgery Saves Lives’ campaign of 2008. Nevertheless, use of the checklist for invasive medical procedures is not yet routine. Such procedures are becoming ever more complex, necessitating multidisciplinary team management and involving higher-risk patients, with the need for general anaesthesia on occasion. As a result, the potential for error increases and the need for a safety checklist has become more apparent. Such a checklist can be modified to provide a framework for specialty-specific safety checks, enhanced team-working and communication for invasive medical procedures. Following an audit of use of the WHO checklist in 20 cases under general anaesthesia in our quaternary referral cardiac catheterisation laboratory, we discovered use of this safety tool was poor (performed/documented: sign in 30%/40%, time out 10%/15%, sign out 10%/15%) and we identified two ‘near miss’ incidents within the audit period. We then developed and implemented a modified WHO checklist for the specific challenges faced in the cardiac catheterisation laboratory. Following a staff education programme, a subsequent audit of 34 cases demonstrated improvement in all sections (performed/documented: sign in 91.2%/82.4%, time out 85.3%/76.5%, sign out 73.5%/64.7%) with no patient safety incidents during the post-intervention audit period. Well-designed, procedural checklists may well prove to be of benefit in other areas of interventional medicine.
KEYWORDS: Checklist, safety, cardiac catheter laboratory
Background
In 1999, the United States Institute of Medicine released the report, To err is human: building a safer health system, which highlighted the fact that medicine is not as safe as it should be, and as many as 98,000 people die in US hospitals each year as a result of preventable medical errors.1 The same situation exists outside of North America: for example, in England, 2,941 incidents resulting in death were logged with the National Patient Safety Agency (NPSA) for the year April 2011 to March 2012.2 The cost to the NHS of adverse incidents is estimated as £1 billion, requiring an additional 3 million bed-days annually.3 Infrequent but serious mistakes occur recurrently over a period of years. These medical errors are often the result of human error, and can be prevented by more effective systems and an improved organisational safety culture.4
Safety checklists in medicine
Since the publication of To err is human, the medical profession has looked to other ‘high stakes’ industries, such as aviation and nuclear power, for strategies to reduce system error and improve patient safety. In these industries, potential errors could have catastrophic consequences and research has focused on both reducing the risk of these events occurring and crisis management. Checklists are fundamental to the aviation industry; they are used to evaluate the mechanical integrity of the plane and its electronic systems on multiple occasions in the cockpit before, during and after the flight.5 In emergency situations, checklists may help to avoid missing critical steps in a highly pressurised environment. Training is carried out to encourage all team members to use checklists and to raise safety concerns without fear of retaliation. Analysis has shown that the strong safety culture in this industry has contributed to a positive outcome in a number of high-profile events.5
A checklist consists of a list of steps each of which must be completed before proceeding to the next step on the list. Its compilation should be evidence-based and it should identify key points that are essential for safe conduct of the procedure. In medical practice, safety checklists are designed to identify a potential error before it results in harm to the patient.6 They ensure that procedures are followed, eliminate reliance on human memory and provide a standardised framework for communication among team members. In addition, they can empower team members to challenge those who are not adhering to the checklist.7
The use of safety checklists in medicine is developing rapidly. One of the first checklists to be widely accepted was for central venous catheter insertion.7 Five clinician-based procedures surrounding catheter care were introduced in combination with a checklist. Together, these interventions resulted in a reduction in catheter-related blood stream infection by 66% (incident rate ratios falling to 0.34 at 18 months after the study). In 2008, Lingard and colleagues introduced a surgical checklist designed to aid communication between surgeons, scrub nurses, anaesthetists and surgical and anaesthetic trainees.8 The mean number of communication failures for each procedure fell from 3.95 before the intervention to 1.31 after the intervention (p<0.001). In 34% of cases, the checklist identified potential problems with the procedure or critical knowledge gaps, or was useful for decision making and follow-up actions.
Development of the WHO surgical safety checklist
Between October 2007 and September 2008, eight hospitals across the world (in Canada, India, Jordan, New Zealand, the Philippines, Tanzania, the UK and the USA) participated in a program called ‘Safe Surgery Saves Lives’, which was introduced by the World Health Organization (WHO).9 The programme implemented a 19-item surgical checklist, known as the World Health Organization Safety Checklist.
The checklist was designed to achieve the ten core standards for safe surgery established by the WHO (Box 1). These included operating on the correct patient at the correct site, recognising and preparing for blood loss, loss of airway or respiratory function, accurately identifying surgical specimens and enabling effective communication and exchange of patient information crucial for the safe conduct of the procedure.
Data were collected pre- and post-intervention. The primary end point was the occurrence of any complication during hospitalisation (up to 30 days). Following the introduction of the checklist, there was a 36% reduction in major complications (from 11% to 7% (p<0.001)) and a 47% reduction in 30-day mortality rate (from 1.5% to 0.8% [p = 0.047]). The reductions in death and complication rates were observed not only in the low-income countries but also at the high-income centres, although there was a more modest reduction at the latter (30-day mortality fell from 0.9% to 0.6%, p = 0.18).
A standard WHO Surgical Safety Checklist (WHO checklist) was released globally in 2008, and in January 2009 the NPSA issued an alert recommending that all NHS organisations ensure that the WHO checklist is completed for every patient undergoing a surgical procedure, including those carried out under local anaesthesia (Fig 1).10 The WHO checklist is used at three set points in the procedural pathway: ‘time in’ occurs prior to the induction of anaesthesia, ‘time out’ prior to skin incision and ‘sign out’ before the patient leaves the operating theatre.
Fig 1.
World Health Organization surgical safety checklist (adapted for England and Wales). ASA = American Society of Anesthesiologists; IV = intravenous. Reproduced with permission from NHS National Patient Safety Agency (2009).10
The checklist ensures a final common pathway, in which the whole team comes together to verify all the key details. It includes an introduction of team members, ensuring that everyone recognises the other individuals present and is aware of what their specific roles are. This is vital where team members may not work regularly with each other and when unfamiliarity may reduce effective communication. The whole team becomes responsible for the safety of the patient, rather than just the lead clinician, and team members are encouraged to express any safety concerns they may have.
More recently, a controlled study from the Netherlands compared the incidence of complications and mortality at six hospitals that used a series of checklists for the whole patient pathway with those at five control hospitals.11 A reduction in complications from 15.4% to 10.6% (p<0.001) and a fall in in-hospital mortality from 1.5% to 0.8% (95% CI, 0.2 to 1.2% reduction) were achieved in the hospitals using the checklists.
Adaptation of the WHO checklist
The WHO advises that the WHO checklist should be adapted both nationally and locally. National adaptation ensures that the checklist reflects the priorities of the healthcare system in which it is designed to work. Accordingly, the NPSA modified the checklist to include sections on venous thromboembolism prophylaxis and the surgical site infection bundle (prophylactic antibiotics, hair removal, patient warming and glycaemic control).10
An example of a national, specialty-specific modification of the WHO checklist is in interventional radiology.12 This checklist asks whether the Ionising Radiation (Medical Exposure) Regulation (IRMER) requirements are met and whether the patient has risk factors for contrast-induced nephropathy. The ‘time out’ phase is only applicable for those under general anaesthesia, so can be skipped in the majority of cases. There have also been a number of other specialty- and procedure-specific national modifications of the WHO checklist, including modifications for obstetrics, cataract surgery and bronchoscopy.13-15
Local adaptation allows the WHO checklist to be both applicable and credible in the environment in which it is designed to be used. Teams are unlikely to engage with a checklist if they feel that many of the points are irrelevant to their environment while other pertinent checks are omitted. It is recommended that the checklist retains its initial three-phase structure, that the elements designed to maintain teamwork and communication remain, and that all members of the team are encouraged to voice concerns prior to skin incision.16
Problems with effective implementation of the WHO checklist include:
disengagement of staff who feel that it causes delay or that it is simply a ‘tick-box’ exercise;17,18 some are concerned that it results in increased anxiety for awake patients, although evidence suggests that this is not the case and a group of obstetric patients undergoing surgery found use of the checklist reassuring19
checks being completed when key individuals are absent17
omission of checklist stages;17 recent studies show that the reduction in 30-day mortality has been smaller than that in the original WHO study and is only demonstrated where the full checklist is completed in its entirety, making full and effective implementation vital.20
Use of patient-safety checklists by medical specialists
The use of checklists in the medical specialties has increased since the original central venous catheter checklist was developed.7 Recently, complications of chest drain insertion were reduced by more than 75% (from 8.3% to 1.5% [p = 0.015]) following the introduction of a ‘pleural checklist’.21 Development and implementation of a venous thromboembolism (VTE) checklist by junior doctors resulted in an increase in the percentage of new patients undergoing risk assessment from 82% to 97% (p = 0.06).22 Furthermore, appropriate prescription of VTE prophylaxis increased significantly from 75% to 98% (p = 0.006). Checklists have also been developed for use on post-take ward rounds and for patient discharge.23,24
Would the WHO checklist have prevented previous medical disasters?
One of the most well-known medical errors in recent history was the case of 70-year-old Graham Reeve, whose sole functioning kidney was removed instead of the diseased one; he died 5 weeks later.25 Although the consent form was completed correctly, the theatre list identified the wrong side. The team did not review the patient pre-operatively on the ward because he was asleep. On transfer to theatre, the imaging was placed back-to-front, and the patient was positioned for a left nephrectomy rather than a right nephrectomy. There are multiple steps in the WHO checklist that would hopefully prevent a similar tragedy occurring today. As with the majority of medical errors, it was not an isolated oversight that resulted in the disaster, but a catalogue of mistakes by multiple individuals. The WHO checklist enables the team to come together for one final check before the procedure starts. This final common check may have averted the catastrophe.
Another well-publicised non-surgical case that might have been adverted by the use of a multidisciplinary, pre-procedure, WHO-style checklist is that of Wayne Jowett. Vincristine, rather than methotrexate, was administered intrathecally to Mr Jowett.26 Again multiple mistakes by multiple individuals were involved. This was the fourteenth case of its kind in the UK since 1985, eleven of which have been fatal. In response to this case, the Department of Health issued guidelines that now include a final common check involving both a member of staff appropriately trained in intrathecal chemotherapy administration and the patient or their guardian.27 There have been no similar errors in the UK since.
When should the WHO surgical safety checklist apply?
The NPSA (now under the auspices of the NHS Commissioning Board Special Health Authority) requires that the WHO checklist be completed for every patient undergoing a surgical procedure.10 The WHO has defined a surgical condition as ‘any condition that requires suture, incision, excision, manipulation, or other invasive procedure that usually, but not always, requires local, regional, or general anesthesia’.28 Invasive procedures requiring written consent, even if not involving surgeons, should be considered to be ‘surgical procedures’ for the purposes of this checklist. We would advocate the development of a specific checklist for invasive medical procedures that can be further adapted for individual subspecialties.
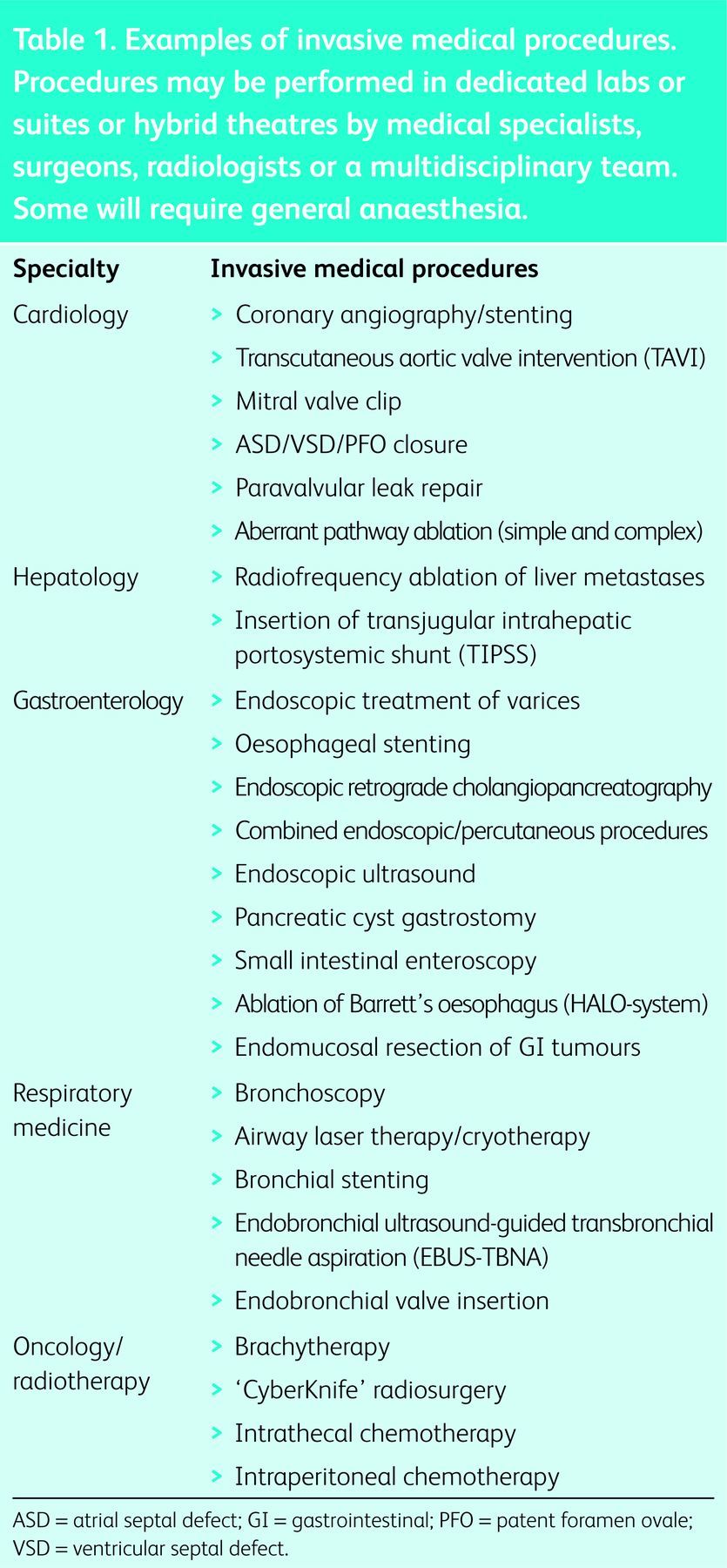
Invasive procedures that are carried out by medical specialists and radiologists are becoming more widespread (Table 1). Not only are these procedures associated with a more rapid recovery and lower cost than surgery, but they can also be offered to patients with multiple comorbidities who are not fit for conventional surgery.
Table 1.
Examples of invasive medical procedures. Procedures may be performed in dedicated labs or suites or hybrid theatres by medical specialists, surgeons, radiologists or a multidisciplinary team. Some will require general anaesthesia.
These ‘non-surgical’ procedures are often carried out under general anaesthesia. Anaesthetists are being asked to provide general anaesthesia for higher-risk patients, for procedures they are less familiar with, in areas of the hospital with which they are less well acquainted. In an era of sub-specialisation, the doctors that perform procedures in endoscopy suites, bronchoscopy suites and catheterisation laboratories may well have never met the patient before the procedure. Furthermore, the team (which may be composed of multiple disciplines) may never have met or worked with each other before. The use of a modified WHO checklist under these circumstances becomes crucial to allow clinicians to communicate and work safely as a team.
Our experience of modifying the WHO checklist
Activity in the cardiac catheterisation laboratory (cath lab) has evolved significantly in recent years. Cardiologists have developed skills and procedures to treat not only diseased coronary arteries, but also a range of other conditions. Transcutaneous aortic valve interventions (TAVI), mitral valve clipping (MitraClip), paravalvular leak repairs, left atrial appendage closures, septal defect repairs and complex electrical ablations are examples of more complex procedures frequently carried out in the cath lab. These procedures may be used as alternatives to conventional surgery for patients for whom the risk of such surgery would be very high.
Standard cardiac procedural checklists have been used for some time but they may not be robust enough in complex cases, particularly when other specialties are involved in patient care and when the patient is under general anaesthesia. For example, although they include risk factors for bleeding and allergy checks, they frequently do not include anaesthetic risks, such as aspiration or equipment checks. Furthermore, they may be completed by a member of staff who is not subsequently present for the procedure, thus crucial patient information may not be highlighted to the team before the procedure begins.
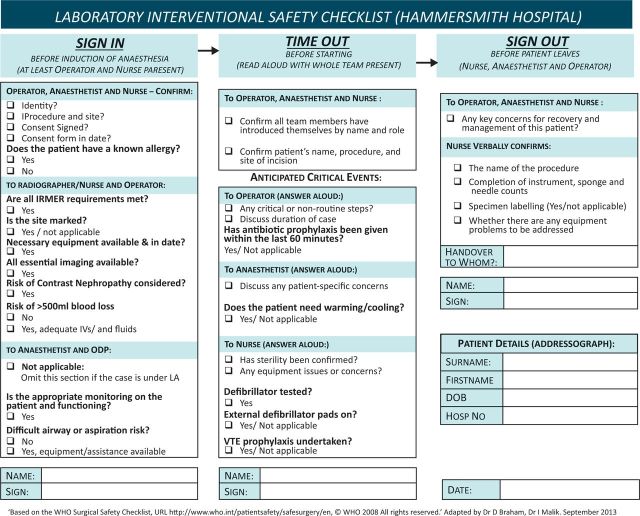
As a quarternary referral centre for structural heart disease and electrophysiology, we perform a large number of cardiac procedures in our unit. With the rapid evolution of services, the multidisciplinary team has had to adapt to new techniques and new environments. The standard WHO checklist (recommended by the NPSA) had been included within the cath lab integrated care pathway (ICP), along with a pre-procedure checklist. Following incident reporting, a number of patient-safety challenges were identified. Those of us who were used to using the WHO checklist in main theatres felt that the use of this checklist was poor in the labs, and that enhanced use of this safety tool could benefit patient safety. We set out to develop a new modified WHO checklist for use in the cath lab (Fig 2). We performed an audit of use of the checklist before and after introduction of the new checklist.
Fig 2.
Modified World Health Organization checklist adapted for the Cardiac Catheterisation Laboratory at the Hammersmith Hospital, Imperial College Healthcare NHS Trust. IRMER = Ionising radiation (medical exposure) regulations; LA = local anaesthesia; VTE = venous thromboembolism.
Baseline audit
We included 20 cath lab cases involving general anaesthesia in our baseline audit. An observer recorded whether the three stages in the Standard WHO Checklist were performed correctly by the team (ie if each step was read aloud and answered aloud by the team). The form was also inspected at the end of the case to see whether the checks were documented to have taken place. The audit form included a free-text section where any patient-safety-related critical incidents that occurred during the case could be recorded.
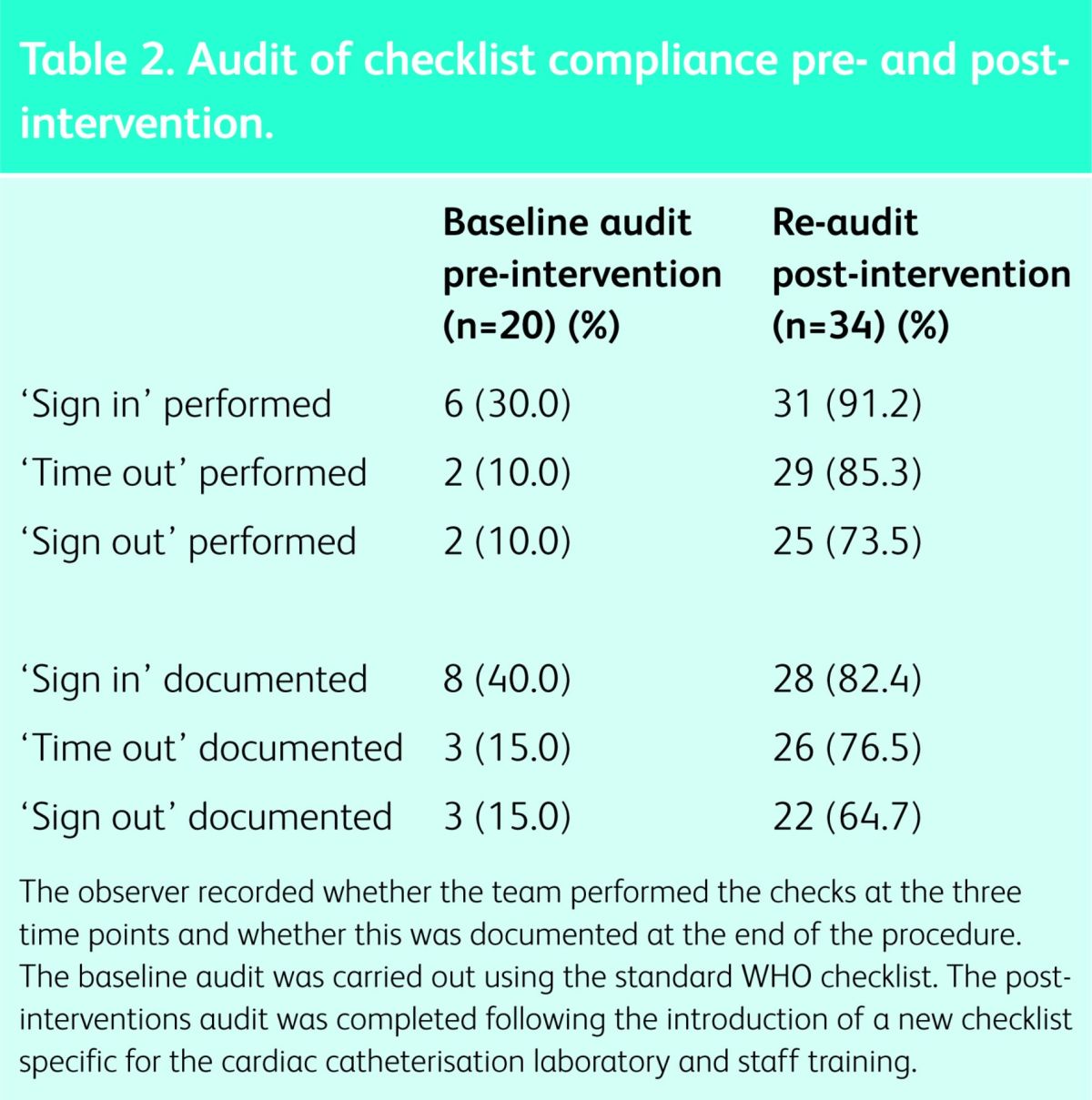
The ‘Sign in’ section was performed in less than one-third of cases and subsequent sections even less frequently (Table 2). All three stages were completed for only two cases (10%). Worryingly, on occasion, the checklist was documented to have been carried out when in fact no team check had taken place. This suggests that the WHO checklist was being treated merely as a ‘tick-box’ exercise. There were two critical incidents during the audit period. In the first of these, a small dose of intravenous midazolam was administered to a patient without the consent form having been checked by the team in the cath lab (‘sign in’ did not take place). Subsequent checks revealed that the consent form had been completed with the patient's name but not actually signed, therefore rendering it invalid. There was no actual patient harm, but the procedure had to be deferred. The other incident was a ‘near miss’ involving an allergy to contrast. The allergy was documented in the pre-procedure checklist but not communicated to the team performing the procedure. No ‘sign in’ took place and contrast was drawn up on the procedure trolley. This was identified by a member of the team and removed prior to starting the procedure. There was no patient harm, but it was felt that these incidents might have been avoided if the WHO checklist had been used.
Table 2.
Audit of checklist compliance pre- and post-intervention.
Reasons for poor use of the WHO checklist
Failure to complete the WHO checklist was perceived to be a result of a degree of confusion as to who should carry out the checklist and when they should do so. Staff expressed the view that the form was focused on traditional surgery – the format and type of questions being less relevant for medical interventions. It was also believed that many important safety checks for this environment were omitted, making the staff feel it was a fruitless exercise that did not add anything to patient safety. For example, checks that the Ionizing Radiation (Medical Exposure) Regulations (IRMER) are met, use of patient cooling (for post-cardiac arrest patients) and whether external defibrillator pads are present (when required) are important pre-procedure checks in this environment that are not included in the standard form. The standard checklist was seen as ‘not fit for purpose’ and there was a reliance on the pre-procedure checklist.
Introduction of the new checklist
In response to the poor initial audit results, we developed a modified WHO checklist that is specific to the cath lab (Fig 2). Engagement of staff was crucial to the adoption of the new checklist, and observations and comments from the multi-disciplinary team relating to the format and use of the standard WHO checklist were taken on board when the new checklist was developed. The cath lab checklist retains the original three-part structure, as recommended by the WHO, but has been adapted so that it includes essential checks specific to this environment and caseload. It was designed to make it clear who should answer each section and how this should occur. It also enabled a focus on some of the local issues raised by critical-incident reporting. Prior to implementation of the new checklist, we facilitated multi-disciplinary training sessions for the team to practise using the new checklist. The issue was also highlighted in clinical governance meetings.
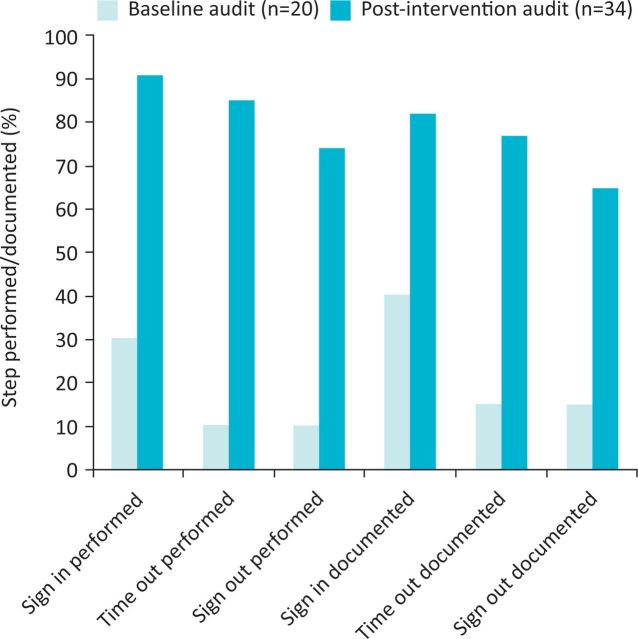
A re-audit involving 34 cases under general anaesthesia took place following implementation of the new checklist. There was an improvement in use of the checklist, compared to use of the standard WHO form in the baseline audit (Table 2, Fig 3). Furthermore, no patient safety incidents were reported during the re-audit period. Despite the improvements in the team's performing the ‘sign in’ section of the checklist, the remaining sections were again utilised less frequently, and all three sections were completed fully in just 25 of the 34 cases (73.5%), leaving further room for improvement. Rates of documentation did not exceed the rates of actual team-performed checks, suggesting the ‘tick-box exercise’ seen in the baseline audit did not occur. It was clear that use increased after encouragement from the senior clinicians and, once implemented, began spreading to all cases in the labs, including more minor ones.
Box 1. World Health Organization's 10 aims for safe surgery.
Fig 3.
Audit of checklist compliance pre- and post-intervention.
Conclusions
Medical procedures are becoming more complex and the patients who undergo them present increasingly high risks. Procedures often require multidisciplinary management and sometimes involve general anaesthesia.
Use of the WHO checklist is aimed at providing a framework for procedural safety and at promoting effective team-working and communication. National and local modifications of the checklist provide the opportunity to highlight the safety issues pertinent to different healthcare systems and specialties.
Implementation of these checklists requires strong leadership and a move away from the clinical autonomy of the lead clinician towards a team approach to patient safety. An attitude change is frequently required and can be difficult to achieve. Staff engagement through communication, training and education is also essential for successful implementation.
The evidence from the traditional surgical specialties is that well-designed, fully implemented, procedural checklists will be of benefit to patients. There is no evidence to suggest that this would not also be the case in procedural medical practice and, in fact, there is evidence to support the use of procedural checklists in medicine. Further research is needed to assess the impact of the introduction of these checklists on procedural morbidity and mortality in the UK.
Through our own experience of introducing a modified three-phase WHO checklist in our cath lab, we aimed to provide a standardised framework for multi-disciplinary communication and patient safety specific to the specialty, and we have demonstrated the improved use of this safety tool. While our re-audit demonstrated fewer patient safety incidents, further work is needed to assess the impact of this safety tool on patient safety.
Acknowledgements
The authors would like to acknowledge the contributions of Dr John Schutzer-Weissmann, Dr Felicity Plaat and Dr Stephen Brett.
References
- 1.Institute of Medicine To err is human: building a safer health system. National Academy of Press, 1999. www.iom.edu/Reports/1999/to-err-is-human-building-a-safer-health-system.aspx [Accessed 30 July 2014]. [Google Scholar]
- 2.National Health Service National reporting and learning system quarterly data workbook up to June 2012. London: NHS, 2013. www.nrls.npsa.nhs.uk/resources/collections/quarterly-data-summaries/?entryid45=135153 [Accessed 30 July 2014]. [Google Scholar]
- 3.Vincent C, Neale G, Woloshynowych M. Adverse incidents in British hospitals: preliminary retrospective record review. Br Med J 2000;322:517–9. 10.1136/bmj.322.7285.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Health An organisation with a memory. Report of an expert group on learning from adverse events in the NHS. London: The Stationery Office, 2000. http://patientsafety.health.org.uk/resources/organisation-memory [Accessed 30 July 2014]. [Google Scholar]
- 5.Eisen LA, Savel RH. What went right: lessons for the intensivist from the crew of US Airways flight 1549. Chest 2009;136:910–7. 10.1378/chest.09-0377 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Patient safety. The checklist effect. www.who.int/patientsafety/implementation/checklists/background/en/index.html [Accessed 30 July 2014]. [Google Scholar]
- 7.Pronovost P, Needham D, Berenholtz S, et al. An Intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725–32. 10.1056/NEJMoa061115 [DOI] [PubMed] [Google Scholar]
- 8.Lingard L, Regehr G, Orsor B, et al. Evaluation of a pre-operative checklist and team briefing among surgeons, nurses and -anaesthesiologists to reduce failures in communication. Arch Surg 2008;143:12–7. 10.1001/archsurg.2007.21 [DOI] [PubMed] [Google Scholar]
- 9.Haynes AB, Weisner TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009;360:491–9. 10.1056/NEJMsa0810119 [DOI] [PubMed] [Google Scholar]
- 10.NHS National Patient Safety Agency WHO surgical safety checklist, 2009. www.nrls.npsa.nhs.uk/resources/?EntryId45=59860 [Accessed 30 July 2014]. [Google Scholar]
- 11.De Vries EN, Prins H, Crolla R, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med 2010;363:1928–37. 10.1056/NEJMsa0911535 [DOI] [PubMed] [Google Scholar]
- 12.Koetser ICJ, de Vries EN, van Delden OM, et al. A checklist to improve patient safety in interventional radiology. Cardiovasc Intervent Radiol 2013;36:312–9. 10.1007/s00270-012-0395-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NHS National Patient Safety Agency WHO surgical safety checklist for maternity case only, 2010. www.nrls.npsa.nhs.uk/resources/?entryid45=83972 [Accessed 30 July 2014]. [Google Scholar]
- 14.NHS National Patient Safety Agency Surgical safety checklist for cataract surgery only, 2010. www.nrls.npsa.nhs.uk/resources/?entryid45=74132 [Accessed 30 July 2014]. [Google Scholar]
- 15.Du Rand IA, Blaikley J, Booton R, et al. BTS guideline for diagnostic flexible bronchoscopy in adults. Thorax 2013;68:S1. 10.1136/thoraxjnl-2013-203618 [DOI] [PubMed] [Google Scholar]
- 16.Safe Surgery 2015 Checklist modification guide/frequently asked questions. www.safesurgery2015.org/checklist-modification.html [Accessed 30 July 2014]. [Google Scholar]
- 17.Vats A, Vincent C, Nagpal K, et al. Practical challenges introducing WHO surgical checklist: UK pilot experience. Br Med J 2010;340:b5433. 10.1136/bmj.b5433 [DOI] [PubMed] [Google Scholar]
- 18.Walker IA, Reshamwalla S, Wilson IH. Surgical safety checklists: do they improve outcomes? Br J Anaesth 2012;109:47–54. 10.1093/bja/aes175 [DOI] [PubMed] [Google Scholar]
- 19.Kerans RJ, Uppal V, Bonner J, et al. The introduction of a surgical checklist in a tertiary referral obstetric centre. BMJ Qual Saf 2011;20:818–22. 10.1136/bmjqs.2010.050179 [DOI] [PubMed] [Google Scholar]
- 20.van Klei WA, Hoff R, van Aarnhem E, et al. Effects of the introduction of the WHO ‘Surgical Safety Checklist’ on in-hospital mortality. Ann Surg 2012;255:44–9. 10.1097/SLA.0b013e31823779ae [DOI] [PubMed] [Google Scholar]
- 21.See KC, Jamil K, Chua AP, et al. Effect of a pleural safety checklist on patient safety in the ultrasound era. Respirology 2013;18:534–9. 10.1111/resp.12033 [DOI] [PubMed] [Google Scholar]
- 22.Colborne NR, Lake DR, Wear KR, Thomson GA. Using a venous thromboembolism checklist significantly improves VTE prevention: a junior doctor led intervention. Int J Clin Pract 2013;67:157–60. 10.1111/ijcp.12059 [DOI] [PubMed] [Google Scholar]
- 23.Herring R, Desai T, Caldwell G. Quality and safety at the point of care: how long should a ward round take? Clin Med 2011;11:20–2. 10.7861/clinmedicine.11-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halasyamani L, Kripalani S, Coleman E, et al. Transition of care for hospitalised elderly patients – development of a discharge checklist for hospitals. J Hosp Med 2006;1:354–60. 10.1002/jhm.129 [DOI] [PubMed] [Google Scholar]
- 25.Dyer O. Doctors suspended for removing wrong kidney. BMJ 2004;328:246. 10.1136/bmj.328.7434.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyer C. Doctor sentenced for manslaughter of leukaemia patient. BMJ 2003;327:697. 10.1136/bmj.327.7417.697-a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Department of Health Health Service Circular. HSC 2008/001 Updated national guidance on the safe administration of intrathecal chemotherapy. London: DH, 2008. http://clsmac70.ndcls.ox.ac.uk/tssg-haematology/lymphoma/safe-it-chemotherapy.pdf [Accessed 30 July 2014]. [Google Scholar]
- 28.Debas HT, Gosselin R, McCord C, Thind A. Surgery. In: Jamison DT, Breman JG, Measham AR, et al.(eds), Disease control priorities in developing countries, 2nd edn. Washington (DC): World Bank, 2006. www.who.int/surgery/SurgeryDebasworldbank.pdf [Accessed 30 July 2014]. [PubMed] [Google Scholar]