Abstract
Since androgen signaling plays a pivotal role in the proliferation and metastasis of prostate cancer, androgen deprivation therapy (ADT) or castration therapy is considered the backbone of treatment for newly diagnosed metastatic prostate cancer. However, almost all men experience disease progression on ADT to a state known as metastatic castration-resistant prostate cancer (mCRPC), which continues to be driven by intratumoral androgen synthesis or androgen receptor signaling. Hence, the extragonadal ablation of androgen synthesis from pregnane precursors holds much promise. An inhibitor of cytochrome P450 17α−hydroxy/17,20-lyase (CYP17) enzymes, abiraterone acetate, has already been approved for men with mCRPC. Newer CYP17 inhibitors continue to be developed which are either more selective or have concomitant inhibitory actions on AR signaling. These include VT-464, orteronel, and galeterone. Herein, we focus on the molecular mechanism of action, efficacy, latest evidence, and clinical potential of CYP17 inhibitors in prostate cancer.
Keywords: abiraterone; androgen biosynthesis; androgen receptors; castration-resistant prostate cancer; cytochrome P450 17α−hydroxy/17,20-lyase inhibitors; galeterone; orteronel; VT-464
Introduction
The cytochrome P450 (CYP) superfamily of enzymes mediates the catalytic conversion of drugs to reactive products that can bind to macromolecules, like proteins and DNA. CYP enzymes account for approximately 75% of the total drug metabolism [Guengerich, 2008]. In addition, they also play a vital role in the synthesis of steroid hormones, cholesterol, and vitamin D metabolism. Fifty-seven human CYPs identified were classified into 18 families and 43 subfamilies, of which CYP families 1, 2, and 3 are mainly responsible for the metabolism of drugs. Evidences support the role of CYPs in tumor formation and inhibition of CYPs has become a key area in the treatment of cancer [Lohr et al. 2004; Bruno and Njar, 2007]. CYP17α hydroxylase/17,20 lyase (CYP17), a pivotal enzyme for androgen synthesis, has been implicated in the pathogenesis of prostate cancer [Vasaitis et al. 2011]. In fact, an increased expression of CYP17 has been demonstrated in prostate carcinoma, which correlated positively with a high-stage, high Gleason score, and short relapse-free time disease [Stigliano et al. 2007].
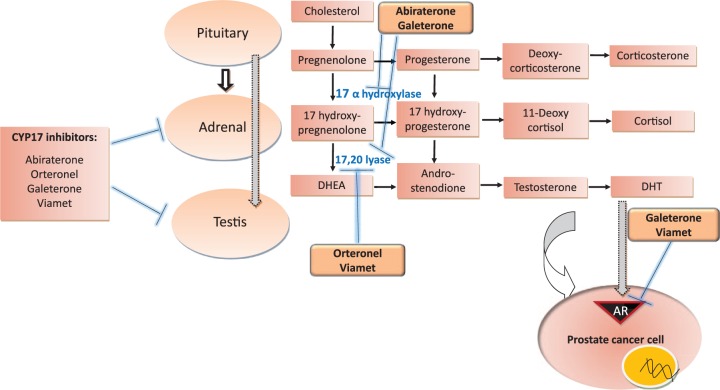
CYP17 enzymes, CYP17 hydroxylase and CYP17,20 lyase, sequentially catalyze the conversion of pregnenolone and progesterone to 17α hydroxypregnenolone and 17α hydroxyprogesterone, which are then further converted to the weak androgens, dehydroepiandrosterone (DHEA) and androstenedione, respectively (Figure 1) [Poole et al. 2014]. Both DHEA and androstenedione, are eventually transformed into testosterone and dihydroxy testosterone (DHT), the most potent androgen. Metastatic prostate cancer is fueled by the androgen axis, and despite the androgen ablation therapy, almost all men with metastatic prostate cancer progress to having castration-resistant prostate cancer (mCRPC), which still maintains its dependence on intratumoral androgen synthesis and androgen receptor (AR) signaling for proliferation. Abiraterone acetate (AA), a CYP17 inhibitor, is the first US Food and Drug Administration (FDA) approved drug of its class for the treatment of mCRPC [Bryce and Ryan, 2012]. The next generation CYP17 inhibitors currently being evaluated in clinical trials for metastatic prostate cancer include orteronel (TAK 700, Takeda Pharmaceuticals, Deerfield, IL, USA), VT-464 (Viamet Pharmaceuticals, Durham, North Carolina, USA), and galeterone (TOK-001, Tokai Pharmaceuticals, Boston, MA, USA).
Figure 1.
Cytochrome P450 (CYP) 17 inhibitors targeting androgen synthesis and androgen receptors (ARs), currently approved and those in advanced stages of clinical development in castration-sensitive or -resistant prostate cancer. Thick arrows denote stimulation, flat lines denote inhibition, and thin arrows denote synthesis. DHEA, dehydroepiandrosterone; DHT, dihydroxy testosterone.
Molecular mechanisms, efficacy, and latest evidence
Abiraterone acetate
AA, a pregnenolone analogue, and its metabolite, abiraterone, are selective inhibitors of the CYP17 enzymes, 17α hydroxylase and 17,20 lyase (Table 1). Recently, it has been shown that a more active form of AA, ∆4 abiraterone (D4A), blocks 17β-hydroxysteroid dehydrogenase and steroid 5α reductase, which are required for DHT synthesis, in addition to CYP17A1 enzymes [Li et al. 2015]. Remarkably, D4A was also shown to have a direct inhibitory effect on AR, comparable to that seen with enzalutamide, a potent AR antagonist. Furthermore, D4A exhibited a higher level of the overall antitumor activity than AA in the xenograft prostate tumors.
Table 1.
Various cytochrome P450 (CYP) 17 inhibitors and their mechanisms of action.
| CYP17 inhibitors | Mechanism of action |
|---|---|
| Abiraterone and D4A | Inhibition of 17α hydroxylase and 17,20 lyase; 17β hydroxysteroid dehydrogenase and steroid 5α reductase; AR antagonism |
| Orteronel (TAK-700) | Selective inhibition of 17,20 lyase over 17α hydroxylase |
| Viamet (VT-464) | Selective inhibition of 17,20 lyase over 17α hydroxylase; AR antagonism |
| Galeterone (TOK-001) | 17α hydroxylase and 17,20 lyase inhibition; AR antagonism; downregulation of AR protein expression |
| CFG920 | Dual CYP17/CYP11B2 inhibitor |
AR, androgen receptor.
In the earlier phase I and II trials, AA was found to be safe and effective in lowering serum androgen levels [Attard et al. 2008; Ryan et al. 2010]. However, a sixfold increase in adrenocorticotropic hormone (ACTH) was observed, leading to secondary mineralocorticoid excess, which precipitated in the form of hypokalemia, fluid retention, and hypertension. To avoid the mineralocorticoid toxicities, a corticosteroid, prednisone, was added on as a concomitant therapy. However, a mineralocorticoid receptor antagonist, eplerenone, in conjunction with AA may preclude the requirement for prednisone [Attard et al. 2008]. This is especially pertinent in those men who have asymptomatic or minimally symptomatic mCRPC, and in whom long-term use of a corticosteroid may not be desirable. A significant increase in the absorption of AA was observed when taken with a high-fat meal [Ryan et al. 2010], and to avoid any dietary variations, the FDA recommends taking AA on an empty stomach.
Based on the results of the COU-AA-301 trial, the FDA approved the use of AA for the treatment of mCRPC in the post-chemotherapy setting in April 2011 [De Bono et al. 2011]. The COU-AA 301 trial observed an overall survival (OS) benefit, increase in time to prostate-specific antigen (PSA) progression and progression-free survival (PFS) in patients in the AA group over the placebo group (median OS, 15.8 versus 11.2 months; median time to PSA progression, 8.5 versus 6.6 months; median radiologic PFS, 5.6 versus 3.6 months). The PSA decline was at least 50% in 29% of the patients in the AA arm compared with 6% in the placebo arm [Fizazi et al. 2012]. Later studies have demonstrated its efficacy in chemotherapy-naïve patients with mCRPC. In a phase III randomized trial with a median follow up of more than 4 years, treatment with AA prolonged OS compared with prednisone alone [34.7 versus 30.3 months; hazard ratio (HR), 0.81; 95% confidence interval (CI), 0.70–0.93; p = 0.0033], suggesting its favorable efficacy and safety profile in chemotherapy-naive patients as well [Ryan et al. 2015].
Though AA is reported to be effective after ketoconazole treatment [Danila et al. 2010; Ryan et al. 2010; Kim et al. 2014], its efficacy is greater in patients who had not received ketoconazole, chemotherapy, or enzalutamide, a novel AR antagonist [Bryce and Ryan, 2012; Cheng et al. 2015; Ryan et al. 2015]. Reports indicate that the benefits of AA on clinical outcomes were increased with concomitant bone-targeted therapy [Saad et al. 2015b]. Further, a systematic review and meta-analysis based on the results from 10 trials, including two phase III trials (COU-AA-301 and COU-AA-302), with 2283 patients (1343 AA; 940 placebo) revealed that AA significantly prolonged the OS, radiographic PFS, and time to progression without any evidence of unexpected toxicity in patients with mCRPC, regardless of prior chemotherapy or not [Zhou et al. 2014]. In addition, an updated analysis of the COU-AA-301 and COU-AA-302 trials suggests a strong association between PSA kinetics and OS in chemotherapy-pretreated and naïve patients [Xu et al. 2015]. Thus, the overall evidence supports the continued use of AA as a standard therapy for mCRPC.
Orteronel
Orteronel (TAK-700) is an oral, nonsteroidal 17,20-lyase inhibitor with higher specificity for 17,20 lyase over 17 hydroxylase; hence it is likely more selective in its mechanism of action compared with AA. In a phase III study, orteronel was evaluated in patients with mCRPC that progressed after docetaxel therapy. One thousand and ninety-nine men were randomly assigned in a 2:1 schedule to receive orteronel 400 mg plus prednisone 5 mg twice daily or placebo plus prednisone 5 mg twice daily, stratified by region (Europe, North America, and non-Europe/North America) [Fizazi et al. 2015]. The results indicated improved radiographic PFS with orteronel–prednisone (median, 8.3 versus 5.7 months; HR, 0.760; 95% CI, 0.653–0.885; p < 0.001). Similarly, PSA 50, i.e. 50% reduction in PSA (25% versus 10%; p<0.001), and time to PSA progression (median, 5.5 versus 2.9 months; p < 0.001) were also significantly different from the placebo–prednisone arm. The median OS, the primary endpoint, did not reach statistical significance in patients with mCRPC post docetaxel [Fizazi et al. 2014], which could be attributed to the fact that TAK-700 is a reversible inhibitor. However, when men were stratified by regions, a significant improvement in OS was seen in men in the non-Europe/North American regions (15.3 versus 10.1 months, p = 0.019), despite having similar baseline clinical and disease characteristics. This discrepancy in OS by region may have been related to the decreased exposure to post-trial treatment with AA and enzalutamide, as these agents were available earlier in North American and European regions.
Similarly, the results from a phase III trial in chemotherapy naïve patients with mCRPC also revealed that treatment with orteronel failed to improve the OS (31.4 months in the orteronel plus prednisone versus 29.5 months in the placebo plus prednisone group; HR, 0.92; 95% CI, 0.79–1.08; p = 0.31) [Saad et al. 2015a]. Nevertheless, the radiographic PFS was prolonged in the orteronel plus prednisone group. Based on these data, orteronel is no longer being developed in the setting of mCRPC, although an ongoing phase III trial through the National Clinical Trials Network continue to explore the potential of orteronel in men with new castration-naïve metastatic prostate cancer.
VT-464
VT-464 is a novel, nonsteroidal CYP17 inhibitor and AR antagonist. VT-464 blocks AR variants F877L and T878A, which have been shown to be associated with resistance to enzalutamide and AA, respectively. VT-464 preferentially inhibits 17,20 lyase over 17α hydroxylase, thus offering an advantage over AA from the perspective of not requiring concomitant therapy with prednisone, owing to its minimal effects on upstream steroid levels [Suzman and Antonarakis, 2014].
Results from the studies on castrate-resistant prostate cancer cell lines and xenograft models that are either enzalutamide responsive or resistant, indicate that VT-464 demonstrated a greater decrease in AR transactivation compared with AA in both enzalutamide-sensitive and -resistant cell lines [Toren et al. 2015]. At gene and protein levels, VT-464 suppressed the AR axis to a greater extent compared with AA. Further, intratumoral androgen levels and PSA decrease trends were significantly lower with VT-464 than with AA, in addition to a more potent tumor growth inhibition. These data suggest greater suppression of the AR axis with VT-464 than AA, which is likely due to its superior selective suppression of androgen synthesis and direct AR antagonism.
Based on these encouraging data, a phase I/II trial (INO-VT-464-CL-001) is being conducted in four subgroups of men with mCRPC: treatment naïve, that is with no prior treatment with AA, enzalutamide, or chemotherapy; prior treatment with AA but not with chemotherapy or enzalutamide; prior treatment with enzalutamide but not with AA or chemotherapy; and prior treatment with both AA and enzalutamide, or chemotherapy. Early results from the INO-VT-464-CL-001 trial [ClinicalTrials.gov identifier: NCT02012920] in mCRPC are promising [De Bono et al. 2015]. Nineteen of 26 treatment-naïve men who received 300–600 mg VT-464 twice daily had PSA responses, ranging from 30% to 90%. More interestingly, preliminary PSA responses in patients with treatment failure indicate a 90% response in a patient who had prior enzalutamide prechemotherapy, and a 50% response in patients who had prior enzalutamide and prior chemotherapy. However, no mineralocorticoid excess syndrome and changes in ACTH responses were observed, despite not using any supplemental steroids.
Galeterone
Galeterone (VN/124-1, TOK-001) is a CYP17 inhibitor with multiple mechanisms of action, including CYP17 inhibition, AR antagonism, and decrease in intratumoral AR levels. Preclinical results indicate that treatment with VN/124-1 caused marked downregulation of AR protein expression, in contrast to treatments with bicalutamide or androgen deprivation therapy (ADT), which may induce upregulation of AR protein expression [Vasaitis et al. 2008]. It also caused a significant reduction in tumor growth compared with AA [Bruno et al. 2011]. It has been suggested that the multifaceted action of galeterone may assist in overcoming the resistance observed with other CYP17 inhibitors [Stein et al. 2014].
In a phase I study of chemonaïve men with mCRPC, galeterone was well tolerated. Of 49 patients, 22% demonstrated a decrease in PSA of more than 50%, while an additional 26% had PSA decline of 30–50% after 12 weeks. No evidence of adrenal mineralocorticoid excess was noted [Montgomery et al. 2012; Taplin et al. 2012]. A phase II trial is currently being undertaken to evaluate the efficacy of galeterone in 144 patients with progressive castration-resistant prostate cancer, stratified to no prior CYP17 inhibitor or enzalutamide, AA-refractory PC, and enzalutamide-refractory mCRPC. The primary endpoints are reduction in PSA and safety.
Clinical potential
Despite the recent advances in the therapeutic regimen, the gain in OS had been modest, and prostate cancer still remains the second leading cause of cancer-related death in men. The implications of novel CYP17 inhibitors in nonmetastatic and metastatic prostate cancer are being explored in ongoing clinical trials (Table 2). Herein, some of the most pertinent studies have been highlighted.
Table 2.
Selected ongoing clinical trials of cytochrome P450 (CYP) 17 inhibitors in metastatic prostate cancer.
| Patient population | Study phase | Intervention/arms | Accrual (N) | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Prostate cancer with a rising PSA or a rising PSA and nodal disease following definitive radical prostatectomy | II | Abiraterone versus abiraterone + degarelix or degarelix alone | Ongoing* (120) | NCT01751451 |
| Progressive chemotherapy-naïve castration-resistant prostate cancer (SPARE) | II | Abiraterone + prednisone + LHRH therapy versus abiraterone + prednisone | Ongoing* (70) | NCT02077634 |
| Metastatic hormone-naïve prostate cancer (PEACE1) | III | ADT ± local RT ± abiraterone acetate | Ongoing* (916) | NCT01957436 |
| Newly diagnosed metastatic sensitive prostate cancer | III | ADT + TAK-700 versus ADT + bicalutamide | Open* (1486) | NCT01809691 |
| CRPC | II | Galeterone | Ongoing (144) | NCT01709734 |
| Patients with metastatic CRPC expressing AR-V7 mRNA | III | Galeterone versus enzalutamide | Open* (148) | NCT02438007 |
| Patients with CRPC who are abiraterone naive or abiraterone resistant | I/II | CFG920 | Ongoing, not recruiting (31) | NCT01647789 |
Currently recruiting.
ADT, androgen deprivation therapy; AR-V7, androgen receptor splice variant 7; CRPC, castration-resistant prostate cancer; LHRH, luteinizing hormone releasing hormone; orteronel, TAK-700.
Abiraterone acetate
A phase II, randomized, three-arm study of AA alone, AA plus degarelix, a Gonadotropin-releasing hormone (GnRH) antagonist, and degarelix alone for patients with a rising PSA or a rising PSA and nodal disease following definitive radical prostatectomy is currently recruiting patients [ClinicalTrials.gov identifier: NCT01751451]. One of the most interesting questions this trial is addressing is whether AA therapy may continue to be efficacious without concomitant gonadal suppression by a GnRH agonist. The primary endpoints are PFS and soft tissue complete response (RECIST), while the secondary outcome measures included PSA response rate, overall quality of life, nonhematologic adverse events, testosterone and luteinizing hormone recovery rates. Further, a correlative tissue analysis where immunohistochemical markers like AR, Phosphatase and tensin homolog (PTEN), Prostate-specific membrane antigen (PSMA), fatty acid synthase, Phospho-AMP-activated protein kinase (AMPK); Phospho-Acetyl-CoA Carboxylase (phospho-ACC), phospho-S6 kinase, phospho-protein kinase B will be assessed.
A crossover phase II study is ongoing to evaluate whether treatment with ADT in combination with AA and prednisone for 8 months controls the disease better than treatment with standard ADT alone in patients with prostate cancer who have PSA progression after prostatectomy or radiotherapy [luteinizing hormone releasing hormone (LHRH) alone versus LHRH plus AA plus prednisone] [ClinicalTrials.gov identifier: NCT01786265]. In this crossover study, upon PSA progression or objective evidence of progressive disease, the participants who had received LHRH agonist alone will receive a combination of LHRH agonist and AA plus prednisone, and those who received the combination therapy will receive LHRH agonist. The primary outcome measure is PSA PFS. This trial is expected to provide insight into the efficacy of an earlier versus delayed therapy with AA in the setting of biochemically recurrent prostate cancer after definitive therapy.
Another interesting question is whether men who have treatment with AA can be spared concomitant therapy with LHRH agonist in the setting of metastatic disease as well. This is being evaluated by an ongoing trial in men with progressive, chemotherapy-naïve, castration-resistant prostate cancer (SPARE) [ClinicalTrials.gov identifier: NCT02077634], in which men are randomized (1:1) to either the current standard regimen of AA plus prednisone plus LHRH therapy (arm A) or AA plus prednisone without a concomitant LHRH agonist (arm B). The primary endpoint is radiographic PFS.
The role of AA is also promising in new hormone-naïve metastatic prostate cancer. This is being addressed in one of the arms of the STAMPEDE trial being conducted in the UK [Sydes et al. 2012], as well as a large, multicenter phase III study in Europe (PEACE 1 study) [ClinicalTrials.gov identifier: NCT01957436]. The PEACE 1 study is comparing the clinical benefit of ADT with or without local radiotherapy, and with or without AA and prednisone, in men with new metastatic hormone-naïve prostate cancer. OS and PFS are the primary outcome measures, while PSA response rate, as defined by an undetectable serum PSA level at 8 months, time to pain progression, and time to chemotherapy are some of the secondary outcomes analyzed.
Orteronel
Although orteronel did not improve OS in phase III trials in a mCRPC setting, given its mechanism of action and clinical data, it continues to hold promise in the treatment of men with new metastatic castration-naïve prostate cancer. S1216 is a NCTN trial sponsored through Southwest Oncology Group (SWOG) [ClinicalTrials.gov identifier: NCT01809691], which is going to randomize 1636 men with newly diagnosed castration-sensitive metastatic prostate cancer to ADT plus orteronel versus ADT plus bicalutamide (Table 2). The primary outcome measure is OS.
The results of these studies are expected to provide evidence for the use of CYP17 inhibitors, early in the course of prostate cancer with biochemical recurrence or with castration-sensitive metastatic disease, along with androgen ablation therapy.
Galeterone
Increased expression of constitutively active AR splice variants lacking the ligand-binding domain has been implicated in the progression of mCRPC, and result in diminished response to treatment with AA, enzalutamide, and taxanes [Antonarakis et al. 2014, 2015]. Preclinical studies have demonstrated superior antitumor efficacy of galeterone over bicalutamide, indicating that it is more potent than castration in the in vivo LAPC4 xenograft, a prostate cancer cell line derived from a lymph node metastasis that expresses wild-type AR and secretes PSA [Vasaitis et al. 2008]. Galeterone (0.15 mmol/kg, twice daily) caused a 93.8% reduction in the mean final LAPC4 xenograft volume compared with the controls. Galeterone exerts its action by disrupting AR signaling through CYP17 lyase inhibition, degradation of AR splice variants, blocking of nuclear translocation and decreased expression of AR dependent genes [Njar and Brodie, 2015]. Given the multitargeted mechanism of action of galeterone, a phase III, randomized study is comparing the efficacy of galeterone with that of enzalutamide in men with mCRPC harboring AR splice variant 7 mRNA (AR-V7) (ARMOR3-SV) [ClinicalTrials.gov identifier: NCT02438007]. Radiographic PFS is the primary outcome measure, while the secondary outcome measures include OS and time to the initiation of chemotherapy.
CFG920
In the emerging line of CYP17 inhibitors, another CYP17A1 inhibitor, CFG920 (Novartis Pharmaceuticals, St. Louis, MO, USA), though in early stages of development, is worth mentioning [Yin et al. 2013; Gomez et al. 2015; Gaul et al. 2015] CFG920 is currently being evaluated in a phase I/II multicenter study for its safety and antitumor activity in patients with mCRPC who are AA naïve or AA resistant [ClinicalTrials.gov identifier: NCT01647789].
Conclusion
In conclusion, abrogating intratumoral androgen synthesis and AR signaling by novel CYP17 inhibitors, as evidenced by the recently available data, have potential in the therapeutic arena of advanced prostate cancer. Ongoing studies are expected to establish new treatment paradigms with the existing agents, as well as lead to the approval of novel CYP inhibitors, all of which will hopefully delay the onset of symptomatic mCRPC and improve survival in a meaningful fashion.
Furthermore, it is important to recognize that multiple other classes of therapy may be emerging for advanced prostate cancer, which may be of interest alone or in combination with CYP17 inhibitors. Whole exome and transcriptome sequencing of mCRPC has revealed a high frequency of clinically relevant entities [Robinson et al. 2015]. Frequent mutations in the BRCA1 and BRCA2 genes may suggest a potential role for PARP inhibitors, and in fact, an ongoing study is exploring the role of abiraterone in combination with the poly ADP ribose polymerase (PARP) inhibitor ABT-888 [ClinicalTrials.gov identifier: NCT01576172]. As another example, patients with PTEN deficiency may be susceptible to phosphoinositide 3-kinase (PI3K) inhibitors [ClinicalTrials.gov identifier: NCT02407054, NCT02215096]. Early phase trials are exploring the combination of novel endocrine therapy with these agents. As multiple CYP inhibitors emerge, it will be critical to explore potential pairings with these novel classes of therapy.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared that there is no conflict of interest.
Contributor Information
Anitha B. Alex, Division of Medical Oncology, Department of Medicine, University of Utah Huntsman Cancer Institute, Salt Lake City, UT, USA
Sumanta K. Pal, Medical Oncology & Experimental Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA
Neeraj Agarwal, Division of Medical Oncology, Department of Medicine, University of Utah Huntsman Cancer Institute, 1950 Circle of Hope, Salt Lake City, UT 84112, USA.
References
- Antonarakis E., Lu C., Luber B., Wang H., Chen Y., Nakazawa M., et al. (2015) Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol 1: 582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis E., Lu C., Wang H., Luber B., Nakazawa M., Roeser J., et al. (2014) AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 371: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard G., Reid A., Yap T., Raynaud F., Dowsett M., Settatree S., et al. (2008) Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol 26: 4563–4571. [DOI] [PubMed] [Google Scholar]
- Bruno R., Njar V. (2007) Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg Med Chem 15: 5047–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno R., Vasaitis T., Gediya L., Purushottamachar P., Godbole A., Ates-Alagoz Z., et al. (2011) Synthesis and biological evaluations of putative metabolically stable analogs of VN/124–1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124–1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids 76: 1268–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce A., Ryan C. (2012) Development and clinical utility of abiraterone acetate as an androgen synthesis inhibitor. Clin Pharmacol Ther 91: 101–108. [DOI] [PubMed] [Google Scholar]
- Cheng H., Gulati R., Azad A., Nadal R., Twardowski P., Vaishampayan U., et al. (2015) Activity of enzalutamide in men with metastatic castration-resistant prostate cancer is affected by prior treatment with abiraterone and/or docetaxel. Prostate Cancer Prostatic Dis 18: 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danila D., Morris M., De Bono J., Ryan C., Denmeade S., Smith M., et al. (2010) Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol 28: 1496–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono J., Logothetis C., Molina A., Fizazi K., North S., Chu L., et al. (2011) Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364: 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bono J., Pezaro C., Gillessen S., Shore N., Nordquist L., Efstathiou E., et al. (2015) The oral CYP17-lyase (L) inhibitor T-464 in patients with CRPC. J Clin Oncol 33: 185. [Google Scholar]
- Fizazi K., Jones R., Oudard S., Efstathiou E., Saad F., De Wit R., et al. (2014) Regional differences observed in the phase 3 trial (ELM-PC 5) with orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel. J Clin Oncol 32: 5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K., Jones R., Oudard S., Efstathiou E., Saad F., De Wit R., et al. (2015) Phase III, randomized, double-blind, multicenter trial comparing orteronel (TAK-700) plus prednisone with placebo plus prednisone in patients with metastatic castration-resistant prostate cancer that has progressed during or after docetaxel-based therapy: ELM-PC 5. J Clin Oncol 33: 723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fizazi K., Scher H., Molina A., Logothetis C., Chi K., Jones R., et al. (2012) Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 13: 983–992. [DOI] [PubMed] [Google Scholar]
- Gaul C., Mistry P., Moebitz H., Perrone M., Gruenenfelder B., Guerreiro N., et al. (2015) Discovery of CFG920, a dual CYP17/CYP11B2 inhibitor, for the treatment of castration resistant prostate cancer. Paper presented at the American Chemical Society, 16–20 August 2015, Boston, MA, USA. [Google Scholar]
- Gomez L., Kovac J., Lamb D. (2015) CYP17a1 inhibitors in castration-resistant prostate cancer. Steroids 95: 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. (2008) Cytochrome P450 and chemical toxicology. Chem Res Toxicol 21: 70–83. [DOI] [PubMed] [Google Scholar]
- Kim W., Zhang L., Wilton J., Fetterly G., Mohler J., Weinberg V., et al. (2014) Sequential use of the androgen synthesis inhibitors ketoconazole and abiraterone acetate in castration-resistant prostate cancer and the predictive value of circulating androgens. Clin Cancer Res 20: 6269–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Bishop A., Alyamani M., Garcia J., Dreicer R., Bunch D., et al. (2015) Conversion of abiraterone to D4a drives anti-tumour activity in prostate cancer. Nature 523: 347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr M., McFadyen M., Murray G., Melvin W. (2004) Cytochrome P450 enzymes and tumor therapy. Mol Cancer Ther 3: 1503; author reply 1503–1504. [PubMed] [Google Scholar]
- Montgomery R., Eisenberger M., Rettig M., Chu F., Pili R., Stephenson J., et al. (2012) Phase I clinical trial of galeterone (TK-001), a multifunctional antiandrogen and CYP 17 inhibitor in castration resistant prostate cancer (CRPC). J Clin Oncol 30: 4665. [Google Scholar]
- Njar V., Brodie A. (2015) Discovery and development of galeterone (TOK-001 or VN/124–1) for the treatment of all stages of prostate cancer.J Med Chem 58: 2077–2087. [DOI] [PubMed] [Google Scholar]
- Poole A., Alva A., Batten J., Agarwal N. (2014) Metastatic castrate resistant prostate cancer: role of androgen signaling inhibitors. In: Thomas Jr C. (ed.), Prostate Cancer: A Multidisciplinary Approach to Diagnosis and Management. New York: Demos Medical Publishing, pp. 337–345. [Google Scholar]
- Robinson D., Van Allen E., Wu Y., Schultz N., Lonigro R., Mosquera J., et al. (2015) Integrative clinical genomics of advanced prostate cancer. Cell 161: 1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C., Smith M., Fizazi K., Saad F., Mulders P., Sternberg C., et al. (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16: 152–160. [DOI] [PubMed] [Google Scholar]
- Ryan C., Smith M., Fong L., Rosenberg J., Kantoff P., Raynaud F., et al. (2010) Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol 28:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad F., Fizazi K., Jinga V., Efstathiou E., Fong P., Hart L., et al. (2015a) Orteronel plus prednisone in patients with chemotherapy-naive metastatic castration-resistant prostate cancer (ELM-PC 4): a double-blind, multicentre, phase 3, randomised, placebo-controlled trial. Lancet Oncol 16: 338–348. [DOI] [PubMed] [Google Scholar]
- Saad F., Shore N., Van Poppel H., Rathkopf D., Smith M., De Bono J., et al. (2015b) Impact of bone-targeted therapies in chemotherapy-naive metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: post hoc analysis of study COU-AA-302. Eur Urol 68: 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M., Patel N., Bershadskiy A., Sokoloff A., Singer E. (2014) Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer. Asian J Androl 16: 387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigliano A., Gandini O., Cerquetti L., Gazzaniga P., Misiti S., Monti S., et al. (2007) Increased metastatic lymph node 64 and CYP17 expression are associated with high stage prostate cancer. J Endocrinol 194: 55–61. [DOI] [PubMed] [Google Scholar]
- Suzman D., Antonarakis E. (2014) Castration-resistant prostate cancer: latest evidence and therapeutic implications. Ther Adv Med Oncol 6: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydes M., Parmar M., Mason M., Clarke N., Amos C., Anderson J., et al. (2012) Flexible trial design in practice – stopping arms for lack-of-benefit and adding research arms mid-trial in stampede: a multi-arm multi-stage randomized controlled trial. Trials 13: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin M., Chu F., Morrison J., Pili R., Rettig M., Stephenson J., et al. (2012) ARMOR1: safety of galeterone (TOK-001) in a phase 1 clinical trial in chemotherapy naive patients with castration-resistant prostate cancer (CRPC). Cancer Res (AACR Meeting) 72: CT-07. [Google Scholar]
- Toren P., Kim S., Pham S., Mangalji A., Adomat H., Guns E., et al. (2015) Anticancer activity of a novel selective CYP17a1 inhibitor in preclinical models of castrate-resistant prostate cancer. Mol Cancer Ther 14: 59–69. [DOI] [PubMed] [Google Scholar]
- Vasaitis T., Belosay A., Schayowitz A., Khandelwal A., Chopra P., Gediya L., et al. (2008) Androgen receptor inactivation contributes to antitumor efficacy of 17α-hydroxylase/17,20-lyase inhibitor 3β-hydroxy-17-(1h-benzimidazole-1-Yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther 7: 2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasaitis T., Bruno R., Njar V. (2011) CYP17 inhibitors for prostate cancer therapy. J Steroid Biochem Mol Biol 125: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Ryan C., Stuyckens K., Smith M., Saad F., Griffin T., et al. (2015) Correlation between prostate-specific antigen kinetics and overall survival in abiraterone acetate-treated castration-resistant prostate cancer patients. Clin Cancer Res 21: 3170–3177. [DOI] [PubMed] [Google Scholar]
- Yin L., Hu Q., Hartmann R. (2013) Recent progress in pharmaceutical therapies for castration-resistant prostate cancer. Int J Mol Sci 14: 13958–13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Liu S., Zhang T., Xia J., Li B. (2014) Abiraterone for treatment of metastatic castration-resistant prostate cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev 15: 1313–1320. [DOI] [PubMed] [Google Scholar]