Abstract
Patients with hormone-refractory prostate cancer often have multiple bone metastases. The resulting bone pain is associated with reduced life quality, increased cost of therapy and impairment of overall survival. Trials with bone-targeting β-emitters have mostly showed an effect on alleviation of bone pain along with prolongation in survival, documented in only a limited number of patients. A randomized phase III trial (ALSYMPCA) using the α-emitter 223RaCl2 (Xofigo®) showed for the first time, a longer overall survival of 3.6 months in treated patients as a sign of an antitumor effect. The time to first skeletal-related events was also significantly longer in the therapy group compared with placebo. Because of the short range of α-emitter, the bone marrow toxicity of radium therapy is low, and so this radionuclide could also be a candidate for combination with chemotherapy. The elimination of 223RaCl2 is mainly through the gastrointestinal tract and side effects are mainly in this area. The procedure is similar to treatment with other bone-seeking agents and consists of six administrations of 50 kBq/kg bodyweight Xofigo®, repeated every 4 weeks. At present Xofigo® is only approved for hormone-refractory prostate cancer.
Keywords: bone metastases, prolongation of survival, prostate cancer, 223RaCl2
Introduction
Skeletal metastases occur in many patients with advanced prostate cancer. Resulting bone pain interferes with the patient’s quality of life and requires effective treatment. Patients with bone metastases commonly endure severe bone pain and this symptom has the biggest impact on quality of life. Though the mechanisms involved in bone pain are poorly understood [Mantyh et al. 2002], one of the important physical factors contributing to pain is thought to be osteolysis (bone breakdown) [Mundy, 2002], especially with infiltration of the bone trabeculae and matrix by tumor osteolysis. Other factors include microfractures and stretching of the periosteum by tumor growth [Serafini, 1994]. Biochemical mechanisms of pain include the stimulation of nerve endings in the endosteum by a variety of chemical mediators, such as bradykinin, prostaglandin, histamine, interleukin and tumor necrosis factor produced by the osteolytic process [Nielsen et al. 1991; Rabbani et al. 1999].
The clinical course for most prostate cancer patients is not very aggressive, even with the presence of multiple skeletal metastases, and there are numerous treatment options currently available to them. Most of them live a long time with their disease and thus, are often suitable candidates for palliative treatment using bone-seeking radionuclide agents. Recent evidence also suggests that their use can result in a prolongation of survival time in patients with multiple bone metastases. In prostate cancer, the balance between resorption and mineralization is impaired, resulting in the overall formation of osteoblastic lesions [Keller et al. 2001], but the resorption by osteoclasts is not completely lost. Thus, increased systemic markers of both bone formation and resorption have been observed in patients with prostate cancer [Scher and Yagoda, 1987]. Patients with bone metastases from prostate cancer are the ideal candidates for therapy with bone-seeking radionuclide agents due to increased bone turnover by the osteoblastic process.
In the treatment of prostate cancer, hormone therapy [or androgen-deprivation therapy (ADT)] is essential. Unfortunately, as prostate cancer advances, it becomes hormone insensitive or castration resistant. At this stage, uncontrolled metastatic bone pain is one of the main symptoms and different strategies are employed to palliate this problem. First-line treatment is analgesic therapy as recommended by the three-step approach postulated by the World Health Organization. The first step for mild to moderate pain includes nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g. aspirin, ibuprofen and naproxen). If the pain persists or increases, a weak opioid (e.g. codeine or naproxen) is added. For persistent or more severe pain, more potent or higher doses of strong opioids are used in step three (morphine, hydromorphone or fentanyl). The efficacy may be improved by concurrent administration of tricyclic antidepressive drugs or phenothiazine [World Health Organization, 1990]. However, strong opioids are associated with nausea, vomiting and constipation, occurring in more than 50% of patients using oral morphine, though these effects are usually treatable. Hallucination and confusion are unusual, but elderly patients may be at an increased risk for these side effects [Portenoy et al. 1994].
Consequently, use of intravenous bisphosphonates to reduce bone loss and prevent skeletal complications has become essential in advanced prostate cancer. Bisphosphonates represent analogues of endogenous pyrophosphates [Lipton, 1997] and induce apoptosis of osteoclasts [Shipman et al. 1997]. The differentiation of the osteoclastic precursor to mature osteoclasts is also inhibited by bisphosphonates [Lowik et al. 1988]. Earlier generations of bisphosphonates (etidronate and clodronate) showed only transient and nonstatistically significant pain relief in placebo-controlled studies [Ernst et al. 2003]. Pamidronate and zoledronic acid are second- and third-generation nitrogen-containing bisphosphonate formulations approved for use in bone metastases [Paes and Serafini, 2010]. They have both demonstrated the ability to reduce skeletal complications and morbidity in patients with cancer [Berenson et al. 2001]. Reports have suggested that RANKL inhibitor, denosumab, significantly reduces the risk of developing first symptomatic skeletal-related events compared with zoledronic acid (20.7 versus 17.1 months) [Smith et al. 2015; Todenhöfer et al. 2015].
Extensive clinical evidence has established bisphosphonates as useful agents for treating bone metastasis associated with breast cancer [Powles et al. 2002]. There is less evidence demonstrating the therapeutic efficacy of bisphosphonates in metastatic prostate cancer, with some trials suggesting no effects from treatment [Mason et al. 2007] and others indicating only a reduction in bone pain [Heidenreich et al. 2002; Weinfurt et al. 2006]. There are also some new nonbisphosphonate candidates for the treatment of bone resorption. Clohisy and colleagues and Morony and colleagues identified osteoprotegerin and its ligands as naturally occurring proteins that inhibit and stimulate osteoclast formation [Clohisy et al. 2000; Morony et al. 2001]. In animal models, osteoprotegerin can inhibit bone lesions caused by a murine adenocarcinoma cell line (C26-DCT). Parathyroid hormone-related proteins (PTHrP) may play an important role in the establishment of osteolytic lesions [Guise et al. 1996; 2002], and their antagonists could be a candidate for therapy. Other interesting agents include endothelin-receptor antagonists, for example, atrasentan. In a placebo-controlled trial, 288 patients with bone metastases from prostate cancer were treated with 2.5 or 10 mg of atrasentan compared with placebo. Atrasentan (10 mg) not only reduces the rise in bone alkaline phosphatase significantly, that is a marker of bone formation, but also reduces the markers of bone resorption compared with placebo [Nelson et al. 2003].
Indications for radiotherapy in bone metastases include pain, risk for pathological fracture and neurological complications arising from spinal cord compression, nerve root pain, or cranial involvement [Janjan, 2006]. About 20% of all radiotherapies are performed for painful bone metastases [Agarawal et al. 2006]. Meta-analysis data have established that more than 40% of treated patients can expect at least 50% pain relief, and fewer than 30% can expect complete pain relief at 1 month [Saarto et al. 2002]. Numerous external beam radiotherapy regimes may be employed in the management of bone pain, included fractionated schedules and single-fraction regimes [Paes and Serafini, 2010].
Single-fraction therapy is perhaps the most useful form of radiotherapy because of its relative convenience for the patient and radiotherapy unit, as well as its lower cost. This treatment can be used for patients with metastases on nonweight-bearing bones, such as the clavicle and the ribs, or on weight-bearing bones in the absence of large lytic lesions, as well as in patients with terminal disease. The recommended dose is 6–8 Gy [Steenland et al. 1999]. Multiple-fraction radiotherapy can be used in patients with lytic lesions in weight-bearing bones or vertebral metastases causing spinal cord or nerve root compression and in the post-surgical treatment of pathological fractures. This strategy allows higher doses of radiation to be administered, leading to a greater reduction in tumor size and longer delay in tumor growth. Commonly used regimes include 30 Gy in ten fractions or 20 Gy in five fractions [Steenland et al. 1999]. Controversy exists regarding whether single- or multiple-fraction radiotherapy is superior [Gaze et al. 1997] with some trials indicating that single-fraction therapy could be as effective (both in pain relief and duration of response) as multiple-fraction therapy [Nielsen et al. 1998; Uppelschoten et al. 1995]. Some trials have demonstrated that higher doses of radiation were more effective, particularly for patients with a relatively good prognosis [Arcangeli et al. 1998; Ratanatharathorn et al. 1999]. Others revealed no differences between the two schedules [Kuban et al. 1989].
Radium-223 dichloride
Xofigo® (Bayer Pharma, Berlin, Germany) is a solution of Radium-223 dichloride (223RaCl2) for intravenous administration in patients with castration-resistant prostate cancer (CRPC) and metastatic bone pain. As an alkaline earth metal, 223RaCl2 mimics the calcium uptake in whole bone and is termed a volume seeker [Liepe, 2009]. In contrast, the radioactive-labelled bisphosphonates are termed surface seekers [Liepe and Kotzerke, 2011]. The α-emitters have a high linear energy transfer and result in a high incidence of DNA double-strand breaks, which lead to a strong tumor-cell-killing effect [Henriksen et al. 2002]. The short track length of 2–10 cell diameters minimizes the cell-damage effect in surrounding healthy tissue, but also reduces the tumor-killing effect to a very short distance in the bone.
Pharmacokinetics and dosimetry
223RaCl2 has a physical half-life of 11.4 days. It undergoes a six-step decay to produce 207lead via a series of α-, β- and γ-emitting daughters (94% as α-emitter, 4% as β-emitter and 2% as γ-emission) [Bruland et al. 2006]. Pharmacokinetic studies in 10 patients with applied activities from 50 to 200 kBq of 223RaCl2 documented a fast clearance from the vascular compartment, with only 14% (range 9–34%), 2% (range 1.3–3.9%), and 0.5% (range 0.4–1.0%) remaining in the plasma immediately, at 4 hours and 24 hours after administration, respectively [Carrasquillo et al. 2013]. In contrast to radiolabeled diphosphonates or 89strontium (89Sr), the excretion is mainly through the intestine (13%ID) and a significantly lower percentage via urine (2%ID). Most of the administered activity was taken up rapidly into bone with greater than 60% of administered activity incorporated by 4 hours [Chittenden et al. 2015].
The extremely low range of α-emitters led to a heterogeneous distribution of cellular-absorbed dose, which is strongly dependent on the distance from the fixed 223RaCl2 in the normal bone structures to the bone metastases. The main radiation to the bone marrow comes from the fixed 223RaCl2 in the trabecular system that is adjacent to the red marrow cells. Thus, the uptake in cortical bone does not contribute significantly to marrow toxicity. Considering this point, the trabecular model was used for calculation of the red marrow-absorbed dose in all patients receiving this therapy. This model assumes two scenarios: that the radiopharmaceutical is in the bone marrow matrix that leads to a lower radiation-absorbed dose; or that the localization is in the endosteal layer that in turn leads to a significantly higher dose. Results from trabecular models differ markedly from standard absorbed-fraction methods. Results suggest that increasing the amount of radioactivity may not substantially increase the risk of marrow toxicity, in contrast to the absorbed-fraction method of dose calculation for a β-emitter [Hobbs et al. 2012]. After a treatment schedule of six administrations with 0.05 MBq/kg bodyweight of 223RaCl2, the absorbed alpha dose to the bone endosteal cells is about 16 Gy and the corresponding absorbed dose to red bone marrow is approximately 1.5 Gy [Lassmann and Nosske, 2013]. Dosimetric studies of bone metastases showed an absorbed dose of 0.7 Gy (range 0.2–1.9 Gy) after first administration of 50 kBq/kg bodyweight of 223RaCl2. It is to be noted that when using 223RaCl2, what could be more important is the relative biological effectiveness (RBE) of α-emitters (RBE = 5), which is approximately 899 mGy/MBq (range 340–2450 mGy/MBq). Thus the calculated dose of relative biological effectiveness (DRBE) for six cycles of 223RaCl2 is approximately 18.9 Gy, which is comparable with DRBE of 10.4 Gy when using samarium-153-EDTMP (153SmEDTMP) or 34.0 Gy using 89SrCl2 [Pacilio et al. 2016].
Clinical studies
Authors of three phase I studies investigated the biodistribution, dosimetry and safety of 223RaCl2 in humans [Nilsson et al. 2004, 2005; Nielsen et al. 2010]. The main goal of the ATI-BC-1 study was to estimate the safety and tolerability with dose escalation of 223RaCl2 in breast and prostate cancer patients. Patients (n = 31) were given a single administration of 46, 93, 163, 213 or 250 kBq/kg bodyweight of 223RaCl2. Even in the high-activity group, no dose-limiting hematologic toxicity was documented. Thrombocytopenia was minor with only grade I toxicity noted, but grade III neutropenia and leukopenia occurred in two and three patients, respectively, with a nadir between the second and fourth week. Nausea and vomiting were more frequently observed in the highest-dosage group induced by nonspecific uptake or excretion of 223RaCl2 in the gut. Rapid blood clearance was documented by a decrease of blood radioactivity from 12% of the administered dose (%ID: percentage injected dose) at 10 min, to 6%ID at 1 hour and to <1%ID at 24 hours in a time period of 48 hours [Nilsson et al. 2005]. The main goal of the two phase II studies was to investigate the possibility of repeated administrations (BC1-02 and BC1-04). In BC1-02, patients with CRPC were assigned to four injections of 50 kBq/kg 223RaCl2 (n = 33) versus placebo (n = 31). Primary endpoints were change in alkaline phosphate (ALP) level and time to first skeletal-related event (SRE). Median relative change in bone ALP from baseline was 66% decrease after 223RaCl2 versus only 9% after placebo 4 weeks after last administration. In addition, a prolonged overall survival (OS) was observed in the 223RaCl2 group (65.3 weeks) versus placebo (46.4 weeks) (p = 0.066) [Nilsson et al. 2007]. Parker and colleagues used dose escalation with three injections of 25 kBq/kg (n = 41), 50 kBq/kg (n = 39), or 80 kBq/kg (n = 42) in 122 patients with CRPC. Slightly extended survival was observed in the groups receiving 50 and 80 kBq/kg, but not in patients receiving 25 kBq/kg of 223RaCl2. [Parker et al. 2013a]. Nilsson and colleagues investigated the effect of single dose of 5, 25, 50, or 100 kBq/kg in 100 CRPC patients. Pain relief in all patients was documented, but a significant decrease of ALP after 4 weeks was noted only with 50 or 100 kBq/kg of 223RaCl2. [Nilsson et al. 2012].
In summary, the phase I and II studies with more than 300 patients demonstrated that multiple administrations with doses up to 100 kBq/kg, or single administration up to 250 kBq/kg of 223RaCl2 was well tolerated. Patients showed pain relief, extended survival and decrease of biochemical markers [ALP and prostate-specific antigen (PSA)] within 12 months after therapy.
ALSYMPCA trial
In this double-blind randomized phase III study (2008–2011) 921 CRPC patients were enrolled. Inclusion criteria included bone pain and at least two bone metastases, without metastases outside the skeleton [Parker et al. 2013a; Sartor et al. 2014]. The study design consisted of six administrations of 50 kBq/kg 223RaCl2 with an interval of 4 weeks between injections. Patients were stratified by the pretherapeutic ALP level (<220 U/l versus ⩾220 U/l) and pretherapeutic bisphosphonate or docetaxel therapy. They were randomly assigned in a 2:1 ratio to the radium or placebo group. That is the first study using bone-seeking agents in bone metastases that used the OS as the primary endpoint, and a sufficient number of treated patients for statistical evaluation. Secondary endpoints were time to first SRE, time to PSA or ALP progress, decrease of ALP after therapy, patient safety using the rate of side effects and the influence on quality of life. In summary, 614 patients in the 223RaCl2 group and 307 patients in the placebo group were evaluated. In the therapy group only 63% of patients were given six administrations of 50 kBq/kg 223RaCl2, and in the placebo group 47% had six administrations of saline injection.
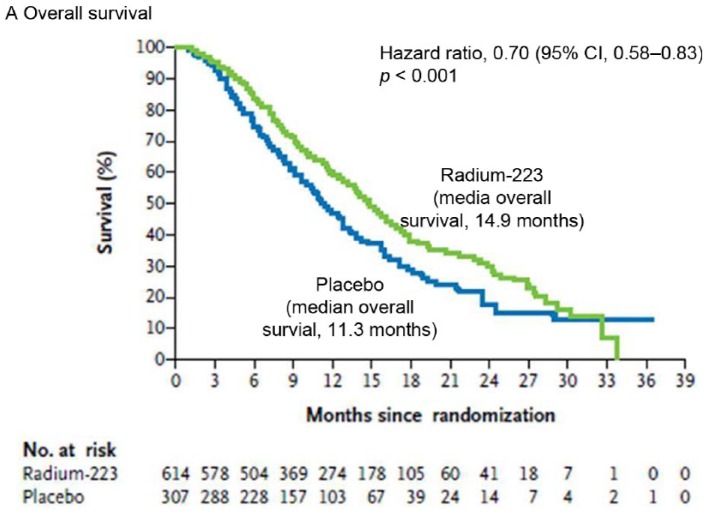
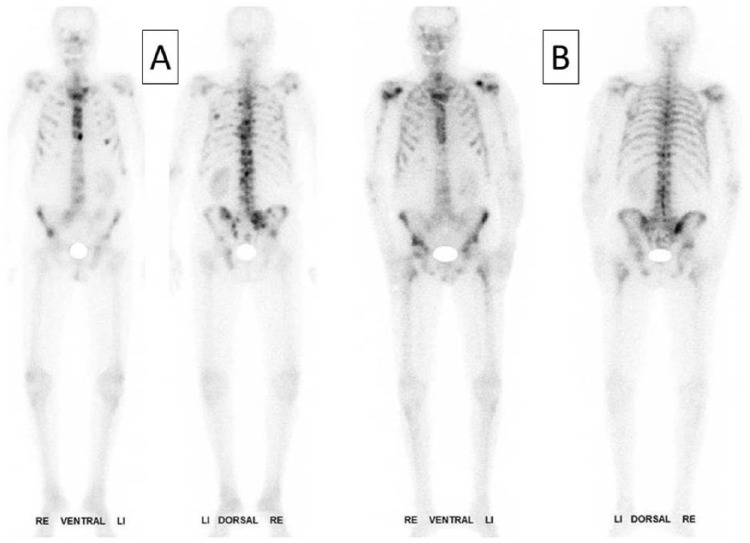
In the ALSYMPCA study, the 223RaCl2 arm had an OS of 14.9 months compared with the placebo arm that had 11.3 months (Figure 1). The authors concluded that 223RaCl2 could extend the OS by 3.6 months in CRPC patients. Patients with widespread bone metastases (ALP level ⩾ 220 U/l) had a more significant prolongation in OS compared with patients having more limited bone metastases (ALP level < 220 U/l). The pretherapeutic opioid, bisphosphonate or docetaxel therapy didn’t have any influence on the therapeutic effect or rate of side effects, except for a slightly higher rate of thrombocytopenia after docetaxel pretherapy [Hoskin et al. 2014]. Significant pain relief was documented up to16 weeks after therapy [Nilsson et al. 2013]. In a single case, a decrease in bone turnover was noted on the follow-up scan 6 months after therapy (Figure 2).
Figure 1.
Time course of overall survival in the ALSYMPCA trial with a prolonged survival of approximately of 3.6 months in therapy group (green upper curve) compared with placebo (blue lower curve) [Parker et al. 2013a].
CI, confidence interval.
Figure 2.
72-year-old man with multiple skeletal metastases and severe pain symptoms (VAS of 5 documented by a 10-step visual analogue scale): (A) pretherapeutic 99mTc-HMDP bone scan with multiple bone metastases in spine, ribs, pelvis, sternum and both proximal femuri and humeri; (B) 99mTc-HMDP bone scan 3 months after four times 223Ra administration with pain relief, significant reduction of bone turnover in the spine, ribs and pelvis (VAS 1).
Radiation protection
Comparing α-emitters with β-radionuclides, problems with radiation exposure for staff are limited. However, there are some problems with the daughters of 223RaCl2. The second step of the decay produces 219radon with a physical T½ of 3.96 seconds that is exhaled by patients; there is a fast decrease in the blood activity within the first hour itself. This leads to a limited radiation exposure of <0.01 mSv, mainly through personal communication. To reduce the radiation exposure from low activity of 219radon in the exhaled air the staff should keep certain distance from patients within the first hour post administration. The last decay from 207thallium to 207lead emits beta rays, which leads to a radiation-exposure dose rate of 1.8 mSv/h, using 6 MBq of 223RaCl2 on the surface of an unshielded syringe (Federal Office for Radiation Protection, 2015). Use of 5 mm perspex shielding around the syringe can limit this radiation exposure significantly. In contrast, the deep-dose equivalent is only 0.3 µSv/h per MBq of 223RaCl2, which is equivalent to a dose rate of 0.03 µsv/minute [Rimpler, 2015].
The multicenter RHAPSODY study [Wanke et al. 2014] was designed to estimate the radiation dose to relatives and caregivers during outpatient therapy with 223RaCl2 in Germany. In this study, the ambient external radiation exposure was measured using standard dose-rate meters at a distance of 1 and 2 m. Excreted Ra-223 was measured in saliva using swabs, and in sweat using skin patches. Wipe tests were taken in the patients’ homes in the restroom and kitchen to identify significant contamination. Preliminary results showed measurable amounts of 223RaCl2 in the range of 10–100 Bq/g in saliva. The skin patch measurements indicated perspiration activity of about 0.02–0.5 Bq/cm2 in the first 24 hours post injection (PI). Contamination in restrooms and kitchens was found to be less than 0.05 Bq/cm2. However, significant amounts of 219Radon were exhaled immediately after injection. Concentrations of more than 2 kBq/l of 219Radon were measured in exhaled air. The authors concluded that the external exposure from the patients can be neglected and it is unlikely that Ra-223 excreted in sweat and saliva or contaminations in the patients’ homes would lead to effective doses exceeding 1 mSv per year for relatives [Liepe, 2015].
Short-term side effects
223RaCl2 in bone pain palliation is well tolerated with a low rate of side effects, especially bone marrow toxicity. The main side effects reported by patients are gastrointestinal, due to the intestinal route of excretion. The most common complaints were nausea and diarrhea in 36% and 25% of patients, respectively. Other symptoms include fatigue (26%), loss of weight (12%) and peripheral edema (13%). The ‘flare’ phenomenon was described in 50% of treated patients. In contrast to β-emitters, marrow suppression is most commonly manifested as anemia, which was observed in 31% of patients. Thrombocytopenia (12%) and neutropenia (5%) were less commonly observed [Parker et al. 2013b; Sartor et al. 2014]. Interestingly, the rates of side effects in the ALSYMPCA study were similar in the placebo- and 223RaCl2-treated groups.
The subgroup analysis in patients with or without previous docetaxel therapy documented a higher incidence of grade 3–4 thrombocytopenia in the patient subgroup who received 223RaCl2 after previous docetaxel therapy, compared with patients receiving placebo and previous docetaxel (9% of 347 patients versus 3% of 171 patients). In contrast, in the 223RaCl2 and placebo groups without previous docetaxel, a similar rate of grade 3–4 thrombocytopenia (3% of 253 patients versus 1% of 130 patients) was observed [Hoskin et al. 2014].
Long-term side effects
There is a long latency time (15–33 years) from radiation exposure to tumor induction [Nekolla et al. 2010]. Hence, radiation-induced tumorigenesis is not expected to be a problem for CRPC patients with bone metastases in light of the reduced life expectancy in these patients.
Actual data concerning long-term side effects using 223RaCl2 are not available. However, 223RaCl2 is an α-emitter and a calcium analogue, comparable with the bone-seeking agent 224RaCl2. Between the 1970s and 1990s, many patients with ankylosing spondylitis were treated using 224RaCl2 [Liepe, 2015]. In 1985, Wick and colleagues published data concerning long-time side effects in 1568 patients with 224RaCl2 therapy and compared these with a control group (ankylosing spondylitis patients without 224RaCl2 therapy) [Wick et al. 1985]. They couldn’t find any differences in mortality or incidence of leukemia or bone tumors between the treated group and controls. However, the same group published another study with different results, reporting 19 cases of leukemia in the exposed group (versus 6.8 cases expected, p < 0.001) compared with 12 cases of leukemia in the control group (versus 7.5 cases expected). Further subclassification of these leukemia cases demonstrated a high incidence of myeloid leukemia in the exposed group (11 cases observed versus 2.9 cases expected, p < 0.001), with a high excess of acute myeloid leukemia (7 cases observed versus 1.8 cases expected, p = 0.003); whereas in the controls the observed cases were within the expected range (4 myeloid leukemia versus 3.1 cases expected) [Wick et al. 2008]. Nekolla and colleagues found a slight increase of tumor incidence in patients treated with 224RaCl2. These cases consisted of mainly young patients (<21 years) with high levels of administered activity, up to 140 MBq [Nekolla et al. 2010].
Current opinion
The treatment of bone metastases with 223RaCl2 is an interesting new approach in the treatment of CRPC patients with bone metastases and shows an extended OS for the first time. However, in contrast to the treatment using β-emitters, the data were limited to one large trial (ALSYMPCA). Metastatic bone pain has been treated with β-emitters since the 1940s, and a significant volume of data exists to support the effectiveness of this modality. More recent reports have suggested that a combination of chemotherapy and bone pain palliation, or β-emitters following two or three cycles of induction chemotherapy (ketoconazole plus doxorubicin alternating with estramustine plus vinblastine) improved the treatment and palliative efficacy. In another study, clinically stable patients with CRPC were randomized to receive weekly doxorubicin with or without 89Sr for 6 weeks. The estimated median survival for patients receiving 89Sr was 27.7 months, compared with 16.8 months for patients who received doxorubicin alone (p = 0.0014) [Tu et al. 2001]. A combination of 89Sr and cisplatin leads to a significantly higher pain relief (91%) compared with 89Sr alone (63%; p < 0.01) [Sciuto et al. 2002]. Additionally, patients with CRPC administered chemotherapy (estramustine or mitoxantrone plus prednisone) within 1 month of 153SmEDTMP therapy demonstrated superior clinical response rates compared with patients who received 153SmEDTMP alone (87.5% versus 53.3%; p = 0.0388). A significantly superior PSA response (p = 0.0235) and median survival time (30 versus 11 months; p = 0.008) were also observed in these patients who were treated with combination therapy [Ricci et al. 2007]. Further promising data have also been reported with combinations of docetaxel and 153SmEDTMP [Fizazi et al. 2009]. Trials with other radionuclides are expected to demonstrate similar effects.
An α-emitter (such as 223RaCl2), with short-range of emission which leads to a bone marrow-sparing effect, in combination with chemotherapy would be a potentially valuable modality to increase the survival of patients with bone metastases and would likely demonstrate reduced bone marrow toxicity compared with current therapies.
Other interesting therapeutic approaches include the combination of systemic radionuclide therapy with bisphosphonates and local-field external-beam radiation. Potential synergistic effects of a radionuclide-bisphosphonate combination have been noted in patients earlier [Soerdjbalie-Maikoe et al. 2002], and systemic radionuclide therapy as an adjuvant to local-field external-beam radiation has demonstrated good therapeutic results. New therapeutic options in the treatment of metastatic bone pain, such as endothelin-1 antagonists [Fizazi et al. 2003], osteoprotegerin, transforming growth factor (TGF)-β, monoclonal antibodies [Kakonen et al. 2002] or fibroplast growth-factor (FGF) inhibitors [Nakamura et al. 1995] offer competition to radionuclide therapies, although synergistic effects are conceivable (particularly with FGF, as evidence has demonstrated radiosensitization following FGF inhibition) and warrant evaluation.
Finally, patients with bone metastases are mainly treated in the advanced stages of disease with severe pain symptoms and high levels of analgesic intake. Consideration should be given to treating patients in the early stages of metastatic cancer. Kraeber-Bodere and colleagues demonstrated a significantly better efficacy of 89Sr in systemic radionuclide therapy for pain relief (p = 0.005), a reduced need for analgesics (p = 0.018) and a longer duration of response (p < 0.0035) in patients with moderate bone involvement compared with more advanced cases [Kraeber-Bodere et al. 2000]. Other investigators have demonstrated similar incremental improvements with early intervention and treatment.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared that there is no conflict of interest.
Contributor Information
Knut Liepe, Department of Nuclear Medicine, GH Hospital Frankfurt (Oder), Müllroser Chaussee 7, 15236 Frankfurt (Oder), Germany.
Ajit Shinto, Department of Nuclear Medicine, Kovai Medical Centre and Hospital, Coimbatore, India.
References
- Agarawal J., Swangsilpa T., Van Der Linden Y., Rades D., Jeremic B., Hoskin P. (2006) The role of external beam radiotherapy in the management of bone metastases. Clin Oncol (R Coll Radiol) 18: 747–760. [DOI] [PubMed] [Google Scholar]
- Arcangeli G., Giovinazzo G., Saracino B., d’Angelo L., Giannarelli D., Micheli A. (1998) Radiation therapy in the management of symptomatic bone metastases: the effect of total dose and histology on pain relief and response duration. Int J Radiat Oncol Biol Phys 42: 1119–1126. [DOI] [PubMed] [Google Scholar]
- Berenson J., Rosen L., Howell A., Porter L., Coleman R., Morley W., et al. (2001) Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer 91: 1191–1200. [DOI] [PubMed] [Google Scholar]
- Biersack H., Palmedo H., Andris A., Rogenhofer S., Knapp F., Guhlke S., et al. (2011) Palliation and survival after repeated (188)Re-HEDP therapy of hormone-refractory bone metastases of prostate cancer: a retrospective analysis. J Nucl Med 52: 1721–1726. [DOI] [PubMed] [Google Scholar]
- Bruland Ø., Nilsson S., Fisher D., Larsen R. (2006) High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 15: 6250s–6257s. [DOI] [PubMed] [Google Scholar]
- Carrasquillo J., O’Donoghue J., Pandit-Taskar N., Humm J., Rathkopf D., Slovin S., et al. (2013) Phase I pharmacokinetic and biodistribution study with escalating doses of ²²³Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging 40: 1384–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden S., Hindorf C., Parker C., Lewington V., Pratt B., Johnson B., et al. (2015) A phase I, open-label study of the biodistribution, pharmacokinetics, and dosimetry of 223Ra-dichloride in patients with hormone-refractory prostate cancer and skeletal metastases. J Nucl Med 56: 1304–1309. [DOI] [PubMed] [Google Scholar]
- Clohisy D., Ramnaraine M., Scully S., Qi M., Van G., Tan H., et al. (2000) Osteoprotegerin inhibits tumor-induced osteoclastogenesis and bone tumor growth in osteopetrotic mice. J Orthop Res 18: 967–976. [DOI] [PubMed] [Google Scholar]
- Ernst D., Tannock I., Winquist E., Venner P., Reyno L., Moore M., et al. (2003) Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol 21: 3335–3342. [DOI] [PubMed] [Google Scholar]
- Federal Office for Radiation Protection (2015) Statement of Federal Office for Radiation Protection (Bfs); 14.01.2015 (Gmbl. 2015, Nr. 8, S. 145). [Google Scholar]
- Fizazi K., Beuzeboc P., Lumbroso J., Haddad V., Massard C., Gross-Goupil M., et al. (2009) Phase II trial of consolidation docetaxel and samarium-153 in patients with bone metastases from castration-resistant prostate cancer. J Clin Oncol 27: 2429–2435. [DOI] [PubMed] [Google Scholar]
- Fizazi K., Yang J., Peleg S., Sikes C., Kreimann E., Daliani D., et al. (2003) Prostate cancer cells-osteoblast interaction shifts expression of growth/survival-related genes in prostate cancer and reduces expression of osteoprotegerin in osteoblasts. Clin Cancer Res 9: 2587–2597. [PubMed] [Google Scholar]
- Gaze M., Kelly C., Kerr G., Cull A., Cowie V., Gregor A., et al. (1997) Pain relief and quality of life following radiotherapy for bone metastases: a randomised trial of two fractionation schedules. Radiother Oncol 45: 109–116. [DOI] [PubMed] [Google Scholar]
- Guise T., Yin J., Taylor S., Kumagai Y., Dallas M., Boyce B., et al. (1996) Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest 98: 1544–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guise T., Yin J., Thomas R., Dallas M., Cui Y., Gillespie M. (2002) Parathyroid hormone-related protein (PTHRP)-(1–139) isoform is efficiently secreted in vitro and enhances breast cancer metastasis to bone in vivo. Bone 30: 670–676. [DOI] [PubMed] [Google Scholar]
- Heidenreich A., Elert A., Hofmann R. (2002) Ibandronate in the treatment of prostate cancer associated painful osseous metastases. Prostate Cancer Prostatic Dis 5: 231–235. [DOI] [PubMed] [Google Scholar]
- Henriksen G., Breistøl K., Bruland Ø., Fodstad Ø., Larsen R. (2002) Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res 62: 3120–3125. [PubMed] [Google Scholar]
- Hobbs R., Song H., Watchman C., Bolch W., Aksnes A., Ramdahl T., et al. (2012) A bone marrow toxicity model for ²²³Ra alpha-emitter radiopharmaceutical therapy. Phys Med Biol 57: 3207–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin P., Sartor O., O’Sullivan J., Johannessen D., Helle S., Logue J., et al. (2014) Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase III ALSYMPCA trial. Lancet Oncol 15: 1397–1406. [DOI] [PubMed] [Google Scholar]
- Janjan N. (2006) Palliation and supportive care in radiation medicine. Hematol Oncol Clin North Am 20: 187–211. [DOI] [PubMed] [Google Scholar]
- Kakonen S., Selander K., Chirgwin J., Yin J., Burns S., Rankin W., et al. (2002) Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem 277: 24571–24578. [DOI] [PubMed] [Google Scholar]
- Keller E., Zhang J., Cooper C., Smith P., McCauley L., Pienta K., et al. (2001) Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev 20: 333–349. [DOI] [PubMed] [Google Scholar]
- Kraeber-Bodere F., Campion L., Rousseau C., Bourdin S., Chatal J., Resche I. (2000) Treatment of bone metastases of prostate cancer with strontium-89 chloride: efficacy in relation to the degree of bone involvement. Eur J Nucl Med 27: 1487–1493. [DOI] [PubMed] [Google Scholar]
- Kuban D., Delbridge T., El-Mahdi A., Schellhammer P. (1989) Half-body irradiation for treatment of widely metastatic adenocarcinoma of the prostate. J Urol 141: 572–574. [DOI] [PubMed] [Google Scholar]
- Lassmann M., Nosske D. (2013) Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med 40: 207–212. [DOI] [PubMed] [Google Scholar]
- Liepe K. (2009) Alpharadin, a 223Ra-based alpha-particle-emitting pharmaceutical for the treatment of bone metastases in patients with cancer. Curr Opin Investig Drugs 10: 1346–1358. [PubMed] [Google Scholar]
- Liepe K. (2015) Radium-223 chloride in bone pain treatment of prostate cancer. Der Nuklearmediziner 38: 131–137. [Google Scholar]
- Liepe K., Kotzerke J. (2011) Internal radiotherapy of painful bone metastases. Methods 55: 258–270. [DOI] [PubMed] [Google Scholar]
- Lipton A. (1997) Bisphosphonates and breast carcinoma. Cancer 80: 1668–1673. [DOI] [PubMed] [Google Scholar]
- Lowik C., Van Der Pluijm G., Van Der Wee-Pals L., Van Treslong-De Groot H., Bijvoet O. (1988) Migration and phenotypic transformation of osteoclast precursors into mature osteoclasts: the effect of a bisphosphonate. J Bone Miner Res 3: 185–192. [DOI] [PubMed] [Google Scholar]
- Mantyh P., Clohisy D., Koltzenburg M., Hunt S. (2002) Molecular mechanisms of cancer pain. Nat Rev Cancer 2: 201–209. [DOI] [PubMed] [Google Scholar]
- Mason M., Sydes M., Glaholm J., Langley R., Huddart R., Sokal M., et al. (2007) Oral sodium clodronate for nonmetastatic prostate cancer–results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (Isrctn61384873). J Natl Cancer Inst 99: 765–776. [DOI] [PubMed] [Google Scholar]
- Morony S., Capparelli C., Sarosi I., Lacey D., Dunstan C., Kostenuik P. (2001) Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res 61: 4432–4436. [PubMed] [Google Scholar]
- Mundy G. (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Hanada K., Tamura M., Shibanushi T., Nigi H., Tagawa M., et al. (1995) Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology 136: 1276–1284. [DOI] [PubMed] [Google Scholar]
- Nekolla E., Walsh L., Spiess H. (2010) Incidence of malignant diseases in humans injected with radium-224. Radiat Res 174: 377–386. [DOI] [PubMed] [Google Scholar]
- Nelson J., Nabulsi A., Vogelzang N., Breul J., Zonnenberg B., Daliani D., et al. (2003) Suppression of prostate cancer induced bone remodeling by the endothelin receptor a antagonist atrasentan. J Urol 169: 1143–1149. [DOI] [PubMed] [Google Scholar]
- Nielsen O., Bentzen S., Sandberg E., Gadeberg C., Timothy A. (1998) Randomized Trial of single dose versus fractionated palliative radiotherapy of bone metastases. Radiother Oncol 47: 233–240. [DOI] [PubMed] [Google Scholar]
- Nielsen O., Munro A., Tannock I. (1991) Bone metastases: pathophysiology and management policy. J Clin Oncol 9: 509–524. [DOI] [PubMed] [Google Scholar]
- Nielsen O., Parker C., Haugen I., Lokna A., Aksnes A., Bolstad B., et al. (2010) Radium-223 chloride, a first-in-class alpha-pharmaceutical with a benign safety profile for patients with castration-resistant prostate cancer (CRPC) and bone metastases: combined analysis of phase I and II clinical trials. J Clin Oncol 28: 4678. [Google Scholar]
- Nilsson S., Balteskard L., Fosså S. (2004) Phase I study of alpharadin2 (223Ra), and a-emitting bone-seeking agent in cancer patients with skeletal metastases. Eur J Nucl Med 31: 370. [Google Scholar]
- Nilsson S., Franzén L., Parker C., Tyrrell C., Blom R., Tennvall J., et al. (2007) Bone-targeted Radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol 8: 587–594. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Franzén L., Parker C., Tyrrell C., Blom R., Tennvall J., et al. (2013) Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of Radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer 11: 20–23. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Larsen R., Fosså S., Balteskard L., Borch K., Westlin J., et al. (2005) First clinical experience with alpha-emitting Radium-223 in the treatment of skeletal metastases. Clin Cancer Res 15: 4451–4459. [DOI] [PubMed] [Google Scholar]
- Nilsson S., Strang P., Aksnes A., Franzèn L., Olivier P., Pecking A., et al. (2012) A randomized, dose-response, multicenter phase II study of Radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer 48: 678–686. [DOI] [PubMed] [Google Scholar]
- Pacilio M., Ventroni G., De Vincentis G., Cassano B., Pellegrini R., Di Castro E., et al. (2016) Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting 223Ra-dichloride. Eur J Nucl Med Mol Imaging 43: 21–33. [DOI] [PubMed] [Google Scholar]
- Paes F., Serafini A. (2010) Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Semin Nucl Med 40: 89–104. [DOI] [PubMed] [Google Scholar]
- Parker C., Nilsson S., Heinrich D., Helle S., O’Sullivan J., Fosså S., et al. (2013a) Alpha emitter Radium-223 and survival in metastatic prostate cancer. N Engl J Med 369: 231–223. [DOI] [PubMed] [Google Scholar]
- Parker C., Pascoe S., Chodacki A., O’Sullivan J., Germá J., O’Bryan-Tear C., et al. (2013b) A randomized, double-blind, dose-finding, multicenter, phase II study of radium chloride (Ra 223) in patients with bone metastases and castration-resistant prostate cancer. Eur Urol 63: 189–197. [DOI] [PubMed] [Google Scholar]
- Parker C., Sartor O. (2013) Radium-223 in prostate cancer. N Engl J Med 369: 1659–1660. [DOI] [PubMed] [Google Scholar]
- Portenoy R., Kornblith A., Wong G., Vlamis V., Lepore J., Loseth D., et al. (1994) Pain in ovarian cancer patients. Prevalence, characteristics, and associated symptoms. Cancer 74: 907–915. [DOI] [PubMed] [Google Scholar]
- Powles T., Paterson S., Kanis J., McCloskey E., Ashley S., Tidy A., et al. (2002) Randomized, placebo-controlled trial of clodronate in patients with primary operable breast cancer. J Clin Oncol 20: 3219–3224. [DOI] [PubMed] [Google Scholar]
- Rabbani S., Gladu J., Harakidas P., Jamison B., Goltzman D. (1999) Over-production of parathyroid hormone-related peptide results in increased osteolytic skeletal metastasis by prostate cancer cells in vivo. Int J Cancer 80: 257–264. [DOI] [PubMed] [Google Scholar]
- Ratanatharathorn V., Powers W., Moss W., Perez C. (1999) Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys 44: 1–18. [DOI] [PubMed] [Google Scholar]
- Ricci S., Boni G., Pastina I., Genovesi D., Cianci C., Chiacchio S., et al. (2007) Clinical benefit of bone-targeted radiometabolic therapy with 153Sm-EDTMP combined with chemotherapy in patients with metastatic hormone-refractory prostate cancer. Eur J Nucl Med 34: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Rimpler A. (2015) External radiation exposure of staff during therapy with Ra-223-dichloride. Der Nuklearmediziner 38: 165–166. [Google Scholar]
- Sartor O., Coleman R., Nilsson S., Heinrich D., Helle S, et al. (2014) Effect of Radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase III, double-blind, randomised trial. Lancet Oncol 15: 738–746. [DOI] [PubMed] [Google Scholar]
- Saarto T., Janes R., Tenhunen M., Kouri M. (2002) Palliative radiotherapy in the treatment of skeletal metastases. Eur J Pain 6: 323–330. [DOI] [PubMed] [Google Scholar]
- Scher H., Yagoda A. (1987) Bone metastases: pathogenesis, treatment, and rationale for use of resorption inhibitors. Am J Med 82: 6–28. [DOI] [PubMed] [Google Scholar]
- Sciuto R., Festa A., Rea S., Pasqualoni R., Bergomi S., Petrilli G., et al. (2002) Effects of low-dose cisplatin on 89Sr therapy for painful bone metastases from prostate cancer: a randomized clinical trial. J Nucl Med 43: 79–86. [PubMed] [Google Scholar]
- Serafini A. (1994) Current status of systemic intravenous radiopharmaceuticals for the treatment of painful metastatic bone disease. Int J Radiat Oncol Biol Phys 30: 1187–1194. [DOI] [PubMed] [Google Scholar]
- Shipman C., Rogers M., Apperley J., Russell R., Croucher P. (1997) Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol 98: 665–672. [DOI] [PubMed] [Google Scholar]
- Smith M., Coleman R., Klotz L., Pittman K., Milecki P., Ng S., et al. (2015) Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 26: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerdjbalie-Maikoe V., Pelger R., Lycklama a, Nijeholt G., Arndt J., Zwinderman A., Papapoulos S., et al. (2002) Strontium-89 (metastron) and the bisphosphonate olpadronate reduce the incidence of spinal cord compression in patients with hormone-refractory prostate cancer metastatic to the skeleton. Eur J Nucl Med Mol Imaging 29: 494–498. [DOI] [PubMed] [Google Scholar]
- Steenland E., Leer J., Van Houwelingen H., Post W., Van Den Hout W., Kievit J., et al. (1999) The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the dutch bone metastasis study. Radiother Oncol 52: 101–109. [DOI] [PubMed] [Google Scholar]
- Todenhöfer T., Stenzl A., Hofbauer L., Rachner T. (2015) Targeting bone metabolism in patients with advanced prostate cancer: current options and controversies. Int J Endocrinol 15(7): 738–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S., Millikan R., Mengistu B., Delpassand E., Amato R., Pagliaro L., et al. (2001) Bone-targeted therapy for advanced androgen-independent carcinoma of the prostate: a randomised phase II trial. Lancet 357: 336–341. [DOI] [PubMed] [Google Scholar]
- Uppelschoten J., Wanders S., De Jong J. (1995) Single-dose radiotherapy (6 Gy): palliation in painful bone metastases. Radiother Oncol 36: 198–202. [DOI] [PubMed] [Google Scholar]
- Wanke C., Pinkert P., Szermerski B., Solle A., Kranert W., Andreeff A., et al. (2014) Design and preliminary results from RAPSODY - a study to estimate the effective dose to relatives of patients treated with Ra-223 dichloride in Germany. Eur J Nucl Med 41: S271. [Google Scholar]
- Weinfurt K., Anstrom K., Castel L., Schulman K., Saad F. (2006) Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Ann Oncol 17: 986–989. [DOI] [PubMed] [Google Scholar]
- Wick R., Chmelevsky D., Gössner W. (1985) 224Ra: risk to bone and haematopoietic tissue in ankylosing spondylitis patients. Strahlentherapie Sonderb 80: 38–44. [PubMed] [Google Scholar]
- Wick R., Nekolla E., Gaubitz M., Schulte T. (2008) Increased risk of myeloid leukaemia in patients with ankylosing spondylitis following treatment with radium-224. Rheumatology (Oxford) 47: 855–859. [DOI] [PubMed] [Google Scholar]
- World Health Organization. (1990) Cancer pain relief and palliative care: Report of a WHO expert committee. World Health Organ Tech Rep Ser 804: 1–75. [PubMed] [Google Scholar]