MIF plays an important role in the initial process of gout via the control of IL-1β.
Keywords: MIF, arthritis, inflammasome, chemokine
Abstract
This study evaluated the role of macrophage migration inhibitory factor in inflammation caused by monosodium urate crystals. The concentration of macrophage migration inhibitory factor was increased in synovial fluid of patients with acute gout, and there was a positive correlation between intra-articular macrophage migration inhibitory factor and IL-1β concentrations. In mice, the injection of monosodium urate crystals into the knee joint increased the levels of macrophage migration inhibitory factor in macrophages and in inflamed tissue. The injection of recombinant macrophage migration inhibitory factor into the joint of mice reproduced the inflammatory response observed in acute gout, including histologic changes, the recruitment of neutrophils, and increased levels of IL-1β and CXCL1. Importantly, the accumulation of neutrophils and the amount IL-1β in the joints were reduced in macrophage migration inhibitory factor-deficient mice when injected with monosodium urate crystals. We observed a similar effect when we blocked macrophage migration inhibitory factor with (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid or anti-macrophage migration inhibitory factor. In addition, the blockade of IL-1R and CXCR2 reduced recombinant macrophage migration inhibitory factor-induced neutrophil recruitment. Mechanistically, recombinant macrophage migration inhibitory factor is important for the synthesis of il1β mRNA in vivo and in isolated macrophages. Altogether, macrophage migration inhibitory factor promotes neutrophil accumulation and is important for IL-1β production, which are 2 crucial events contributing to the pathogenesis of acute gout.
Introduction
Gout is an ancient disease caused by the deposition of MSU crystals in the joints [1]. This disease is directly correlated with a lavish lifestyle, including excessive consumption of alcohol and purine-rich foods, and by genetic factors that regulate uric acid metabolism, a byproduct of purine degradation [2]. The incidence of gout has increased over the past few decades [3]. Hyperuricemia is necessary but not sufficient to cause gout attacks and joint disease. During the acute phase of gout, deposition of MSU crystals triggers an extremely painful inflammatory attack in the affected joints, accompanied by edema and erythema of the overlying skin. The persistence of the stimulus contributes to the appearance of tophi, joint limitation, joint swelling, and deformity, characteristic clinical features of the chronic stage of gout [4].
Resident cells interact with MSU crystals and initiate the production of several inflammatory mediators [5,6]. It is well known that MSU crystals trigger NLRP3 inflammasome activation, culminating in the release of the active form of IL-1β, a major cytokine driving inflammatory changes in gout [7]. The influx of neutrophils into the joint is a hallmark of an acute gout attack [8]. Experimentally, it has been shown that neutrophil recruitment that occurs in gout is dependent on the chemokine receptor CXCR2, and the production of the CXCR2-binding molecule, CXCL1, is driven by IL-1β [9]. Once in the joint, neutrophils release a series of inflammatory mediators that contribute to joint inflammation and pain [10].
MIF was one of the first cytokines described and was identified in a delayed-type hypersensitivity reaction [11]. MIF has important functions in innate immunity and is expressed constitutively in immune cells, which release MIF after stimulation with microbial products and proinflammatory cytokines [12]. MIF appears to contribute to the pathogenesis of certain acute and chronic inflammatory diseases, including rheumatoid arthritis [13]. MIF is known to up-regulate the cytokines IL-8, the cognate ligand of CXCR2, and IL-1β [14]. In the present study, we investigated whether MIF could be involved in the inflammatory cascade and injury that follow the injection of MSU crystals in the knee joint of mice.
METHODS
Collection of SF
SF was obtained from 12 patients diagnosed with gout by the identification of MSU in SF. The definition of the patient population is shown in Table 1. The SF was harvested using a sterile syringe with the needle injected into the knee joint using a standardized anterolateral portal technique, strictly avoiding hemarthrosis. SF was collected in tubes containing heparin, and cell counts were performed within 24 h of aspiration. An aliquot of these samples was centrifuged, and supernatants were collected for MIF and IL-1β measurements. All patients provided informed consent to participate the study, which was approved by the Ethics Committee of the University Hospital of Ribeirão Preto, University of São Paulo (Brazil; Protocol No. 4971/2012).
TABLE 1.
Gouty patients’ characteristics
| Characteristic | Value |
|---|---|
| Number of patients | 12 |
| Gender (female/male) | 0/12 |
| Age (yr; means ± sd) | 54.36 ± 12.66 |
| Total cells in SF (mean ± sd) | 26,268.18 ± 31,205/mm3 |
| Neutrophils (%) in SF | 83.09 ± 21.02 |
Animals
Eight- to 10-wk-old male C57BL/6J (WT) mice were purchased from the Centro de Bioterismo of the UFMG; Minas Gerais, Brazil). Experiments with Mif−/− mice in the C57BL/6 background [15] were performed in Universidade Federal do Rio de Janeiro (Brazil). All animals were maintained with filtered water and food ad libitum and kept in a controlled environment. Experiments received prior approval by the Animal Ethics Committee of UFMG (Protocol No. 165/2008 and 2/2015).
Articular inflammation
Mice were placed under anesthesia (60:5 mg/kg ketamine:xylazine, i.p.; Syntec, São Paulo, Brazil) and were injected with human rMIF (100 ng/cavity; PeproTech, Rocky Hill, NJ, USA) or MSU crystals (100 µg/cavity, prepared as described previously [9]) into the tibiofemoral joint. In a different set of experiments, mice received an injection of an inhibitor of MIF activity (ISO-1; 50 µg/cavity; Calbiochem, San Diego, CA, USA) into the tibiofemoral joint; an antagonist of CXCR2 (Reparixin, 30 mg/kg, s.c.; Dompé, Milan, Italy); anti-MIF IgG1 mAb (1 mg/kg; clone NIHIII.D.9); nonimmune IgG1 isotype control (1 mg/kg); or IL-1ra (5 mg/kg, i.p.; Biogen, Cambridge, MA, USA) before the injections of MSU crystals or rMIF. Inflammatory parameters were evaluated at different time points after injection of rMIF (6 and 15 h) or MSU crystals (1, 3, 6, and 15 h). Mice were euthanized in a CO2 chamber, followed by cervical dislocation, and the articular cavity was washed with PBS containing 3% BSA (2 × 5 µl) for cell counts. Periarticular tissue was removed from the joints for cytokine evaluation. Leukocyte numbers in the articular cavity were determined in a Neubauer chamber after staining with Turk’s solution. Differential counts were performed in Shandon CytoSpin III (Thermo Shandon, Frankfurt, Germany) preparations by evaluating the percentage of each leukocyte on a slide stained with May Grunwald-Giemsa [9].
Evaluation of hypernociception
Evaluation of mechanical hypernociception was performed as described previously [16], using an electronic pressure meter (Insight Instruments, Ribeirão Preto, São Paulo, Brazil). The flexion-elicited withdrawal threshold was used to infer behavioral responses associated with pain. Results are expressed as the change in withdrawal threshold (in grams).
Cytokine determination
Periarticular tissues were collected and homogenized in PBS containing antiproteases [17]. Samples were centrifuged, and the supernatant was used for cytokine concentrations, in accordance with the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Real-time PCR
Total RNA was isolated from synovial tissue using TRIzol reagent (Ambion, Life Technologies, Thermo Fisher Scientific, Grand Island, NY, USA) and from BMDMs using an RNeasy mini kit (Qiagen, Hilden. Germany). Real-time PCR quantitative mRNA analyses were performed on a 7500 Fast Real-Time PCR system using Power SYBR Green PCR Master Mix (Applied Biosystems, Thermo Fisher Scientific) after reverse transcription reactions of 1 µg RNA using SuperScript III Reverse Transcriptase (Invitrogen, Life Technologies, Thermo Fisher Scientific). The relative level of gene expression was determined by the comparative threshold cycle method, as described by the manufacturer, whereby data for each sample were normalized to a GAPDH constitutive gene and expressed as a fold change compared with control. The following primer pairs were used: for gapdh, 5′-ACG GCC GCA TCT TCT TGT GCA-3′ (forward) and 5′-CGG CCA AAT CCG TTC ACA CCG A-3′ (reverse); for il1β, 5′-CTA CAG GCT CCG AGA TGA ACA AC-3′ (forward) and 5′-TCC ATT GAG GTG GAG AGC TTT C-3′ (reverse); for mif, 5′-CAGAACCGCAACTACAGTAAGC-′3 (forward) and 5′-GGTGGATAAACACAGAACACTACG-′3 (reverse).
In vitro study
Macrophages were derived from bone marrow of C57BL/6 mice. Bone marrow cells were removed from femurs and tibias of the animals and cultured as described [18]. At d 10, BMDMs were completely differentiated for further experimental procedures. Cells were stimulated with 1 µg/ml LPS from Escherichia coli (Sigma-Aldrich, St. Louis, MO, USA) or 200 ng/ml rMIF for 1 h [19] for the expression of il1β mRNA (real-time PCR). Cells were also stimulated with 300 µg/ml MSU crystals for 1, 3, and 6 h, and the supernatant was collected to measure MIF by ELISA.
Confocal microscopy
Mice were injected with saline and MSU crystals (100 µg/cavity), and 6 h later, the articular cavity was washed for cell counts. Cells (5 × 105) were used to performed Shandon CytoSpin III (Thermo Shandon). The cytocentrifuged slides were fixed for 30 min, and a nonspecific receptor was blocked, adding anti-mouse CD16/32 (Fc block; BD Biosciences, San Jose, CA, USA). For surface staining, we used anti-F4/80-PE antibody (BD Biosciences) overnight. Then, cells were treated with 1× permeabilization wash (Cytofix/Cytoperm kit; BD Biosciences) and were intracellularly stained with rabbit anti-MIF (Cell Sciences, Canton, MA, USA) overnight. Then, the secondary anti-rabbit IgG-Alexa Fluor 488 (Life Technologies, Thermo Fisher Scientific) was added for 30 min. Images were obtained using C2 Eclipse Ti confocal microscope (Nikon Instruments, Melville, NY, USA). Fluorescence intensity was measured off-line using Volocity software 6.3 (PerkinElmer, Waltham, MA, USA), and the fluorescence profile was assessed using ImageJ software (NIH, Bethesda, MD, USA).
Histologic analysis
Samples were fixed in 10% buffered formalin (pH 7.4), decalcified for 28 d in 14% EDTA, embedded in paraffin, sectioned, and stained with H&E. Two sections of knee joints were microscopically examined by a single pathologist and scored in a blinded manner. The histologic score was modified from the arthritis index described previously by Queiroz-Junior et al. [20]. The parameters evaluated were: severity of synovial hyperplasia, intensity and extension of inflammatory infiltrate, vascular hyperemia, presence of inflammatory cells in the synovial cavity, and changes in tissue architecture. These criteria ranged a score with 8 points.
Statistical analysis
All results are presented as the means ± sem. The analysis of the difference between 2 groups was performed by Student’s t test. Normalized data of 3 or more groups were analyzed by one-way ANOVA, and differences between groups were assessed using the Newman-Keuls post-test. Pearson correlation was used for correlation in normalized data. Statistical significance is detailed in figure legends. Calculations were performed using Prism 5.0 software for Windows (GraphPad Software, La Jolla, CA, USA).
RESULTS
The concentration of MIF is increased in SF of gouty patients and in periarticular tissue of mice injected with MSU crystals
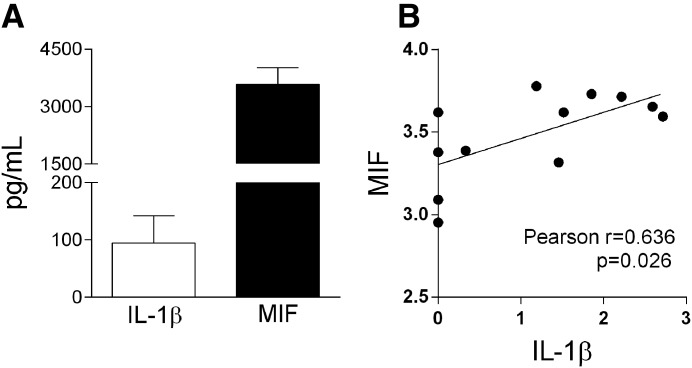
The concentration of MIF was initially evaluated in the SF of patients with gout. Patients had typical acute clinical presentation associated with the finding of MSU crystals and high percentage of neutrophils in the SF (Table 1). The concentration of MIF and IL-1β was elevated in the SF of patients, and there was a positive correlation between these 2 cytokines (r = 0.636, P = 0.026; Fig. 1A and B).
Figure 1. Patients with gout present an increase level of MIF.
SF was obtained from patients with acute gout. MIF and IL-1β were quantified by ELISA (A). Pearson correlation analysis shows the association of levels of MIF with IL-1β: r = 0.636; P = 0.026 (B).
The injection of MSU crystals into the knee joint of mice also increased the concentration of MIF in periarticular tissue when compared with vehicle-injected joints (Fig. 2A). We have shown previously that joint inflammation peaks between 6 and 15 h after injection of MSU crystals in the knee joint of mice [9]. The concentration of MIF was already elevated at 1 h after injection of MSU crystals and kept elevated throughout the observation period (Fig. 2A). Likewise, the mRNA of mif was increased in synovial tissue of mice after 1 h following the injection of MSU crystals in the knee (Fig. 2B). By confocal microscopy, we identified that macrophages recovered from the synovial cavity had MIF in their cytoplasm under basal conditions, and there was an increase of MIF detection in these cells when we previously injected MSU crystals in the knee joint (Fig. 2C and D). Furthermore, there was an increase of MIF protein in the supernatant of BMDM when stimulated with MSU crystals (Fig. 2E).
Figure 2. MSU crystals increase the production of MIF in a murine model of gout.
Mice received intra-articular injection of MSU crystals (100 µg/cavity), and periarticular tissue was collected in different times to quantify MIF by ELISA (A). Synovial tissue was collected 1 h after the injection of MSU crystals to quantify MIF by quantitative PCR. V, Vehicle group (B). Control groups received saline (Sal) injection (10 µl). Macrophages recovered from the synovial cavity, 6 h after the injection of saline or MSU crystals, were intracellularly stained for MIF, and the images were obtained by confocal microscopy (C). The mean fluorescence intensity (MFI) of MIF along the cell was measured off-line using Volocity software 6.3 (PerkinElmer), and the fluorescence profile was assessed using ImageJ (NIH; D). BMDMs (5 × 105/well) were stimulated with MSU crystals (300 μg/ml, and MIF proteins were analyzed in the supernatant by ELISA (E). Bars show the means ± sem results in 5 mice/group. *P < 0.05 versus saline group; **P < 0.01 versus saline group and ***P < 0.001 versus saline group.
The injection of rMIF induces joint inflammation
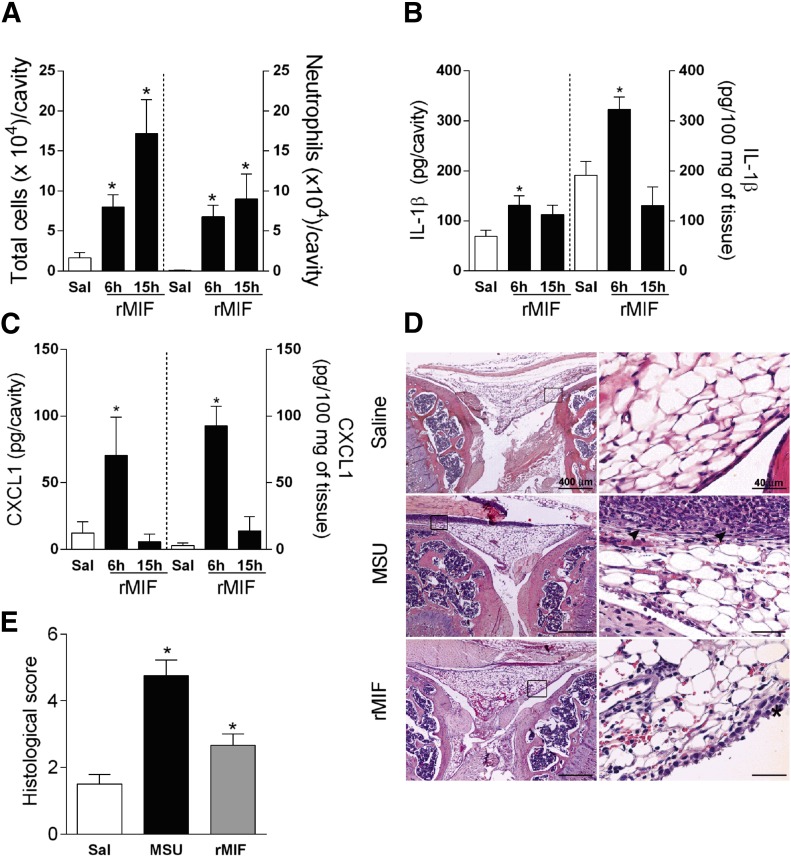
To verify whether MIF could induce joint inflammation akin to what has been observed after injection of MSU crystals [9], we injected 100 ng rMIF into the knee joint of mice. As seen in Fig. 3, the injection of rMIF promoted the recruitment of leukocytes, 6 and 15 h later (Fig. 3A), and neutrophils were the majority of infiltrating cells (Fig. 3A). Moreover, rMIF induced the expression of IL-1β and CXCL1 in the joint cavity and periarticular tissue (Fig. 3B and C), mainly at 6 h after injection. Histologic analyses revealed that the injection of rMIF into the knee joint (6 h) induced a mild diffuse infiltrate of neutrophils in the synovial tissue, hemorrhagic areas, and light hyperplasia (Fig. 3D). As expected, the injection of MSU crystals induced significant inflammation in the knee joint. Changes were similar to those observed after injection of rMIF but were more intense (Fig. 3E). Thus, MIF per se can cause joint inflammation that mimics several parameters observed after injection of MSU crystals.
Figure 3. rMIF induces neutrophil recruitment and cytokine production in vivo.
Mice received intra-articular injection of rMIF (100 ng/cavity), and total cell and neutrophil accumulation was evaluated 6 and 15 h after the injection (A). Levels of the cytokine IL-1β and CXCL1 into the articular cavity (B) and periarticular tissue (C). The histopathological evaluation of tissue damage (D) and histologic score (E) was investigated 6 h after the challenge with MSU crystals or rMIF. (D) The arrowheads indicate the inflammatory infiltrate, and the asterisk shows hyperplasia of synovial membrane. Control groups received saline injection (10 µl). Bars show the means ± sem results in 5 mice/group. *P < 0.05 versus saline group (ANOVA followed by Newman-Keuls post-test).
MIF is an important cytokine that mediates joint inflammation in gout
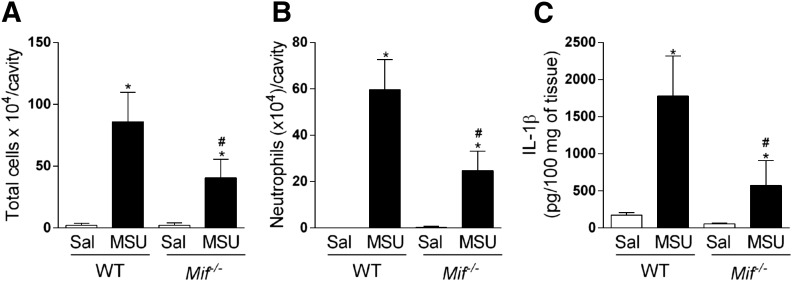
We analyzed joint inflammation induced by injection of MSU crystals in the knee joint of WT and Mif−/− mice. There was significant accumulation of leukocytes, mainly neutrophils, in the cavity, 15 h after the challenge of WT mice when compared with saline-injected joints. However, neutrophil influx was greatly reduced in the joint of Mif−/− mice after the injection of MSU crystals (Fig. 4A and B). Moreover, there were significantly lower amounts of IL-1β in periarticular tissues of Mif−/− mice (Fig. 4C).
Figure 4. Mif−/− mice have reduced inflammatory response induced by MSU crystals.
Mif−/− mice were injected with MSU crystals (100 µg/cavity), and leukocyte recruitment to articular cavity was evaluated 15 h after injection (A and B). Levels of IL-1β were measured in periarticular tissue (C). Control groups received saline injection (10 µl). Bars show the means ± sem results in 5 mice/group. *P < 0.05 versus saline group; #P < 0.05 versus MSU group (ANOVA followed by Newman-Keuls post-test).
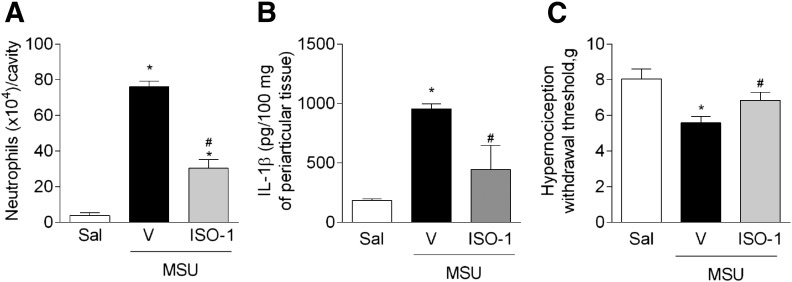
Next, we used 2 pharmacological strategies to inhibit the functions of MIF: ISO-1, a small molecule that inhibits the activity of MIF, and an anti-MIF mAb. Mice pretreated with ISO-1 (50 µg/cavity) showed reduction of the recruitment of neutrophils, 15 h after the injection of MSU crystals when compared with saline-treated mice (Fig. 5A). In a similar manner, mice pretreated with anti-MIF (1 mg/kg, i.p.) showed decreased neutrophil accumulation into the joint after injection of MSU crystals when compared with IgG isotype-treated (1 mg/kg, i.p.) mice (mean ± sem: saline, 0.3 ± 0.3; MSU crystals + IgG, 43.3 ± 10.0; MSU crystals + anti-MIF, 15.0 ± 6.0 × 104 neutrophils/cavity; n = 5, *P < 0.05 compared to saline group, #P < 0.05 compared to MSU crystals + IgG group). Importantly, MIF seemed to contribute to MSU crystal-induced joint dysfunction. Mice treated with anti-MIF had reduced hypernociception following the injection of MSU crystals compared with vehicle-treated mice (mean ± sem: saline, 10.1 ± 0.8; MSU crystals + IgG, 5.6 ± 0.5; MSU crystals + anti-MIF, 7.6 ± 0.2 g; n = 5, *P < 0.05 compared to saline group, #P < 0.05 compared to MSU crystals + IgG group). The blockade of MIF with ISO-1 reduced the levels of IL-1β detected in periarticular tissue (Fig. 5B), as seen in Mif−/− mice. Furthermore, the blockade of MIF activity also reduced hypernociception induced by injection of MSU crystals (Fig. 5C).
Figure 5. The blockage of MIF reduces inflammatory response induced by MSU crystals.
Mice were treated with ISO-1 (50 µg, intra-articularly), 1 h before the injection of MSU crystals, and the analyses were evaluated 15 h after the injection of MSU crystals. Accumulation of neutrophils into the joint (A). The levels of the cytokine IL-1β in periarticular tissue were measured by ELISA (B). Paw withdrawal threshold assessment (C). Control groups received saline injection (10 µl). Bars show the means ± sem results in 5 mice/group. *P < 0.05 versus saline group; #P < 0.05 versus vehicle group (mice only challenged with MSU crystals; ANOVA followed by Newman-Keuls post-test).
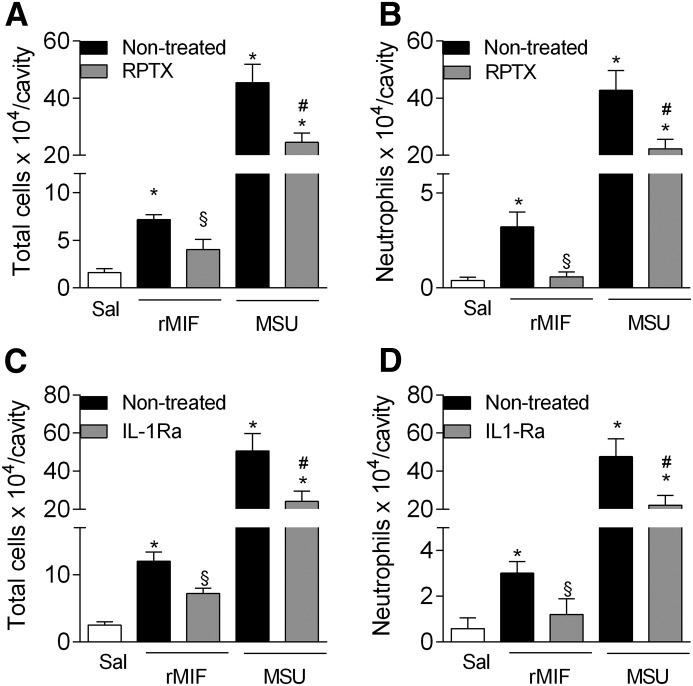
MIF contributes to MSU crystal-induced inflammation by a mechanism dependent on IL-1R and CXCR1/2 activities
Here, our results suggest that MIF induces the recruitment of neutrophils in the joint via the production of IL-β and CXCL1. Therefore, we investigated whether the 2 pathways were relevant for the proinflammatory functions of MIF in acute gouty arthritis in mice. To assess this, we treated mice with Reparixin, an allosteric inhibitor of CXCR1/2, or IL-1Ra, an antagonist at the IL-1βR, before the intra-articular injection of rMIF or MSU crystals. Both treatments significantly reduced the number of total cells and neutrophils recruited to the joint cavity after injection of rMIF (Fig. 6A–D). As shown previously [9], these treatments also reduced the number of neutrophils recruited in response to injection of MSU crystals.
Figure 6. Accumulation of neutrophils in the joint is dependent on CXCL1 and IL-1β.
Mice were treated with Reparixin (RPTX; 30 mg/kg, s.c.) or IL-1Ra (5 mg/kg, i.p.), 30 min before the injection of MSU crystals (100 µg/cavity) or rMIF (100 ng/cavity), and accumulation of leukocytes (A and C), mainly neutrophils (B and D), in cavity was evaluated. Control groups received saline injection (10 µl). Bars show the means ± sem results in 5 mice/group. *P < 0.05 versus saline group; #P < 0.05 versus MSU group; §P < 0.05 versus rMIF group (ANOVA followed by Newman-Keuls post-test).
MIF contributes to inflammation in acute gout by the induction of IL-1β mRNA synthesis
Two signals are required to release the mature form of IL-1β: the first that drives pro-IL-1β mRNA and protein synthesis and a second signal that activates the inflammasome and consequent pro-IL-1β protein processing [21]. The injection of rMIF into the tibiofemoral joint increased the expression of il1b mRNA, 1 h later in the synovial tissue compared with the saline-injected group (Fig. 7A). Likewise, the injection of MSU crystals increased the expression of il1b mRNA in synovial tissue. The treatment with the MIF inhibitor ISO-1 significantly reduced the expression of il1b mRNA (Fig. 7B) induced by MSU crystal injection (3 h after MSU crystals). Therefore, our in vivo results clearly show that control of il1b mRNA expression by MIF may underlie its proinflammatory actions in this model of gout. Consistent with these in vivo results, rMIF induced an increase in the expression of il1b mRNA by BMDM (Fig. 7C).
Figure 7. MIF contributes to gouty inflammation through IL-1β synthesis.
Mice received injection of rMIF (100 ng/cavity) to evaluate levels of il1β mRNA in synovial tissue, 1 h later (A). Mice was treated with ISO-1 (50 µg/cavity), 10 min before the injection of MSU crystals (100 µg/cavity), and il1β mRNA levels, 3 h after the challenge was assessed (B). BMDMs were treated with rMIF (200 ng/ml) or LPS (1 µg/ml) for 1 h. The mRNA was extracted from cells for IL-1β measurement (C). Control groups received saline injection (10 µl). Bars show the means ± sem results in 5 mice/group. *P < 0.05 versus saline group; ***P < 0.001 versus saline group; #P < 0.05 versus vehicle group (mice only challenged with MSU crystal; ANOVA followed by Newman-Keuls post-test and Student’s t test).
DISCUSSION
Previous studies evaluating levels of MIF in human SF have suggested that MIF has a relevant role in the pathogenesis of juvenile idiopathic arthritis [22], OA [23], and rheumatoid arthritis [24]. In our study population, levels of MIF could be detected in all samples and correlated positively with levels of IL-1β. Increased levels of IL-1β and a high percentage of neutrophils in the SF of patients with acute gout have been described previously [25] and corroborate our findings. This is the first demonstration of the correlation between MIF and IL-1β in the joint of gouty patients. In our study, the number of patients did not allow for categorized levels of MIF according to severity or disease progression. However, our study suggests that this may be of interest in future studies with more patients with various degrees of disease severity. We had no access to samples from nonarthritic individuals, and levels of MIF, which were used as a control for our gouty population, were similarly elevated in samples of OA patients (data not shown). A previous study showed that levels of MIF in SF were higher in patients with OA than in control individuals [23], suggesting that levels of MIF in our study indeed should be elevated when compared with background levels.
To investigate more closely the role of MIF in gout, we used a murine model of acute gout that relied on the injection of MSU crystals in the tibiofemoral joint. This injection produces an inflammatory response similar to an acute gout attack, with an accumulation of neutrophils in the joint cavity and increased levels of proinflammatory cytokines and pain [9]. We demonstrate herein that the injection of MSU crystals increased the levels of mRNA and protein MIF in the joints at an early time, which agrees with the measurement of the cytokine in SF. As previously described, macrophages are important sources of MIF [26]. Here, we confirm that result and showed that MSU crystals increase the production of MIF by BMDMs and macrophages recovered from MSU crystal-injected joints.
MIF is known to have chemotactic actions for different cell types, including monocytes, T and B cells, and neutrophils and eosinophils [19, 27–30]. MIF is considered to play a role in acute and chronic immunoinflammatory conditions, including rheumatoid arthritis, atherosclerosis, and lupus erythematosus [31]. MIF is released by mature blood and tissue neutrophils under TNF stimulation and has a positive feedback on neutrophil recruitment in different systems [32]. In our experiments, injection of MIF promoted neutrophil recruitment when injected into the tibiofemoral joint of mice. Of note, the influx of neutrophils into the joint is a hallmark of an acute gout attack and contributes to articular damage and pain [8]. Moreover, we show that rMIF induces a mild increase in histologic score and increases intra-articular levels of the cytokine IL-1β and the chemokine CXCL1—molecules that are known to contribute to the recruitment of neutrophils to the joint [9].
Different studies have shown that the absence of MIF or its blockade by antibodies reduced the recruitment of leukocytes under inflammatory conditions [29, 30, 33], including autoimmune arthritis [34–36]. It was demonstrated previously that the small molecule ISO-1 inhibits MIF tautomerase activity, suppressing its proinflammatory activities [37]. Here, we used 2 pharmacological strategies to block MIF: the small molecule MIF antagonist ISO-1 and anti-MIF mAb to target the endogenous MIF activity and suppress inflammation in vivo [37, 38]. Moreover, we also used Mif−/− mice to characterize better the role of MIF in the model. Our results demonstrate that the absence of MIF greatly reduced neutrophil recruitment induced by injection of MSU crystals. Moreover, ISO-1 treatment reduced the hypernociception associated with crystal-induced inflammation. Interestingly, MIF has been reported to be associated with the development of inflammatory pain induced by thermal sensitivity in mice [39]. In our system, we have shown previously that MSU crystal-induced hypernociception was dependent on the influx of neutrophils [9]. Therefore, early MIF production after injection of MSU crystals drives neutrophil influx and neutrophil-dependent inflammatory pain.
We also demonstrate that the accumulation of neutrophils induced by MIF in the joint cavity is dependent on IL-1β and CXCL1. Previous studies have shown that the association that IL-1β drives CXCL1 and consequent CXCR2-dependent recruitment of neutrophils [7, 9, 17, 36]. The inhibition of either receptor was able to reduce the influx of neutrophils and associated pain induced by MSU crystals [9]. Furthermore, it has been demonstrated that there was a reduction of neutrophil migration induced by CXCL1 in Mif−/− mice [36], and cells from these genetic deficient mice are hyporesponsive to IL-1β activation [40].
It has been shown previously that the cytokine IL-1β is a critical mediator of neutrophil influx and pain induced by injection of MSU crystals [9, 10]. IL-1β is firstly produced in its immature form, called pro-IL-1β, which is then cleaved and released in its active form by the NLRP3 inflammasome assembled in response to recognition of MSU crystals by specific cells. Moreover, the production of CXCL1 is driven by NLRP3 inflammasome in this model [9]. Here, MIF increased the levels of IL-1β and CXCL1 in the inflamed joints. This is consistent with the ability of MIF to induce proinflammatory mediators in other conditions [29, 40–42]. More importantly, blockade of MIF action with ISO-1 was associated with inhibition of IL-1β levels in response to injection of MSU crystals. ISO-1 prevented the increase of pro-il1β mRNA induced by the MSU crystals in vivo and in vitro, suggesting that control of IL-1β production is a major mechanism by which MIF controls inflammation in the context of MSU crystal-induced inflammation and probably, in acute gout. This mechanism is consistent with other in vitro studies showing that il1β mRNA was up-regulated by MIF in synovial fibroblasts [14]. Moreover, our results corroborate findings of Santos et al. [36] that showed decreased expression of il1β mRNA in synovial tissue of Mif−/− mice in K/BxN serum transfer arthritis.
In conclusion, we showed in this study that MIF plays a major role in MSU crystal-induced inflammation in mice. The mechanism by which MIF controls inflammation induced by MSU crystals is dependent on its ability to control the production of il1β mRNA. MIF levels in human SF are strongly correlated with increased levels of IL-1β, which corroborates our findings in mice. Altogether, MIF appears to be a very important mediator in the initial process of inflammation in gout, mimicking an acute gout attack, and could be a target to control the excessive inflammation triggered by the deposition of MSU crystals in the joints.
AUTHORSHIP
I.G., D.G.S., M.T.B., M.M.T., and F.A.A. designed research, analyzed data, and wrote the paper. I.G., A.C.F.D., L.D.T., I.P.S.R., C.M.Q.-J., V.V.C., A.C.R., L.P.S., and F.A.A. performed experiments and analyzed data. R.D.R.O., P.L.-J., R.B., and L.L. provided essential materials for the development of work.
ACKNOWLEDGMENTS
The authors thank the funding agencies, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil); Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Brazil); and European Community's Seventh Framework Programme (FP7-2007-2013, TIMER consortium), under Grant Agreement No. HEALTH-F4-2011-281608, for financial support. R.B. and L.L. are supported by U.S. National Institutes of Health Grant NIH-AR049610. The authors thank Ilma Marçal for technical assistance.
Glossary
- BMDM
bone marrow-derived macrophage
- IL-1-ra
IL-1R antagonist
- ISO-1
(S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid
- MIF
macrophage migration inhibitory factor
- Mif−/−
macrophage migration inhibitory factor-deficient mice
- MSU
monosodium urate
- NLRP3
neuronal apoptosis inhibitor protein, class 2 transcription activator of the MHC, heterokaryon incompatibility, and telomerase-associated protein 1 (NACHT), leucine-rich repeat (LRR), and pyrin domain (PYD) domain-containing protein 3
- OA
osteoarthritis
- rMIF
recombinant macrophage migration inhibitory factor
- SF
synovial fluid
- UFMG
Universidade Federal de Minas Gerais
- WT
wild-type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Nuki G., Simkin P. A. (2006) A concise history of gout and hyperuricemia and their treatment. Arthritis Res. Ther. 8 (Suppl 1), S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson P. C., Horsburgh S. (2014) Gout: joints and beyond, epidemiology, clinical features, treatment and co-morbidities. Maturitas 78, 245–251. [DOI] [PubMed] [Google Scholar]
- 3.Roddy E., Choi H. K. (2014) Epidemiology of gout. Rheum. Dis. Clin. North Am. 40, 155–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Ruiz F., Castillo E., Chinchilla S. P., Herrero-Beites A. M. (2014) Clinical manifestations and diagnosis of gout. Rheum. Dis. Clin. North Am. 40, 193–206. [DOI] [PubMed] [Google Scholar]
- 5.Martin W. J., Walton M., Harper J. (2009) Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 60, 281–289. [DOI] [PubMed] [Google Scholar]
- 6.Reber L. L., Marichal T., Sokolove J., Starkl P., Gaudenzio N., Iwakura Y., Karasuyama H., Schwartz L. B., Robinson W. H., Tsai M., Galli S. J. (2014) Mast cell-derived IL-1beta contributes to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 66, 2881–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241. [DOI] [PubMed] [Google Scholar]
- 8.Popa-Nita O., Naccache P. H. (2010) Crystal-induced neutrophil activation. Immunol. Cell Biol. 88, 32–40. [DOI] [PubMed] [Google Scholar]
- 9.Amaral F. A., Costa V. V., Tavares L. D., Sachs D., Coelho F. M., Fagundes C. T., Soriani F. M., Silveira T. N., Cunha L. D., Zamboni D. S., Quesniaux V., Peres R. S., Cunha T. M., Cunha F. Q., Ryffel B., Souza D. G., Teixeira M. M. (2012) NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout. Arthritis Rheum. 64, 474–484. [DOI] [PubMed] [Google Scholar]
- 10.Mitroulis I., Kambas K., Ritis K. (2013) Neutrophils, IL-1β, and gout: is there a link? Semin. Immunopathol. 35, 501–512. [DOI] [PubMed] [Google Scholar]
- 11.David J. R. (1966) Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 56, 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calandra T., Roger T. (2003) Macrophage migration inhibitory factor: a regulator of innate immunity. Nat. Rev. Immunol. 3, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leech M., Metz C., Hall P., Hutchinson P., Gianis K., Smith M., Weedon H., Holdsworth S. R., Bucala R., Morand E. F. (1999) Macrophage migration inhibitory factor in rheumatoid arthritis: evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheum. 42, 1601–1608. [DOI] [PubMed] [Google Scholar]
- 14.Onodera S., Nishihira J., Koyama Y., Majima T., Aoki Y., Ichiyama H., Ishibashi T., Minami A. (2004) Macrophage migration inhibitory factor up-regulates the expression of interleukin-8 messenger RNA in synovial fibroblasts of rheumatoid arthritis patients: common transcriptional regulatory mechanism between interleukin-8 and interleukin-1beta. Arthritis Rheum. 50, 1437–1447. [DOI] [PubMed] [Google Scholar]
- 15.Bozza M., Satoskar A. R., Lin G., Lu B., Humbles A. A., Gerard C., David J. R. (1999) Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J. Exp. Med. 189, 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachs D., Coelho F. M., Costa V. V., Lopes F., Pinho V., Amaral F. A., Silva T. A., Teixeira A. L., Souza D. G., Teixeira M. M. (2011) Cooperative role of tumour necrosis factor-α, interleukin-1β and neutrophils in a novel behavioural model that concomitantly demonstrates articular inflammation and hypernociception in mice. Br. J. Pharmacol. 162, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coelho F. M., Pinho V., Amaral F. A., Sachs D., Costa V. V., Rodrigues D. H., Vieira A. T., Silva T. A., Souza D. G., Bertini R., Teixeira A. L., Teixeira M. M. (2008) The chemokine receptors CXCR1/CXCR2 modulate antigen-induced arthritis by regulating adhesion of neutrophils to the synovial microvasculature. Arthritis Rheum. 58, 2329–2337. [DOI] [PubMed] [Google Scholar]
- 18.Gomes M. T., Campos P. C., Oliveira F. S., Corsetti P. P., Bortoluci K. R., Cunha L. D., Zamboni D. S., Oliveira S. C. (2013) Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J. Immunol. 190, 3629–3638. [DOI] [PubMed] [Google Scholar]
- 19.Klasen C., Ohl K., Sternkopf M., Shachar I., Schmitz C., Heussen N., Hobeika E., Levit-Zerdoun E., Tenbrock K., Reth M., Bernhagen J., El Bounkari O. (2014) MIF promotes B cell chemotaxis through the receptors CXCR4 and CD74 and ZAP-70 signaling. J. Immunol. 192, 5273–5284. [DOI] [PubMed] [Google Scholar]
- 20.Queiroz-Junior C. M., Madeira M. F., Coelho F. M., Costa V. V., Bessoni R. L., Sousa L. F., Garlet G. P., Souza Dda. G., Teixeira M. M., Silva T. A. (2011) Experimental arthritis triggers periodontal disease in mice: involvement of TNF-α and the oral microbiota. J. Immunol. 187, 3821–3830. [DOI] [PubMed] [Google Scholar]
- 21.Martinon F., Burns K., Tschopp J. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 10, 417–426. [DOI] [PubMed] [Google Scholar]
- 22.Meazza C., Travaglino P., Pignatti P., Magni-Manzoni S., Ravelli A., Martini A., De Benedetti F. (2002) Macrophage migration inhibitory factor in patients with juvenile idiopathic arthritis. Arthritis Rheum. 46, 232–237. [DOI] [PubMed] [Google Scholar]
- 23.Liu M., Hu C. (2012) Association of MIF in serum and synovial fluid with severity of knee osteoarthritis. Clin. Biochem. 45, 737–739. [DOI] [PubMed] [Google Scholar]
- 24.Onodera S., Tanji H., Suzuki K., Kaneda K., Mizue Y., Sagawa A., Nishihira J. (1999) High expression of macrophage migration inhibitory factor in the synovial tissues of rheumatoid joints. Cytokine 11, 163–167. [DOI] [PubMed] [Google Scholar]
- 25.Scanu A., Oliviero F., Ramonda R., Frallonardo P., Dayer J. M., Punzi L. (2012) Cytokine levels in human synovial fluid during the different stages of acute gout: role of transforming growth factor β1 in the resolution phase. Ann. Rheum. Dis. 71, 621–624. [DOI] [PubMed] [Google Scholar]
- 26.Calandra T., Bernhagen J., Mitchell R. A., Bucala R. (1994) The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 179, 1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernhagen J., Krohn R., Lue H., Gregory J. L., Zernecke A., Koenen R. R., Dewor M., Georgiev I., Schober A., Leng L., Kooistra T., Fingerle-Rowson G., Ghezzi P., Kleemann R., McColl S. R., Bucala R., Hickey M. J., Weber C. (2007) MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 13, 587–596. [DOI] [PubMed] [Google Scholar]
- 28.Gregory J. L., Morand E. F., McKeown S. J., Ralph J. A., Hall P., Yang Y. H., McColl S. R., Hickey M. J. (2006) Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J. Immunol. 177, 8072–8079. [DOI] [PubMed] [Google Scholar]
- 29.De Souza H. S., Tortori C. A., Lintomen L., Figueiredo R. T., Bernardazzi C., Leng L., Bucala R., Madi K., Buongusto F., Elia C. C., Castelo-Branco M. T., Bozza M. T. (2015) Macrophage migration inhibitory factor promotes eosinophil accumulation and tissue remodeling in eosinophilic esophagitis. Mucosal Immunol. 8, 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magalhaes E. S., Paiva C. N., Souza H. S., Pyrrho A. S., Mourao-Sa D., Figueiredo R. T., Vieira-de-Abreu A., Dutra H. S., Silveira M. S., Gaspar-Elsas M. I., Xavier-Elsas P., Bozza P. T., Bozza M. T. (2009) Macrophage migration inhibitory factor is critical to interleukin-5-driven eosinophilopoiesis and tissue eosinophilia triggered by Schistosoma mansoni infection. FASEB J. 23, 1262–1271. [DOI] [PubMed] [Google Scholar]
- 31.Santos L. L., Morand E. F. (2009) Macrophage migration inhibitory factor: a key cytokine in RA, SLE and atherosclerosis. Clin. Chim. Acta 399, 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Daryadel A., Grifone R. F., Simon H. U., Yousefi S. (2006) Apoptotic neutrophils release macrophage migration inhibitory factor upon stimulation with tumor necrosis factor-alpha. J. Biol. Chem. 281, 27653–27661. [DOI] [PubMed] [Google Scholar]
- 33.Magalhães E. S., Mourao-Sa D. S., Vieira-de-Abreu A., Figueiredo R. T., Pires A. L., Farias-Filho F. A., Fonseca B. P., Viola J. P., Metz C., Martins M. A., Castro-Faria-Neto H. C., Bozza P. T., Bozza M. T. (2007) Macrophage migration inhibitory factor is essential for allergic asthma but not for Th2 differentiation. Eur. J. Immunol. 37, 1097–1106. [DOI] [PubMed] [Google Scholar]
- 34.Gregory J. L., Leech M. T., David J. R., Yang Y. H., Dacumos A., Hickey M. J. (2004) Reduced leukocyte-endothelial cell interactions in the inflamed microcirculation of macrophage migration inhibitory factor-deficient mice. Arthritis Rheum. 50, 3023–3034. [DOI] [PubMed] [Google Scholar]
- 35.Leech M., Metz C., Santos L., Peng T., Holdsworth S. R., Bucala R., Morand E. F. (1998) Involvement of macrophage migration inhibitory factor in the evolution of rat adjuvant arthritis. Arthritis Rheum. 41, 910–917. [DOI] [PubMed] [Google Scholar]
- 36.Santos L. L., Fan H., Hall P., Ngo D., Mackay C. R., Fingerle-Rowson G., Bucala R., Hickey M. J., Morand E. F. (2011) Macrophage migration inhibitory factor regulates neutrophil chemotactic responses in inflammatory arthritis in mice. Arthritis Rheum. 63, 960–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lubetsky J. B., Dios A., Han J., Aljabari B., Ruzsicska B., Mitchell R., Lolis E., Al-Abed Y. (2002) The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J. Biol. Chem. 277, 24976–24982. [DOI] [PubMed] [Google Scholar]
- 38.Al-Abed Y., Dabideen D., Aljabari B., Valster A., Messmer D., Ochani M., Tanovic M., Ochani K., Bacher M., Nicoletti F., Metz C., Pavlov V. A., Miller E. J., Tracey K. J. (2005) ISO-1 binding to the tautomerase active site of MIF inhibits its pro-inflammatory activity and increases survival in severe sepsis. J. Biol. Chem. 280, 36541–36544. [DOI] [PubMed] [Google Scholar]
- 39.Alexander J. K., Cox G. M., Tian J. B., Zha A. M., Wei P., Kigerl K. A., Reddy M. K., Dagia N. M., Sielecki T., Zhu M. X., Satoskar A. R., McTigue D. M., Whitacre C. C., Popovich P. G. (2012) Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Exp. Neurol. 236, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toh M. L., Aeberli D., Lacey D., Yang Y., Santos L. L., Clarkson M., Sharma L., Clyne C., Morand E. F. (2006) Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J. Immunol. 177, 4818–4825. [DOI] [PubMed] [Google Scholar]
- 41.Assunção-Miranda I., Bozza M. T., Da Poian A. T. (2010) Pro-inflammatory response resulting from sindbis virus infection of human macrophages: implications for the pathogenesis of viral arthritis. J. Med. Virol. 82, 164–174. [DOI] [PubMed] [Google Scholar]
- 42.Assunção-Miranda I., Amaral F. A., Bozza F. A., Fagundes C. T., Sousa L. P., Souza D. G., Pacheco P., Barbosa-Lima G., Gomes R. N., Bozza P. T., Da Poian A. T., Teixeira M. M., Bozza M. T. (2010) Contribution of macrophage migration inhibitory factor to the pathogenesis of dengue virus infection. FASEB J. 24, 218–228. [DOI] [PubMed] [Google Scholar]