Primary hematopoietic cells express Aldh3b1 and Aldh1a1, enzymes, which synthesize ATRA from vitamin A, yet do not synthesize intracellular ATRA in vivo.
Keywords: aldehyde dehydrogenase, ATRA, retinoid receptor, Aldh3b1
Abstract
In vivo pathways of natural retinoid metabolism and elimination have not been well characterized in primary myeloid cells, even though retinoids and retinoid receptors have been strongly implicated in regulating myeloid maturation. With the use of a upstream activation sequence-GFP reporter transgene and retrovirally expressed Gal4-retinoic acid receptor α in primary mouse bone marrow cells, we identified 2 distinct enzymatic pathways used by mouse myeloid cells ex vivo to synthesize retinoic acid receptor α ligands from free vitamin A metabolites (retinyl acetate, retinol, and retinal). Bulk Kit+ bone marrow progenitor cells use diethylaminobenzaldehyde-sensitive enzymes, whereas bone marrow-derived macrophages use diethylaminobenzaldehyde-insensitive enzymes to synthesize natural retinoic acid receptor α-activating retinoids (all-trans retinoic acid). Bone marrow-derived macrophages do not express the diethylaminobenzaldehyde-sensitive enzymes Aldh1a1, Aldh1a2, or Aldh1a3 but instead, express Aldh3b1, which we found is capable of diethylaminobenzaldehyde-insensitive synthesis of all trans-retinoic acid. However, under steady-state and stimulated conditions in vivo, diverse bone marrow cells and peritoneal macrophages showed no evidence of intracellular retinoic acid receptor α-activating retinoids, despite expression of these enzymes and a vitamin A-sufficient diet, suggesting that the enzymatic conversion of retinal is not the rate-limiting step in the synthesis of intracellular retinoic acid receptor α-activating retinoids in myeloid bone marrow cells and that retinoic acid receptor α remains in an unliganded configuration during adult hematopoiesis.
Introduction
Retinoid receptors are nuclear hormone receptors that are dynamically regulated during terminal myeloid maturation and influence hematopoietic stem cell self-renewal and differentiation [1–12]. There are 2 types of retinoid receptors—RARs and RXRs—both of which have 3 subtypes (α, β, γ). RARs heterodimerize with RXR, and this heterodimer recognizes RA response elements in promoter/enhancer sequences of target genes. Retinoid receptor transcriptional activity is ligand dependent: in the absence of ligand, RAR/RXR heterodimers bind to corepressors (e.g., nuclear receptor corepressor and silencing mediator for retinoid and thyroid hormone receptors); in the presence of ligand, retinoid receptors release corepressors, recruit coactivators, and activate transcription activity [13]. ATRA is thought to be the principal natural ligand for RARA and is a critical therapy for the treatment of patients with APL (a leukemia with pathognomonic expression of the fusion oncoprotein PML-RARA) [14–18]. However, the enzymatic pathways that regulate natural retinoids have not been well defined in primary hematopoietic cells.
ATRA is synthesized from vitamin A through 2 sequential steps. Vitamin A (principally, protein-bound serum retinol) is oxidized by ADHs to yield retinal (there are many isoforms that appear redundant). Retinal is then irreversibly oxidized by an ALDH to generate retinoic acid [19, 20]. At least 19 different human ALDHs have been identified. Among them, ALDH1A1, ALDH1A2, and ALDH1A3 have been shown to oxidize all trans-retinal to ATRA with high affinity (Km is <0.1 µM, 0.7 µM, 0.2 µM, respectively) [21–24] and have been implicated as the rate-limiting step in ATRA synthesis [25, 26]. Intracellular levels of ATRA are also regulated by elimination via P450 enzymes (principally, CYP26 family) [27].
Multiple ALDH inhibitors have been characterized. In specific, DEAB is an ALDH inhibitor commonly used as a negative control in the Aldefluor assay, which is used for identification, evaluation, and isolation of stem and progenitor cells based on their expression of ALDH. DEAB behaves as a classic substrate for ALDH3A1, as a covalent inhibitor for ALDH2 and ALDH1A2, and as an intermediate between substrate and inhibitor for ALDH1A1, ALDH1A3, ALDH1B1, and ALDH5A1 [28]. More recently, WIN 18446 was found to be a potent reversible inhibitor of ALDH1A1 and ALDH1A3 (IC50 = 102 ± 2 nM and 187 ± 1 nM) and an efficient, time-dependent inhibitor (kinact = 22.0 ± 2.4 h-1, KI = 1026 ± 374 nM; where kinact = maximal inactivation and KI = concentration at 50% Kinact) of ALDH1A2 [29].
To understand the synthesis and metabolism of naturally occurring RARA ligands in primary mouse bone marrow cells, we used a UAS-GFP reporter mouse, which we described recently [30]. The UAS promoter (upstream-activating sequence) is recognized by the yeast Gal4 transcription factor but not by mammalian proteins [31]. When we transduce UAS-GFP bone marrow cells with retroviruses, which express a fusion of the Gal4 DNA-binding domain and the RARA ligand-binding domain, cells with intracellular RARA ligands activate the GFP reporter.
We found that immature mouse Kit+ bone marrow progenitor cells (Kit+ progenitor cells) and mature mouse BMMφ used different pathways to synthesize active RARA ligands. The former uses canonical ALDH pathways that are inhibited by DEAB (likely, Aldh1a1, Aldh1a2, and Aldh1a3), whereas BMMφ use DEAB-insensitive enzymes. Through expression-array profiling, we identified Aldh3b1 as a likely candidate and found that overexpression of this gene in 293T cells could synthesize ATRA and specifically activate the UAS-GFP/Gal4-RARA reporter. However, in vivo, we observed no evidence of naturally occurring retinoids in bone marrow cells or in peritoneal macrophages, despite a vitamin A-sufficient diet, suggesting that the synthesis of intracellular RARA ligands is tightly controlled in hematopoietic cells but that expression of these enzymes is not the rate-limiting step for in vivo retinoid synthesis.
MATERIALS AND METHODS
Reagents
ATRA, RyA, retinol, retinal, daidzin, cyanamide, DEAB, citral, 5FU, phenylhydrazine, neuraminidase, octanal, NAD+, and NADH were from Sigma-Aldrich (St. Louis, MO, USA). Disulfram was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Imject Alum was from Thermo Fisher Scientific (Grand Island, NY, USA). BMS493, recombinant mouse Scf, Flt3, IL-3, Tpo, Liarozole, and Talarozole were purchased from R&D Systems (Minneapolis, MN, USA). Ro41-5253 was purchased from Enzo (Farmingdale, NY, USA). M-CSF was produced as described [32]. G-CSF was from Amgen (Millstadt, IL, USA). Mouse CD117 MicroBeads were purchased from Miltenyi Biotec (San Diego, CA, USA). Restriction enzymes were purchased from New England BioLabs (Ipswich, MA, USA) WIN 18446 was from Tocris (Minneapolis, MN, USA). Rabbit polyclonal anti-ALDH3B1 was from Abcam (Cambridge, MA, USA) and used at 1:200 with anti-rabbit-IgG HRP-linked antibody from Cell Signaling Technology (Danvers, MA, USA) at 1:500. Viromer Green was purchased from OriGene (Rockville, MD, USA).
Constructs
The plasmids pCMX-Gal4-RARA and pCMX-Gal4-RXRA were generous gifts from Dr. Christopher K. Glass (University of California at San Diego, CA, USA) and Mercedes Ricote (Centro Nacional de Investigaciones Cardiovasculares, Madrid, Spain). The plasmid pPAX6m-Gal4-VP16 was a gift from Thomas Perlmann (Ludwig Cancer Center, Stockholm, Sweden). MSCV-Gal4-RARA-IC has been described previously [30]. Human ALDH3B1 plasmids were kind gifts from Akio Kihara (Hokkaido University, Sapporo, Japan) [33]. MSCV-STRA6-IRES-Cerulean was generated by cloning the NheI/XhoI Cerulean fragment from Addgene plasmid 15214 into MSCV-IRES-YFP, replacing the YFP sequence. STRA6 cDNA was a kind gift from Marcin Golczak (Case Western Reserve University, Cleveland, Ohio), and the XmnI/XhoI fragment containing the entire cDNA was blunt cloned into MSCV-IRES-Cerulean. The insertion was confirmed to be identical to the STRA6 reference sequences in the Ensembl data base. Aldh3b1, Aldh7a1, Aldh8a1, Aldh9a1, Akr1a4, Akr1b8, and 2810405K02Rik expression constructs were purchased from OriGene.
Mice
UAS-GFP mice were bred as described and maintained on standard chow (PicoLab 20, which contains 15 IU/gm vitamin A; LabDiet, St. Louis, MO, USA) [30]. ATRA was administered by oral gavage, daily for 3 d, and suspended in sterile corn oil, 50 µg/mouse, 100 µg/mouse, or 200 µg/mouse. G-CSF (300 µg/kg/d × 3 d), Phenylhydrazine (60 mg/kg once), Neuraminidase (0.05 U/mouse once), 5FU (4.5 mg/mouse once), or injections of 100 μg (4-hydroxy-3-nitrophenyl) acetyl-chicken γ globulin in alum were administered as described previously [34–42]. There were 5 mice each in control and experiment groups. Each experiment was done at least twice. All of the mice used for experiments were 6–8 wk old. Both genders were used. As gender-specific differences were not observed, the data were combined. All of the mice were cared for in the Experimental Animal Center of Washington University School of Medicine. The Washington University Animal Studies Committee approved all animal experiments.
Retrovirus production and transduction
Calcium chloride transfection and low-passage 293T cells were used for the virus package, as described [43]. 293T cells (5 × 106) were seeded in 10 cm dishes in DMEM (high glucose) + 10% FBS, 18∼24 h before transfection, and grown to ∼80% confluence before the transfection. MSCV-Gal4-RARA-IC or MSCV-Gal4-RXRA-IC (12 µg), 8 µg Ecopak, and 155 µl 2 M CaCl2 were added to H2O to 1.25 ml, mixed by gentle vortex, and drop-wise added 1.25 ml 2× HEPES-buffered saline (273.8 mM NaCl, 9.4 mM KCl, 42 mM HEPES, 11.1 mM glucose, 0.4 mM Na2HPO4 7 H2O, pH 7.05) while vortex. This mixture was incubated at room temperature for 20 min and then drop-wise, added the mixture onto 293T cells. After 12 h, the medium was changed and virus collected at 48 and 72 h after transfection. Virus was aliquoted and stored at −80°C. Fresh, 48 h virus was used for bone marrow transplant experiments, and frozen virus was used for in vitro experiments.
Transduction of Kit+ cells.
Femurs, tibias, and pelvises were isolated from 6- to 8-wk-old UAS-GFP mice. Bone marrow cells were collected by centrifuging bones at 6000 rpm for 2 min. RBCs were lysed by ammonium-chloride-potassium buffer (NH4Cl 150 mM, KHCO3, 10 mM, Na2EDTA, 0.1 mM) on ice for 5 min. Kit+ cells were isolated by MACS using an Automacs Pro (Miltenyi Biotec), per the manufacturer’s protocol. Kit+ cells (1.5 million)/well were plated in a 12-well plate in progenitor cell expansion medium (RPMI 1640 + 15% FBS + 50 ng/ml Scf + 25 ng/ml Flt3 + 10 ng/ml IL-3 + 10 ng/ml Tpo + 50 µM 2-ME) overnight and transduced with MSCV-Gal4-RARA-IC retrovirus by spinfection with 10 µg/ml polybrene and 10 mM HEPES at 2400 rpm, 30°C for 90 min in an Eppendorf 5810R centrifuge. Cells were maintained in progenitor cell expansion medium, with or without drugs unless otherwise stated, and analyzed after 72 h.
Transduction of BMMφ.
Mouse bone marrow Kit− cells were isolated as above and grown in macrophage differentiation media (α-MEM + 10% FBS + 50 ng/ml M-CSF) for 4 d. Adherent macrophages were harvested and plated into a Petri 6-well plate in macrophage differentiation media, 5 × 105/well. BMMφ were transduced with MSCV-Gal4-RARA-IC retrovirus by spinfection with 10 µg/ml polybrene and 10 mM HEPES at 2400 rpm, 30°C for 90 min in an Eppendorf 5810R centrifuge. Cells were maintained in macrophage differentiation medium, with or without drugs unless otherwise stated, and analyzed after 72 h.
Transplantation.
At d 1, Kit+ cells were collected and grown in progenitor cell expansion media, as above. At d 2, Kit+ bone marrow cells were transduced with fresh, 48 h MSCV-Gal4-RARA-IC retrovirus, as above. Recipient mice were treated with 1100 cGy total-body irradiation and were given antibiotic water. At d 3, Kit+ bone marrow cells were transduced with fresh, 48 h MSCV-Gal4-RARA-IC retrovirus, as above, a second time. Two hours later, ∼1 million cells/mouse were administered via tail-vein injection. After 6 wk engraftment, transplant mice were treated as indicated and analyzed.
Flow cytometry
GFP and mCherry were detected on a FACScan or Gallios instrument. These 2 machines have different dynamic ranges for GFP detection, accounting for differences in the calculated MFI. FACScan instruments have 5 decades of GFP detection but cannot detect mCherry; these data are presented as percent GFP+ cells without normalization for mCherry.
In vitro ALDH assay
FLAG-mouse ALDH3B1 (3×) and 3× FLAG-human ALDH3B1 were expressed in 293T cells using calcium phosphate transfection, as above. 293T cells, BMMφ, and Kit+ progenitor cells were lysed with radioimmunoprecipitation assay buffer (50 mM Tris-HCl, NaCl 150 mM, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA). Protein was quantified by a bicinchoninic acid quantification kit (Thermo Fisher Scientific).
The assay was performed as described [33]. Octanal was used as substrate. Protein (1 µl) was incubated with 500 µM NAD+ and Octanal (1, 5, 10, 33, 50, and 100 µM) in 50 µl buffer B [50 mM Tris-HCl (pH 8.5), 150 mM NaCl, 10% glycerol, and 0.1% Triton X-100] at 37°C. The reaction was monitored by measuring the fluorescence of the NADH product (excitation at 356 nm and emission at 460 nm) using Synergy 2 (BioTek, Winooski, VT, USA). A linear, standard curve was obtained from 0, 5, 10, 25, and 100 µM NADH and used to quantify the NADH produced in the assay.
Liquid chromatography/MS/MS analysis of ATRA
Retinoids were extracted from media or cell pellets by methanol extraction. Media (200 µl) were mixed with 300 µl methanol and 10 ng 14,15-DHET-d11 standard (Cayman Chemical, Ann Arbor, MI, USA), vortexed and centrifuged for 5 min, and the supernatant was diluted with 100 µl water and analyzed. Cell pellets were mixed with 100 µl water, 400 µl methanol, and 10 ng 14,15-DHET-d11 standard; vortexed; and centrifuged for 5 min, and the supernatant was diluted with 300 µl water and analyzed. Three-point ATRA (Sigma-Aldrich) calibration standard samples (0.2, 0.8, and 10 ng), containing the internal standard (10 ng), were also prepared for the absolute quantification. Diluted urine and calibration samples (50 µl) were injected onto a C18 column (BetaSil 100 × 2.1 mm, 3 µm; Thermo Electron, Thermo Fisher Scientific, Keystone, PA, USA),connected to an online trapping LC-20AD HPLC system (Shimadzu, Kyoto, Japan), interfaced with an API 4000 triple-quadruple mass spectrometer (Applied Biosystems, Foster City, CA, USA) for ATRA analysis. Acetonitrile and water containing 10 mM ammonium acetate were used for chromatographic separation of ATRA and the internal standard. The solvent gradient was at 70% acetonitrile for 2 min and then programmed to 99% acetonitrile for 5 min at a flow rate of 0.7 ml/min. Negative ion electrospray multiple reaction monitoring mode (monitoring Q1/Q3 ions: 299/255 for ATRA and 348/207 for 14,15-DHET-d11) was controlled by Analyst software (Applied Biosystems) for quantification of ATRA.
Knockdown ALDH3B1 in BMMφ
On d 1, bone marrow macrophages were plated at 100,000 cells/well in 24-well plates in 200 µl. A 300 µl mixture of retrovirus containing 0.25 µl polybrene and 7.5 µl HEPES was added and cells centrifuged as above. After 1 h of incubation, cells were transfected with Aldh3b1 siRNA. Viromer Green (2.25 µl) was mixed with 410 µl solution F (provided by manufacturer). siRNA (4.5 µl; 20 µM) was mixed with 28 µl solution F. These 2 dilutions were then mixed and incubated for 10 min at room temperature, and 70 µl was added in triplicate wells to cells in 500 µl media. Four hours later, an additional 250 µl media was added. On d 3, retinal was added to 100 nM. On d 5, cells were collected for analysis.
RT-PCR
RNA was extracted using Trizol, and cDNA was generated using Superscript II, per the manufacturer's recommendations (Invitrogen, Thermo Fisher Scientific). PCR was performed using a SYBR Green PCR kit (Qiagen, Valencia, CA, USA) and a StepOnePlus Real-Time PCR System (Applied Biosystems). Primers used are described in Table 1.
TABLE 1.
Primers used
| Gene | Primer sequence | Gene | Primer sequence |
|---|---|---|---|
| Aldh1a1-F | gcactcaatggtgggaaagt | Aldh4a1-F | ggcttttctgctgcattctc |
| Aldh1a1-R | aatgtttaccacgccaggag | Aldh4a1-R | agagaggaggtgtgcttcca |
| Aldh1a2-F | ccattggagtgtgtggacag | Aldh5a1-F | cactgtggtggtgaaacctg |
| Aldh1a2-R | gtccaagtcagcatctgcaa | Aldh5a1-R | tgtttgagcaaacgcaagtc |
| Aldh1a3-F | gtggagttcgccaagaagag | Aldh6a1-F | gggaagactcttgctgatgc |
| Aldh1a3-R | cactgctgctgtgagtccat | Aldh6a1-R | cacaaagctgattgccttga |
| Aldh1a7-F | ctgtgggaacactgtggttg | Aldh7a1-F | ggaaggtcactgctgagagg |
| Aldh1a7-R | tctgaccctggtggaagaac | Aldh7a1-R | agtggcaagcagcctaccta |
| Aldh1b1-F | tgaggtaggccacctgattc | Aldh8a1-F | ctggctgatgtactggagca |
| Aldh1b1-R | gtagcccaggattcgttcaa | Aldh8a1-R | ccaggcagtcacagaagtca |
| Aldh1L1-F | ggcagaagctgacgtttttc | Aldh9a1-F | ctagcagctggggtcttcac |
| Aldh1L1-R | cacagctccttcacctcctc | Aldh9a1-R | ctaatgacccaaagcctgga |
| Aldh3a1-F | gcgtggtccttgtcataggt | Aldh16a1-F | ttcccaggagtccttccttt |
| Aldh3a1-R | caaggtgacaggggtcagat | Aldh16a1-R | tgctccgtgcctttctagtt |
| Aldh3b1-F | gtgagaagcctggatgaagc | Aldh18a1-F | atggttaccgctttggactg |
| Aldh3b1-R | cgactggagtagggtgggta | Aldh18a1-R | gttggaaacgctgaggagag |
| Stra6-F | gttcaggtctggcagaaagc | Oaz1-F | agtcagcgggatcacagtct |
| Stra6-R | ccacctggtaagtggctgtt | Oaz1-R | ccaagaaagctgaaggttcg |
Expression array profiling
Expression profiling was done using Affymetrix exon 1.0 arrays, as described previously [44]. Data have been deposited in Gene Expression Omnibus (GSE76060).
Data analysis
Flow cytometry data were analyzed with FlowJo software version 9 (Tree Star, Ashland, OR, USA). Statistical analysis was performed using Prism (GraphPad Software, San Diego, CA, USA) and Excel (Microsoft, Seattle, WA, USA). t-Test and ANOVA tests were performed, as appropriate. All studies were performed in triplicate, unless otherwise indicated. Error bars represent sd. Data points without error bars have sd below GraphPad’s limit to display.
RESULTS
DEAB-sensitive and -insensitive pathways of retinoid synthesis
To determine pathways used for retinoid synthesis in different mouse bone marrow cells, we applied an assay using a UAS-GFP reporter and a coexpressed fusion of the DNA-binding domain from the yeast Gal4 transcription factor and the ligand-binding domain from RARA (Gal4-RARA). This fusion recognizes the UAS promoter, which is not activated by mammalian transcription factors.
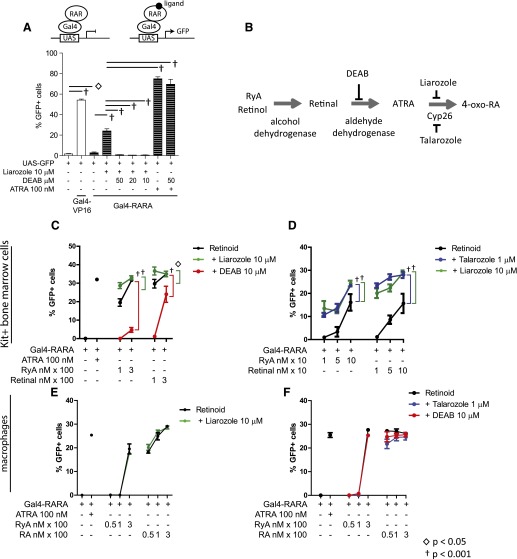
Initial studies with the UAS-GFP reporter were performed in a cell line that could be easily transfected—293T. We found that cotransfection of the UAS-GFP reporter and Gal4-RARA plasmids resulted in a modest but reproducible increase in GFP (Fig. 1A, column 3 vs. 1). Inhibition of P450 by liarozole treatment augmented the GFP reporter activity (Fig. 1A, column 4), and the ALDH antagonist DEAB abrogated the effects of liarozole (Fig. 1A,, columns 5–7). This suggests that in 293T cells, natural RARA ligands are synthesized via enzymatic conversion of serum-available vitamin A, and their intracellular levels are balanced through a process of simultaneous synthesis and then elimination via P450 enzymes, as has been published previously (Fig. 1B, schema) [27].
Figure 1. Activation of Gal4-RARA by natural retinoids.
(A) 293T cells were transfected, as indicated; treated with liarozole, DEAB, or ATRA; and analyzed for GFP expression after 72 h. (B) Model of stepwise ATRA synthesis and effect of inhibitors. (C and D) Activation of UAS-GFP reporter by MSCV-Gal4-RARA-IC in Kit+ progenitor cells. (E and F) Activation of UAS-GFP reporter by MSCV-Gal4-RARA-IC in BMMφ. In each case, cells were prepared, transduced with MSCV-Gal4-RARA-IC, and then treated as indicated for 72 h before analysis of GFP. Error bars represent sd of triplicate experiments.
To determine whether primary hematopoietic cells use similar retinoid synthesis and elimination pathways, we used UAS-GFP transgenic mice [30]. We initially transduced MACS-selected Kit+ bone marrow progenitor cells (Kit+ progenitor cells) from UAS-GFP mice ex vivo with MSCV-Gal4-RARA-IC. These cells represent a heterogeneous population of myeloid and erythroid progenitor cells that can be maintained ex vivo in Scf, IL-3, Flt3, and Tpo and retain high numbers of CFUs after 1 wk in culture (∼1 in 10 cells forms colonies in myeloid methylcellulose). We found that when grown in 15% FCS, these cells did not synthesize sufficient intracellular RARA ligands to induce the GFP reporter, even with liarozole treatment, although they did respond to exogenous ATRA (Fig. 1C, columns 1 and 2, and data not shown). This system is highly sensitive to available ATRA, with an EC50 of 0.36 ± 0.14 nM [30]. Serum levels of vitamin A (principally retinol) are ∼1 µM in humans and mice, with lower concentrations of RyA (∼200 nM) and retinal (∼300 nM) [45–51]. We supplemented the medium with different forms of vitamin A, which resulted in a dose-dependent response (Fig. 1C and D). This response could be augmented by inhibition of P450 activity (liarozole treatment, green) and was inhibited by DEAB treatment (red; Fig. 1C and D). Similar results were observed using a second P450 inhibitor with greater Cyp26 specificity (talarozole, blue; Fig. 1D). These results demonstrate that primary Kit+ progenitor cells are capable of synthesizing transcriptionally active retinoids when supplied with exogenous, free vitamin A metabolites, RyA, and retinal.
We repeated these studies using a second primary cell type—BMMφ—which unlike the Kit+ progenitor cells, are terminally differentiated and have undergone cell-cycle arrest. When BMMφ were transduced with MSCV-Gal4-RARA-IC, we observed that they could synthesize RARA ligands from exogenously added RyA and retinal (50–300 nM) but again, could not make use of serum-available forms of vitamin A (Fig. 1E and F). However, this process was insensitive to DEAB (Fig. 1F, red), liarozole (Fig. 1E, green), and talarozole (Fig. 1F, blue), suggesting that BMMφ use alternative enzymatic pathways to synthesize and eliminate transcriptionally active retinoids from vitamin A metabolites.
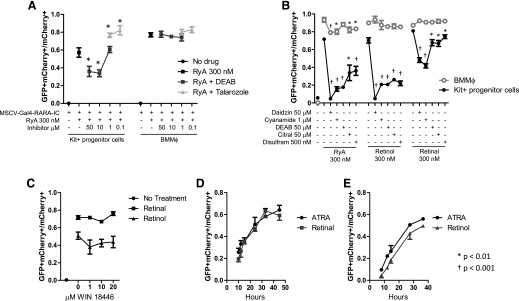
To validate these results, we treated Kit+ progenitor cells and BMMφ in parallel and again, observed that DEAB inhibited retinoid synthesis in Kit+ progenitors but not in BMMφ (Fig. 2A). With the use of a series of ALDH inhibitors, we found that each could inhibit synthesis of RARA ligands from 3 different vitamin A precursors (RyA, retinol, and retinal) in the Kit+ progenitor cells, but they had a very limited effect on retinoid synthesis in BMMφ (Fig. 2B). A new compound—WIN 18446—has recently shown strong inhibition activity of all 3 ALDH1A family enzymes [29]. Synthesis of RARA ligands from free retinal and retinol in BMMφ was resistant to WIN 18446, as well (Fig. 2C). These data strongly suggested that BMMφ use alternative enzymatic pathways other than the ALDH1A family to synthesize active RARA ligands.
Figure 2. Differential sensitivity of pathways leading to natural Gal4-RARA-activating ligands in Kit+ progenitor cells and in BMMφ.
(A and B) Kit+ progenitor cells and BMMφ were transduced with MSCV-Gal4-RARA-IC retrovirus and treated as indicated for 72 h before analysis of GFP within cells expressing mCherry. (C) BMMφ were transduced with MSCV-Gal4-RARA-IC and treated with WIN 18466, as indicated for 72 h before analysis of GFP within cells expressing mCherry. (D and E) BMMφ were transduced with MSCV-Gal4-RARA-IC and treated with 300 nM of indicated retinoids for 72 h before analysis of GFP within cells expressing mCherry. Error bars represent sd of triplicate experiments.
To investigate the kinetics of the active RARA ligand formation in BMMφ, we compared the rate of UAS-GFP reporter activation in BMMφ treated with retinol, retinal, or ATRA (each 300 nM; Fig. 2D and E). We observed similar kinetics of UAS-GFP reporter activation among ATRA, retinal, and retinol in BMMφ, suggesting that the enzymatic conversion of retinal and retinol must occur on a time scale that is faster than the sensitivity of the ex vivo assay.
We assessed a second mature myeloid cell population ex vivo by differentiating Kit+ progenitor cells into granulocytes in the presence of G-CSF and Scf (Supplemental Fig. 1A). We found that these cells had an intermediate phenotype compared with BMMφ; we observed no difference with liarozole treatment and only a mild reduction with DEAB treatment.
Expression of ALDHs in primary bone marrow cells
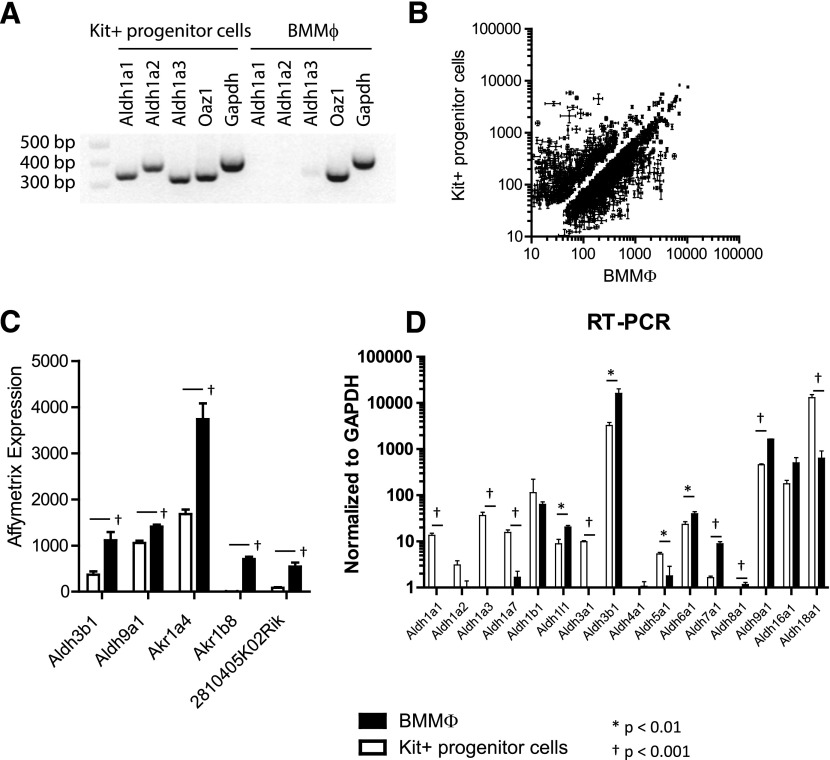
With the use of RT-PCR, we assessed RNA levels of the 3 ALDH1A enzymes described to have retinaldehyde dehydrogenase activity and to be DEAB and WIN 18446 sensitive [29, 52, 53]. Not surprisingly, we detected expression of Aldh1a1, Aldh1a2, and Aldh1a3 in Kit+ progenitor cells but not in BMMφ (Fig. 3A). We examined an extended panel of Aldh genes by RT-PCR in Kit+ progenitor cells and in ex vivo-derived granulocytes and noted similar expression levels of Aldh1a1 but reduced expression of Aldh1a2 and Aldh1a3, consistent with the intermediate sensitivity to DEAB that we observed previously (Supplemental Fig. 1B).
Figure 3. Differentially expressed enzyme transcripts in Kit+ progenitors and BMMφ.
(A) Semiquantitative RT-PCR analysis of Aldh1a1, Aldh1a2, and Aldh1a3 and 2 control transcripts, ornithine decarboxylase antizyme 1 (Oaz1) and Gapdh, in Kit+ progenitor cells and BMMφ. (B) Affymetrix expression array of Kit+ progenitor cells vs. BMMφ. (C) Affymetrix expression results for 5 transcripts with differential expression between Kit+ progenitor cells and BMMφ. Data normalized to chip mean of 1500. (D) Quantitative RT-PCR analysis of Aldh genes in Kit+ progenitor cells vs. BMMφ. In all studies, error bars represent sd of triplicate experiments.
To identify additional enzymes that might contribute to DEAB-insensitive retinoid synthesis in BMMφ, we compared gene expression in Kit+ progenitor cells with BMMφ by Affymetrix array profiling. We identified 1217 genes that were annotated as enzymes or as involved in enzymatic pathways and were differentially expressed in BMMφ vs. Kit+ progenitor cells (P < 0.01; Fig. 3B). We hand curated this list and identified 5 genes that were more highly expressed in BMMφ, annotated with lipid-binding capacity and dehydrogenase function, or were largely uncharacterized (Fig. 3C). None of these had been implicated previously in retinaldehyde dehydrogenase activity, although ALDH3B1 has been shown to oxidize lipid-derived aldehydes generated in the plasma membrane [33] and is known to be expressed in kidney, liver, cerebral astrocytes, and histocytes [54, 55]. We validated the expression difference of Aldh3b1 and Aldh9a1 using quantitative RT-PCR and included an extended panel of additional Aldh genes (Fig. 3D). Again, Aldh1a1, Aldh1a2, and Aldh1a3 were noted to be expressed at lower levels in BMMφ vs. Kit+ progenitor cells.
Identification of the enzyme required in ATRA synthesis in BMMφ
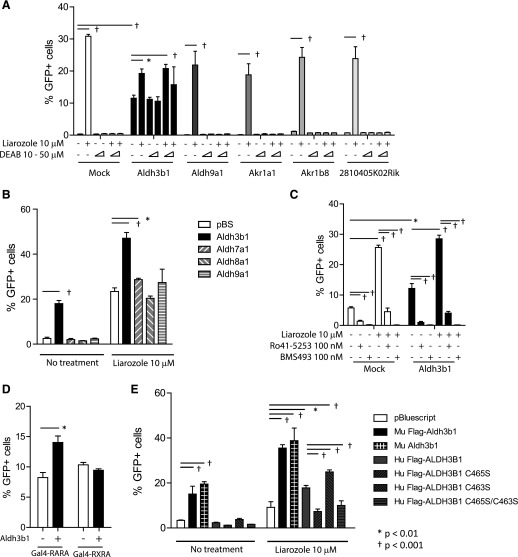
To identify enzymes further that might synthesize RARA active ligands in BMMφ, we generated UGN/FAP cells. When treated with liarozole, these cells induced GFP expression, and the effect of liarozole was abrogated by DEAB, as expected (Fig. 4A, column 2 vs. columns 5 and 6). Transfection of an Aldh3b1 expression vector led to DEAB-insensitive Gal4-RARA transactivation and GFP expression, whereas transfection of Aldh9a1, Akr1a1, Akr1b8, or 2810405K02Rik did not (expression plasmids for Akr1a4 were not commercially available, and we tested Akr1a1, which is closely related and has a human homolog, whereas Akr1a4 does not; Fig. 4A). Two additional Aldhs that showed differential but low expression in macrophages (Fig. 3D) was also examined (Aldh7a1 and Aldh8a1). When triple transfected with the UAS-GFP reporter and the Gal4-RARA expression vector into 293T cells, only Aldh7a1 modestly augmented GFP output and only in the presence of liarozole (Fig. 4B).
Figure 4. Aldh3b1 expression associated with Gal4-RARA activation and ATRA synthesis.
(A) Stable 293T UGN/FAP cells were transiently transfected with indicated expression vectors, treated as indicated, and analyzed, 72 h later. (B) 293T cells were transfected with UAS-GFP reporter, Gal4-RARA expression plasmid, and indicated Aldh expression plasmids and treated as indicated. pBS, pBluescript. (C) Stable 293T UGN/FAP cells were transiently transfected with Aldh3b1 expression plasmid and treated with liarozole or with RARA antagonists, as indicated. (D) 293T cells were transiently transfected with the UAS-GFP reporter and expression plasmids for Aldh3b1, Gal4-RARA, or Gal4-RXRA, as indicated. (E) 293T cells were transfected with UAS-GFP reporter plasmids, Gal4-RARA expression plasmids, and indicated Aldh3b1 plasmids. Mu, Murine; Hu, human. Cells were treated with or without liarozole for 72 h and assessed by flow cytometry. Error bars indicate sd of triplicate experiments.
The effects of Aldh3b1 transfection were inhibited by 2 different RARA antagonists (Ro41-5253 and BMS493; Fig. 4C). Furthermore, Aldh3b1 cotransfection increased the activity of Gal4-RARA but not Gal4-RXRA, suggesting that Aldh3b1 cotransfection results in synthesis of natural RARA-specific ligands that do not nonspecifically cross-react to transactivate the closely related Gal4-RXRA (Fig. 4D). We also compared the activity of mouse Aldh3b1 and human ALDH3B1 in RARA ligand production. We observed that human ALDH3B1 had less activity, and the C465S mutation that prevents enzyme prenylation also prevented synthesis of RARA ligands in 293T cells (Fig. 4E) [33].
ALDH activity of Aldh3b1 in vitro
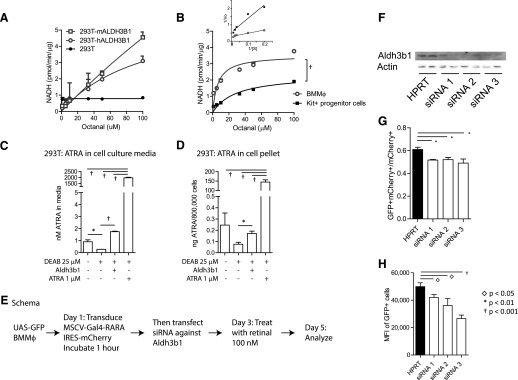
We investigated the ALDH activity of mouse Aldh3b1 and human ALDH3B1 in vitro. We overexpressed mouse Aldh3b1 and human ALDH3B1 in 293T cells. We detected the ALDH activity of the protein by monitoring the fluorescence of the NADH product (excitation at 356 nm and emission at 460 nm) in increasing concentrations of octanal, as described previously [33]. We observed that mouse Aldh3b1 (Vmax = 4.55 ± 0.019) had higher ALDH activity than human ALDH3B1 (Vmax = 3.1 ± 0.017) when using octanal as a substrate (Fig. 5A). We also detected endogenous mouse Aldh3b1 activity by use of the Kit+ progenitor cell lysate and BMMφ lysate (Fig. 5B). We observed higher Vmax in BMMφ vs. Kit+ progenitor cells (3.5 ± 0.27 vs. 2.4 ± 0.25) and lower Km (6.5 ± 2.1 vs. 28.9 ± 8; Fig. 5B, inset, Lineweaver-Burk plot). These data are suggestive of lower concentrations of ALDH enzymes with lower affinity for octanal in Kit+ progenitor cells vs. BMMφ and are consistent with higher expression of Aldh3b1 in BMMφ vs. Kit+ progenitor cells. We performed MS on media from UGN/FAP stable 293T cells transfected with mouse Aldh3b1 expression plasmid. We found that nanomolar concentrations of ATRA could be detected in the media of untreated UGN/FAP cells, which decreased with DEAB treatment, and that Aldh3b1 transfection abrogated the effects of DEAB treatment and resulted in restoration of ATRA levels (Fig. 5C). Importantly, the concentrations we observed match the dynamic range of RARA dissociation constant using radiolabeled ATRA (0.2 nM) [56] and the MSCV-Gal4-RARA-IC EC50 we have observed in Kit+ bone marrow cells ex vivo [30]. Similar results were observed when cell pellets were analyzed for intracellular ATRA (Fig. 5D).
Figure 5. Aldh3b1 activity.
(A) Mouse (m)Aldh3b1 and human (h)ALDH3B1 were transfected into 293T cells and ALDH activity assessed in total protein lysates using octanal as a substrate. (B) Endogenous mouse Aldh3b1 activity was assessed in total protein lysates from BMMφ and Kit+ progenitor cells using octanal as substrate (Lineweaver-Burk plot is inset). Error bars indicate sd of duplicates. (C) MS determination of ATRA concentrations in cell-culture media from 293T stable UGN/FAP cells transfected with Aldh3b1 and treated with DEAB or ATRA. (D) MS of ATRA concentrations in total cells transfected in C. (E) Schema of knockdown Aldh3b1 in BMMφ. (F) Western blot of BMMφ transfected with siRNA targeting Aldh3b1. HPRT, Hypoxanthine phosphoribosyltransferase. (G) Percentage of GFP+ BMMφ after knockdown Aldh3b1. (H) GFP MFI of BMMφ after knockdown Aldh3b1. Error bars indicate sd of triplicate measurements.
Aldh3b1 knockdown affects synthesis of RARA active ligands in BMMφ
To study the function of endogenous Aldh3b1 in BMMφ, we knocked down Aldh3b1 using siRNA (Fig. 5E; schema). Results from a Western blot assay demonstrated incomplete reduction in Aldh3b1 expression (Fig. 5F). Following retroviral transduction of MSCV-Gal4-RARA-IC and treatment with retinal, we observed a reduction in the percentage of GFP+ cells and in the MFI of GFP on a per-cell basis (Fig. 5G and H), suggesting that Aldh3b1 contributes directly to RARA ligand synthesis in mouse BMMφ.
Expression patterns of Aldh3b1, Aldh1a1, Aldh1a2, and Aldh1a3
We assessed the expression of Aldh3b1, Aldh1a1, Aldh1a2, and Aldh1a3 in mouse and human hematopoietic cells using previously published datasets (Supplemental Figs. 2 and 3) [57–59]. We found that Aldh3b1 was dynamically regulated during myelopoiesis, with higher expression in mature granulocytes in mice and in promyelocytes in humans, and that it was also easily detected in diverse human leukemias by RNA-Seq, with highest expression in M4 myelomonocytic and M5 monocytic leukemia (Supplemental Fig. 2). In contrast, Aldh1a1 was expressed predominantly in KLS stem/progenitor cells and in megakaryocyte-erythroid progenitor cells in mice and in human CD34+ cells, with down-regulation during myeloid maturation and low but detectable expression in diverse acute myeloid leukemias (Supplemental Fig. 3A, D, and G). Aldh1a2 and Aldh1a3 tend to be expressed at lower levels in mouse and human bone marrow cells and undergo less dynamic regulation. Thus, in hematopoietic cells, Aldh3b1 expression is distinct from Aldh1a1, Aldh1a2, or Aldh1a3.
Absence of natural RARA ligands in hematopoietic cells in vivo
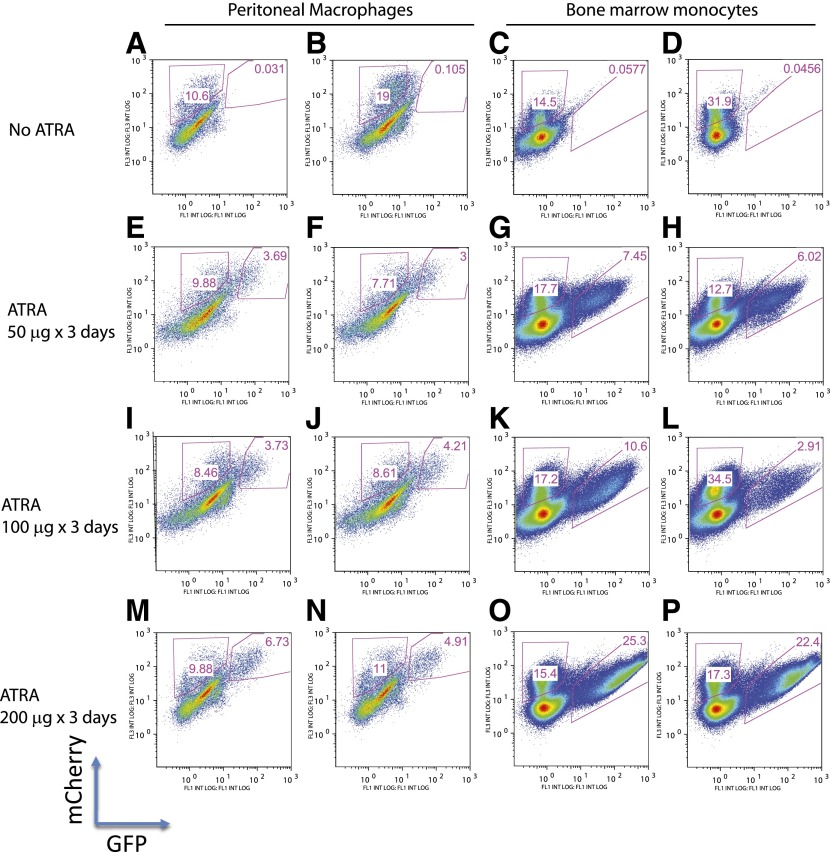
To evaluate whether natural concentrations of vitamin A are sufficient to induce in vivo retinoid synthesis in macrophages and bone marrow granulocytes (cells with the highest Aldh3b1 expression), we transduced UAS-GFP bone marrow Kit+ cells with MSCV-Gal4-RARA-IC and transplanted these cells into lethally irradiated mice. After 6 wk engraftment, we analyzed peritoneal macrophages and bone marrow monocytes (Gr1+CD11b+). We observed populations of mCherry+ cells that had been transduced with MSCV-Gal4-RARA-IC but only rare GFPdim cells, suggesting that intracellular retinoids are absent in these cells in vivo (Fig. 6A–D). As a positive control, we treated mice daily for 3 d with ATRA in corn oil by gavage (the doses 50, 100, and 200 µg/d are approximately equivalent to 7.5, 15, and 30 mg/m2). We observed a response beginning with the lowest dose and a dose-dependent increase in the GFP MFI and GFP percentage (Fig. 6E–P).
Figure 6. In vivo analysis of natural retinoids in myeloid cells.
UAS-GFP bone marrow Kit+ cells were transduced with MSCV-Gal4-RARA-IC and transplanted into lethally irradiated recipient mice. After 6 wk engraftment, mice were untreated or treated with ATRA by daily gavage for 3 d. Peritoneal macrophages and bone marrow monocytes (Gr1+CD11b+) were then analyzed for GFP expression within cells that express mCherry. Two representative mice are shown, from a total of 5 mice analyzed with each treatment. FL1/3, Fluorescence 1/3. INT LOG, Integrated log scale.
Consistent with these findings, ex vivo Kit+ progenitor cells and BMMφ were unable to synthesize RARA ligands from available precursors when cultured in 15% FCS, whereas 293T cells were able to do so (Figs. 1 and 2). Thus, even though BMMφ and Kit+ bone marrow cells express enzymes involved in retinoid synthesis and use these enzymes to synthesize retinoids ex vivo when exposed to free vitamin A precursors at expected serum-available concentrations, this does not translate into significant concentrations of intracellular retinoids in vivo in mice maintained on a vitamin A-sufficient diet.
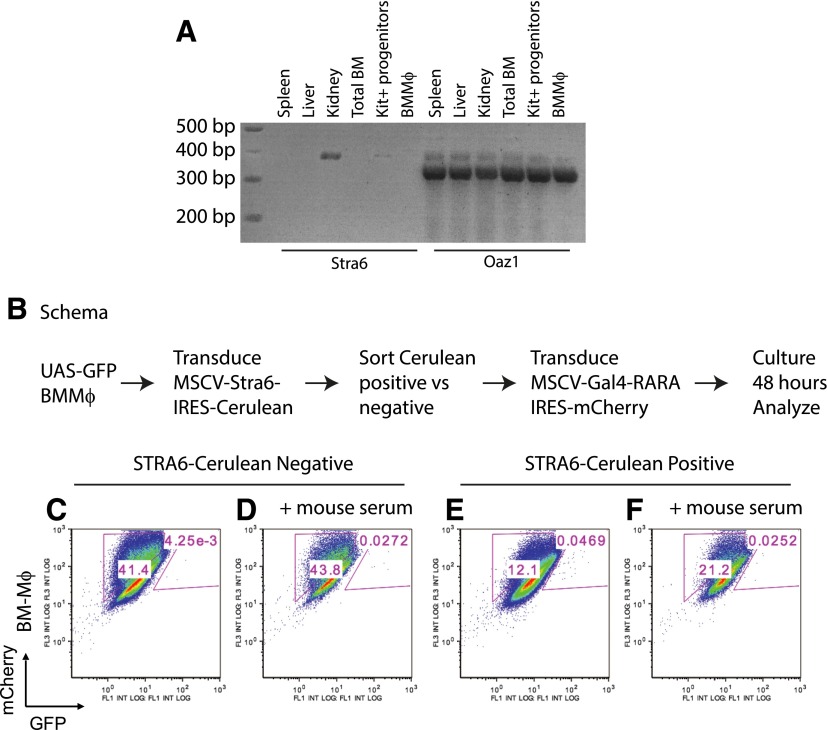
STRA6 has been implicated as an important transporter of retinol/retinol-binding protein 4 complexes [60–62]. Like other groups, we observed that Stra6 could be detected easily in kidney tissue by RT-PCR but was nearly absent in BMMφ (Fig. 7A) [63]. Therefore, we determined whether low expression of this transporter might act as the rate-limiting step for ATRA synthesis in BMMφ. We transduced UAS-GFP BMMφ with MSCV-STRA6-IRES-Cerulean, sorted Cerulean-positive and -negative cells, and then transduced these cells with MSCV-Gal4-RARA-IC (Fig. 7B, schema). We found that simple overexpression of STRA6 was not sufficient to enable intracellular ATRA synthesis using available vitamin A precursors in the FCS (Fig. 7C vs. E). Furthermore, we observed no effect following treatment with additional mouse serum (Fig. 7D vs. F).
Figure 7. Stra6 in BMMφ.
(A) Semiquantitative RT-PCR of Stra6 and Oaz1 in RNA extracted from spleen, liver, kidney, total bone marrow (BM) cells, and Kit+ progenitor cells and in BMMφ. PCR was performed with 50 ng total RNA starting material/well and 34 cycles. Expected fragment size Stra6, 379 bp; Oaz1, 332 bp. (B) Schema of BMMφ transduction and evaluation. (C and D) After transduction with MSCV-STRA6-IRES-Cerulean, Cerulean-negative BMMφ were transduced with Gal4-RARA-IC and treated with or without mouse serum for 48 h before evaluation. (E and F) Cerulean-positive BMMφ were transduced with MSCV-Gal4-RARA-IC and treated with or without mouse serum for 48 h before evaluation. Representative data of 2 independent experiments.
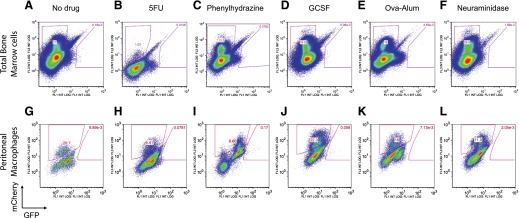
Finally, we evaluated whether hematopoietic stress, in the form of recovery from lineage-restricted stimulation, resulted in activation of necessary pathways for intracellular synthesis of ATRA (or other natural RARA ligands) in hematopoietic cells. We transduced UAS-GFP bone marrow Kit+ cells with MSCV-Gal4-RARA-IC, as before, and transplanted these into lethally irradiated recipient mice. Following engraftment, cohorts of 3 or more mice were treated with the following: 5FU to induce pancytopenia and stem cell expansion; phenylhydrazine to induce hemolytic anemia and erythroid progenitor expansion; G-CSF to induce granulopoiesis, myeloid expansion, and stem cell mobilization; inflammatory triggered granulopoiesis via Ova-alum immunization; or neuraminidase to induce thrombocytopenia and megakaryocyte expansion. We found that none of these hematopoietic stimuli resulted in hematopoietic intracellular ATRA synthesis or synthesis of other RARA ligands (Fig. 8).
Figure 8. Hematopoietic stress does not induce natural RARA ligand synthesis in hematopoietic cells.
UAS-GFP bone marrow cells were transduced with MSCV-Gal4-RARA-IC and transplanted into recipient mice. After engraftment, mice were treated with 5FU, phenylhydrazine, G-CSF, Ova-alum, or neuraminidase. Bone marrow cells and peritoneal macrophages collected after 2 d (neuraminidase), 3 d (phenylhydrazine, G-CSF, and Ova-alum), or 9 d (5FU). Similar results were observed on d 3, 6, and 12 after 5FU. Representative data of at least 3 separately treated recipient mice.
DISCUSSION
RARA is the target of at least 10 fusion proteins that lead to APL [14–16], and RARA expression increases dramatically during myeloid maturation [1]. Many of the X-RARA fusions have been proposed to act by reduced sensitivity to retinoid-dependent differentiation programs (a dominant-negative effect) [64–66]. However, enzymatic pathways required for retinoid synthesis have not been defined in primary hematopoietic cells.
In this study, we found that 2 different enzymatic pathways can be used for the synthesis of ATRA: DEAB-sensitive enzymes (Aldh1a1, Aldh1a2, and Aldh1a3) are principally used in dividing hematopoietic progenitor cells, whereas mature BMMφ use a DEAB-insensitive enzyme. Russo et al. [67] had noted previously that ALDH1A1 inhibition only led to a partial inhibition of retinal-induced maturation of HL60 promyelocytic cells, suggesting that alternative enzymes might exist to metabolize vitamin A derivatives in myeloid cells. We identified Aldh3b1 as an addition enzyme capable of ATRA synthesis in myeloid cells.
The expression of Aldh1a1, Aldh1a2, Aldh1a3, and Aldh3b1 is distinct but not mutually exclusive (Supplemental Figs. 2 and 3). Of note, the effect of diverse ALDH inhibitors on retinoid synthesis in Kit+ progenitor cells was statistically significant but was rarely complete (Fig. 2B), suggesting that these cells used predominantly DEAB-sensitive enzymes in retinoid synthesis, although low activity of DEAB-insensitive enzymes cannot be ruled out, and low-level expression of Aldh3b1 was also noted in these cells (Fig. 3C and D). Several ALDH inhibitors exhibited a small but reproducible effect on RyA in BMMφ, consistent with low but detectable levels of Aldh1a3 in BMMφ (Figs. 2B and 3A).
The RNA expression profile showed that Aldh3b1 is also highly expressed in ex vivo-derived granulocytes and in mature neutrophils (Supplemental Figs. 1B and 2). RARA ligand synthesis from retinal demonstrated an intermediate phenotype, with partial sensitivity to DEAB, consistent with Aldh3b1 expression, and retained expression of Aldh1a1, which we observed (Supplemental Fig. 1).
We observed differential expression of 3 other ALDH enzymes in BMMφ vs. Kit+ progenitor cells (Aldh7a1, Aldh8a1, and Aldh9a1). Whereas it is possible that these enzymes also contribute to ATRA synthesis in BMMφ, several observations counter this possibility. ALDH7A1 and ALDH8A1 are inhibited by compounds to which BMMφ were largely resistant (DEAB, citral, and disulfram) [68, 69]. Aldh7A1 has been implicated in lysine catabolism, and the active site is unlikely to tolerate longer-chain fatty acids, such as retinal, whereas Aldh8a1 has low activity with retinal as a substrate [70]. Consistent with these previous findings, we observed little effect of Aldh7a1, Aldh8a1, or Aldh9a1 when cotransfected into a RARA ligand reporter assay (Fig. 4), collectively suggesting that these enzymes are unlikely to participate in RARA ligand synthesis.
Alternative enzymatic pathways must also be considered. The intracellular RA level is balanced by synthesis and elimination as shown in Fig. 1B, schema. After retinol enters a cell, besides being oxidized by ADHs, it could be esterified by the enzyme lecithin:retinol acyltranferase [46] or diacylglycerol O-acyltransferase 1[71]. However, our RNA profiling and real-time PCR data showed that these genes were not differentially expressed in Kit+ progenitor cells and BMMφ (Supplemental Fig. 4B, and data not shown). Finally, ATRA is known to be eliminated when oxidized by the CYP26 family. We found that Cyp26 was expressed at low levels and was not differentially expressed between the Kit+ progenitor cells and BMMφ in our expression profiling data (Supplemental Fig. 4A).
ALDH enzymes have been implicated previously as the rate-limiting step in ATRA synthesis [25, 26]. However, despite expressing the necessary enzymes required for converting RyA, retinol, or retinal to ATRA, primary hematopoietic cells could not generate RARA ligands from vitamin A precursors available in FCS or mouse serum ex vivo or in vivo in mice fed a vitamin A-sufficient diet (Figs. 1C–F, each column 1, and 6A–D).
In vivo vitamin A is largely protein bound, whereas the RyA, retinol, and retinal that we used to treat cells ex vivo were added as free molecules into the tissue-culture medium. Therefore, the rate-limiting step of intracellular retinoid synthesis may be the expression of retinoid-binding proteins and the channels necessary for the intracellular transport of protein-bound retinol. Under homeostatic conditions in vivo, these pathways are sufficiently inactive (or absent) to result in the absence of intracellular retinoids in diverse hematopoietic cells. Simple overexpression of STRA6 was insufficient to enable ATRA synthesis from serum-available vitamin A precursors in BMMφ ex vivo (Fig. 7), and we could identify no physiologic stress that induced the necessary pathways required for vitamin A transport and subsequent intracellular ATRA synthesis in hematopoietic cells in vivo (Fig. 8).
Further evidence, suggesting that ALDH enzymes are not the rate-limiting step in ATRA synthesis in hematopoietic cells, is obtained by analyzing the expression patterns of ALDH genes in APLs (a leukemia that is sensitive to ATRA). ALDH1A1 and ALDH3B1 are expressed in APLs; we observed low but detectable expression of Aldh3b1 in mouse cathepsin G-PML-RARA leukemias (Affymetrix expression range 234–894; average 557; Supplemental Fig. 2B, column 1) and significant expression in human APLs (RNA-Seq range 5.1–22.29 fpkms; average 12.7; Supplemental Fig. 2D, column 4). Likewise ALDH1A1, ALDH1A2, and ALDH1A3 transcripts are consistently detected in human APL (RNA-Seq range 0.009–4.6 fpkms, average 0.7; range 0–0.7, average 0.16; and range 0–1, average 0.3, respectively; Supplemental Fig. 3G–I). As these leukemias are highly sensitive to ATRA (which causes differentiation and loss of self renewal), the expression of these enzymes further suggests that they are not the rate-limiting step in retinoid synthesis, and alternative mechanisms must be limiting the intracellular availability of vitamin A in hematopoietic cells in vivo.
High levels of ALDH gene expression and activity have been correlated with hematopoietic stem cells [25, 26, 53, 72–75]. Observations that ATRA can augment stem cell function ex vivo [8, 76] and that Aldh1a1, Aldh1a2, and Aldh1a3 are expressed at lower levels in mature BMMφ vs. heterogeneous Kit+ progenitor cell populations might suggest that the stem cell-associated function of ALDH might occur through ATRA synthesis. However, our data suggest that hematopoietic cells (including KLS stem/progenitor cells) exist within a retinoid-deplete environment (Fig. 6A and ref. [30]). Likewise, Aldh3b1 expression is largely restricted to granulocytes and macrophages (Supplemental Fig. 2), yet this does not result in intracellular ATRA synthesis in vivo (Figs. 6 and 8). Therefore, the stem-associated function of ALDH and the granulocyte-associated function of Aldh3b1 are not likely to be through ATRA generation. Alternatively, other groups have suggested that these enzymes may play important roles protecting cells against oxidative stress [77, 78].
In sum, we have found that at least 2 distinct enzymatic pathways may be used in primary hematopoietic cells to synthesize ATRA from vitamin A metabolites. First, mouse bone marrow progenitor cells predominantly use a set of DEAB-sensitive enzymes (Aldh1a1, Aldh1a2, and Aldh1a3), whereas mature macrophages use an alternative, DEAB-insensitive pathway. We have identified Aldh3b1 as a likely candidate and shown that it is capable of ATRA synthesis in tissue culture. Although these enzymes are expressed in primary bone marrow cells, we found that this did not result in the synthesis of active intracellular RARA ligands in diverse hematopoietic cells, unless the cells were exposed to free vitamin A (RyA, retinol, or retinal). This suggests that in hematopoietic cells, the rate-limiting step in retinoid synthesis is likely to involve additional enzymes that facilitate the intracellular transport and release of vitamin A. Finally, even though RARA is dynamically regulated during hematopoietic cell differentiation and involved in diverse fusion oncoproteins, these studies suggest that hematopoietic cells lack intracellular retinoids and that adult hematopoietic RARA functions principally through ligand-independent activity.
AUTHORSHIP
H.N., G.H., H.F., and J.S.W. planned and performed experiments and wrote the manuscript.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) K99/R00 HL103975-03 (to J.S.W.). The Siteman Cancer Center is supported, in part, by a NIH National Cancer Institute Cancer Center Support grant (#P30 CA91842). The authors thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital (St. Louis, MO, USA) for the use of the embryonic stem cell core, which facilitated UAS-GFP embryonic stem cell transfection, selection, and isolation, and for the use of the flow cytometry core. The authors also thank High-Throughput Screening Center at Washington University School of Medicine.
Glossary
- 5FU
5-fluorouracil
- ADH
alcohol dehydrogenase
- ALDH
aldehyde dehydrogenase
- APL
acute promyelocytic leukemia
- ATRA
all-trans retinoic acid
- BMMϕ
bone marrow-derived macrophage
- Cyp26
cytochrome P450 family 26
- DEAB
diethylaminobenzaldehyde
- fpkms
fragments per kilobase of exon per million fragments mapped
- IRES
internal ribosome entry site
- KLS
Kit+Lin−Sca+
- Km
Michaelis constant
- MFI
mean fluorescence intensity
- MS/MS
tandem mass spectrometry
- MSCV
mouse stem cell virus
- MSCV-Gal4-RARA-IC
mouse stem cell virus-Gal4-retinoic acid receptor α-internal ribosome entry site-mCherry
- PML
promyelocytic leukemia
- RAR
retinoic acid receptor
- RARA
retinoic acid receptor α
- RNA-Seq
RNA-sequencing
- RXR
retinoid X receptor
- RXRA
retinoid X receptor α
- RA
retinoic acid
- RyA
retinyl acetate
- Scf
stem cell factor
- siRNA
small interfering RNA
- Stra6
stimulated by retinoic acid 6
- Tpo
thrombopoietin
- UAS
upstream activation sequence (recognized by the Gal4 DNA-binding domain)
- UGN/FAP
293T stable cell line with upstream activation sequence-GFP phosphoglycerate kinase-neomycin and Flag-Gal4-retinoic acid receptor α phosphoglycerate kinase puromycin cassettes stably integrated
- Vmax
maximum velocityYFP - yellow fluorescent protein
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
SEE CORRESPONDING EDITORIAL ON PAGE 791
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Welch J. S., Yuan W., Ley T. J. (2011) PML-RARA can increase hematopoietic self-renewal without causing a myeloproliferative disease in mice. J. Clin. Invest. 121, 1636–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oren T., Sher J. A., Evans T. (2003) Hematopoiesis and retinoids: development and disease. Leuk. Lymphoma 44, 1881–1891. [DOI] [PubMed] [Google Scholar]
- 3.Purton L. E. (2007) Roles of retinoids and retinoic acid receptors in the regulation of hematopoietic stem cell self-renewal and differentiation. PPAR Res. 2007, 87934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans T. (2005) Regulation of hematopoiesis by retinoid signaling. Exp. Hematol. 33, 1055–1061. [DOI] [PubMed] [Google Scholar]
- 5.Mullen E. M., Gu P., Cooney A. J. (2007) Nuclear receptors in regulation of mouse ES cell pluripotency and differentiation. PPAR Res. 2007, 61563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kastner P., Lawrence H. J., Waltzinger C., Ghyselinck N. B., Chambon P., Chan S. (2001) Positive and negative regulation of granulopoiesis by endogenous RARalpha. Blood 97, 1314–1320. [DOI] [PubMed] [Google Scholar]
- 7.Kastner P., Chan S. (2001) Function of RARalpha during the maturation of neutrophils. Oncogene 20, 7178–7185. [DOI] [PubMed] [Google Scholar]
- 8.Purton L. E., Dworkin S., Olsen G. H., Walkley C. R., Fabb S. A., Collins S. J., Chambon P. (2006) RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J. Exp. Med. 203, 1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safi R., Muramoto G. G., Salter A. B., Meadows S., Himburg H., Russell L., Daher P., Doan P., Leibowitz M. D., Chao N. J., McDonnell D. P., Chute J. P. (2009) Pharmacological manipulation of the RAR/RXR signaling pathway maintains the repopulating capacity of hematopoietic stem cells in culture. Mol. Endocrinol. 23, 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewamitta S. R., Joseph C., Purton L. E., Walkley C. R. (2014) Erythroid-extrinsic regulation of normal erythropoiesis by retinoic acid receptors. Br. J. Haematol. 164, 280–285. [DOI] [PubMed] [Google Scholar]
- 11.Rőszer T., Menéndez-Gutiérrez M. P., Cedenilla M., Ricote M. (2013) Retinoid X receptors in macrophage biology. Trends Endocrinol. Metab. 24, 460–468. [DOI] [PubMed] [Google Scholar]
- 12.Roszer T., Menéndez-Gutiérrez M. P., Lefterova M. I., Alameda D., Núñez V., Lazar M. A., Fischer T., Ricote M. (2011) Autoimmune kidney disease and impaired engulfment of apoptotic cells in mice with macrophage peroxisome proliferator-activated receptor gamma or retinoid X receptor alpha deficiency. J. Immunol. 186, 621–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurokawa R., DiRenzo J., Boehm M., Sugarman J., Gloss B., Rosenfeld M. G., Heyman R. A., Glass C. K. (1994) Regulation of retinoid signalling by receptor polarity and allosteric control of ligand binding. Nature 371, 528–531. [DOI] [PubMed] [Google Scholar]
- 14.De Braekeleer E., Douet-Guilbert N., De Braekeleer M. (2014) RARA fusion genes in acute promyelocytic leukemia: a review. Expert Rev. Hematol. 7, 347–357. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y., Li S., Zhou C., Li C., Ru K., Rao Q., Xing H., Tian Z., Tang K., Mi Y., Wang B., Wang M., Wang J. (2014) TBLR1 fuses to retinoid acid receptor α in a variant t(3;17)(q26;q21) translocation of acute promyelocytic leukemia. Blood 124, 936–945. [DOI] [PubMed] [Google Scholar]
- 16.Yin C. C., Jain N., Mehrotra M., Zhagn J., Protopopov A., Zuo Z., Pemmaraju N., DiNardo C., Hirsch-Ginsberg C., Wang S. A., Medeiros L. J., Chin L., Patel K. P., Ravandi F., Futreal A., Bueso-Ramos C. E. (2015) Identification of a novel fusion gene, IRF2BP2-RARA, in acute promyelocytic leukemia. J. Natl. Compr. Canc. Netw. 13, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenk T., Stengel S., Zelent A. (2014) Unlocking the potential of retinoic acid in anticancer therapy. Br. J. Cancer 111, 2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giguere V., Ong E. S., Segui P., Evans R. M. (1987) Identification of a receptor for the morphogen retinoic acid. Nature 330, 624–629. [DOI] [PubMed] [Google Scholar]
- 19.Guo Y., Brown C., Ortiz C., Noelle R. J. (2015) Leukocyte homing, fate, and function are controlled by retinoic acid. Physiol. Rev. 95, 125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchitti S. A., Brocker C., Stagos D., Vasiliou V. (2008) Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin. Drug Metab. Toxicol. 4, 697–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Penzes P., Napoli J. L. (1996) Cloning of a cDNA encoding an aldehyde dehydrogenase and its expression in Escherichia coli. Recognition of retinal as substrate. J. Biol. Chem. 271, 16288–16293. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida A., Hsu L. C., Davé V. (1992) Retinal oxidation activity and biological role of human cytosolic aldehyde dehydrogenase. Enzyme 46, 239–244. [DOI] [PubMed] [Google Scholar]
- 23.Niederreither K., Subbarayan V., Dollé P., Chambon P. (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 21, 444–448. [DOI] [PubMed] [Google Scholar]
- 24.Graham C. E., Brocklehurst K., Pickersgill R. W., Warren M. J. (2006) Characterization of retinaldehyde dehydrogenase 3. Biochem. J. 394, 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duester G., Mic F. A., Molotkov A. (2003) Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem. Biol. Interact. 143-144, 201–210. [DOI] [PubMed] [Google Scholar]
- 26.Molotkov A., Duester G. (2003) Genetic evidence that retinaldehyde dehydrogenase Raldh1 (Aldh1a1) functions downstream of alcohol dehydrogenase Adh1 in metabolism of retinol to retinoic acid. J. Biol. Chem. 278, 36085–36090. [DOI] [PubMed] [Google Scholar]
- 27.Ross A. C., Zolfaghari R. (2011) Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu. Rev. Nutr. 31, 65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan C. A., Parajuli B., Buchman C. D., Dria K., Hurley T. D. (2015) N,N-diethylaminobenzaldehyde (DEAB) as a substrate and mechanism-based inhibitor for human ALDH isoenzymes. Chem. Biol. Interact. 234, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold S. L., Kent T., Hogarth C. A., Schlatt S., Prasad B., Haenisch M., Walsh T., Muller C. H., Griswold M. D., Amory J. K., Isoherranen N. (2015) Importance of ALDH1A enzymes in determining human testicular retinoic acid concentrations. J. Lipid Res. 56, 342–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu H., Chacko J., Hadwiger G., Welch J. S. (2015) Absence of natural intracellular retinoids in mouse bone marrow cells and implications for PML-RARA transformation. Blood Cancer J. 5, e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi J. S., Desai-Yajnik V., Greene M. E., Raaka B. M., Samuels H. H. (1995) The ligand-binding domains of the thyroid hormone/retinoid receptor gene subfamily function in vivo to mediate heterodimerization, gene silencing, and transactivation. Mol. Cell. Biol. 15, 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeshita S., Kaji K., Kudo A. (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 15, 1477–1488. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura T., Naganuma T., Abe K., Nakahara K., Ohno Y., Kihara A. (2013) Substrate specificity, plasma membrane localization, and lipid modification of the aldehyde dehydrogenase ALDH3B1. Biochim. Biophys. Acta 1831, 1395–1401. [DOI] [PubMed] [Google Scholar]
- 34.Schuettpelz L. G., Borgerding J. N., Christopher M. J., Gopalan P. K., Romine M. P., Herman A. C., Woloszynek J. R., Greenbaum A. M., Link D. C. (2014) G-CSF regulates hematopoietic stem cell activity, in part, through activation of Toll-like receptor signaling. Leukemia 28, 1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenbaum A. M., Link D. C. (2011) Mechanisms of G-CSF-mediated hematopoietic stem and progenitor mobilization. Leukemia 25, 211–217. [DOI] [PubMed] [Google Scholar]
- 36.Vannucchi A. M., Bianchi L., Cellai C., Paoletti F., Carrai V., Calzolari A., Centurione L., Lorenzini R., Carta C., Alfani E., Sanchez M., Migliaccio G., Migliaccio A. R. (2001) Accentuated response to phenylhydrazine and erythropoietin in mice genetically impaired for their GATA-1 expression (GATA-1(low) mice). Blood 97, 3040–3050. [DOI] [PubMed] [Google Scholar]
- 37.Spivak J. L., Toretti D., Dickerman H. W. (1973) Effect of phenylhydrazine-induced hemolytic anemia on nuclear RNA polymerase activity of the mouse spleen. Blood 42, 257–266. [PubMed] [Google Scholar]
- 38.Stenberg P. E., Levin J., Baker G., Mok Y., Corash L. (1991) Neuraminidase-induced thrombocytopenia in mice: effects on thrombopoiesis. J. Cell. Physiol. 147, 7–16. [DOI] [PubMed] [Google Scholar]
- 39.Li X., Slayton W.B. (2013) Molecular mechanisms of platelet and stem cell rebound after 5-fluorouracil treatment. Exp. Hematol. 41, 635–645.e3. [DOI] [PubMed] [Google Scholar]
- 40.Donowitz G. R., Quesenberry P. (1986) 5-Fluorouracil effect on cultured murine stem cell progeny and peripheral leukocytes. Exp. Hematol. 14, 207–214. [PubMed] [Google Scholar]
- 41.Ueda Y., Yang K., Foster S. J., Kondo M., Kelsoe G. (2004) Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J. Exp. Med. 199, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cain D. W., Snowden P. B., Sempowski G. D., Kelsoe G. (2011) Inflammation triggers emergency granulopoiesis through a density-dependent feedback mechanism. PLoS One 6, e19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G. G., Calvo K. R., Pasillas M. P., Sykes D. B., Häcker H., Kamps M. P. (2006) Quantitative production of macrophages or neutrophils ex vivo using conditional Hoxb8. Nat. Methods 3, 287–293. [DOI] [PubMed] [Google Scholar]
- 44.Welch J. S., Klco J. M., Varghese N., Nagarajan R., Ley T. J. (2011) Rara haploinsufficiency modestly influences the phenotype of acute promyelocytic leukemia in mice. Blood 117, 2460–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furr H. C. (2004) Analysis of retinoids and carotenoids: problems resolved and unsolved. J. Nutr. 134, 281S–285S. [DOI] [PubMed] [Google Scholar]
- 46.Clagett-Dame M., Knutson D. (2011) Vitamin A in reproduction and development. Nutrients 3, 385–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckhoff C., Collins M. D., Nau H. (1991) Human plasma all-trans-, 13-cis- and 13-cis-4-oxoretinoic acid profiles during subchronic vitamin A supplementation: comparison to retinol and retinyl ester plasma levels. J. Nutr. 121, 1016–1025. [DOI] [PubMed] [Google Scholar]
- 48.Van Bennekum A. M., Wei S., Gamble M. V., Vogel S., Piantedosi R., Gottesman M., Episkopou V., Blaner W. S., Blaner W. S. (2001) Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J. Biol. Chem. 276, 1107–1113. [DOI] [PubMed] [Google Scholar]
- 49.Liu L., Gudas L. J. (2005) Disruption of the lecithin:retinol acyltransferase gene makes mice more susceptible to vitamin A deficiency. J. Biol. Chem. 280, 40226–40234. [DOI] [PubMed] [Google Scholar]
- 50.Underwood B. A., Loerch J. D., Lewis K. C. (1979) Effects of dietary vitamin A deficiency, retinoic acid and protein quantity and quality on serially obtained plasma and liver levels of vitamin A in rats. J. Nutr. 109, 796–806. [DOI] [PubMed] [Google Scholar]
- 51.Kane M. A., Folias A. E., Napoli J. L. (2008) HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal. Biochem. 378, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreb J. S., Ucar D., Han S., Amory J. K., Goldstein A. S., Ostmark B., Chang L. J. (2012) The enzymatic activity of human aldehyde dehydrogenases 1A2 and 2 (ALDH1A2 and ALDH2) is detected by Aldefluor, inhibited by diethylaminobenzaldehyde and has significant effects on cell proliferation and drug resistance. Chem. Biol. Interact. 195, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma A. C., Chung M. I., Liang R., Leung A. Y. (2010) A DEAB-sensitive aldehyde dehydrogenase regulates hematopoietic stem and progenitor cells development during primitive hematopoiesis in zebrafish embryos. Leukemia 24, 2090–2099. [DOI] [PubMed] [Google Scholar]
- 54.Marchitti S. A., Orlicky D. J., Vasiliou V. (2007) Expression and initial characterization of human ALDH3B1. Biochem. Biophys. Res. Commun. 356, 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marchitti S. A., Orlicky D. J., Brocker C., Vasiliou V. (2010) Aldehyde dehydrogenase 3B1 (ALDH3B1): immunohistochemical tissue distribution and cellular-specific localization in normal and cancerous human tissues. J. Histochem. Cytochem. 58, 765–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. (1993) Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc. Natl. Acad. Sci. USA 90, 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wartman L. D., Larson D. E., Xiang Z., Ding L., Chen K., Lin L., Cahan P., Klco J. M., Welch J. S., Li C., Payton J. E., Uy G. L., Varghese N., Ries R. E., Hoock M., Koboldt D. C., McLellan M. D., Schmidt H., Fulton R. S., Abbott R. M., Cook L., McGrath S. D., Fan X., Dukes A. F., Vickery T., Kalicki J., Lamprecht T. L., Graubert T. A., Tomasson M. H., Mardis E. R., Wilson R. K., Ley T. J. (2011) Sequencing a mouse acute promyelocytic leukemia genome reveals genetic events relevant for disease progression. J. Clin. Invest. 121, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cancer Genome Atlas Research Network (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 368, 2059–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Payton J. E., Grieselhuber N. R., Chang L. W., Murakami M., Geiss G. K., Link D. C., Nagarajan R., Watson M. A., Ley T. J. (2009) High throughput digital quantification of mRNA abundance in primary human acute myeloid leukemia samples. J. Clin. Invest. 119, 1714–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amengual J., Zhang N., Kemerer M., Maeda T., Palczewski K., Von Lintig J. (2014) STRA6 is critical for cellular vitamin A uptake and homeostasis. Hum. Mol. Genet. 23, 5402–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muenzner M., Tuvia N., Deutschmann C., Witte N., Tolkachov A., Valai A., Henze A., Sander L. E., Raila J., Schupp M. (2013) Retinol-binding protein 4 and its membrane receptor STRA6 control adipogenesis by regulating cellular retinoid homeostasis and retinoic acid receptor α activity. Mol. Cell. Biol. 33, 4068–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amengual J., Golczak M., Palczewski K., von Lintig J. (2012) Lecithin:retinol acyltransferase is critical for cellular uptake of vitamin A from serum retinol-binding protein. J. Biol. Chem. 287, 24216–24227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norseen J., Hosooka T., Hammarstedt A., Yore M. M., Kant S., Aryal P., Kiernan U. A., Phillips D. A., Maruyama H., Kraus B. J., Usheva A., Davis R. J., Smith U., Kahn B. B. (2012) Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and Toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell. Biol. 32, 2010–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsai S., Bartelmez S., Sitnicka E., Collins S. (1994) Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 8, 2831–2841. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y. A., Shen K., Ishida Y., Wang Y., Kakizuka A., Brooks S. C. (2005) Induction of murine leukemia and lymphoma by dominant negative retinoic acid receptor alpha. Mol. Carcinog. 44, 252–261. [DOI] [PubMed] [Google Scholar]
- 66.Du C., Redner R. L., Cooke M. P., Lavau C. (1999) Overexpression of wild-type retinoic acid receptor alpha (RARalpha) recapitulates retinoic acid-sensitive transformation of primary myeloid progenitors by acute promyelocytic leukemia RARalpha-fusion genes. Blood 94, 793–802. [PubMed] [Google Scholar]
- 67.Russo J., Barnes A., Berger K., Desgrosellier J., Henderson J., Kanters A., Merkov L. (2002) 4-(N,N-Dipropylamino)benzaldehyde inhibits the oxidation of all-trans retinal to all-trans retinoic acid by ALDH1A1, but not the differentiation of HL-60 promyelocytic leukemia cells exposed to all-trans retinal. BMC Pharmacol. 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo M., Gates K. S., Henzl M. T., Tanner J. J. (2015) Diethylaminobenzaldehyde is a covalent, irreversible inactivator of ALDH7A1. ACS Chem. Biol. 10, 693–697. [DOI] [PubMed] [Google Scholar]
- 69.Amico D., Barbui A. M., Erba E., Rambaldi A., Introna M., Golay J. (2003) Differential response of human acute myeloid leukemia cells to gemtuzumab ozogamicin in vitro: role of Chk1 and Chk2 phosphorylation and caspase 3. Blood 101, 4589–4597. [DOI] [PubMed] [Google Scholar]
- 70.Lin M., Zhang M., Abraham M., Smith S. M., Napoli J. L. (2003) Mouse retinal dehydrogenase 4 (RALDH4), molecular cloning, cellular expression, and activity in 9-cis-retinoic acid biosynthesis in intact cells. J. Biol. Chem. 278, 9856–9861. [DOI] [PubMed] [Google Scholar]
- 71.Shih M. Y., Kane M. A., Zhou P., Yen C. L., Streeper R. S., Napoli J. L., Farese R. V. Jr. (2009) Retinol esterification by DGAT1 is essential for retinoid homeostasis in murine skin. J. Biol. Chem. 284, 4292–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Storms R. W., Trujillo A. P., Springer J. B., Shah L., Colvin O. M., Ludeman S. M., Smith C. (1999) Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc. Natl. Acad. Sci. USA 96, 9118–9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muzio G., Maggiora M., Paiuzzi E., Oraldi M., Canuto R. A. (2012) Aldehyde dehydrogenases and cell proliferation. Free Radic. Biol. Med. 52, 735–746. [DOI] [PubMed] [Google Scholar]
- 74.Ginestier C., Hur M. H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., Jacquemier J., Viens P., Kleer C. G., Liu S., Schott A., Hayes D., Birnbaum D., Wicha M. S., Dontu G. (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chute J. P., Muramoto G. G., Whitesides J., Colvin M., Safi R., Chao N. J., McDonnell D. P. (2006) Inhibition of aldehyde dehydrogenase and retinoid signaling induces the expansion of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 103, 11707–11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Purton L. E., Bernstein I. D., Collins S. J. (2000) All-trans retinoic acid enhances the long-term repopulating activity of cultured hematopoietic stem cells. Blood 95, 470–477. [PubMed] [Google Scholar]
- 77.Marchitti S. A., Brocker C., Orlicky D. J., Vasiliou V. (2010) Molecular characterization, expression analysis, and role of ALDH3B1 in the cellular protection against oxidative stress. Free Radic. Biol. Med. 49, 1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makia N. L., Bojang P., Falkner K. C., Conklin D. J., Prough R. A. (2011) Murine hepatic aldehyde dehydrogenase 1a1 is a major contributor to oxidation of aldehydes formed by lipid peroxidation. Chem. Biol. Interact. 191, 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.