Abstract
AMP deaminase (AMPD; EC 3.5.4.6) is encoded by a multigene family in mammals. The AMPD1 gene is expressed at high levels in skeletal muscle, where this enzyme is thought to play an important role in energy metabolism. Deficiency of AMPD activity in skeletal muscle is associated with symptoms of a metabolic myopathy. Eleven unrelated individuals with AMPD deficiency were studied, and each was shown to be homozygous for a mutant allele characterized by a C----T transition at nucleotide 34 (codon 12 in exon 2) and at nucleotide 143 (codon 48 in exon 3). The C----T transition at codon 12 results in a nonsense mutation predicting a severely truncated AMPD peptide. Consistent with this prediction, no immunoreactive AMPD1 peptide is detectable in skeletal muscle of these patients. This mutant allele is found in 12% of Caucasians and 19% of African-Americans, whereas none of the 106 Japanese subjects surveyed has this mutant allele. We conclude from these studies that this mutant allele is present at a sufficiently high frequency to account for the 2% reported incidence of AMPD deficiency in muscle biopsies. The restricted distribution and high frequency of this doubly mutated allele suggest it arose in a remote ancestor of individuals of Western European descent.
Full text
PDF

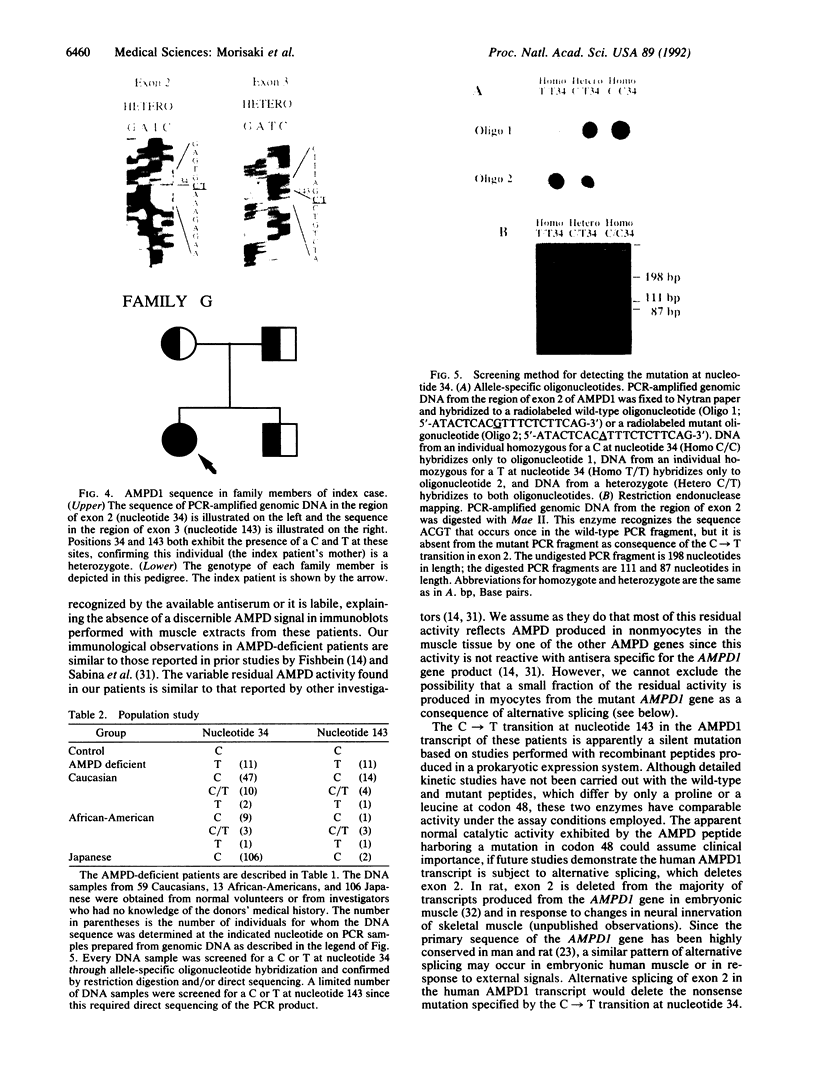

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DiMauro S., Miranda A. F., Hays A. P., Franck W. A., Hoffman G. S., Schoenfeldt R. S., Singh N. Myoadenylate deaminase deficiency--muscle biopsy and muscle culture in a patient with gout. J Neurol Sci. 1980 Aug;47(2):191–202. doi: 10.1016/0022-510x(80)90003-9. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N., Armbrustmacher V. W., Griffin J. L. Myoadenylate deaminase deficiency: a new disease of muscle. Science. 1978 May 5;200(4341):545–548. doi: 10.1126/science.644316. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N., Davis J. I., Nagarajan K., Winkert J. W., Foellmer J. W. Immunologic distinction of human muscle adenylate deaminase from the isoenzyme(s) in human peripheral blood cells: implications for myoadenylate deaminase deficiency. Arch Biochem Biophys. 1980 Dec;205(2):360–364. doi: 10.1016/0003-9861(80)90118-6. [DOI] [PubMed] [Google Scholar]
- Fishbein W. N. Myoadenylate deaminase deficiency: inherited and acquired forms. Biochem Med. 1985 Apr;33(2):158–169. doi: 10.1016/0006-2944(85)90024-9. [DOI] [PubMed] [Google Scholar]
- Gertler P. A., Jacobs R. P. Myoadenylate deaminase deficiency in a patient with progressive systemic sclerosis. Arthritis Rheum. 1984 May;27(5):586–590. doi: 10.1002/art.1780270518. [DOI] [PubMed] [Google Scholar]
- Goebel H. H., Bardosi A., Conrad B., Kuhlendahl H. D., DiMauro S., Rumpf K. W. Myoadenylate deaminase deficiency. Klin Wochenschr. 1986 Apr 1;64(7):342–347. doi: 10.1007/BF01711958. [DOI] [PubMed] [Google Scholar]
- Hayes D. J., Summers B. A., Morgan-Hughes J. A. Myoadenylate deaminase deficiency or not? Observations on two brothers with exercise-induced muscle pain. J Neurol Sci. 1982 Jan;53(1):125–136. doi: 10.1016/0022-510x(82)90086-7. [DOI] [PubMed] [Google Scholar]
- Heller S. L., Kaiser K. K., Planer G. J., Hagberg J. M., Brooke M. H. McArdle's disease with myoadenylate deaminase deficiency: observations in a combined enzyme deficiency. Neurology. 1987 Jun;37(6):1039–1042. doi: 10.1212/wnl.37.6.1039. [DOI] [PubMed] [Google Scholar]
- Kaletha K., Nowak G. Myoadenylate deaminase deficiency studies on normal and deaminase-deficient skeletal muscle. Clin Chim Acta. 1990 Oct 15;190(3):147–155. doi: 10.1016/0009-8981(90)90168-r. [DOI] [PubMed] [Google Scholar]
- Kar N. C., Pearson C. M. Muscle adenylate deaminase deficiency. Report of six new cases. Arch Neurol. 1981 May;38(5):279–281. doi: 10.1001/archneur.1981.00510050045005. [DOI] [PubMed] [Google Scholar]
- Kelemen J., Rice D. R., Bradley W. G., Munsat T. L., DiMauro S., Hogan E. L. Familial myoadenylate deaminase deficiency and exertional myalgia. Neurology. 1982 Aug;32(8):857–863. doi: 10.1212/wnl.32.8.857. [DOI] [PubMed] [Google Scholar]
- Lally E. V., Friedman J. H., Kaplan S. R. Progressive myalgias and polyarthralgias in a patient with myoadenylate deaminase deficiency. Arthritis Rheum. 1985 Nov;28(11):1298–1302. doi: 10.1002/art.1780281115. [DOI] [PubMed] [Google Scholar]
- Marquetant R., Desai N. M., Sabina R. L., Holmes E. W. Evidence for sequential expression of multiple AMP deaminase isoforms during skeletal muscle development. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2345–2349. doi: 10.1073/pnas.84.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercelis R., Martin J. J., Dehaene I., de Barsy T., Van den Berghe G. Myoadenylate deaminase deficiency in a patient with facial and limb girdle myopathy. J Neurol. 1981;225(3):157–166. doi: 10.1007/BF00313744. [DOI] [PubMed] [Google Scholar]
- Mineo I., Clarke P. R., Sabina R. L., Holmes E. W. A novel pathway for alternative splicing: identification of an RNA intermediate that generates an alternative 5' splice donor site not present in the primary transcript of AMPD1. Mol Cell Biol. 1990 Oct;10(10):5271–5278. doi: 10.1128/mcb.10.10.5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki T., Sabina R. L., Holmes E. W. Adenylate deaminase. A multigene family in humans and rats. J Biol Chem. 1990 Jul 15;265(20):11482–11486. [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Yamada Y., Watanabe T. Distribution of AMP-deaminase isozymes in rat tissues. Eur J Biochem. 1978 Jun 15;87(2):297–304. doi: 10.1111/j.1432-1033.1978.tb12378.x. [DOI] [PubMed] [Google Scholar]
- Perryman M. B., Kerner S. A., Bohlmeyer T. J., Roberts R. Isolation and sequence analysis of a full-length cDNA for human M creatine kinase. Biochem Biophys Res Commun. 1986 Nov 14;140(3):981–989. doi: 10.1016/0006-291x(86)90732-1. [DOI] [PubMed] [Google Scholar]
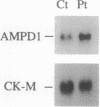
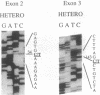
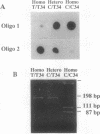
- Sabina R. L., Fishbein W. N., Pezeshkpour G., Clarke P. R., Holmes E. W. Molecular analysis of the myoadenylate deaminase deficiencies. Neurology. 1992 Jan;42(1):170–179. doi: 10.1212/wnl.42.1.170. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Morisaki T., Clarke P., Eddy R., Shows T. B., Morton C. C., Holmes E. W. Characterization of the human and rat myoadenylate deaminase genes. J Biol Chem. 1990 Jun 5;265(16):9423–9433. [PubMed] [Google Scholar]
- Sabina R. L., Ogasawara N., Holmes E. W. Expression of three stage-specific transcripts of AMP deaminase during myogenesis. Mol Cell Biol. 1989 May;9(5):2244–2246. doi: 10.1128/mcb.9.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabina R. L., Swain J. L., Olanow C. W., Bradley W. G., Fishbein W. N., DiMauro S., Holmes E. W. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J Clin Invest. 1984 Mar;73(3):720–730. doi: 10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Shumate J. B., Katnik R., Ruiz M., Kaiser K., Frieden C., Brooke M. H., Carroll J. E. Myoadenylate deaminase deficiency. Muscle Nerve. 1979 May-Jun;2(3):213–216. doi: 10.1002/mus.880020309. [DOI] [PubMed] [Google Scholar]
- Sinkeler S. P., Joosten E. M., Wevers R. A., Oei T. L., Jacobs A. E., Veerkamp J. H., Hamel B. C. Myoadenylate deaminase deficiency: a clinical, genetic, and biochemical study in nine families. Muscle Nerve. 1988 Apr;11(4):312–317. doi: 10.1002/mus.880110406. [DOI] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]