CD40-deficient mice fail to induce conventional CD4+ cells in adipose tissue associated with decreased markers of antigen presentation in ATMs.
Keywords: inflammation, antigen presenting cells (APC), CD40L
Abstract
Obesity activates both innate and adaptive immune responses in adipose tissue, but the mechanisms critical for regulating these responses remain unknown. CD40/CD40L signaling provides bidirectional costimulatory signals between antigen-presenting cells and CD4+ T cells, and CD40L expression is increased in obese humans. Therefore, we examined the contribution of CD40 to the progression of obesity-induced inflammation in mice. CD40 was highly expressed on adipose tissue macrophages in mice, and CD40/CD40L signaling promoted the expression of antigen-presenting cell markers in adipose tissue macrophages. When fed a high fat diet, Cd40-deficient mice had reduced accumulation of conventional CD4+ T cells (Tconv: CD3+CD4+Foxp3−) in visceral fat compared with wild-type mice. By contrast, the number of regulatory CD4+ T cells (Treg: CD3+CD4+Foxp3+) in lean and obese fat was similar between wild-type and knockout mice. Adipose tissue macrophage content and inflammatory gene expression in fat did not differ between obese wild-type and knockout mice; however, major histocompatibility complex class II and CD86 expression on adipose tissue macrophages was reduced in visceral fat from knockout mice. Similar results were observed in chimeric mice with hematopoietic Cd40-deficiency. Nonetheless, neither whole body nor hematopoietic disruption of CD40 ameliorated obesity-induced insulin resistance in mice. In human adipose tissue, CD40 expression was positively correlated with CD80 and CD86 expression in obese patients with type 2 diabetes. These findings indicate that CD40 signaling in adipose tissue macrophages regulates major histocompatibility complex class II and CD86 expression to control the expansion of CD4+ T cells; however, this is largely dispensable for the development of obesity-induced inflammation and insulin resistance in mice.
Introduction
Chronic adipose tissue inflammation has long been implicated in obesity-associated diseases, such as cardiovascular disease, type 2 diabetes, and metabolic syndrome. Adipose tissue inflammation involves activation of both innate and adaptive immune responses [1, 2]. MHC class II–restricted signals from macrophages and dendritic cells contribute to T cell activation in response to obesogenic cues and contribute to metabolic dysfunction [3–5]. ATMs and dendritic cells can function as APCs and provide critical cues to generate proinflammatory signals that include activation of adipose tissue CD4+ T cells toward a Th1 and effector/memory phenotype [6–8]. Obesity is also associated with the induction of CD8+ T cells in adipose tissue and a decrease in Tregs that contribute to a proinflammatory environment [9, 10]. Based on this, there has been increasing interest in understanding how APC–T cell cross talk contributes to activation of an adaptive immune response and adipose tissue inflammation in obesity.
Costimulatory signals from APCs are important for sustaining T cell activation, and recent studies have aimed to elucidate the roles of costimulatory molecules in adipose tissue inflammation because these may be novel therapeutic targets for mitigating obesity-induced metabolic inflammation. Costimulatory receptors, such as CD40 and the B7 complex (CD80/CD86), are induced in adipose tissue of obese humans and animal models [11–13]. However, the role of these costimulatory molecules in the development of adipose tissue inflammation and metabolic dysfunction remains cloudy. Some studies have identified a beneficial role for costimulation in maintaining a Treg pool, both systemically and in adipose tissue, which is protective against obesity-induced inflammation and metabolic disease [5, 13]. In many other instances, loss of costimulation has been shown to alter energy use and inflammation in animal models [14–19].
CD40 is a surface glycoprotein, is expressed in hematopoietic and nonhematopoietic cells, and is activated by CD40L/CD154 expressed on T cells and by soluble CD40L [20, 21]. CD40-CD40L signaling has an important role in the regulation of APC function and the ability to stimulate adaptive immunity [22]. In APCs, CD40 activation induces the expression of MHC II, CD80, and CD86 [23] and enhances cytokine and chemokine production [24]. Relevant to obesity-research, serum levels of soluble CD40L are highly correlated with obesity and metabolic syndrome [25, 26]. Rodent studies indicate that CD40 deficiency prevents vascular inflammation and atherosclerosis [17, 27, 28]. Importantly, CD40 deficiency has been shown to block the proinflammatory M1 activation of macrophages [28], and CD40 has been identified as a marker of ATMs in obesity [11].
Several studies have examined the role of CD40 in obesity-induced inflammation, yet it remains unclear whether CD40/CD40L signaling has protective or deleterious roles in modulating adipose tissue inflammation and metabolic homeostasis [16, 17, 29, 30]. CD40 is expressed on leukocytes and adipose tissue stromal cells and adipocytes [12, 15, 31], which have multiple roles in the regulation of adipose tissue metabolism. CD40 deficiency in T cells potentiated adipose tissue inflammation during obesity, suggesting a protective function for CD40 signaling [29, 30]. By contrast, a chemical blockade of CD40-TRAF6 signaling, but not signaling through TRAF2/3/5, protected mice from obesity-induced insulin resistance and hepatosteatosis [17, 19]. Obviously, variable results in these studies may be attributed to differences in the diet used to induce obesity, to environmental conditions, to differences in gut microbiota, or to the genetic background strains. Nonetheless, a consensus for the role of CD40 in obesity-induced adipose tissue inflammation has not been reached.
Our group is interested in the nature and function of APCs in adipose tissue, and we have found that ATMs are the primary functional APC in fat that promote obesity-induced inflammation [3, 4]. Here, we performed an independent evaluation of the importance of CD40 in obesity-induced metainflammation to determine whether CD40/CD0L signaling contributed to ATM–T cell cross talk in obese adipose tissue. We observed that Cd40−/− (KO) mice had impaired induction of conventional CD4+ T cells (Tconv) in adipose tissue with obesity, without any significant changes in Treg or CD8+ T cells. This was associated with a decrease in the expression of MHC class II and CD86 on ATMs in obese KO mice. Bone marrow chimeras generated from Cd40−/− donors also had reduced Tconv numbers during dietary obesity. These findings indicate that CD40 signaling in ATMs regulates MHC II and CD86 expression to control CD4+ T cell numbers in fat.
MATERIALS AND METHODS
Mice and BMT studies
CD40−/− (B6.129P2-Cd40tm1Kik/J; C57BL/6Ncr background) mice and congenic C57BL/6J mice (CD45.1 and CD45.2) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Lines were established and maintained at the University of Michigan (Ann Arbor, Michigan, USA). All animal procedures complied with the Guide for the Care and Use of Laboratory Animals from the Institute of Laboratory Animal Research (Washington, DC, USA), and the University Committee on Use and Care of Animals at the University of Michigan approved all animal protocols. Beginning at 8 wk of age, male, WT C57BL/6J and CD40−/− (KO) mice were fed an ND (4.5% fat; PMI Nutrition International, St. Louis, MO, USA) or an HFD (60% fat; Research Diets, New Brunswick, NJ, USA) to induce obesity.
BMTs were performed as described [32]. Male recipient WT CD45.1 mice (8 wk old) were exposed to a single dose of lethal irradiation (900 Rad) 4 h before receiving 1 × 107 bone marrow cells (i.v.) from either WT (CD45.2) or KO (CD45.2) male donor mice. Reconstitution was confirmed 6 wk after transplantation by monitoring CD45.1 (recipient) vs. CD45.2 (donor) expression on blood leukocytes by flow cytometry. Diet studies were initiated 6 wk posttransfer (14 wk old), and BMT-WT and BMT-KO mice were fed either ND or HFD for 20 wk.
Isolation of SVCs for immunophenotyping and cell culture
Adipose tissue SVCs were isolated, as previously described [33]. For cell culture experiments, SVCs were isolated from epididymal fat pads of male WT mice fed HFD for 16 wk. SVCs were enriched for CD45+ leukocytes using mouse CD45 microbeads (Miltenyi Biotec, San Diego, CA, USA) and plated at 1 × 106 cells/ml in DMEM containing 10% heat-inactivated FBS. After 3 h, nonadherent cells were removed, and fresh medium was added. Adherent cells were cultured overnight before being treated with recombinant mouse CD40 ligand (CD40L; R&D Systems, Minneapolis, MN, USA) for 6 h.
Flow cytometry analysis
Blood leukocytes were prepared as described [34]. Single-cell suspensions were prepared from spleen and thymus by pressing tissues through a 40-μm cell strainer (BD Falcon; BD Biosciences, Franklin Lakes, NJ, USA). SVCs, blood leukocytes, splenocytes, and thymocytes were suspended in PBS/0.5% BSA (107 cells/ml) and incubated in Fc block (rat anti-mouse CD16/CD32; eBioscience, San Diego, CA, USA) for 15 min on ice. Cells were stained with the indicated antibodies for 30 min at 4°C in the dark. Antibodies used for flow cytometry are provided in Supplemental Table 1. Foxp3 staining kits (eBioscience) were used according to the manufacturer’s instructions. Stained cells were washed twice in PBS, fixed in 0.1% paraformaldehyde, and analyzed on a FACSCanto II Flow Cytometer (BD Biosciences) using FlowJo software (version 9.4; Tree Star, Ashland, OR, USA).
Isolation of T cells, stimulation, and staining for CD154 (CD40L)
SVCs were isolated from epididymal fat pads of male WT mice fed an HFD for 16 wk. SVCs were enriched for CD4+ leukocytes using mouse CD4 microbeads (Miltenyi Biotec) and plated at 1 × 106 cells/ml in RMPI 1640 supplemented with 10% FBS, 10 mM HEPES, 1 mM glutamine, 1 mM sodium pyruvate, and 50 μm 2-mercaptoethanol. CD4+ splenocytes were also isolated and treated in parallel as controls. CD4+ T cells were stimulated with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 2 h and were then treated with monensin (2 μM) for an additional 1 h. T cells were collected, fixed, and permeabilized before staining with CD4 and CD154 antibodies.
Gene expression analysis
Quantitative RT-PCR in mice was performed as described [34]. Adipose tissue was flash frozen in liquid nitrogen and stored at −80°C until analysis. Relative gene expression was determined using the 2−ΔΔCT method after normalizing to Arbp expression. Sequences for PCR primers are provided online (Supplemental Table 2). Gene expression of human adipose tissue was assessed using TaqMan probes (Applied Biosystems, Foster City, CA, USA).
Metabolic tests
Body weights were measured weekly during experimental periods. Blood glucose levels (mg/dl) were measured using a glucometer (One Touch Ultra; Bayer, Whippany, NJ, USA). Mice were fasted for 6 h (10:00 AM–4:00 PM) before GTTs or ITTs were performed. For GTTs, d-glucose (0.7 g/kg body weight) was administered (i.p.), and blood glucose levels were measured at 0, 15, 30, 45, 60, 90, and 120 min after injection. For ITTs, human insulin (1 unit/kg of body weight; Humulin R; Eli Lilly, Indianapolis, IN, USA) was injected i.p., and blood glucose levels (mg/dl) were measured 0, 15, 30, 45, and 60 min after injection. Plasma insulin levels were measured using an ultrasensitive mouse insulin ELISA kit (Crystal Chem, Downers Gove, IL, USA). HOMA-IR values were calculated as fasting insulin (pM/L) × fasting glucose (mM/L)/22.5.
Human adipose tissue samples
Participants (n = 20) were recruited from the University of Michigan Bariatric Surgery Program (clinical characteristics are provided in Supplemental Table 3). All participants in this cohort were female. Recruitment and study protocols were approved by the institutional review board at the University of Michigan Medical School. Omental adipose tissue biopsies were collected at the time of surgery, flash frozen, and stored at −80°C before analysis. Patients with a prior diagnosis of diabetes or taking one or more diabetic medications at the time of surgery were considered to be diabetic (n = 9). Patients were scored for presence or absence of physician-diagnosed metabolic syndrome criteria for hypertension and hyperlipidemia.
Statistical analysis
Data are presented as means ± sem. GraphPad Prism software (version 5.01; GraphPad Software, La Jolla, CA, USA) was used for statistical analysis. Differences between groups were determined using unpaired, 2-tailed Student’s t tests or 1-way ANOVA with Dunnett’s post hoc test. P < 0.05 was considered significant. The Pearson correlation coefficient was used to determine the relationship between genes expressed in human adipose tissue samples.
RESULTS
CD40/CD40L expression in adipose tissue of mice
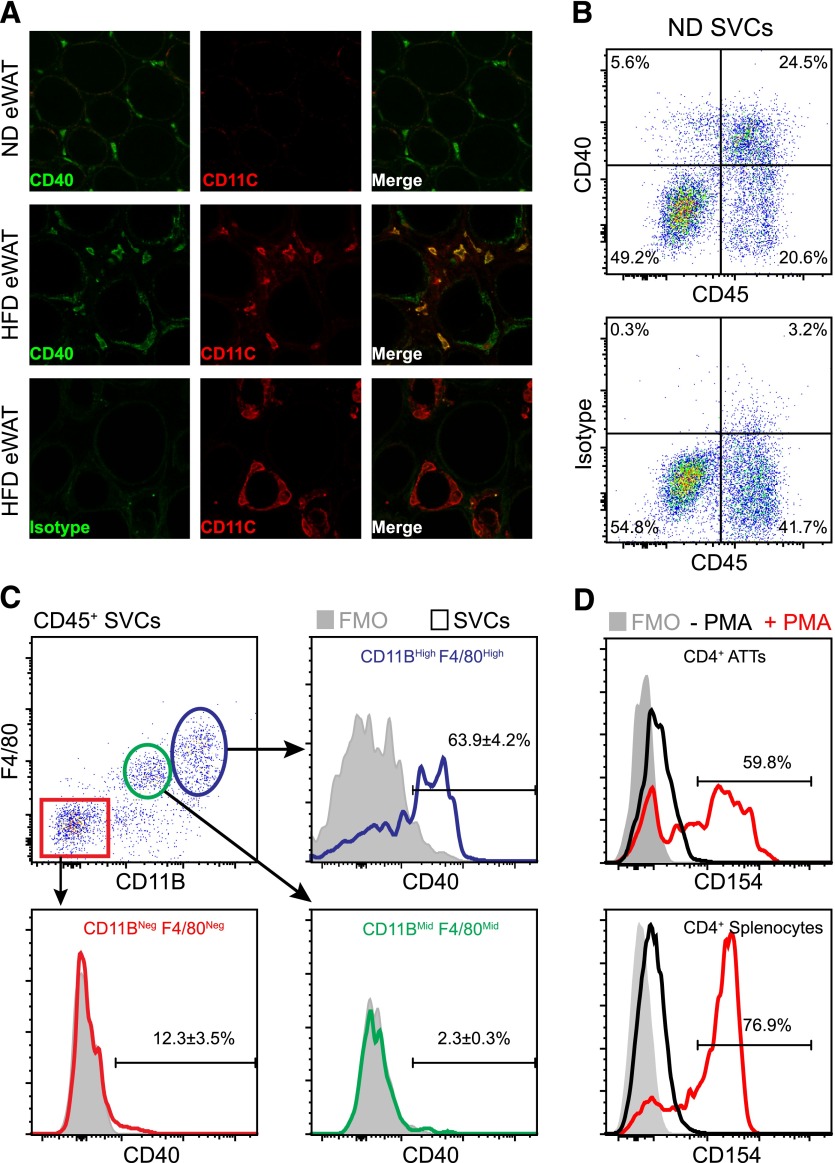
We have previously shown that Cd40 is expressed in FAC-purified ATMs and that obesity and inflammatory signals induce Cd40 expression in ATMs [3]. Given the diverse range of cell types known to express Cd40 in adipose tissue, we used confocal microscopy and flow cytometry analysis to better delineate the expression of CD40 protein in situ. By confocal microscopy, CD40 was detectable on ATMs in the visceral/eWAT from lean mice fed an ND (Fig. 1A; upper panel). CD40 expression was detected in both CD11c+ (M1) and CD11c− (M2) ATMs in obese fat after feeding HFD for 20 wk (Fig. 1A; middle panel). Low-level CD40 immunoreactivity was observed on adipocytes by this method, but signals were significantly lower than on leukocytes, suggesting that ATMs are the primary cell type expressing CD40 protein in adipose tissue.
Figure 1. CD40/CD40L expression in visceral adipose tissue of mice.
Male C57BL/6 mice were fed ND or HFD for 20 wk to induce obesity. (A) Confocal microscopy images demonstrating CD40 (green) and CD11c (red) expression on ATMs in eWAT from lean (ND) and obese (HFD) mice. Staining with rat isotype (IgG2aκ) was used as a control. (B) Representative scatterplots demonstrating CD40 expression on adipose tissue leukocytes (CD45+) in eWAT from lean mice. (C) Surface expression of CD40 on ATMs (blue, CD45+CD11BhighF4/80High), CD11BMidF4/80Mid SVCs (green), and non-ATMs (red, CD45+CD11B−F4/80−) in eWAT from ND mice. Fluorescence minus one staining (FMO) (−CD40 mAb; shaded) is shown. (D) Histogram showing CD154 (CD40L) expression levels on unstimulated (− PMA; black) and stimulated (+ PMA; red) CD4+ ATTs isolated from eWAT of HFD-fed mice. Splenocytes were isolated and treated in parallel as a positive control (lower histogram). (B–D) The percentage (%; means ± sem) of cells in each gate is indicated.
We next examined CD40 expression on SVCs in eWAT from lean mice by flow cytometry. We found that CD40 is expressed almost exclusively by CD45+ SVCs (Fig. 1B). ATMs (CD45+ CD11BHigh F4/80High) were identified as the predominant CD40+ leukocyte population in eWAT, and ∼64% of ATMs expressed high levels of CD40 (Fig. 1C). Approximately 12% of CD45+ CD11BNeg F4/80Neg SVCs expressed detectable levels of CD40 (Fig. 1C); this compartment contains B and T adipose tissue lymphocytes. A small percentage (∼2%) of CD45+ CD11BMid F4/80Mid SVCs expressed CD40 (Fig. 1C); these were likely putative adipose tissue dendritic cells, based on other reports [35]. Collectively, these data indicate that CD40 expressing ATMs are a major constituent of the stromal vascular compartment in eWAT from mice.
To support the model that CD40/CD40L signaling contributes to interactions between ATMs and adipose tissue T cells, we assessed expression of CD154/CD40L on CD4+ ATT cells. CD154 expression on CD4+ T cells is transient and is detectable only in activated T cells in many conditions [36–38]. We were unable to detect CD154 on freshly isolated CD4+ ATTs (data not shown); however, when we stimulated isolated CD4+ ATTs and splenocytes from HFD-fed mice with PMA/ionomycin ex vivo, approximately one-half of the CD4+ ATTs expressed CD154 (Fig. 1D). Taken together, these data suggest that CD40/CD40L signaling molecules may contribute to cross talk between ATMs and ATTs in adipose tissue.
CD40L signaling induces the expression of APC genes in ATMs
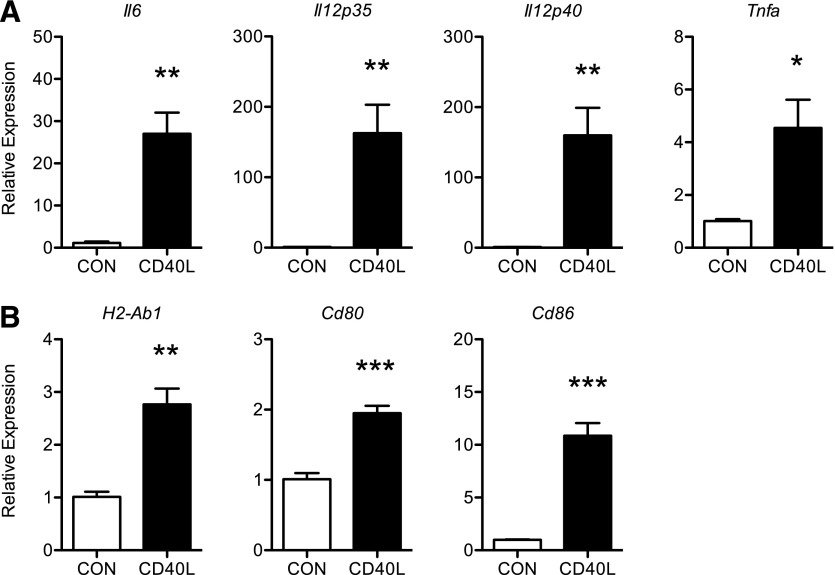
In mice, alternative splicing of the pre-Cd40 mRNA generates multiple CD40 isoforms, with and without intact intracellular domains that convey signaling [34, 39]. Notably, these spliced variants are not easily distinguishable by the quantitative PCR strategy that we used to examine Cd40 expression in previous studies. Therefore, to determine whether CD40 expressed on ATMs is signaling competently, we treated cultured ATMs with soluble recombinant mouse CD40L and evaluated the expression of a number of CD40-regulated genes that are associated with APC function. CD40L treatment induced robust increases in Il6, Il12p35, Il12p40, and Tnfa mRNA levels in ATMs from lean mice (Fig. 2A). The expression of H2-Ab1, the major MHC class II loci in C57BL/6J mice, as well as costimulatory molecules Cd80 and Cd86, was also induced by CD40L (Fig. 2B). These findings indicate that CD40 activation may have a role in potentiating APC function of ATMs.
Figure 2. CD40L signaling induces the expression of APC genes in ATMs.
SVCs were isolated from the eWAT of HFD mice and enriched for ATMs by positive selection (CD45+ microbeads) and plastic adherence. ATMs were cultured overnight and then treated with soluble recombinant mouse CD40L (10 μg/ml) for 6 h. (A–B) Relative expression of inflammatory and APC genes in the absence (CON) or presence of CD40L (n = 4 individual mice/treatment). Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001.
CD40 is required from maximal accumulation of CD4+ ATTs, but not ATMs, in response to diet-induced obesity
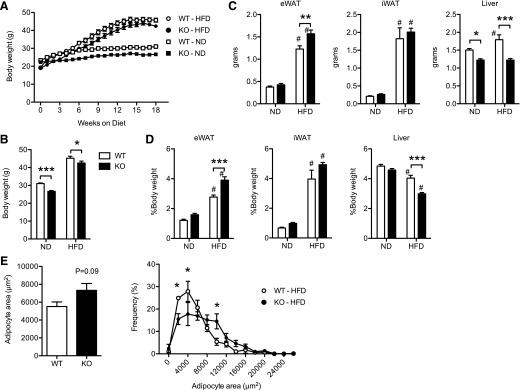
To determine the role that CD40 has in obesity-induced adipose tissue inflammation, we fed WT and Cd40−/− (KO) mice an HFD for 20 wk to induce obesity. Both WT and KO mice gained weight on the HFD, but KO mice gained less weight in response to HFD compared with WT mice (weight gain: WT, 26.6 ± 3.3g; KO, 23.3 ± 2.5g; P = 0.03) (Fig. 3A–B). Metabolic phenotyping did not detect significant differences in energy expenditure, energy consumption, or locomotor activity that could explain the differences in body weights between the 2 genotypes on either ND or HFD (data not shown). Despite this difference in total body weight, HFD KO mice had larger eWAT depots and smaller livers compared with WT mice (Fig. 3C–D), suggesting improved efficiency of nutrient storage in visceral eWAT depots. By contrast, there were no significant differences in subcutaneous/iWAT in KO and WT mice. Mean adipocyte size did not differ significantly between KO and WT mice (Fig. 3E). However, differences in adipocyte size distribution were noted, with more small adipocytes in the WT mice and more moderate-sized adipocytes in the KO mice.
Figure 3. HFD-induced weight gain, but not visceral adiposity, is reduced in CD40-KO mice.
(A) Body weight gain (g) in male WT and KO mice fed ND or HFD for 18 wk (n = 8–11 mice/group). (B) Body weights in male WT and KO mice after 20 wk of diet exposure (n = 8–11 mice/group). (C) eWAT, iWAT, and liver weight (g) in WT and KO mice fed ND and HFD for 20 wk (n = 8–11 mice/group). (D) eWAT, iWAT, and liver weight expressed as a percentage of body weight. (E) Assessment of adipocyte area and size distribution in obese WT and KO mice. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001. #P < 0.05 vs. ND-fed mice.
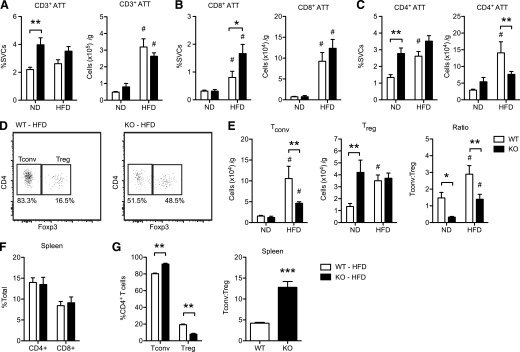
We next evaluated whether CD40 has a role in the regulation of ATTs and ATMs in eWAT during HFD-induced obesity. For this, we used flow cytometry to identify and quantify ATT and ATM populations (see Supplemental Fig. 1 for gating strategies). Data are presented both as the percentage of SVCs and as the total cell counts normalized to total fat pad weight to account for the adipose tissue hypertrophy seen in HFD-fed mice. In lean ND-fed mice, KO mice had an increase in the proportion of total CD3+ lymphocytes in eWAT (Fig. 4A). When normalized to adipose tissue weight, HFD induced a similar expansion of total lymphocytes in eWAT in both WT and KO mice. ND-fed mice had a similar proportion of CD8+ lymphocytes in eWAT, but KO mice had more CD4+ lymphocytes (Fig. 4B–C). This difference was lost when normalized to fat mass. HFD exposure induced similar accumulation of CD8+ lymphocytes in both genotypes. However, increases in CD4+ ATTs were seen in HFD-fed WT mice, but not in KO mice. When normalized to fat mass, HFD-fed KO mice had a significant reduction in the number of CD4+ ATTs compared with WT mice (Fig. 4C). These changes in T cells were specific to eWAT because KO and WT mice had similar numbers of blood (data not shown) and splenic CD4+ and CD8+ T cells in both diet conditions (Fig. 4F).
Figure 4. CD40 is required from maximal accumulation of CD4+ ATTs in response to diet-induced obesity.
WT and KO mice were fed ND and HFD, respectively, for 20 wk (n = 5–8 mice/group). ATTs were identified and quantified in eWAT by flow cytometry (see Supplement Fig. 1 for gating strategy). (A) Quantitation of CD3+ ATTs. Data are expressed as the percentage of total SVCs and total cells/gram of adipose (eWAT) tissue. (B–C) Quantitation of CD3+CD8+ ATTs, and CD3+CD4+ ATTs. (D) Representative flow plots demonstrating Tconv (Foxp3−) and Treg (Foxp3+) CD4+ ATTs in eWAT from WT and KO mice fed an HFD. Percentage of CD4+ ATTs in each gate is indicated. (E) Quantification of Tconv (CD3+CD4+Foxp3−) and Treg (CD3+CD4+Foxp3+) ATTs. Ratio of Tconv:Treg ATTs was also determined. (F) Quantification of CD4+ T cells and CD8+ T cells in spleens from HFD-fed mice. G: Quantification of Tconv and Treg cells, and ratio of Tconv:Treg in spleens from HFD-fed mice. Data are means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001. #P < 0.05 vs. ND-fed mice.
We next evaluated the distribution of CD4+ ATTs in eWAT by assessing Foxp3 expression to differentiate Tconv (CD3+CD4+Foxp3−) and Tregs (CD3+CD4+Foxp3+) (Fig. 4D). In ND-fed mice, there were no difference in Tconv, but there was a significant increase in the number of Tregs in eWAT in the lean KO mice (Fig. 4E). HFD exposure induced an increase in the number of Tconvs in eWAT of WT mice, whereas obese KO mice had significantly fewer Tconvs (Fig. 4E). In contrast, Treg content in eWAT was comparable in both HFD-fed KO and WT mice (Fig. 4E). In both diet conditions, the ratio of Tconvs:Tregs in eWAT was significantly decreased in KO mice (Fig. 4E). Again, this effect was specific for adipose tissue because KO mice had slightly more splenic Tconvs and fewer Tregs compared with WT mice (Fig. 4G). These data suggest that, in lean states, CD40 may serve to limit Treg numbers in adipose tissues. With HFD exposure, the expansion of a subset of Tconvs in eWAT is dependent on CD40, which may differ from other secondary lymphoid organs.
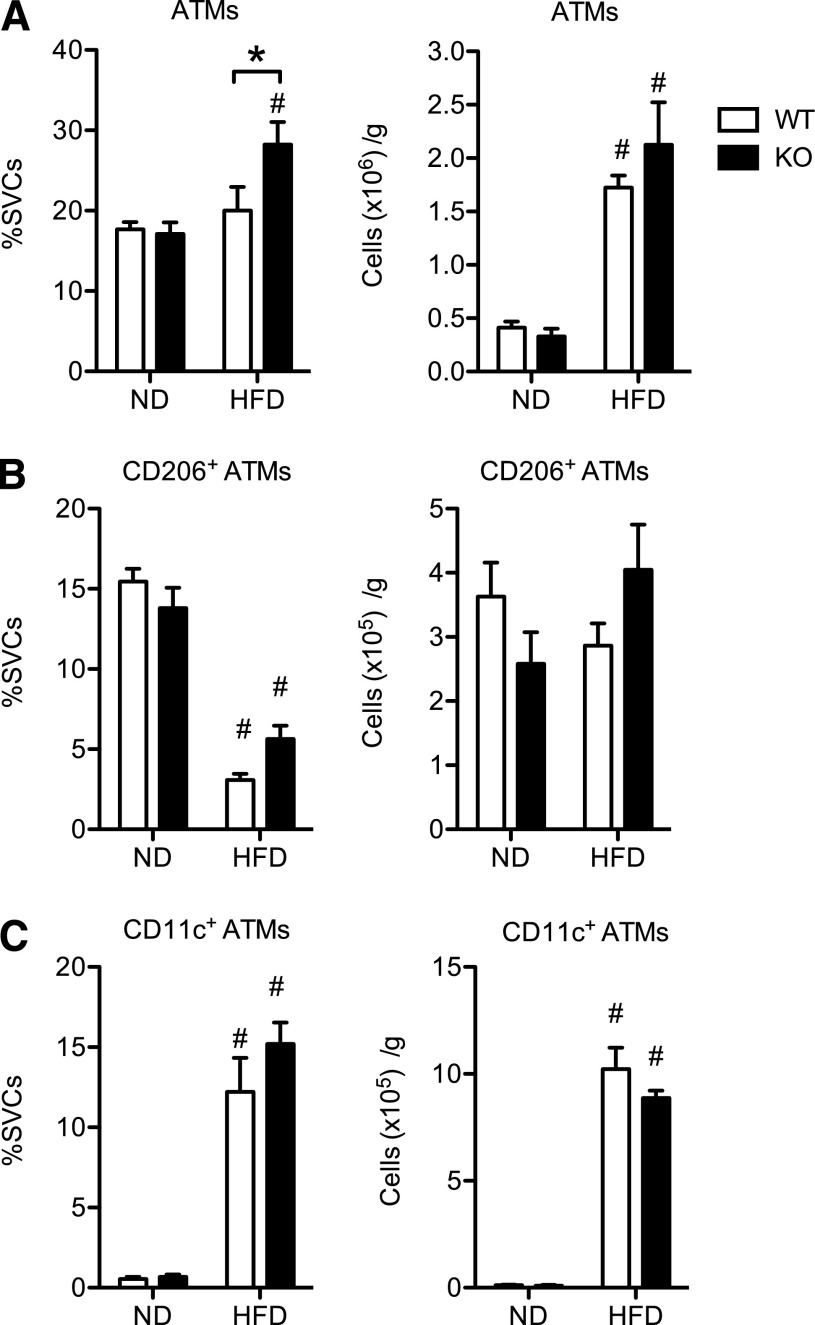
We next assessed the influence of CD40 deficiency on ATM content in eWAT. There were no differences in total ATM content in eWAT from lean mice between genotypes (Fig. 5A). With HFD, both WT and KO mice had significant increases in ATMs, but there was no difference in ATM numbers between obese WT and KO mice. In both WT and KO mice, there was a similar reduction in the proportion of resident CD206+ ATMs and an increase in the number of CD11c+ ATMs in eWAT in response to HFD (Fig. 4B–C).
Figure 5. CD40 deficiency does affect ATM infiltration into visceral fat of mice during diet-induced obesity.
WT and KO mice were fed ND and HFD for 20 wk (n = 8–11 mice/group). ATMs (CD11BHighF4/80High) were quantified in eWAT by flow cytometry (see Supplement Fig. 2 for gating strategy). (A) Quantification of ATMs. Data are expressed as the percentage of total SVCs and total cells/gram of adipose (eWAT) tissue. (B) Quantification of CD206+ (M2-like) ATMs. (C) Quantification of CD11c+ (M1-like) ATMs. Data are means ± sem. *P < 0.05. #P < 0.05 vs. ND-fed mice.
We assessed the expression levels of genes implicated in adipose tissue inflammation (Ccl2, Tnfa, Il10, Nos2, Il12p35, Il12p40, and Ifng). Irrespective of diet, there was no significant difference in the expression levels of Ccl2, Tnfa, Il12p35, and Il12p40 between WT and KO mice (Supplemental Fig. 2A–B). Il10, Nos2, and Ifng expression levels were lower in eWAT from KO mice fed ND, but the expression of these genes was not different after HFD (Supplemental Fig. 3A–B). Collectively, these data indicate that CD40 is required for the maximal expansion of a pool of CD4+ Tconvs, but not for the recruitment of CD11c+ ATMs or the regulation of inflammatory genes in eWAT with HFD-induced obesity.
CD40 regulates MHC class II expression on ATMs during HFD-induced obesity
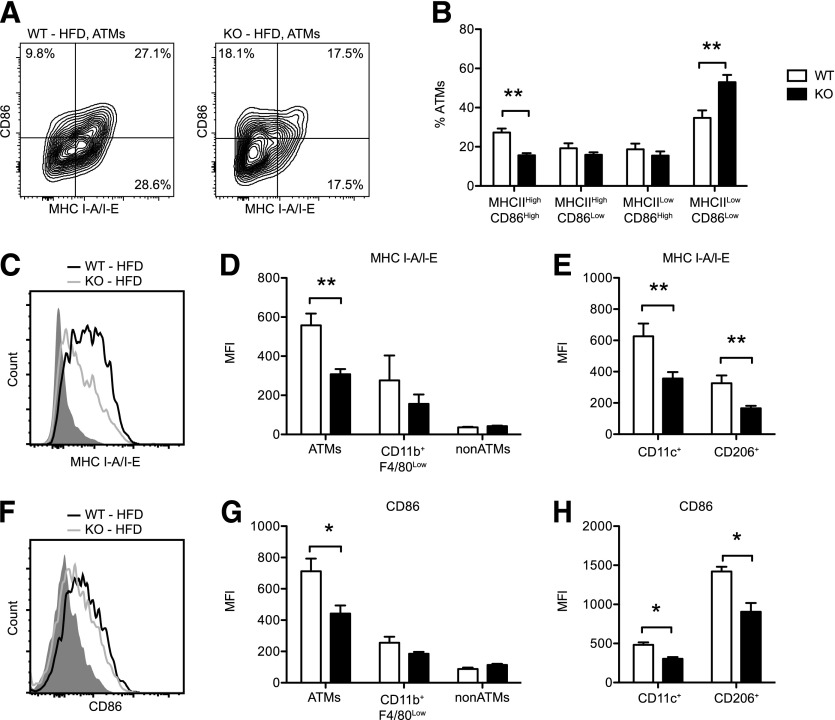
MHC class II expression is induced on ATMs in eWAT during diet-induced obesity [3] and plays a pivotal role in regulating ATT numbers in mice [4]. Given our observations that CD40L stimulated H2-Ab1 and Cd86 expression in cultured ATMs (Fig. 2), we examined the expression of these molecules on ATMs in HFD-fed obese WT and KO mice. By gene expression analysis, CD40-deficiency was associated with a modest, but not statistically significant reduction in H2-Ab1 expression in total eWAT from lean and obese KO mice (Supplemental Fig. 2C). Flow cytometry analysis of ATMs from HFD-fed mice revealed that KO mice had a marked reduction in the frequency of MHC IIHigh CD86High ATMs and a corresponding increase in the frequency of MHC IILow CD86Low ATMs compared with WT mice (Fig. 6A–B). Compared with WT mice, the expression of MHC II and CD86 expression was also reduced on ATMs from KO mice (Fig. 6C–D and F–G). No differences in MHC II and CD86 expression were observed in CD11b+F4/80Low (putative adipose tissue dendritic cells) or CD11b−F4/80− (non-ATMs) between obese WT and KO mice. Specific reductions in MHC II and CD86 expression were observed in both CD11c+ (recruited M1-like) and CD206+ (resident M2-like) ATMs (Fig. 6E and H). These findings indicate that CD40 is required for maximal MHC II and CD86 expression on ATMs during HFD-induced obesity. This reduction in MHC II and CD86 expression likely contributes to the impaired accumulation of Tconv ATTs with diet-induced obesity in KO mice.
Figure 6. CD40 regulates MHC class II expression on ATMs during HFD-induced obesity.
WT and KO mice were fed HFD for 20 wk. ATMs were analyzed by flow cytometry (see Supplement Fig. 2 for gating strategy). (A) Representative contour plots showing CD86 and MHC II (MHC I-A/I-E) expression on ATMs from WT and KO mice. The percentage (%) of cells in each gate is indicated. (B) Distribution (% of ATMs) of MHC IIHigh CD86High, MHC IIHigh CD86Low, MHC IILow CD86High, and MHC IILow CD86Low ATMs in eWAT from WT and KO mice fed HFD (n = 5–8 mice/group). (C) Representative histograms showing surface expression of MHC II (I-A/I-E) on ATMs from WT (black line) and KO (gray line) mice fed an HFD. Isotype staining is indicated in the shaded histogram. (D) MHC I-A/I-E expression (mean fluorescence intensity [MFI]) on ATMs, CD11BHighF4/80High ATMs, CD11B+F4/80Low SVCs, and Non-ATMs (CD11B−F4/80−) after HFD exposure (n = 5–8 mice/group). E. Surface expression (MFI) of MHC I-A/I-E on CD11c+ (M1-like) and CD206+ (M2-like) ATMs from WT and KO mice after HFD (n = 5–8 mice/group). (F) Representative histograms showing surface expression of CD86 on ATMs from WT (black line) and KO (gray line) mice fed an HFD. Isotype staining is indicated in the shaded histogram. (G) CD86 expression (MFI) on ATMs, CD11BHighF4/80High ATMs, CD11B+F4/80Low SVCs, and non-ATMs (CD11B−F4/80−) after HFD exposure (n = 5–8 mice/group). (H) Surface expression (MFI) of CD86 on CD11c+ (M1-like) and CD206+ (M2-like) ATMs from WT and KO mice after HFD (n = 5–8 mice/group). Data are means ± sem. *P < 0.05, **P < 0.01.
Hematopoietic CD40 promotes MHC class II expression on ATMs and accumulation of Tconv ATTs during HFD-induced obesity
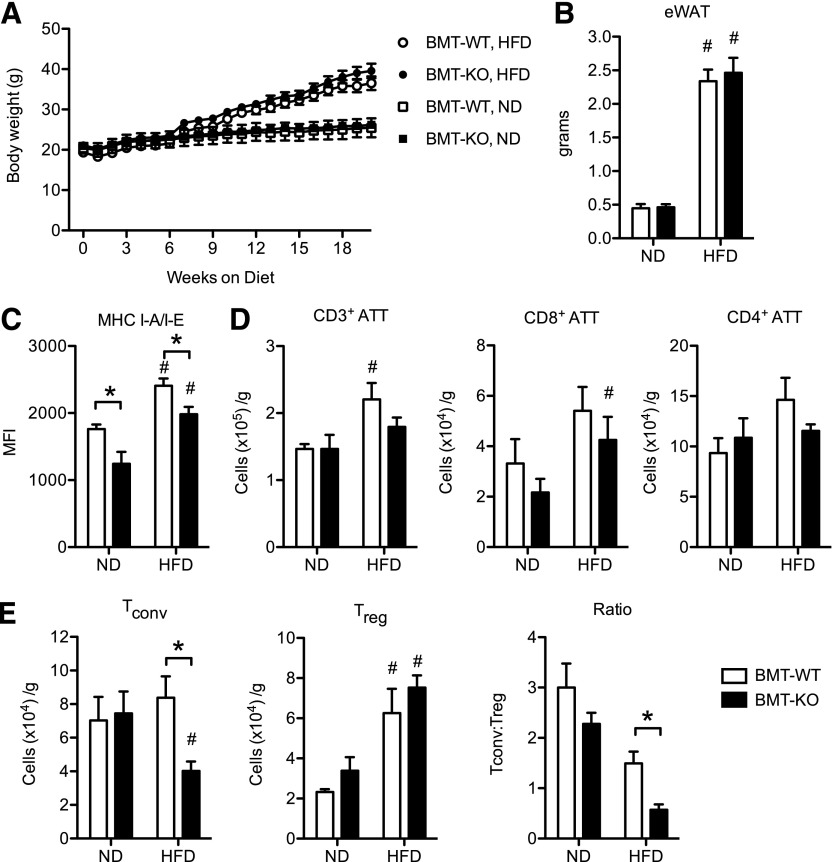
Given that CD40 is expressed on multiple cell types, we sought to evaluate the specific role of Cd40 in hematopoietic cells on adipose tissue inflammation. BMT experiments were performed by reconstituting WT recipients with marrow from WT and KO donors to generate chimeric mice with (BMT-WT) or without (BMT-KO) CD40 expression on hematopoietic cells. Six weeks after transplantation, BMT-WT and BMT-KO mice were fed an ND or an HFD for 20 wk to induce obesity. BMT-WT and BMT-KO mice had similar leukocyte chimerism in bone marrow, spleen, eWAT, and blood leukocyte compartments (as a percentage of their total leukocytes) after reconstitution (Supplemental Fig. 3). BMT-WT and BMT-KO mice did not differ in weight in both ND- and HFD-fed conditions (Fig. 7A). Obese BMT-KO and BMT-WT mice had comparable eWAT weight (Fig. 7B) and similar numbers of ATMs (data not shown). When we evaluated MHC II expression on ATMs from eWAT, we observed reduced MHC II expression on ATMs in both ND- and HFD-fed BMT-KO mice compared with BMT-WT mice (Fig. 7C). This indicates that CD40 deficiency leads to a cell-autonomous decrease in MHCII expression in ATMs.
Figure 7. Hematopoietic CD40 promotes MHC class II expression on ATMs and accumulation of Tconv ATTs during HFD-induced obesity.
Bone marrow cells from donor WT or KO mice were transplanted into lethally irradiated, male, WT recipients to generate mice with (BMT-WT) or without (BMT-KO) CD40 expression on hematopoietic cells. Both groups were fed an ND or HFD for 20 wk. (A) Body weight gain in BMT-WT and BMT-KO mice fed an ND (n = 4 mice/group) or an HFD (n = 7 mice/group). (B) eWAT weight (g). (C) MHC I-A/I-E expression (mean fluorescence intensity [MFI]) on ATMs from BMT-WT and BMT-KO mice fed an ND or an HFD for 20 wk. (D) Quantification of CD3+, CD3+CD8+, and CD3+CD4+ ATTs in eWAT. (E) Quantification of Tconv and Treg CD3+CD4+ ATTs and the ratio of Tconv:Treg ATTs in eWAT. Data are means ± sem. *P < 0.05. #P < 0.05 vs. ND-fed mice.
When ATTs were examined, no significant quantitative differences in total CD3+, CD3+CD8+, or CD3+CD4+ ATTs were observed between obese BMT-KO and BMT-WT mice (Fig. 7D). However, Tconv ATTs were significantly reduced in eWAT from obese BMT-KO mice compared with BMT-WT mice (Fig. 7E). By contrast, the number of Treg ATTs was comparable between BMT-KO and BMT-WT mice fed either ND or HFD (Fig. 7E). The overall ratio of Tconvs:Tregs was reduced in BMT-KO, HFD mice compared with obese, BMT-WT mice. These observations with Tconv phenocopy observations in whole-body KO mice and suggest that hematopoietic CD40 signaling is required for accumulation of Tconv ATTs with diet-induced obesity.
Whole-body, but not hematopoietic, CD40 deficiency improves insulin sensitivity during HFD-induced obesity
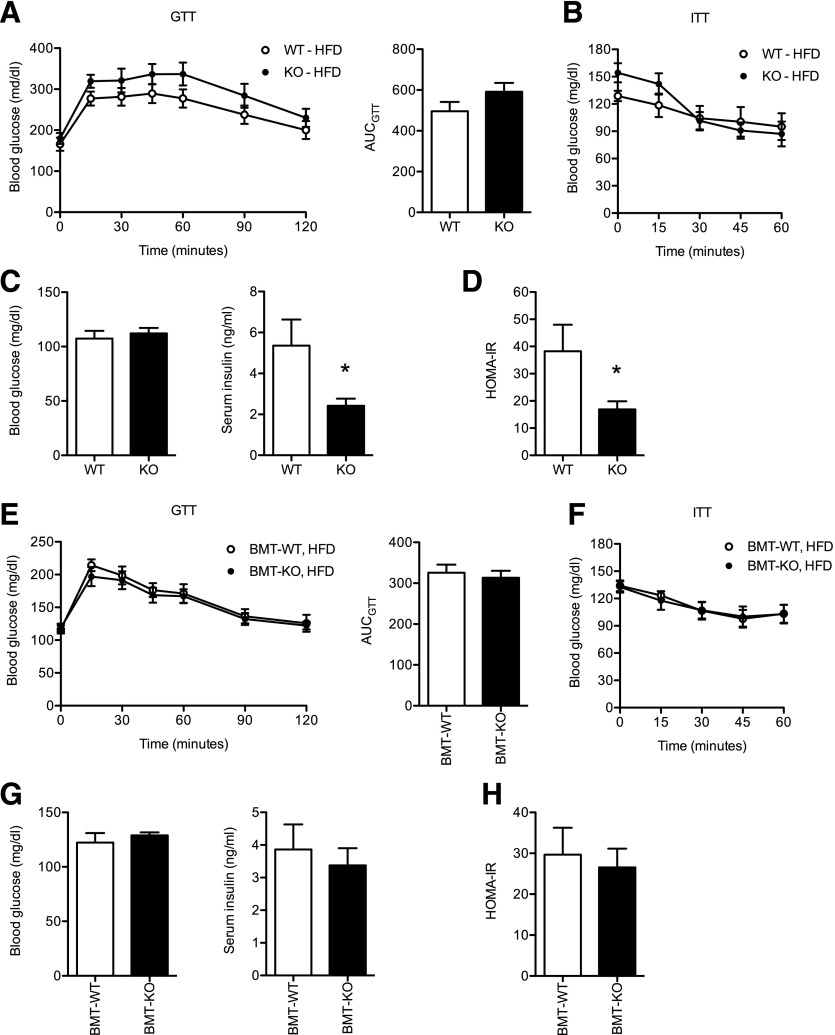
We evaluated metabolic status of the mice generated in these experiments by assessing glucose tolerance and insulin sensitivity. Whole-body obese KO and WT mice had similar impairments in glucose tolerance and insulin sensitivity as assessed by GTT and ITT (Fig. 8A–B). However, although HFD-fed KO and WT mice had similar fasting glucose levels (Fig. 8C), obese KO mice had lower serum insulin levels and subsequently lower HOMA-IR scores compared with obese WT controls (Fig. 8D). This is consistent with an increase of in vivo insulin sensitivity in KO mice. However, when the bone marrow chimeras were analyzed, no significant differences in glucose tolerance, insulin tolerance, fasting glucose or insulin levels, or HOMA-IR were observed BMT-KO and BMT-WT mice (Fig. 8E–H). These data suggest that CD40 signaling in nonhematopoietic cells promotes whole-body insulin resistance.
Figure 8. Whole-body, but not hematopoietic, CD40 deficiency modestly improves insulin resistance during HFD-induced obesity.
(A) Glucose excursion curve and area under the curve (AUC) analysis for GTT performed on WT and KO mice after 15 wk of HFD exposure (n = 8 WT mice; n = 11 KO mice). (B) Glucose excursion curve for ITT performed on WT and KO mice after 18 wk of HFD exposure (n = 8 WT mice; n = 8 KO mice). (C) Fasting (6 h) blood glucose and serum insulin levels in WT and KO mice after 19 wk on HFD (n = 8 WT mice; n = 8 KO mice). (D) HOMA-IR calculated for WT and KO male mice after 19 wk on HFD. (E) GTT and AUC analysis for BMT-WT and BMT-KO mice after 15 wk of HFD exposure (n = 7 mice/group). (F) ITT performed on BMT-WT and BMT-KO mice after 18 wk of HFD exposure (n = 7 mice/group). (G) Fasting (6 h) blood glucose and serum insulin levels in BMT-WT and BMT-KO mice after 19 wk on HFD (n = 7 mice/group). (H) HOMA-IR calculated for BMT-WT and BMT-KO male mice after 19 wk on HFD. Data are means ± sem. *P < 0.05.
Coordinated regulation of CD40 and CD80/CD86 gene expression in omental adipose tissue from obese diabetic patients
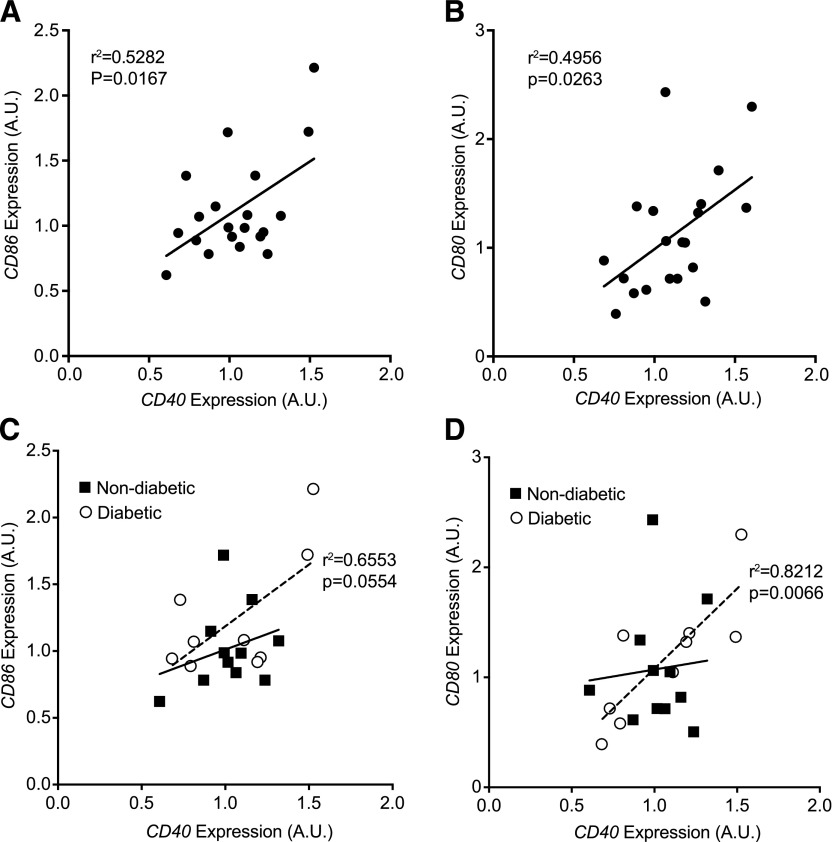
To examine the relevance of CD40 in obese human adipose tissue, we evaluated CD40, CD80, and CD86 gene expression in omental adipose tissue biopsies from a cohort of bariatric surgery patients (n = 20; clinical characteristics in Supplemental Table 3). There was no significant association with body-mass index and expression of any of these genes in this population (data not shown). However, significant correlations were observed between CD40 and the expression of both CD80 and CD86 in the entire cohort of obese patients (Fig. 9A–B). The patients were then subdivided based on the diagnosis of type 2 diabetes (nondiabetic, n = 11; diabetic n = 9). No significant differences in age or body-mass index were observed between the nondiabetic and diabetic groups. There were no differences in absolute expression of CD40, CD80, and CD86 between diabetic and nondiabetic groups (data not shown). However, the expression of CD40 and CD86 was marginally correlated (r = 0.6553; P = 0.0554) in the diabetic group, and there was a significant positive correlation (r = 0.8212; P = 0.0066) between CD40 and CD80 expression in omental fat from obese diabetic patients (Fig. 9C-D). By contrast, correlation between CD40 and CD80 or CD86 expression did not reach significance in the nondiabetic group. Thus, the association between CD40 and CD80/CD86 expression in omental fat from this obese cohort is driven primarily by a strong coordinate regulation of these genes in obese diabetic patients. These data demonstrate a coordinated regulation of costimulatory genes in obese human adipose tissue, which may be a useful biomarker for distinguishing metabolically healthy and unhealthy obese individuals.
Figure 9. CD40 expression is positively correlated with expression of costimulatory genes (CD80, CD86) in omental adipose tissue of obese diabetic patients.
RNA was isolated from omental adipose tissue biopsies from obese patients (n = 20) undergoing bariatric surgery. Expression of CD40, CD80, and CD86 was assessed by quantitative real-time PCR, and correlation analysis was performed. (A–B) Correlation analysis (Pearson) in the entire cohort (n = 20). (C–D) Correlation analysis of patients with the diagnosis of type 2 diabetes (diabetic; n = 9) and nondiabetic obesity (n = 11). Pearson correlation coefficients and P-value are shown for the diabetic group. Correlations within the nondiabetic group were not significant.
DISCUSSION
The emerging concept that activation of the adaptive immune system links obesity to metabolic disease has led our group and others to examine the role of leukocyte cross talk in adipose tissue inflammation [14, 40–43]. T cell costimulation is important in APC–T cell communication, and these signals are often critical nodes in the regulation of both Treg and effector/memory Tconv activity and homeostasis [18, 44, 45]. Because the presence or absence of appropriate costimulatory signals likely influences the magnitude of adipose tissue inflammation in obesity, many recent studies have examined the role of costimulatory molecules in this process with somewhat mixed results. This is likely due to the complexity of costimulatory receptors and ligands that are expressed on the surface of both leukocytes and nonleukocytes in adipose tissue. Additionally, soluble costimulatory molecules, such as CD40L and CD137L, are increased in obesity and provide another level of costimulatory regulation [15, 25, 46].
Here, we examined the role of CD40 in the regulation of adipose tissue inflammation in diet-induced obesity. We found that Cd40−/− mice have decreased adipose tissue Tconv relative to Tregs with obesity, which is mainly dependent on CD40 expression in hematopoietic cells. CD40 deficiency was also associated with reduced MHC II and CD86 expression on both M1-like (CD11c+) and M2-like (CD206+) ATMs in eWAT Cd40−/− mice. Because ATMs are the primary APC in adipose tissue [3, 4], the APC functionality of ATMs is at least partially dependent on CD40 signaling to generate and maintain of Tconv in eWAT. Certainly, the reductions in Tconv content in obese Cd40−/− mice parallels our recent observations that macrophage-specific deletion of MHCII in mice blunts the accumulation of Tconv in fat with obesity [4]. Notably, the increase in Tregs in lean whole-body KO mice was not recapitulated in BMT-KO mice, suggesting that hematopoietic and nonhematopoietic CD40 may have divergent effects on the regulation of Treg content in adipose tissue. Our data are in line with a report that shows an increase in Tregs in Cd40−/− mice [16, 47] but has not been the case in other studies [17, 30]. These findings are also in agreement with the observation that CD40L deficient mice have increased Tregs and show protection from insulin resistance with obesity [14]. Nonetheless, we observed that these differences are specific to adipose tissue, which is in line with the unique nature of adipose tissue Tregs and their unique functional capacity compared with other induced Treg populations (e.g., spleen) [48, 49].
Changes in Tconv content have not been observed in other studies that have mainly focused on CD8+ cells and Tregs. There is significant variation in how ATT content in adipose tissue is reported in the literature, which makes it difficult to compare our results with other studies. Studies report ATT cells as total cells per adipose tissue fat pad, percentage of adipose tissue leukocytes, or after normalizing to fat pad weight [16, 17, 30, 47]. This remains a somewhat controversial issue, and there is no clear, standardized measure of adipose tissue leukocyte content. For example, we found that HFD-fed Cd40−/− mice harbor more CD8+ ATTs as a percentage of SVCs, which is in line with other reports; however, when CD8+ ATT content was normalized to adipose tissue mass, there was no statistically significant difference between obese KO and WT mice. Given the massive expansion in adipose tissue weight with HFD feeding and the increase in leukocytes within the SVCs, we feel that some degree of normalization is required. In our view, reporting ATT cells as a percentage of the all SVCs and total ATTs per gram adipose tissue are the most informative for understanding the leukocyte dynamics, so we present both here.
Despite a reduction in Tconvs, we observed only mild differences in metabolism based on glucose or insulin sensitivity in whole-body Cd40 deficient mice and no changes in BM chimeras made from Cd40−/− donors. Whole-body disruption of Cd40 in C57BL/6 mice offered modest protection from HFD-induced weight gain and was associated with mild improvement in insulin sensitivity, as determined by decreased circulating insulin levels and improved HOMA-IR. This weight difference was not observed in BM chimeras generated with Cd40−/− donors. These observations differ from other studies that have shown both protection and aggravation of insulin resistance in Cd40−/− mice [16, 17, 30]. Different husbandry and diet conditions among laboratories may contribute to this variation, or it may relate to studies demonstrating divergent effects of TRAF signaling pathways downstream of CD40 activation, with CD40–TRAF6 signaling promoting insulin resistance and CD40 signaling through TRAF2/3/5 having a protective effect on metabolism [17, 19]. Certainly, CD40 expression on nonhematopoietic cells is an important contributor. The lack of significant metabolic changes in our obese KO mice parallels the observation that ATM content, ATM phenotype, and the expression of inflammatory cytokines were not different when compared with HFD-fed WT mice. Given that ATMs are potent effectors of both innate and acquired signals in adipose tissue, it appears that the activation of ATMs in KO mice may be largely independent of CD40.
The coregulation of CD40 and costimulatory genes was recapitulated in human visceral adipose tissue samples, where we observed positive correlations between CD40 and CD80/CD86 expression in obese patients with type 2 diabetes. This observation agrees with gene expression profiling studies that have revealed associations between APC genes and obesity and metabolic disease in humans [50] and the ability of CD40 receptor activation to induced CD80/86 expression [51]. Few data exist on the expression of these genes in adipose tissue of diabetic patients, and cellular analyses have produced mixed results. CD80+ and CD86+ ATMs were shown to be negatively associated with insulin resistance in human samples [13]. Others have reported CD86 expression on ATMs to be an M1 macrophage marker that does not change significantly with obesity [52]. At the same time, CD86 is a member of a macrophage-enriched metabolic network of genes shown to be increased in adipose tissue during metabolic disease [53–55].
Overall, our studies reveal a novel role for CD40 signaling in hematopoietic cells. CD40 signaling promotes the APC functionality of ATMs by controlling MHC II and CD86 expression. We propose that this has an important role in translating obesogenic cues into an acquired immune response associated with the expansion of Tconvs in adipose tissue. However, in this study, CD40-dependent generation of Tconvs did not have a significant effect on the development of insulin resistance, which is likely attributed to a sustained activation profile for ATMs. This does not exclude the possible use of inhibitors of the CD40/CD40L pathway as a possible therapeutic strategy for metabolic disease but suggests additional pathways to interrogate in the process.
AUTHORSHIP
D.L.M. conceived the study, designed and performed experiments, analyzed data, and wrote the manuscript. K.E.O., T.A.M., K.W.C., J.L.D., K.S., and R.W.O. performed experiments, acquired and analyzed data, and contributed to the discussion. C.E.M., R.W.O., and C.N.L. contributed to the discussion. C.N.L. also wrote the manuscript, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the analysis.
ACKNOWLEDGMENTS
This work was supported by research grants from the U.S. National Institutes of Health (NIH) (Grants DK090262 [C.N.L], DK095050 [R.W.O], DK097449 [R.W.O], and DK093954 [C.E.M]), the American Diabetes Association (Grant 7-12-CD-08 to C.N.L.), and the Indiana Health Clarian Values Research Fund (C.E.M.). D.L.M. was supported Grants DK091976 and DK100515 from NIH/National Institute of Diabetes and Digestive and Kidney Disease. K.S. was supported by an institutional K12 training grant from NIH (HD028820) and a Pediatric Endocrine Society Research Fellowship Award. This worked used Core Services from the Michigan Nutrition and Obesity Research Center (NIH Grant DK089503 to the University of Michigan). We thank Drs. Mark Kaplan and Olufolakemi Awe, Indiana University School of Medicine, for providing technical assistance in detecting CD154 expression in restimulated CD4+ T cells, and Lynn Geletka and Dr. Gabriel Martinez-Santibanez, University of Michigan, for helpful discussions.
Glossary
- ATM
adipose tissue macrophage
- ATT
adipose tissue T cell,
- BMT
bone marrow transplant
- eWAT
epididymal white adipose tissue
- GTT
glucose tolerance test
- HFD
high fat diet
- HOMA-IR
homeostatic model assessment of insulin resistance
- iWAT
inguinal white adipose tissue
- ITT
insulin tolerance test
- KO
Cd40 knockout
- MFI
median fluorescent intensity
- MHC II
major histocompatibility complex, class II
- ND
normal diet
- SVC
stromal vascular cell
- Tconv
conventional T cell
- Treg
regulatory T cell
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Mathis D. (2013) Immunological goings-on in visceral adipose tissue. Cell Metab. 17, 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant R. W., Dixit V. D. (2015) Adipose tissue as an immunological organ. Obesity (Silver Spring) 23, 512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris D. L., Cho K. W., Delproposto J. L., Oatmen K. E., Geletka L. M., Martinez-Santibanez G., Singer K., Lumeng C. N. (2013) Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes 62, 2762–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho K. W., Morris D. L., Delproposto J. L., Geletka L., Zamarron B., Martinez-Santibanez G., Meyer K. A., Singer K., O'Rourke R. W., Lumeng C. N. (2014) An MHC II-dependent activation loop between adipose tissue macrophages and CD4+ T cells controls obesity-induced inflammation. Cell Rep. 9, 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolodin D., van Panhuys N., Li C., Magnuson A. M., Cipolletta D., Miller C. M., Wagers A., Germain R. N., Benoist C., Mathis D. (2015) Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21, 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer S., Chan Y., Paltser G., Truong D., Tsui H., Bahrami J., Dorfman R., Wang Y., Zielenski J., Mastronardi F., Maezawa Y., Drucker D. J., Engleman E., Winer D., Dosch H. M. (2009) Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H., Youm Y. H., Vandanmagsar B., Ravussin A., Gimble J. M., Greenway F., Stephens J. M., Mynatt R. L., Dixit V. D. (2010) Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 185, 1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaughlin T., Liu L. F., Lamendola C., Shen L., Morton J., Rivas H., Winer D., Tolentino L., Choi O., Zhang H., Hui Yen Chng M., Engleman E. (2014) T-cell profile in adipose tissue is associated with insulin resistance and systemic inflammation in humans. Arterioscler. Thromb. Vasc. Biol. 34, 2637–2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feuerer M., Herrero L., Cipolletta D., Naaz A., Wong J., Nayer A., Lee J., Goldfine A. B., Benoist C., Shoelson S., Mathis D. (2009) Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura S., Manabe I., Nagasaki M., Eto K., Yamashita H., Ohsugi M., Otsu M., Hara K., Ueki K., Sugiura S., Yoshimura K., Kadowaki T., Nagai R. (2009) CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920. [DOI] [PubMed] [Google Scholar]
- 11.Aron-Wisnewsky J., Tordjman J., Poitou C., Darakhshan F., Hugol D., Basdevant A., Aissat A., Guerre-Millo M., Clément K. (2009) Human adipose tissue macrophages: M1 and M2 cell surface markers in subcutaneous and omental depots and after weight loss. J. Clin. Endocrinol. Metab. 94, 4619–4623. [DOI] [PubMed] [Google Scholar]
- 12.Poggi M., Jager J., Paulmyer-Lacroix O., Peiretti F., Gremeaux T., Verdier M., Grino M., Stepanian A., Msika S., Burcelin R., de Prost D., Tanti J. F., Alessi M. C. (2009) The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia 52, 1152–1163. [DOI] [PubMed] [Google Scholar]
- 13.Zhong J., Rao X., Braunstein Z., Taylor A., Narula V., Hazey J., Mikami D., Needleman B., Rutsky J., Sun Q., Deiuliis J. A., Satoskar A. R., Rajagopalan S. (2014) T-cell costimulation protects obesity-induced adipose inflammation and insulin resistance. Diabetes 63, 1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poggi M., Engel D., Christ A., Beckers L., Wijnands E., Boon L., Driessen A., Cleutjens J., Weber C., Gerdes N., Lutgens E. (2011) CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler. Thromb. Vasc. Biol. 31, 2251–2260. [DOI] [PubMed] [Google Scholar]
- 15.Chatzigeorgiou A., Phieler J., Gebler J., Bornstein S. R., Chavakis T. (2013) CD40L stimulates the crosstalk between adipocytes and inflammatory cells. Horm. Metab. Res. 45, 741–747. [DOI] [PubMed] [Google Scholar]
- 16.Guo C. A., Kogan S., Amano S. U., Wang M., Dagdeviren S., Friedline R. H., Aouadi M., Kim J. K., Czech M. P. (2013) CD40 deficiency in mice exacerbates obesity-induced adipose tissue inflammation, hepatic steatosis, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 304, E951–E963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatzigeorgiou A., Seijkens T., Zarzycka B., Engel D., Poggi M., van den Berg S., van den Berg S., Soehnlein O., Winkels H., Beckers L., Lievens D., Driessen A., Kusters P., Biessen E., Garcia-Martin R., Klotzsche-von Ameln A., Gijbels M., Noelle R., Boon L., Hackeng T., Schulte K.-M., Xu A., Vriend G., Nabuurs S., Chung K. J., Willems van Dijk K., Rensen P. C., Gerdes N., de Winther M., Block N. L., Schally A. V., Weber C., Bornstein S. R., Nicolaes G., Chavakis T., Lutgens E. (2014) Blocking CD40-TRAF6 signaling is a therapeutic target in obesity-associated insulin resistance [published correction in Proc. Natl. Acad. Sci. U. S. A. (2014) 111, 464]. Proc. Natl. Acad. Sci. U. S. A. 111, 2686–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatzigeorgiou A., Chung K. J., Garcia-Martin R., Alexaki V. I., Klotzsche-von Ameln A., Phieler J., Sprott D., Kanczkowski W., Tzanavari T., Bdeir M., Bergmann S., Cartellieri M., Bachmann M., Nikolakopoulou P., Androutsellis-Theotokis A., Siegert G., Bornstein S. R., Muders M. H., Boon L., Karalis K. P., Lutgens E., Chavakis T. (2014) Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology 60, 1196–1210. [DOI] [PubMed] [Google Scholar]
- 19.Van den Berg S. M., Seijkens T. T., Kusters P. J., Zarzycka B., Beckers L., den Toom M., Gijbels M. J., Chatzigeorgiou A., Weber C., de Winther M. P., Chavakis T., Nicolaes G. A., Lutgens E. (2015) Blocking CD40-TRAF6 interactions by small-molecule inhibitor 6860766 ameliorates the complications of diet-induced obesity in mice. Int. J. Obes. 39, 782–790. [DOI] [PubMed] [Google Scholar]
- 20.Castle B. E., Kishimoto K., Stearns C., Brown M. L., Kehry M. R. (1993) Regulation of expression of the ligand for CD40 on T helper lymphocytes. J. Immunol. 151, 1777–1788. [PubMed] [Google Scholar]
- 21.Schönbeck U., Libby P. (2001) CD40 signaling and plaque instability. Circ. Res. 89, 1092–1103. [DOI] [PubMed] [Google Scholar]
- 22.Fujii S., Liu K., Smith C., Bonito A. J., Steinman R. M. (2004) The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 199, 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella M., Scheidegger D., Palmer-Lehmann K., Lane P., Lanzavecchia A., Alber G. (1996) Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 184, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caux C., Massacrier C., Vanbervliet B., Dubois B., Van Kooten C., Durand I., Banchereau J. (1994) Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 180, 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unek I. T., Bayraktar F., Solmaz D., Ellidokuz H., Sisman A. R., Yuksel F., Yesil S. (2010) The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clin. Med. Res. 8, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unek I. T., Bayraktar F., Solmaz D., Ellidokuz H., Yuksel F., Sisman A. R., Yesil S. (2010) Enhanced levels of soluble CD40 ligand and C-reactive protein in a total of 312 patients with metabolic syndrome. Metabolism 59, 305–313. [DOI] [PubMed] [Google Scholar]
- 27.Mach F., Schönbeck U., Sukhova G. K., Atkinson E., Libby P. (1998) Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 394, 200–203. [DOI] [PubMed] [Google Scholar]
- 28.Lutgens E., Lievens D., Beckers L., Wijnands E., Soehnlein O., Zernecke A., Seijkens T., Engel D., Cleutjens J., Keller A. M., Naik S. H., Boon L., Oufella H. A., Mallat Z., Ahonen C. L., Noelle R. J., de Winther M. P., Daemen M. J., Biessen E. A., Weber C. (2010) Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J. Exp. Med. 207, 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolf D., Jehle F., Michel N. A., Bukosza E. N., Rivera J., Chen Y. C., Hoppe N., Dufner B., Rodriguez A. O., Colberg C., Nieto L., Rupprecht B., Wiedemann A., Schulte L., Peikert A., Bassler N., Lozhkin A., Hergeth S. P., Stachon P., Hilgendorf I., Willecke F., von Zur Mühlen C., von Elverfeldt D., Binder C. J., Aichele P., Varo N., Febbraio M. A., Libby P., Bode C., Peter K., Zirlik A. (2014) Coinhibitory suppression of T cell activation by CD40 protects against obesity and adipose tissue inflammation in mice. Circulation 129, 2414–2425. [DOI] [PubMed] [Google Scholar]
- 30.Yi Z., Stunz L.L., Bishop G.A. (2014) CD40-mediated maintenance of immune homeostasis in the adipose tissue microenvironment. Diabetes 63, 2751–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Q. Q., Yan C. F., Lin R., Zhang J. Y., Wang W. R., Yang L. N., Zhang K. F. (2012) SIRT1 regulates TNF-α-induced expression of CD40 in 3T3-L1 adipocytes via NF-κB pathway. Cytokine 60, 447–455. [DOI] [PubMed] [Google Scholar]
- 32.Singer K., Morris D. L., Oatmen K. E., Wang T., DelProposto J., Mergian T., Cho K. W., Lumeng C. N. (2013) Neuropeptide Y is produced by adipose tissue macrophages and regulates obesity-induced inflammation [published correction in PLoS One (2013);8]. PLoS One 8, e57929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho K. W., Morris D. L., Lumeng C. N. (2014) Flow cytometry analyses of adipose tissue macrophages. Methods Enzymol. 537, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris D. L., Oatmen K. E., Wang T., DelProposto J. L., Lumeng C. N. (2012) CX3CR1 deficiency does not influence trafficking of adipose tissue macrophages in mice with diet-induced obesity. Obesity (Silver Spring) 20, 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertola A., Ciucci T., Rousseau D., Bourlier V., Duffaut C., Bonnafous S., Blin-Wakkach C., Anty R., Iannelli A., Gugenheim J., Tran A., Bouloumié A., Gual P., Wakkach A. (2012) Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61, 2238–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy M., Waldschmidt T., Aruffo A., Ledbetter J. A., Noelle R. J. (1993) The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 151, 2497–2510. [PubMed] [Google Scholar]
- 37.Yellin M. J., Sippel K., Inghirami G., Covey L. R., Lee J. J., Sinning J., Clark E. A., Chess L., Lederman S. (1994) CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J. Immunol. 152, 598–608. [PubMed] [Google Scholar]
- 38.Chattopadhyay P. K., Yu J., Roederer M. (2006) Live-cell assay to detect antigen-specific CD4+ T-cell responses by CD154 expression. Nat. Protoc. 1, 1–6. [DOI] [PubMed] [Google Scholar]
- 39.Tone M., Tone Y., Fairchild P. J., Wykes M., Waldmann H. (2001) Regulation of CD40 function by its isoforms generated through alternative splicing. Proc. Natl. Acad. Sci. U. S. A. 98, 1751–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winer S., Winer D. A. (2012) The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol. Cell Biol. 90, 755–762. [DOI] [PubMed] [Google Scholar]
- 41.Seijkens T., Kusters P., Chatzigeorgiou A., Chavakis T., Lutgens E. (2014) Immune cell crosstalk in obesity: a key role for costimulation? Diabetes 63, 3982–3991. [DOI] [PubMed] [Google Scholar]
- 42.DeFuria J., Belkina A. C., Jagannathan-Bogdan M., Snyder-Cappione J., Carr J. D., Nersesova Y. R., Markham D., Strissel K. J., Watkins A. A., Zhu M., Allen J., Bouchard J., Toraldo G., Jasuja R., Obin M. S., McDonnell M. E., Apovian C., Denis G. V., Nikolajczyk B. S. (2013) B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. U. S. A. 110, 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lutgens E., Poggi M., Weber C. (2010) CD40L-CD40 fuel ignites obesity. Thromb. Haemost. 103, 694–695. [DOI] [PubMed] [Google Scholar]
- 44.Huynh A., Zhang R., Turka L. A. (2014) Signals and pathways controlling regulatory T cells. Immunol. Rev. 258, 117–131. [DOI] [PubMed] [Google Scholar]
- 45.Jiang X., Sun W., Guo D., Cui Z., Zhu L., Lin L., Tang Y., Wang X., Liang J. (2011) Cardiac allograft acceptance induced by blockade of CD40-CD40L costimulation is dependent on CD4+CD25+ regulatory T cells. Surgery 149, 336–346. [DOI] [PubMed] [Google Scholar]
- 46.Missiou A., Wolf D., Platzer I., Ernst S., Walter C., Rudolf P., Zirlik K., Köstlin N., Willecke F. K., Münkel C., Schönbeck U., Libby P., Bode C., Varo N., Zirlik A. (2010) CD40L induces inflammation and adipogenesis in adipose cells--a potential link between metabolic and cardiovascular disease. Thromb. Haemost. 103, 788–796. [DOI] [PubMed] [Google Scholar]
- 47.Wolf D., Jehle F., Michel N. A., Bukosza E. N., Rivera J., Chen Y. C., Hoppe N., Dufner B., Rodriguez A. O., Colberg C., Nieto L., Rupprecht B., Wiedemann A., Schulte L., Peikert A., Bassler N., Lozhkin A., Hergeth S. P., Stachon P., Hilgendorf I., Willecke F., von Zur Mühlen C., von Elverfeldt D., Binder C. J., Aichele P., Varo N., Febbraio M. A., Libby P., Bode C., Peter K., Zirlik A. (2014) Co-inhibitory suppression of T Cell activation by CD40 protects from obesity and adipose tissue inflammation in mice. Circulation 129, 2414–2425. [DOI] [PubMed] [Google Scholar]
- 48.Cipolletta D., Cohen P., Spiegelman B. M., Benoist C., Mathis D. (2014) Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. Proc. Natl. Acad. Sci. U. S. A. 112, 482–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feuerer M., Hill J. A., Kretschmer K., von Boehmer H., Mathis D., Benoist C. (2010) Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc. Natl. Acad. Sci. U. S. A. 107, 5919–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deng T., Lyon C. J., Minze L. J., Lin J., Zou J., Liu J. Z., Ren Y., Yin Z., Hamilton D. J., Reardon P. R., Sherman V., Wang H. Y., Phillips K. J., Webb P., Wong S. T., Wang R. F., Hsueh W. A. (2013) class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 17, 411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ranheim E. A., Kipps T. J. (1993) Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J. Exp. Med. 177, 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer M., Yao-Borengasser A., Unal R., Rasouli N., Gurley C. M., Zhu B., Peterson C. A., Kern P. A. (2010) Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am. J. Physiol. Endocrinol. Metab. 299, E1016–E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang X., Deignan J. L., Qi H., Zhu J., Qian S., Zhong J., Torosyan G., Majid S., Falkard B., Kleinhanz R. R., Karlsson J., Castellani L. W., Mumick S., Wang K., Xie T., Coon M., Zhang C., Estrada-Smith D., Farber C. R., Wang S. S., van Nas A., Ghazalpour A., Zhang B., Macneil D. J., Lamb J. R., Dipple K. M., Reitman M. L., Mehrabian M., Lum P. Y., Schadt E. E., Lusis A. J., Drake T. A. (2009) Validation of candidate causal genes for obesity that affect shared metabolic pathways and networks. Nat. Genet. 41, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen Y., Zhu J., Lum P. Y., Yang X., Pinto S., MacNeil D. J., Zhang C., Lamb J., Edwards S., Sieberts S. K., Leonardson A., Castellini L. W., Wang S., Champy M. F., Zhang B., Emilsson V., Doss S., Ghazalpour A., Horvath S., Drake T. A., Lusis A. J., Schadt E. E. (2008) Variations in DNA elucidate molecular networks that cause disease. Nature 452, 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schadt E. E., Lamb J., Yang X., Zhu J., Edwards S., Guhathakurta D., Sieberts S. K., Monks S., Reitman M., Zhang C., Lum P. Y., Leonardson A., Thieringer R., Metzger J. M., Yang L., Castle J., Zhu H., Kash S. F., Drake T. A., Sachs A., Lusis A. J. (2005) An integrative genomics approach to infer causal associations between gene expression and disease. Nat. Genet. 37, 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]