Abstract
Treatment strategies for metastatic colorectal cancer (mCRC) patients have undergone dramatic changes in the past decade and despite improved patient outcomes, there still exist areas for continued development. The introduction of targeted agents has provided clinicians with additional treatment options in mCRC, however, results have been mixed at best. These novel therapies were designed to interfere with specific molecules involved in the cellular carcinogenesis pathway and ultimately deliver a more focused treatment. Currently, their use in mCRC has been limited primarily as an adjunct to conventional chemotherapy regimens. This review explores the relevant cell-signaling networks in colorectal cancer, provides focus on the current targeted agent armamentarium approved for use in mCRC and explores the usefulness of predictive mCRC biomarkers.
Keywords: biomarkers, colorectal cancer, signaling, targeted therapy
Introduction
Colorectal cancer (CRC) represents a significant health issue as it is the most common gastrointestinal (GI) tract cancer worldwide with over 1.2 million new diagnoses each year [Ferlay et al. 2010]. It is the third most common cancer diagnosis in both men and women [Siegel et al. 2012]. Each year, there are over 520,000 people newly diagnosed with colorectal cancer in the western world [Ferlay et al. 2010]. Between 35–50% of those diagnosed will die from colorectal cancer, making it the second leading cause of cancer deaths affecting both sexes [US Cancer Statistics Working Group, 2009; Ferlay et al. 2010; Siegel et al. 2012]. Curative approaches are limited in a large proportion of patients as nearly 25% will present with metastatic disease and 40–50% of those diagnosed initially with early-stage disease will eventually develop metastatic disease [Ferlay et al. 2007; Siegel et al. 2012].
The American Joint Committee on Cancer (AJCC) reports the overall 5-year survival for colorectal cancer at 65.2% [O’Connell et al. 2004]. Early-stage disease has a more favorable prognosis and patients are frequently cured with surgical resection alone. Unfortunately most patients with advanced or metastatic disease are not suitable for resection and treatment is part of a palliative, rather than curative, approach. In such a setting, the treatment objectives are to delay disease progression, prolong survival and maintain quality of life.
Despite decades of research and some promising discoveries, the mainstay of metastatic colorectal cancer (mCRC) treatment remains based on cytotoxic chemotherapy agents such as irinotecan or oxaliplatin combined with a fluoropyrimidine and leucovorin (FOLFIRI or FOLFOX regimens) that have both shown modest outcomes when used as first-line therapy [Goldberg et al. 2004; Meyerhardt and Mayer, 2005; Tournigand et al. 2004]. When 5-fluorouracil (5-FU) and leucovorin were the only therapeutic options, the survival for patients with mCRC was between 10 to 12 months [Erlichman et al. 1992; Piedbois, 1998]. The addition of irinotecan or oxaliplatin increased overall survival (OS) to 18 months [Goldberg et al. 2004]. The addition of targeted therapies over the past 10 years has improved OS in mCRC to between 20 to 24 months [Fuchs et al. 2008; Saltz et al. 2008; Tabernero et al. 2007; Van-Cutsem et al. 2008]. Due the heterogeneous nature of cancer, a number of patients receive targeted treatments with little or no benefit to them [Simon, 2008]. Further analysis of patient nonresponders has led to the discovery of some common genetic alterations in the cancer genome that highlights the need for a more personalized approach [Stuart and Sellers, 2009]. Increased toxicity and treatment costs associated with targeted therapies have further necessitated the identification of diagnostic tools to select for patients who will benefit from such treatments. Currently, our available biomarkers are limited to identifying the patients for whom treatment is not suited, instead of those who would benefit from treatment [Schrag, 2004; Strimpakos et al. 2009; Workman et al. 2006].
Multiple critical protein-encoding genes and pathways are believed to be responsible for tumorigenesis [Cancer and Atlas, 2012]. Colorectal tumors contain a median 76 mutations, with, on average, 15 of these affecting candidate cancer genes [Vecchione et al. 2011; Wood et al. 2007]. Increased understanding of the genetic and genomic changes in CRC has helped direct therapies and predict response, as evident in patients with KRAS and BRAF mutations [Sclafani et al. 2013; Vaughn et al. 2011]. Genetic and epigenetic errors in signal-transduction pathways lead to malignant transformations and have thus emerged as key candidates for molecular-targeted therapies [Tan et al. 2009].
There are seven Food and Drug Administration (FDA)-approved targeted therapies in mCRC (Figure 1): the large-molecule monoclonal antibodies (mAbs) (bevacizumab, cetuximab, panitumumab and ramucirumab), a recombinant fusion protein (ziv-aflibercept), a small molecule inhibitor (regorafenib) and a nucleoside analog (trifluridine/tipiracil) [Grothey et al. 2013; Hurwitz et al. 2004; Van-Cutsem et al. 2010a, 2010b, 2012]. This article reviews the recent advances and evidence related to the employment of the FDA-approved targeted therapies in mCRC and explores the available biomarkers [NCI, 2015].
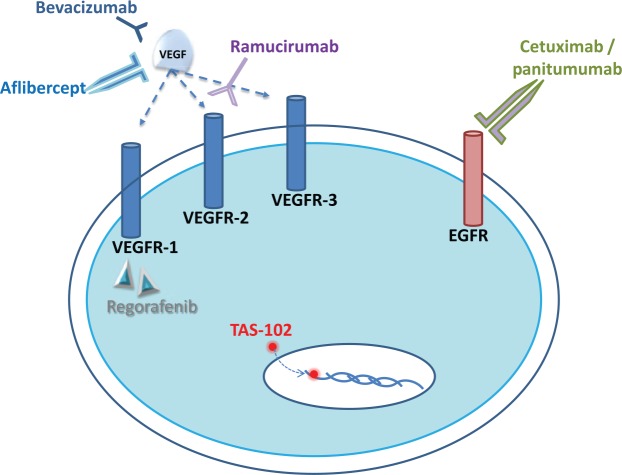
Figure 1.
Schematic of an endothelial cell depicting VEGFR-1, VEGFR-2, and VEGFR-3 and the mechanisms of action of the antiangiogenic agents bevacizumab, aflibercept, ramucirumab and regorafenib. Bevacizumab and aflibercept bind to VEGF and interrupt the interaction with VEGF receptors. Regorafenib is a small-molecule multi-kinase inhibitor of which targets include VEGFR-1 and VEGFR-3. TAS-102 consists of trifluridine that is incorporated into DNA, inducing DNA dysfunction, including DNA strand breakage. Cetuximab and panitumumab are anti-EGFR treatments that result in disruption of the MAP kinase pathway.
EGFR, endothelial growth-factor receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth-factor receptor; DNA, deoxyribonucleic acid; MAP, a protein kinase signaling pathway.
Targeting receptors in colorectal cancer
Vascular endothelial growth factor
Angiogenesis is essential for the normal physiological functions of tissues, however, it also represents a critical process for tumor growth, survival and metastasis [Risau, 1997]. Tumor cells require an extensive supply of new blood vessels to sustain their rapid growth and spread [Tanigawa et al. 1997]. Tumor vascularization occurs through the formation of new vessels from the preexisting vasculature or by insertion of interstitial tissue columns into the lumen of preexisting vessels [Hubbard and Grothey, 2010]. Numerous signaling molecules have been identified in promoting angiogenesis, including vascular endothelial growth factor (VEGF), ephrin, angiopoietin, platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF) [Folkman and Klagsbrun, 1987; Takahashi et al. 1996; Yancopoulos et al. 2000]. Among these molecules, VEGF is the most important regulator of the angiogenic process identified to date and has shown markedly increased expression in advanced colorectal tumors [Ferrara et al. 2003; Shibuya, 2011; Takahashi et al. 1995]. Rapidly dividing tumor cells outgrow their blood supply, creating a hypoxic and nutrient-deficient microenvironment, leading to activation of the hypoxia-inducible factor (HIF) system [Pugh and Ratcliffe, 2003; Tonini et al. 2003]. HIF is a critical regulatory factor in the upregulation of VEGF and numerous other proangiogenic mediators (FGF, PIGF and PDGF) from the preexisting vasculature [Eichholz et al. 2010; Hoeben et al. 2004; Wek and Staschke, 2010]. There are multiple ligands and receptors in the VEGF/VEGF-receptor (VEGFR) axis required for specific binding and the resultant activation of multiple signaling networks [Shibuya, 2001]. VEGF binding initiates a cascade of signaling processes that promote endothelial cell proliferation and migration, remodeling of the extracellular matrix, and increased vascular permeability and dilatation [Ferrara et al. 2003]. In addition to this, VEGF has been linked to endothelial progenitor cells involved in neovasculogenesis [George et al. 2011]. VEGF is therefore an attractive target when designing and developing drugs to restrict tumor angiogenesis. Numerous anti-VEGF/VEGFR-targeted therapies have demonstrated their potential to inhibit angiogenesis and tumor growth in the preclinical setting [Hicklin and Ellis, 2005].
VEGF (also known as VEGF-A) and its glycoprotein homologues (VEGF-B, VEGF-C, VEGF-D and PIGF) form a subfamily within the PDGF family of growth factors [Meyer et al. 1999; Neufeld et al. 1999; Shibuya, 2011]. VEGF and its family members mediate their angiogenic effects through differential binding to the three VEGF receptors (VEGFR-1, VEGFR-2, and VEGFR-3) [Matthews et al. 1991; Shibuya et al. 1990]. VEGF has been identified as the most important regulator of blood vessel formation [Ferrara, 1997; Hicklin and Ellis, 2005]. It is a multifunctional cytokine commonly expressed by tumor cells [Dvorak, 2002]. VEGF binds to both VEGFR-1 and VEGFR-2, inducing endothelial-cell migration and proliferation, in addition to increasing microvascular dilatation, permeability and neovascularization in cancer and other disease processes [Dvorak, 2002; Ferrara et al. 2003]. VEGFR-1 and VEGFR-2 are cell-surface-receptor tyrosine kinases (RTKs) expressed predominantly by vascular endothelial cells that activate downstream intracellular kinase-mediated signaling sequences after ligand binding [Hicklin and Ellis, 2005]. Both of these receptors act as signaling molecules during vascular development and have important roles in physiological and pathological angiogenesis in contrast to VEGFR-3, which mainly functions as a regulator of lymphangiogenesis through which it has been linked to promoting metastases [Alitalo and Carmeliet, 2002; Mustonen and Alitalo, 1995; Nathanson, 2003; Roberts et al. 2006].
The important role of VEGF-A and its receptor VEGFR-2 in tumor angiogenesis has led to a large amount of research and drug development in mCRC and other malignancies. Therapeutic agents such as bevacizumab and regorafenib have been developed with activity against the VEGF system, either by targeting its ligands, cell-surface receptors or receptor kinases.
Epidermal growth-factor receptor
The epidermal growth-factor receptor (EGFR) has emerged as a captivating therapeutic target due to its key roles in both the regulation of important normal cellular processes and in cancer pathophysiology. EGFR was one of the first growth-factor receptors to be identified and extensively studied [Cohen, 1975]. It is a ubiquitous transmembrane glycoprotein belonging to the ErbB/HER family of receptors, of which it is one of four structurally related receptor tyrosine kinases (RTKs) [Robinson et al. 2000]. These include EGFR (or ErbB-1/HER-1), ErbB-2 (HER-2), ErbB-3 (HER-3) and ErbB-4 (HER-4) [Casalini et al. 2004].
Ligand binding to the EGFR’s extracellular domain triggers receptor homo- or heterodimerization and subsequent autophosphorylation within its cytoplasmic domain [Scagliotti et al. 2004]. Phosphorylation occurs on specific tyrosine residues and creates binding sites for proteins that serve as adaptors of downstream proteins involved in signal transduction [Cohen et al. 1981]. Activated signal pathways include RAS/RAF/MAPK, PI3K/AKT, phospholipase C and JAK2/STAT3 [Fiske et al. 2009; Hynes and Lane, 2005; Yarden and Sliwkowski, 2001]. Stimulation of these pathways promotes processes responsible for tumor cell growth, proliferation, migration, survival and invasion [Citri and Yarden, 2006; Fischer et al. 2003]. There are over 10 ligands identified that bind to EGFR, ErbB-3 and ErbB-4. These include epidermal growth factor (EGF), transforming growth-factor alpha (TGF-α), heparin-binding EGF, amphiregulin, betacellulin, epiregulin, and neuregulin [Hynes and Lane, 2005; Salomon et al. 1995; Yarden and Sliwkowski, 2001]. Of these ligands, EGF and TGF-α are thought to be the most important as they selectively bind to EGFR [Jones et al. 1999].
EGFR expression is associated with solid tumor growth and is a common component of various malignancies including colorectal, lung, breast, and head and neck [Bonner et al. 2010; Nicholson et al. 2001; Pirker et al. 2009; Spaulding and Spaulding, 2002]. Inappropriate activation of EGFR can occur from receptor or ligand overexpression, gene mutation or amplification and loss of regulatory mechanisms [Kuan et al. 2001; Moscatello et al. 1996; Pedersen et al. 2005]. Abnormal EGFR activity initiates and promotes processes responsible for tumor growth and progression, including cell proliferation and maturation, angiogenesis, invasion, metastasis, and inhibition of apoptosis [Nicholson et al. 2001; Rocha-Lima et al. 2007; Yarden and Sliwkowski, 2001].
Receptor tyrosine kinase
RTKs are primary mediators of the signal transduction pathways mediating critical cellular processes, such as survival, differentiation and proliferation [Blume-Jensen and Hunter, 2001; ElShamy, 2005]. There are 58 identified RTKs with approximately 20 different classes including the VEGFR, EGFR, Her2/neu (c-erbB2), and c-Kit (stem-cell-factor receptor) [Lemmon and Schlessinger, 2010; Robinson et al. 2000]. RTKs are cell-surface allosteric enzymes consisting of a single transmembrane domain that separates an intracellular kinase domain from an extracellular ligand-binding domain [Cadena and Gill, 1992]. Tyrosine kinase activation occurs following ligand binding to the extracellular domain that drives receptor homo- or heterodimerization and autophosphorylation of the receptor complex [Casalini et al. 2004]. The phosphorylated receptor complex acts as a site for signaling proteins to assemble, leading to activation of signaling pathways such as RAS/RAF/MAPK, PI3/AKT, STAT3, and protein kinase C [Bogdan and Klämbt, 2001; Schlessinger, 2000]. Intracellular mediators in these pathways transduce signals into the nucleus, affecting DNA synthesis and cell division as well as a variety of cellular processes [Blume-Jensen and Hunter, 2001]. Growth factors or somatic mutations can effect inappropriate RTK activation, consequently promoting tumor-cell proliferation and growth [Arora and Scholar, 2005]. Tyrosine kinases have been the target of biological agents such as mAbs that can interfere with RTK activation or by small-molecule inhibitors that target the intracellular adenosine triphosphate (ATP)-binding site domain.
Targeted therapies
Over the past 10 years, the number of targeted agents used in various malignancies has increased dramatically. Currently there are seven FDA approved targeted agents in mCRC with many more in development and in clinical trials [Chu, 2012]. These targeted agents fall under the broad classification of mAbs, fusion proteins and small molecule inhibitors.
Monoclonal antibodies
MAbs were the first class of targeted agents proven to provide further benefit to patients with mCRC. Currently there are three FDA-approved monoclonal-antibody agents and they act by either binding to the ligand (e.g. bevacizumab) or the extracellular domain of a receptor (e.g. cetuximab and panitumumab) which inhibits tyrosine kinase signal-transduction pathways necessary for cancer development [Cohen et al. 2005].
Angiogenesis inhibition through molecular-targeted therapy has been researched for decades with the rationale that disruption of the VEGF–VEGFR axis might prove beneficial in cancer therapy [Folkman et al. 1971]. Antibody blockade of VEGF-A was first demonstrated in the early 1990s to suppress human-tumor growth in nude mice [Kim et al. 1993]. The antibody treatment selectively suppressed VEGF-A originating from the tumor and impressively showed significant inhibition of tumor growth without chemotherapy [Kim et al. 1993]. Clinical trials with anti-VEGF agents have not been as successful as demonstrated in the murine model, however, they have proven beneficial when in combination with standard chemotherapy regimens.
Bevacizumab
Bevacizumab (Avastin, Genentech/Roche, CA, US) is a recombinant, humanized monoclonal antibody that binds directly to all major isoforms of VEGF-A, forming a protein complex that prevents further binding to VEGF receptors [Ferrara et al. 2004]. This neutralizes VEGF signal transduction through both VEGFR-1 and VEGFR-2 and inhibits endothelial cell proliferation and angiogenesis [Ellis, 2006]. Combining an anti-VEGF agent with standard cytotoxic chemotherapy regimens enhances the suppressive effect on tumor-cell growth and the induction of apoptosis in an additive manner [Ellis, 2006]. It also stabilizes tumor vasculature and decreases its hydrostatic pressure, which improves systemic delivery of the chemotherapy agents [Ellis, 2006].
In 2004, the FDA approved bevacizumab as a first-line agent for patients with mCRC based on the results of a randomized, double-blind clinical trial of 813 patients. Bevacizumab, when administered intravenously in conjunction with the IFL regimen (irinotecan, 5-FU bolus, and leucovorin), had a significantly longer median OS than the IFL plus placebo (20.3 versus 15.6 months; Table 1). Bevacizumab plus IFL was associated with increased median progression-free survival (PFS) (10.6 versus 6.2 months), increased response rate (RR) (44.8% versus 34.8%), and longer duration of response (10.4 versus 7.1 months) [Hurwitz et al. 2004]. In 2006, results from the Eastern Cooperative Oncology Group Study (E3200) led to its approval as a second-line treatment in patients with previously treated mCRC. Following the failure of a prior irinotecan-containing regimen, patients who then received bevacizumab and FOLFOX had increased OS (from 10.8 to 12.9 months; Table 1) and PFS (from 4.7 to 7.3 months; Table 1) [Giantonio et al. 2007]. Subsequent studies have validated the addition of bevacizumab to FOLFOX or FOLFIRI regimens in untreated mCRC patients due to their improved RR and PFS [Fuchs et al. 2008; Saltz et al. 2008]. The most recent FDA approval for bevacizumab was in 2013 for use in combination with a fluoropyrimidine and either irinotecan- or oxaliplatin-based chemotherapy in mCRC patients whose disease had progressed while on a first-line bevacizumab-containing regimen. This decision was based on a large randomized international clinical trial (ML18147), which had 820 patients randomly assigned chemotherapy alone or chemotherapy in combination with beva- cizumab. The bevacizumab plus chemotherapy group had a significant improvement in OS compared with chemotherapy alone (11.2 versus 9.8 months; Table 1) [Bennouna et al. 2013]. There was also a significant improvement in median PFS which increased from 4.0 to 5.7 months with bevacizumab (Table 1) [Bennouna et al. 2013].
Table 1.
FDA-approved therapeutic monoclonal antibodies used in metastatic colorectal cancer.
| Drug | Class | Target | Study (year) | 1st or 2nd line | Regimen | Marker | Improvement (months) |
|---|---|---|---|---|---|---|---|
| Bevacizumab | mAb | VEGF-A | (2004) Hurwitz et al. [2004] | 1st | IFL | None | OS (15.6–20.3) |
| Bevacizumab | mAb | VEGF-A | E3200 (2006) Giantonio et al. [2007] | 2nd (failure of irinotecan regimen) | FOLFOX | None | OS (10.8–12.9) PFS (4.7–7.3) |
| Bevacizumab | mAb | VEGF-A | ML18147 (2013) Bennouna et al. [2013] | 2nd (progressed with bevacizumab regimen) | FOLFOX or FOLFIRI | KRAS WT | OS (9.8–11.2) PFS (4.0–5.7) |
| Cetuximab | mAb | EGFR | BOND (2004) Cunningham et al. [2004] | 2nd (failure of irinotecan regimen) | FOLFIRI | None | TSR (22.9%) TGD (4.1) |
| Cetuximab | mAb | EGFR | BOND (2004) Cunningham et al. [2004] | 2nd (intolerant of irinotecan) | Mono tx | None | TSR (10.8%) TGD (1.5) |
| Cetuximab | mAb | EGFR | CRYSTAL (2012)Van-Cutsem et al. [2007] | 1st line (KRAS WT) | FOLFIRI | KRAS WT | PFS (8.4–9.9) |
| Panitumumab | mAb | EGFR | (2006) Giusti et al. [2007] | 2nd (failure of FOLFOX/ FOLFIRI) | BSC | None | PFS (7.3–8.0 weeks) OS (0–10%) |
| Panitumumab | mAb | EGFR | PRIME (2010) Douillard et al. [2010] | FOLFOX4 | KRAS WT | PFS (8.0–9.6) | |
| Ramucirumab | mAb | VEGF-R2 | RAISE Tabernero et al. [2015] | 2nd (progressed with bevacizumab, oxaliplatin and a fluoropyrimidine) | FOLFIRI | None | OS (11.7–13.3) PFS (4.5–5.7) |
EGFR, endothelial growth-factor receptor; VEGFR, vascular endothelial growth factor receptor; FOLFIR, chemotherapy regimen that includes FOL – Folinic acid (leucovorin, calcium folinate or FA), F – Fluorouracil (5FU), IRI – Irinotecan hydrochloride; FOLFOX4, chemotherapy regimen that includes FOL – Folinic acid (leucovorin, calcium folinate or FA), F – Fluorouracil (5FU), OX (Oxaliplatin); IFL, chemotherapy regimen that includes I (Irinotecan), F (Fluorouracil (5FU)), L (Leucovorin); mAb, monoclonal antibody; KRAS Kirsten ras proto-oncogene; WT wild type.
Treatment with bevacizumab is relatively safe but there are some risks. Early clinical trials suggested that treatment with bevacizumab alone or with chemotherapy resulted in an increased incidence of thrombosis, bleeding, proteinuria, and hypertension [Gordon et al. 2001; Kabbinavar and Hurwitz, 2003; Yang et al. 2003]. Hurwitz and colleagues found similar adverse effects in mCRC patients receiving bevacizumab therapy but also noted there was a large incidence of patients developing grade 3 hypertension (requiring treatment) [Hurwitz et al. 2004]. A recent meta-analysis on the safety of bevacizumab therapy in patients with advanced cancer concluded that there was a slightly higher risk for any severe (grade 3 or 4) adverse event compared with chemotherapy alone [Geiger-Gritsch et al. 2010].
Cetuximab and panitumumab
Cetuximab (Erbitux, ImClone, NJ, US) and panitumumab (Vectibix, Amgen, CA, US) are mAbs with FDA approval for use in mCRC. They differ from bevacizumab in their mechanism of action by targeting EGFR, which is associated with tumor progression and a worse prognosis in mCRC and other GI tract malignancies [Kaklamanis and Gatter, 1992; Yasui et al. 1988]. Cetuximab is a chimeric human-murine immunoglobulin (IgG1), whereas panitumumab (IgG2) is fully humanized and therefore believed to have less cellular cytotoxicity [Kimura et al. 2007; Saltz et al. 2006]. Cetuximab and panitumumab bind specifically to EGFR on both normal and tumor cells, and competitively inhibit the binding of EGF, TGF-α and other ligands [Baselga, 2001]. Both mAbs block downstream signaling by binding to the EGFR’s extracellular domain, which prevents further ligand binding, sterically hinders dimerization with other RTKs and induces EGFR degradation [Cohen et al. 2005; Li et al. 2005; Saltz et al. 2006]. Blocking EGFR activation and subsequent impairment of downstream signaling (RAS-RAF-MAP kinase pathway) results in inhibition of cell growth, induction of apoptosis, decreased matrix metalloproteinase (MMPs) and VEGF production [Vincenzi et al. 2010].
There are numerous oncogenic mutations present in CRC which have contributed to the lack of clinical success with targeted therapies in some patient cohorts. Intrinsic or acquired resistances from mutations can lead to a significant variability in clinical response. Identification of the KRAS gene mutation as a marker of impending failure of EGFR-targeted therapy was the first large step in tailoring treatment of individuals [Amado et al. 2008; Khambata-Ford et al. 2007; Lievre et al. 2008; Normanno et al. 2009]. The RAS family comprises some small GTPases (hydrolase enzymes that bind and hydrolyze guanosine triphosphate) that are integral constituents of signaling networks contributing to a multitude of vital cellular processes [Bos, 1989]. Frequent oncogenic mutations are found in members of the RAS subfamily (KRAS, NRAS, and HRAS), which lead to tumor development [Fernández-Medarde and Santos, 2011]. KRAS is a critical mediator of EGFR-induced signaling. Activation of EGFR recruits proteins to the cell membrane and causes KRAS to become activated, which results in signaling through the PI3-K/AKT and MAPK (also known as ERK) pathways [Schubbert et al. 2007]. KRAS mutants are unable to hydrolyze RAS-GTP to RAS-GDP and thus cannot be restrained, leading to EGFR-independent activation [Schubbert et al. 2007].
KRAS mutations have been detected in 40–45% of CRC samples with a high grade of concordance between primary and metastatic sites [Loupakis et al. 2009; Vaughn et al. 2011]. NRAS and HRAS mutations are less commonly found in CRC (1–3% of samples) [Irahara et al. 2010; Vaughn et al. 2011]. Most KRAS mutations are missense and affect codons 12 and 13 of exon 2 [Amado et al. 2008; Hayashi et al. 1995]. The mutation at codon 12 is the most prevalent (80% versus 20%) and oncogenic of the two [Guerrero et al. 2000]. More recently, KRAS mutations on codons 61 and 146, and exons 3 and 4 have also been reported to decrease anti-EFGR therapy [Douillard et al. 2013; Heinemann et al. 2014; Loupakis et al. 2009]. In addition to KRAS, there is strong evidence to support BRAF and NRAS mutations inhibiting the effect of anti-EGFR therapy [De Roock et al. 2010]. The BRAF mutation has been shown to be a strong negative prognostic factor in CRC [Eklof et al. 2013]. The BRAF gene encodes a serine threonine protein kinase which is directly activated by KRAS and leads to stimulation of the MAPK pathway [Di Fiore et al. 2010; Wan et al. 2004]. The average prevalence of BRAF mutations in colorectal cancer is an estimated 9.6%, with the valine-to-glutamic-acid-amino-acid (V600E) substitution being the most common [Davies et al. 2002; Safaee Ardekani et al. 2012]. BRAF mutations are considered mutually exclusive with KRAS mutations, as concomitant tumor mutations are extremely rare [Sahin et al. 2013]. In a pooled analysis of the CRYSTAL and OPUS randomized clinical trials, BRAF mutations were found to be a marker of poor prognosis but not an effective biomarker predictor in patients treated with anti-EGFR mAbs [Bokemeyer et al. 2012]. NRAS is a proto-oncogene from the RAS family and its mutations on exon 2, 3, and 4 have been shown to be effective predictors of anti-EGFR resistance [Douillard et al. 2013; Heinemann et al. 2014]. PIK3CA mutations on exon 9 and 20 often coexist with KRAS mutations and are associated with poor survival in patients treated with anti-EGFR therapy [Perrone et al. 2009; Wu et al. 2013].
Anti-EGFR mAbs therefore have minimal if not harmful results in patients with KRAS mutations due to their EGFR-independent activation of oncogenic signaling cascades [Benvenuti et al. 2007]. The CRYSTAL study, along with the supportive cetuximab studies, have clearly demonstrated that the presence of KRAS mutations negatively affects the anti-EGFR therapies [Chau and Cunningham, 2009; Dahabreh et al. 2011]. This finding led to National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology and the American Society for Clinical Oncology (ASCO) guidelines to recommend restricting anti-EGFR agents to mCRC patients with a wild-type KRAS allele [Allegra et al. 2009; Jimeno et al. 2009].
The prognostic potential of KRAS mutations in mCRC and its impact on the effectiveness of chemotherapy or anti-VEGF inhibition remains undefined. The KRAS pathway has previously been shown to upregulate angiogenic factors and recently, a study demonstrated KRAS mutant cells to express higher levels of VEGF-A [Downward, 2003; Figueras et al. 2013; Zhang et al. 2001]. Retrospective analysis of clinical benefit from bevacizumab in patients with wild- or mutant-type KRAS tumors has found comparable benefits in PFS and OS [Hurwitz et al. 2009].
Both anti-EGFR treatments appear to be well tolerated, with a low incidence of grade 3 or 4 adverse events. The most common adverse event with cetuximab was an acneiform rash. Other adverse events normally associated with cetuximab therapy include infusion reactions, cardiac events, and hypomagnesemia, as observed in the wild-type KRAS populations of the CRYSTAL, OPUSS and CA225025 trials [Hubbard and Alberts, 2013]. The most common adverse events with panitumumab use were skin rash, hypomagnesemia, paronychia, fatigue, abdominal pain, nausea, and diarrhea [Giusti et al. 2007].
In 2004, cetuximab became the first anti-EGFR mAb approved by the FDA for use in mCRC. It was approved as a second-line therapy for use in irinotecan-refractory or intolerant patients with EGFR-expressing tumors. Approval was based on a randomized, two-arm phase II clinical trial (BOND study) of 329 patients. Cetuximab combined with irinotecan significantly improved RRs (22.9% versus 10.8%; Table 1) and time to progression (TTP) (4.1 versus 1.5 months; Table 1) compared with cetuximab alone [Cunningham et al. 2004]. The results demonstrated that interfering with EGFR signaling can resensitize tumors that are refractory to irinotecan. In 2012, the FDA expanded its approval of cetuximab for use as a first-line treatment in patients with KRAS wild type (mutation negative), EGFR-expressing mCRC. The decision was based on retrospective analyses according to KRAS mutation status of tumor samples from patients enrolled in the CRYSTAL trial and two supportive studies (CA225025 and OPUS). The addition of cetuximab to chemotherapy or best supportive care (BSC) resulted in improved OS, PFS and objective response rate (ORR) in patients with KRAS wild-type tumors [Bokemeyer et al. 2012]. The use of cetuximab in patients with KRAS mutant tumors provided no benefit, and even potential harm.
The CRYSTAL (cetuximab combined with irinotecan in first-line therapy for mCRC) trial was a phase III open-label, randomized, multicenter study that included 1217 patients (irrespective of KRAS status) who had not received prior chemotherapy for mCRC. A significant improvement in median PFS was observed for the cetuximab plus FOLFIRI arm compared with the FOLFIRI only arm (8.9 versus. 8.1 months) [Van-Cutsem et al. 2007]. There were minor but not significant differences in the median OS (19.6 versus 18.5 months) and the ORR (46% versus 38%) in both trial arms [Van-Cutsem et al. 2007]. However, following retrospective analyses of patient subsets for KRAS status, the results were more favorable in the KRAS wild-type patients given cetuximab. An updated survival analysis in 2011 further supported the addition of cetuximab to FOLFIRI as first-line therapy in patients with KRAS wild-type as these patients had increased median PFS (9.9 versus 8.4 months; Table 1), median OS (23.5 versus 20.0 months) and ORR (57.3% versus 39.7%) compared with FOLFIRI alone [Van-Cutsem et al. 2011]. The patients with KRAS mutations did not benefit from the addition of cetuximab as they had no improvement in median PFS (8.1 versus 7.5 months), OS (15.3 versus 15.8 months) and ORR (31.0% versus 45.0 %) compared with FOLFIRI alone [Van-Cutsem et al. 2011].
CA225025 was an open-label randomized trial that compared cetuximab plus BSC with BSC alone in 572 patients with previously treated EGFR-expressing mCRC. Among patients with wild-type KRAS, cetuximab significantly increased median OS (8.6 versus 5.0 months) and PFS (3.8 versus 1.9 months). No benefits were observed in the mutant KRAS patients treated with cetuximab.
OPUS (oxaliplatin and cetuximab in first-line treatment of mCRC) was a phase II open-label, randomized study that compared FOLFOX-4 (fluorouracil, leucovorin, and oxaliplatin) plus cetuximab versus FOLFOX-4 alone in 337 untreated EGFR-expressing mCRC patients [Bokemeyer et al. 2009]. KRAS wild-type patients who received cetuximab plus FOLFOX-4 had increased ORR (57% versus 34%) and PFS (8.3 versus 7.2 months) compared with those receiving only FOLFOX-4 [Bokemeyer et al. 2011]. Median survival time was improved with cetuximab plus FOLFOX-4 but it was not statistically significant (22.8 versus 18.5 months) [Bokemeyer et al. 2011]. Patients with KRAS mutations who received cetuximab plus FOLFOX-4 had a decreased ORR (34% versus 53%) and PFS (5.5 versus 8.6 months) compared with those receiving FOLFOX-4 alone [Bokemeyer et al. 2011].
A recent comprehensive meta-analysis examined the effect of anti-EGFR mAbs in mCRC patients expressing wild-type KRAS compared with mutant KRAS [Vale et al. 2012]. A total of 10 out of 14 RCTs identified had available KRAS status. As expected, there was a positive effect on PFS when anti-EGFR mAbs were used in patients with wild-type KRAS-expressing tumors but not in the mutant KRAS patients. The PFS benefits were confined to trials combining mAbs alongside 5FU-based chemotherapy. There was also no evidence of a PFS benefit when anti-EGFR mAbs were given with bevacizumab.
In 2006, the FDA provided accelerated approval to panitumumab (Vectibix) for the treatment of patients with EGFR-expressing, mCRC with disease progression on or following a FOLFOX/FOLFIRI-containing regimen. The approval was based on the findings of a single, open-label, multinational phase III study that randomized 463 patients to receive panitumumab plus BSC or BSC alone. The median PFS was significantly greater in patients receiving panitumumab compared with BSC alone (8.0 versus 7.3 weeks; Table 1) [Giusti et al. 2007]. The ORR also favored panitumumab (10.0% versus 0%; Table 1). There were 19 partial responses (8%) with a median duration of 17 weeks among the panitumumab group. Retrospective analysis of the study provided further evidence to the importance of KRAS status as clinical benefit was specific to patients with wild-type KRAS tumors given panitumumab monotherapy. The median PFS in the wild-type KRAS group treated with panitumumab was 12.3 weeks compared with 7.3 weeks for BSC [Amado et al. 2008]. Panitumumab RRs were also improved in the wild-type KRAS group (17% versus 0%). There was no difference in OS between the two study arms, likely due to the crossover design.
The PRIME (panitumumab randomized trial in combination with chemotherapy for metastatic colorectal cancer to determine efficacy) study examined the efficacy and safety of panitumumab in combination with FOLFOX-4. This was a multicenter phase III trial that enrolled 1183 patients with no prior chemotherapy for mCRC. In the wild-type KRAS group, panitumumab plus FOLFOX-4 significantly improved PFS compared with FOLFOX-4 (9.6 versus 8.0 months; Table 1) and nonsignificantly improved the median OS (23.9 versus 19.7 months) [Douillard et al. 2010]. In the mutant KRAS group, panitumumab plus FOLFOX-4 had a negative effect on both PFS and median OS compared with FOLFOX-4 (15.5 versus 19.3 months).
A meta-analysis in 2011 of four randomized clinical studies found significant clinical benefit for panitumumab-based therapy in wild-type KRAS mCRC patients following prior chemotherapy exposure [Ibrahim and Abouelkhair, 2011]. There was an associated 42% improvement in PFS when panitumumab was used as a second-line therapy but no benefit in the first-line setting [Ibrahim and Abouelkhair, 2011].
Both cetuximab and panitumumab are indicated for the treatment of EGFR-expressing, mCRC. Panitumumab approval is for patients with disease progression while on, or following a FOLFOX/FOLFIRI-containing regimen, whereas cetuximab is for use with FOLFIRI as a first-line treatment and also in patients who are irinotecan intolerant or refractory. Panitumumab approval was based on its improvement of PFS, while cetuximab approval was based on ORR. Neither anti-EGFR agent demonstrated a statistically significant benefit in OS, representing a change in the accepted endpoints of a treatment, as previous new agents required an improvement in OS to gain FDA approval [Berlin et al. 2006; Tabernero et al. 2007].
Ramucirumab
Ramucirumab (Cyramza; Eli Lilly and Co., Indianapolis, IN, US) became the latest FDA-approved mAb on 24 April 2015 [Goel and Sun, 2015]. It is now indicated in combination with FOLFIRI for the treatment of patients with mCRC whose disease has progressed on a first-line bevacizumab-, oxaliplatin- and fluoropyrimidine-containing regimen [Tabernero et al. 2015]. Ramucirumab is a recombinant human monoclonal IgG1 antibody that binds and blocks further activity of the human VEGF-R2 with its ligands. Approval was based on the RAISE trial which was a randomized, double-blind, multinational trial enrolling patients with mCRC that progressed during or within 6 months of discontinuation of bevacizumab-, oxaliplatin- and fluoropyrimidine-based combination chemotherapy [Tabernero et al. 2015]. The clinical trial consisted of 1072 patients who were randomly allocated (1:1) to receive FOLFIRI plus placebo or FOLFIRI plus ramucirumab (n = 536 per arm) as an intravenous infusion every two weeks. The primary efficacy endpoint of the study was OS. A statistically significant OS improvement was observed in patients receiving FOLFIRI plus ramucirumab compared with those receiving FOLFIRI plus placebo (13.3 versus 11.7 months; Table 1). PFS was also significantly improved in patients who received ramucirumab in combination with FOLFIRI (5.7 versus 4.5 months; Table 1). The infusion was generally well tolerated, however, thyroid dysfunction was noted in 2.6% of patients.
Fusion proteins
Ziv-aflibercept
In 2012, the FDA approved ziv-aflibercept (Zaltrap; Sanofi and Regeneron Pharmaceuticals, Inc., Tarrytown, NY, US) for the treatment of mCRC that has progressed following an oxaliplatin-containing regimen. Ziv-aflibercept (previously known as aflibercept) is a recombinant fusion protein consisting of VEGF-binding sections from the extracellular domains of human VEGFR-1 and VEGFR-2 attached to the Fc portion of human IgG1 immunoglobulin [Wang and Lockhart, 2012]. Ziv-aflibercept binds to and inactivates circulating VEGF, VEGF-B and PlGF ligands, preventing their interaction with VEGF receptors [Holash et al. 2002]. FDA approval was based on the VELOUR trial, an international randomized double-blind study in which 1226 patients received FOLFIRI with either ziv-aflibercept or placebo [Van-Cutsem et al. 2012]. These patients all had disease progression during or within 6 months of receiving oxaliplatin-based chemotherapy with or without bevacizumab. A significant improvement in OS (13.5 versus 12.1 months; Table 2), PFS (6.9 versus 4.7 months; Table 2) and RR (20% versus 11%; Table 2) was observed in patients receiving the FOLFIRI plus zib-aflibercept regimen compared with the placebo cohort [Van-Cutsem et al. 2012]. Further subgroup analysis found the addition of ziv-aflibercept to FOLFIRI had a trend of increased OS and PFS, regardless of prior bevacizumab use [Allegra et al. 2012].
Table 2.
US Food and Drug Administration (FDA)-approved therapeutic targeted inhibitors used in metastatic colorectal cancer.
| Drug | Class | Target | Study (year) | 1st or 2nd line | Regimen | Marker | Improvement |
|---|---|---|---|---|---|---|---|
| Aflibercept | Fusion Ab | VEGF ligand | VELOUR (2012) Van-Cutsem et al. [2012] | 2nd (failure of oxaliplatin) | FOLFIRI | None | OS (12.1–13.5) PFS (4.7–6.9) RR (11–22%) |
| Regorafenib | Multikinase | VEGF TIE2 |
CORRECT (2012) Grothey et al. [2013] | 3rd (failure of standard therapies) | BSC | None | OS (5–6.4) PFS (1.7–2.0) RR (15–44%) |
| Trifluridine/tipiracil | Nucleoside analog | DNA | RECOURSE (2015) Mayer et al. [2015] | 3rd (failure of standard therapies + biological | None | OS (5.3–7.1) PFS (1.7–2.0] |
DNA, deoxyribonucleic acid; VEGF, vascular endothelial growth factor; BSC, best supportive care; FOLFIRI, irinotecan or oxaliplatin combined with a fluoropyrimidine and leucovorin; OS, overall survival; PFS, progression-free survival; RR, response rate.
Small-molecule inhibitors
mAbs target circulating growth factors or receptors on the cell exterior whereas small-molecule inhibitors block cell signaling pathways from within. These inhibitors primarily compete with ATP for the ATP-binding site in the hinge region of the kinase receptor by mimicking the hydrogen bonds formed by the adenine ring of ATP [Liu and Gray, 2006]. Other compounds allosterically inhibit the catalytic activity by binding outside the active site [Zhang et al. 2009]. Small-molecule inhibitors can either target a single receptor only, such as gefitinib (targets EGFR only), or they can target multiple receptors, as in the use of sorafenib (which targets VEGFR, PDGFR, c-kit, Raf, flt-3 and RET) [Ranson et al. 2002; Yau et al. 2009]. The most successful use of tyrosine kinase inhibitors in clinical practice has been with gastrointestinal stromal tumors (GISTs) and the inhibition of c-Kit. Most solid tumors have multiple genetic alterations in specific proteins affecting a number of signaling networks making it difficult to target with single inhibitors.
Regorafenib
Regorafenib (BAY 73-4506; Bayer Pharma AG, Berlin, Germany) is an oral multikinase small-molecule inhibitor that blocks several protein kinases involved in tumor growth and angiogenesis which include VEGFR-1, VEGFR-2, VEGFR-3, TIE2, RET, KIT, PDGFR and FGFR [Bhargava and Robinson, 2011; Wilhelm et al. 2011]. Additionally, it disrupts the downstream tumor-signaling cascades by binding to the serine/threonine-specific protein kinase BRAF in the MAPK pathway responsible for stimulating cell growth [Wilhelm et al. 2011]. In 2012, regorafenib became the first FDA-approved small-molecule inhibitor for use in mCRC when combined with FOLFIRI. This was based on the results of a pivotal phase III, multinational trial called CORRECT, which randomized 760 patients to receive BSC plus either regorafenib or placebo. All the patients had already progressed during or within 3 months of their last standard approved therapies. Regorafenib displayed an increased median OS (6.4 versus 5 months; Table 2), PFS (2.0 versus 1.7 months; Table 2) and RR (44% versus 15%; Table 2) [Grothey et al. 2013]. The 1.4 month increase in OS equates a 23% reduction in risk of death in a patient population with a very poor prognosis and few options.
Nucleoside analog
TAS-102 is a combination of trifluridine and tipiracil (LONSURF; Taiho Oncology, Inc., Princeton, NJ, US), the most recent targeted agent to gain FDA approval on 22 September 2015. It is indicated in the treatment of patients with mCRC who have previously been treated with fluoropyrimidine-, oxaliplatin-, and irinotecan-based chemotherapy, an anti-VEGF biologic product, and an anti-EGFR mAb, if RAS is wild type [Mayer et al. 2015]. The drug is an oral combination therapy consisting of trifluridine (a thymidine-based nucleoside analog), plus tipiracil hydrochloride (a novel thymidine phosphorylase inhibitor) [Lenz et al. 2015]. TAS-102 is a dual-targeting formulation, with its major mechanism of action through trifluridine being incorporated into DNA during DNA synthesis, thereby causing DNA dysfunction and damage [Peters, 2015]. The thymidine phosphorylase inhibitor (tipiracil) prevents the degradation of trifluridine.
Approval was based on a multicenter, double-blind, placebo-controlled trial (RECOURSE study) involving 800 patients with previously treated mCRC [Mayer et al. 2015]. The two arms of the study had patients receiving trifluridine/tipiracil (n = 534) plus BSC or matching placebo (n = 266) plus BSC. The inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) status of 0 or 1, absence of brain metastasis, and absence of ascites requiring drainage in the 4 weeks leading to treatment.
A statistically significant improvement in OS was demonstrated in the trifluridine/tipiracil compared with the placebo arm (7.1 versus 5.3 months; Table 2). PFS was also improved in patients randomly allocated to receive trifluridine/tipiracil (2.0 versus 1.7 months; Table 2).
The most common adverse drug reactions or laboratory abnormalities were neutropenia (38%), anemia (18%), and thrombocytopenia (5%) [Mayer et al. 2015].
Combination therapies
Anti-VEGF anti-EGFR
Paul Ehrlich’s magic bullet theory has been realized to some extent with selective-binding agents but the effects are not as overwhelming as anticipated [Winau et al. 2004]. The vision of targeted cancer therapies have not reached their full potential; in part due to the complexity of multiple and often redundant molecular pathways that promote oncogenic cellular processes [Tortora et al. 2008]. Therefore, it is rationalized that multiple-targeted agents may be required to selectively inhibit the numerous tumor pathways [Johnson and Dippold, 1989]. Preclinical studies had suggested that combined blockade of both VEGF and EGFR may be beneficial [Jung et al. 2002; Shaheen et al. 2001]. Dual targeting of VEGF and EGFR, two functionally linked and closely related targets could interfere with the molecular feedback loops responsible for acquired resistance and potentially increase the antitumor effects of the individual agents [Saltz et al. 2007].
This theory was supported with the results of BOND-2 (bevacizumab and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer), a randomized, phase II feasibility study of 83 irinotecan-refractory mCRC patients. It demonstrated that the triple combination of irinotecan, cetuximab, and bevacizumab achieved better results in irinotecan-refractory mCRC compared with only cetuximab and bevacizumab. The triple-therapy arm had increased time to tumor progression (7.3 versus 4.9 months), objective RR (37% versus 20%) and OS (14.5 versus 11.4 months) [Saltz et al. 2007].
Further studies would not support the good anti-VEGF/EGFR results seen in BOND-2. The CAIRO-2 study, a large multi-institutional clinical trial conducted in the Netherlands, had 755 patients with previously untreated mCRC randomly assigned to receive bevacizumab plus CAPOX (capecitabine and oxaliplatin), or the same regimen accompanied by cetuximab [Tol et al. 2009]. Surprisingly, the addition of cetuximab worsened median PFS (10.7 versus 9.4 months) and subset analysis demonstrated no improved outcome in patients with wild-type KRAS [Tol et al. 2009]. There was even a significant detrimental effect in PFS (8.1 versus 10.5 months) to patients with mutated KRAS receiving bevacizumab and cetuximab [Tol et al. 2009]. The incidence of adverse events was similar in both treatment groups after the exclusion of cetuximab-related adverse cutaneous effects.
A similar negative outcome was reported in the Panitumumab Advanced Colorectal Cancer Evaluation (PACCE) trial in which previously untreated mCRC patients were randomly assigned to receive chemotherapy (FOLFOX or FOLFIRI) and bevacizumab, either alone or accompanied by panitumumab. The addition of panitumumab to the FOLFOX group reduced both the median PFS (10.0 versus 11.4 months) and the median OS (19.4 versus 24.5 months) [Hecht et al. 2009]. A similar pattern was observed in the smaller FOLFIRI cohort, although the differences were not statistically significant. The PACCE trial was prematurely discontinued due to the negative results and increased adverse events (skin toxicity, diarrhea, infections and pulmonary embolism) in the panitumumab group. There is no obvious reason for the negative effect observed by the combination of an anti-VEGF and anti-EGFR mAbs with standard chemotherapy regimens.
The encouraging results observed in anti-VEGF/EGFR preclinical studies were not validated when examined in randomized trials. The failure of combined targeted therapies illustrates the difficulties and level of understanding we have of molecular oncology. It is possible that there is some interaction between the two antibodies and cytotoxic chemotherapy which negatively affected outcomes in PACCE and CAIRO-2 [Blanke, 2009].
Conclusion
Treatment options for mCRC continue to emerge, however, there remains a number of challenges to overcome. The complicated signaling pathways and network cross-talk involved in tumorigenesis must be more effectively targeted. There is also the dynamic tumor microenvironment, genetic instabilities and host immune responses to be better understood. Further development of therapies aimed at membrane receptors, intracellular signaling molecules and other protein kinase targets is ongoing. All of these potential targets demonstrate the complexity of cancer and showcase the unlikelihood of finding a ‘magical bullet’ therapy that will work for all patients. Some promising breakthroughs have been made researching the role of HER2 amplification and microsatellite instability in mCRC patients. As we move forward, further progress in identifying new targeted therapies with associated predictive biomarkers is essential.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared that there is no conflict of interest.
Contributor Information
Andrew Moriarity, St James’s Hospital, Surgical Oncology, St James’s St, Dublin 8, Ireland.
Jacintha O’Sullivan, St James’s Hospital, Dublin, Ireland.
John Kennedy, St James’s Hospital, Dublin, Ireland.
Brian Mehigan, St James’s Hospital, Dublin, Ireland.
Paul McCormick, St James’s Hospital, Dublin, Ireland.
References
- Alitalo K., Carmeliet P. (2002) Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1: 219–227. [DOI] [PubMed] [Google Scholar]
- Allegra C., Jessup J., Somerfield M., Hamilton S., Hammond E., Hayes D., et al. (2009) American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27: 2091–2096. [DOI] [PubMed] [Google Scholar]
- Amado R., Wolf M., Peeters M., Van Cutsem E., Siena S., Freeman D., et al. (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26: 1626–1634. [DOI] [PubMed] [Google Scholar]
- Arora A., Scholar E. (2005) Role of tyrosine kinase inhibitors in cancer therapy. J Pharmacol Exp Ther 315: 971–979. [DOI] [PubMed] [Google Scholar]
- Baselga J. (2001) The EGFR as a target for anticancer therapy–focus on cetuximab. Eur J Cancer 37: S16–S22. [DOI] [PubMed] [Google Scholar]
- Bennouna J., Sastre J., Arnold D., Österlund P., Greil R., Van Cutsem E., et al. (2013) Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase III trial. Lancet Oncol 14: 29–37. [DOI] [PubMed] [Google Scholar]
- Benvenuti S., Sartore-Bianchi A., Di Nicolantonio F., Zanon C., Moroni M., Veronese S., et al. (2007) Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67: 2643–2648. [DOI] [PubMed] [Google Scholar]
- Berlin J., Neubauer M., Swanson P. (2006) Panitumumab antitumor activity in patients (pts) with metastatic colorectal cancer (mCRC) expressing ⩾10% epidermal growth factor receptor (EGFr). J Clin Oncol 24: 3548.16877720 [Google Scholar]
- Bhargava P., Robinson M. (2011) Development of second-generation VEGFR tyrosine kinase inhibitors: current status. Curr Oncol Rep 13: 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke C. (2009) Dual-antibody therapy in advanced colorectal cancer: gather ye rosebuds while ye may. J Clin Oncol 27: 655–658. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P., Hunter T. (2001) Oncogenic kinase signalling. Nature 411: 355–365. [DOI] [PubMed] [Google Scholar]
- Bogdan S., Klämbt C. (2001) Epidermal growth factor receptor signaling. Curr Biol 11: R292–R295. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C., Bondarenko I., Hartmann J., de Braud F., Schuch G., Zubel A., et al. (2011) Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 22: 1535–1546. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C., Bondarenko I., Makhson A., Hartmann J., Aparicio J., de Braud F., et al. (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27: 663–671. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C., Van Cutsem E., Rougier P., Ciardiello F., Heeger S., Schlichting M., et al. (2012) Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 48: 1466–1475. [DOI] [PubMed] [Google Scholar]
- Bonner J., Harari P., Giralt J., Cohen R., Jones C., Sur R., et al. (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase III randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 11: 21–28. [DOI] [PubMed] [Google Scholar]
- Bos J. (1989) Ras oncogenes in human cancer: a review. Cancer Res 49: 4682–4689. [PubMed] [Google Scholar]
- Cadena D., Gill G. (1992) Receptor tyrosine kinases. FASEB J 6: 2332–2337. [DOI] [PubMed] [Google Scholar]
- Cancer T., Atlas G. (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalini P., Iorio M., Galmozzi E., Ménard S. (2004) Role of HER receptors family in development and differentiation. J Cell Physiol 200: 343–350. [DOI] [PubMed] [Google Scholar]
- Chau I., Cunningham D. (2009) Treatment in advanced colorectal cancer: what, when and how? Br J Cancer 100: 1704–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. (2012) An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin Colorectal Cancer 11: 1–13. [DOI] [PubMed] [Google Scholar]
- Citri A., Yarden Y. (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7: 505–516. [DOI] [PubMed] [Google Scholar]
- Cohen S. (1975) Human epidermal growth factor: isolation and chemical and biological properties. Proc Natl Acad Sci 72: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., King L. (1981) Epidermal growth factor-receptor-protein kinase interactions. Prog Clin Biol Res 66: 557–567. [PubMed] [Google Scholar]
- Cohen S., Cohen R., Meropol N. (2005) Targeting signal transduction pathways in colorectal cancer–more than skin deep. J Clin Oncol 23: 5374–5385. [DOI] [PubMed] [Google Scholar]
- Cunningham D., Humblet Y., Siena S., Khayat D., Bleiberg H., Santoro A., et al. (2004) cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351: 337–345. [DOI] [PubMed] [Google Scholar]
- Dahabreh I., Terasawa T., Castaldi P., Trikalinos T. (2011) Systematic review: anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med 154: 37–49. [DOI] [PubMed] [Google Scholar]
- Davies H., Bignell G., Cox C., Stephens P., Edkins S., Clegg S., et al. (2002) Mutations of the BRAF gene in human cancer. Nature 417: 949–954. [DOI] [PubMed] [Google Scholar]
- De Roock W., Claes B., Bernasconi D., De Schutter J., Biesmans B., Fountzilas G., et al. (2010) Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 11: 753–762. [DOI] [PubMed] [Google Scholar]
- Di Fiore F., Sesboüé R., Michel P., Sabourin J., Frebourg T. (2010) Molecular determinants of anti-EGFR sensitivity and resistance in metastatic colorectal cancer. Br J Cancer 103: 1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard J., Oliner K., Siena S., Tabernero J., Burkes R., Barugel M., et al. (2013) Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369: 1023–1034. [DOI] [PubMed] [Google Scholar]
- Douillard J., Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., et al. (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28: 4697–4705. [DOI] [PubMed] [Google Scholar]
- Downward J. (2003) Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3: 11–22. [DOI] [PubMed] [Google Scholar]
- Dvorak H. (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20: 4368–4380. [DOI] [PubMed] [Google Scholar]
- Eichholz A., Merchant S., Gaya A. (2010) Anti-angiogenesis therapies: their potential in cancer management. Onco Targets Ther 3: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklof V., Wikberg M., Edin S., Dahlin A., Jonsson B., Öberg Å. (2013) The prognostic role of KRAS, BRAF, PIK3CA and PTEN in colorectal cancer. Br J Cancer 108: 2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. (2006) Mechanisms of action of bevacizumab as a component of therapy for metastatic colorectal cancer. Semin Oncol 33: S1–S7. [DOI] [PubMed] [Google Scholar]
- ElShamy W. (2005) Epithelial to mesenchymal transition, cell surface receptors activation and intracellular communications in cancer metastasis. Cancer Ther 3: 443–460. [Google Scholar]
- Erlichman C., Carlson R., Valone F., Labianca R., Park R., Hospital M., et al. (1992) Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: evidence in terms of response rate. Advanced Colorectal Cancer Meta-Analysis Project. J Clin Oncol 10: 896–903. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Autier P., Boniol M., Heanue M., Colombet M., Boyle P. (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18: 581–592. [DOI] [PubMed] [Google Scholar]
- Ferlay J., Shin H., Bray F., Forman D., Mathers C., Parkin D. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- Fernández-Medarde A., Santos E. (2011) Ras in cancer and developmental diseases. Genes Cancer 2: 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. (1997) The biology of vascular endothelial growth factor. Endocr Rev 18: 4–25. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Gerber H., LeCouter J. (2003) The biology of VEGF and its receptors. Nat Med 9: 669–676. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Hillan K., Gerber H., Novotny W. (2004) Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391–400. [DOI] [PubMed] [Google Scholar]
- Figueras A., Arbos M., Quiles M., Viñals F., Germà J., Capellà G. (2013) The impact of KRAS mutations on VEGF-A production and tumour vascular network. BMC Cancer 13: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer O., Hart S., Gschwind A., Ullrich A. (2003) EGFR signal transactivation in cancer cells. Biochem Soc Trans 31: 1203–1208. [DOI] [PubMed] [Google Scholar]
- Fiske W., Threadgill D., Coffey R. (2009) ERBBs in the gastrointestinal tract: recent progress and new perspectives. Exp Cell Res 315: 583–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. (1987) Angiogenic factors. Science 235: 442–447. [DOI] [PubMed] [Google Scholar]
- Folkman J., Merler E., Abernathy C., Williams G. (1971) Isolation of a tumor factor responsible for angiogenesis. J Exp Med 133: 275–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs C., Marshall J., Barrueco J. (2008) Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: updated results from the BICC-C study. J Clin Oncol 26: 689–690. [DOI] [PubMed] [Google Scholar]
- Geiger-Gritsch S., Stollenwerk B., Miksad R., Guba B., Wild C., Siebert U. (2010) Safety of bevacizumab in patients with advanced cancer: a meta-analysis of randomized controlled trials. Oncologist 15: 1179–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George A., Bangalore-Prakash P., Rajoria S., Suriano R., Shanmugam A., Mittelman A., et al. (2011) Endothelial progenitor cell biology in disease and tissue regeneration. J Hematol Oncol 4: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giantonio B., Catalano P., Meropol N., O’Dwyer P., Mitchell E., Alberts S., et al. (2007) Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 25: 1539–1544. [DOI] [PubMed] [Google Scholar]
- Giusti R., Shastri K., Cohen M., Keegan P., Pazdur R. (2007) FDA drug approval summary: panitumumab (Vectibix). Oncologist 12: 577–583. [DOI] [PubMed] [Google Scholar]
- Goel G., Sun W. (2015) Ramucirumab, another anti-angiogenic agent for metastatic colorectal cancer in second-line setting–its impact on clinical practice. J Hematol Oncol 8: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R., Sargent D., Morton R., Fuchs C., Ramanathan R., Williamson S., et al. (2004) A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 22: 23–30. [DOI] [PubMed] [Google Scholar]
- Gordon M., Margolin K., Talpaz M., Sledge G., Holmgren E., Benjamin R., et al. (2001) Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19: 843–850. [DOI] [PubMed] [Google Scholar]
- Grothey A., Van Cutsem E., Sobrero A., Siena S., Falcone A., Ychou M., et al. (2013) Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase III trial. Lancet 381: 303–312. [DOI] [PubMed] [Google Scholar]
- Guerrero S., Casanova I., Farré L., Mazo A., Capellà G., Mangues R. (2000) K-ras codon 12 mutation induces higher level of resistance to apoptosis and predisposition to anchorage-independent growth than codon 13 mutation or proto-oncogene overexpression. Cancer Res 60: 6750–6756. [PubMed] [Google Scholar]
- Hayashi N., Ito I., Yanagisawa A., Kato Y., Nakamori S., Imaoka S., et al. (1995) Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet 345: 1257–1259. [DOI] [PubMed] [Google Scholar]
- Hecht J., Mitchell E., Chidiac T., Scroggin C., Hagenstad C., Spigel D., et al. (2009) A randomized phase IIIb trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 27: 672–680. [DOI] [PubMed] [Google Scholar]
- Heinemann V., von Weikersthal L., Decker T., Kiani A., Vehling-Kaiser U., Al-Batran S. (2014) T FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-2): a randomised, open-label, phase III trial. Lancet Oncol 15: 1065–1075. [DOI] [PubMed] [Google Scholar]
- Hicklin D., Ellis L. (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23: 1011–1027. [DOI] [PubMed] [Google Scholar]
- Hoeben A., Landuyt B., Highley M., Wildiers H., Van Oosterom A., De Bruijn E. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549–580. [DOI] [PubMed] [Google Scholar]
- Holash J., Davis S., Papadopoulos N., Croll S., Ho L., Russell M., et al. (2002) VEGF-trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA 99: 11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J., Alberts S. (2013) Alternate dosing of cetuximab for patients with metastatic colorectal cancer. Gastrointest Cancer Res 6: 47–55. [PMC free article] [PubMed] [Google Scholar]
- Hubbard J., Grothey A. (2010) Antiangiogenesis agents in colorectal cancer. Curr Opin Oncol 22: 374–380. [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- Hurwitz H., Yi J., Ince W., Novotny W., Rosen O. (2009) The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 14: 22–28. [DOI] [PubMed] [Google Scholar]
- Hynes N., Lane H. (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5: 341–354. [DOI] [PubMed] [Google Scholar]
- Ibrahim E., Abouelkhair K. (2011) Clinical outcome of panitumumab for metastatic colorectal cancer with wild-type KRAS status: a meta-analysis of randomized clinical trials. Med Oncol 28: S310–S317. [DOI] [PubMed] [Google Scholar]
- Irahara N., Baba Y., Nosho K., Shima K., Yan L., Dias-Santagata D., et al. (2010) NRAS mutations are rare in colorectal cancer. Diagn Mol Pathol 19: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimeno A., Messersmith W., Hirsch F., Franklin W., Eckhardt S. (2009) KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol 27: 1130–1136. [DOI] [PubMed] [Google Scholar]
- Johnson J., Dippold W. (1989) Realizing Paul Ehrlich’s magic bullets. J Cancer Res Clin Oncol 115: 494–495. [Google Scholar]
- Jones J., Akita R., Sliwkowski M. (1999) Binding specificities and affinities of egf domains for ErbB receptors. FEBS Lett 447: 227–231. [DOI] [PubMed] [Google Scholar]
- Jung Y., Mansfield P., Akagi M., Takeda A., Liu W., Bucana C., et al. (2002) Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer 38: 1133–1140. [DOI] [PubMed] [Google Scholar]
- Kabbinavar F., Hurwitz H. (2003) Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol 21: 60–65. [DOI] [PubMed] [Google Scholar]
- Kaklamanis L., Gatter K. (1992) Interleukin-4 receptor and epidermal growth factor receptor expression in colorectal cancer. Br J cancer 66: 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khambata-Ford S., Garrett C., Meropol N., Basik M., Harbison C., Wu S., et al. (2007) Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 25: 3230–3237. [DOI] [PubMed] [Google Scholar]
- Kim K., Li B., Winer J., Armanini M., Gillett N., Phillips H., et al. (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362: 841–844. [DOI] [PubMed] [Google Scholar]
- Kimura H., Sakai K., Arao T., Shimoyama T., Tamura T., Nishio K. (2007) Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci 98: 1275–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan C., Wikstrand C., Bigner D. (2001) EGF mutant receptor vIII as a molecular target in cancer therapy. Endocr Relat Cancer 8: 83–96. [DOI] [PubMed] [Google Scholar]
- Lemmon M., Schlessinger J. (2010) Cell signaling by receptor tyrosine kinases. Cell 141: 1117–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz H., Stintzing S., Loupakis F. (2015) TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev 41: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Schmitz K., Jeffrey P., Wiltzius J., Kussie P., Ferguson K. (2005) Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7: 301–311. [DOI] [PubMed] [Google Scholar]
- Lievre A., Bachet J., Boige V., Cayre A., Le Corre D., Buc E., et al. (2008) KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 26: 374–379. [DOI] [PubMed] [Google Scholar]
- Liu Y., Gray N. (2006) Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol 2: 358–364. [DOI] [PubMed] [Google Scholar]
- Loupakis F., Ruzzo A., Cremolini C., Vincenzi B., Salvatore L., Santini D. (2009) KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 101: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews W., Jordan C., Gavin M., Jenkins N., Copeland N., Lemischka I. (1991) A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci 88: 9026–9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R., Van Cutsem E., Falcone A., Yoshino T., Garcia-Carbonero R., Mizunuma N., et al. (2015) Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 372: 1909–1919. [DOI] [PubMed] [Google Scholar]
- Meyer M., Clauss M., Lepple-Wienhues A., Waltenberger J., Augustin H., Ziche M., et al. (1999) A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (flt-1) receptor tyrosine kinases. EMBO J 18: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhardt J., Mayer R. (2005) Systemic therapy for colorectal cancer. N Engl J Med 352: 476–487. [DOI] [PubMed] [Google Scholar]
- Moscatello D., Montgomery R., Sundareshan P., McDanel H., Wong M., Wong A. (1996) Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene 13: 85–96. [PubMed] [Google Scholar]
- Mustonen T., Alitalo K. (1995) Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol 129: 895–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson S. (2003) Insights into the mechanisms of lymph node metastasis. Cancer 98: 413–423. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute (NCI). (2015) Drugs Approved for Colon and Rectal Cancer. Bethesda, MD: NCI. [Google Scholar]
- Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13: 9–22. [PubMed] [Google Scholar]
- Nicholson R., Gee J., Harper M. (2001) EGFR and cancer prognosis. Eur J Cancer 37: S9–S15. [DOI] [PubMed] [Google Scholar]
- Normanno N., Tejpar S., Morgillo F., De Luca A., Van Cutsem E., Ciardiello F. (2009) Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol 6: 519–527. [DOI] [PubMed] [Google Scholar]
- O’Connell J., Maggard M., Ko C. (2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst 96: 1420–1425. [DOI] [PubMed] [Google Scholar]
- Pedersen M., Pedersen N., Damstrup L., Villingshøj M., Sønder S., Rieneck K., et al. (2005) Analysis of the epidermal growth factor receptor specific transcriptome: effect of receptor expression level and an activating mutation. J Cell Biochem 96: 412–427. [DOI] [PubMed] [Google Scholar]
- Perrone F., Lampis A., Orsenigo M., Di Bartolomeo M., Gevorgyan A., Losa M., et al. (2009) PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol 20: 84–90. [DOI] [PubMed] [Google Scholar]
- Peters G. (2015) Therapeutic potential of TAS-102 in the treatment of gastrointestinal malignancies. Ther Adv Med Oncol 7: 340–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedbois P. (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16: 301–308. [DOI] [PubMed] [Google Scholar]
- Pirker R., Pereira J., Szczesna A., von Pawel J., Krzakowski M., Ramlau R., et al. (2009) Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 373: 1525–1531. [DOI] [PubMed] [Google Scholar]
- Pugh C., Ratcliffe P. (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684. [DOI] [PubMed] [Google Scholar]
- Ranson M., Hammond L., Ferry D., Kris M., Tullo A., Murray P., et al. (2002) ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol 20: 2240–2250. [DOI] [PubMed] [Google Scholar]
- Risau W. (1997) Mechanisms of angiogenesis. Nature 386: 671–674. [DOI] [PubMed] [Google Scholar]
- Roberts N., Kloos B., Cassella M., Podgrabinska S., Persaud K., Wu Y., et al. (2006) Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res 66: 2650–2657. [DOI] [PubMed] [Google Scholar]
- Robinson D., Wu Y., Lin S. (2000) The protein tyrosine kinase family of the human genome. Oncogene 19: 5548–5557. [DOI] [PubMed] [Google Scholar]
- Rocha-Lima C., Soares H., Raez L., Singal R. (2007) EGFR targeting of solid tumors. Cancer Control 14: 295–304. [DOI] [PubMed] [Google Scholar]
- Safaee Ardekani G., Jafarnejad S., Tan L., Saeedi A., Li G. (2012) The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One 7: e47054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin I., Kazmi S., Yorio J., Bhadkamkar N., Kee B., Garrett C. (2013) Rare though not mutually exclusive: a report of three cases of concomitant KRAS and BRAF mutation and a review of the literature. J Cancer 4: 320–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D., Brandt R., Ciardiello F., Normanno N. (1995) Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol 19: 183–232. [DOI] [PubMed] [Google Scholar]
- Saltz L., Clarke S., Diaz-Rubio E., Scheithauer W., Figer A., Wong R., et al. (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26: 2013–2019. [DOI] [PubMed] [Google Scholar]
- Saltz L., Easley C., Kirkpatrick P. (2006) Panitumumab. Nat Rev Drug Discov 5: 987–988. [DOI] [PubMed] [Google Scholar]
- Saltz L., Lenz H., Kindler H., Hochster H., Wadler S., Hoff P., et al. (2007) Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 25: 4557–4561. [DOI] [PubMed] [Google Scholar]
- Scagliotti G., Selvaggi G., Novello S., Hirsch F. (2004) The biology of epidermal growth factor receptor in lung cancer. Clin Cancer Res 10: 4227s–4232s. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. (2000) Cell signaling by receptor tyrosine kinases. Cell 103: 211–225. [DOI] [PubMed] [Google Scholar]
- Schrag D. (2004). The price tag on progress – chemotherapy for colorectal cancer. N Engl J Med 351: 317–319. [DOI] [PubMed] [Google Scholar]
- Schubbert S., Shannon K., Bollag G. (2007) Hyperactive Ras in developmental disorders and cancer. Nat Rev Cancer 7: 295–308. [DOI] [PubMed] [Google Scholar]
- Sclafani F., Gullo G., Sheahan K., Crown J. (2013) BRAF mutations in melanoma and colorectal cancer: a single oncogenic mutation with different tumour phenotypes and clinical implications. Crit Rev Oncol Hematol 87: 55–68. [DOI] [PubMed] [Google Scholar]
- Shaheen R., Ahmad S., Liu W., Reinmuth N., Jung Y., Tseng W., et al. (2001) Inhibited growth of colon cancer carcinomatosis by antibodies to vascular endothelial and epidermal growth factor receptors. Br J Cancer 85: 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M. (2001) Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct 26: 25–35. [DOI] [PubMed] [Google Scholar]
- Shibuya M. (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2: 1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Yamaguchi S., Yamane A., Ikeda T., Tojo A., Matsushime H., et al. (1990) Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5: 519–524. [PubMed] [Google Scholar]
- Siegel R., Naishadham D., Jemal A. (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29. [DOI] [PubMed] [Google Scholar]
- Simon R. (2008) The use of genomics in clinical trial design. Clin Cancer Res 14: 5984–5993. [DOI] [PubMed] [Google Scholar]
- Spaulding D., Spaulding B. (2002) Epidermal growth factor receptor expression and measurement in solid tumors. Semin Oncol 29: 45–54. [DOI] [PubMed] [Google Scholar]
- Strimpakos A., Syrigos K., Saif M. (2009) Pharmacogenetics and biomarkers in colorectal cancer. Pharmacogenomics J 9: 147–160. [DOI] [PubMed] [Google Scholar]
- Stuart D., Sellers W. (2009) Linking somatic genetic alterations in cancer to therapeutics. Curr Opin Cell Biol 21: 304–310. [DOI] [PubMed] [Google Scholar]
- Tabernero J., Van Cutsem E., Diaz-Rubio E., Cervantes A., Humblet Y., Andre T., et al. (2007) Phase II trial of cetuximab in combination with fluorouracil, leucovorin, and oxaliplatin in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 25: 5225–5232. [DOI] [PubMed] [Google Scholar]
- Tabernero J., Yoshino T., Cohn A., Obermannova R., Bodoky G., Garcia-Carbonero R., et al. (2015) Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase III study. Lancet Oncol 16: 499–508. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Bucana C., Liu W., Yoneda J., Kitadai Y., Cleary K., et al. (1996) Platelet-derived endothelial cell growth factor in human colon cancer angiogenesis: role of infiltrating cells. JNCI J Natl Cancer Inst 88: 1146–1151. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kitadai Y., Bucana C., Cleary K., Ellis L. (1995) Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 55: 3964–3968. [PubMed] [Google Scholar]
- Tan D., Thomas G., Garrett M., Banerji U., de Bono J., Kaye S., et al. (2009) Biomarker-driven early clinical trials in oncology. Cancer J 15: 406–420. [DOI] [PubMed] [Google Scholar]
- Tanigawa N., Amaya H., Matsumura M., Lu C., Kitaoka A., Matsuyama K., et al. (1997) Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res 57: 1043–1046. [PubMed] [Google Scholar]
- Tol J., Koopman M., Cats A., Rodenburg C., Creemers G., Schrama J., et al. (2009) Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 360: 563–572. [DOI] [PubMed] [Google Scholar]
- Tonini T., Rossi F., Claudio P. (2003) Molecular basis of angiogenesis and cancer. Oncogene 22: 6549–6556. [DOI] [PubMed] [Google Scholar]
- Tortora G., Ciardiello F., Gasparini G. (2008) Combined targeting of EGFR-dependent and VEGF-dependent pathways: rationale, preclinical studies and clinical applications. Nat Clin Pract Oncol 5: 521–530. [DOI] [PubMed] [Google Scholar]
- Tournigand C., André T., Achille E., Lledo G., Flesh M., Mery-Mignard D., et al. (2004) FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 22: 229–237. [DOI] [PubMed] [Google Scholar]
- US Cancer Statistics Working Group. (2009) United States Cancer Statistics: 1999–2005 Incidence and Mortality Web-based Report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention & National Cancer Institute; Atlanta, Georgia. [Google Scholar]
- Vale C., Tierney J., Fisher D., Adams R., Kaplan R., Maughan T., et al. (2012) Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev 38: 618–625. [DOI] [PubMed] [Google Scholar]
- Van-Cutsem E., Köhne C., Láng I., Folprecht G., Nowacki M., Cascinu S., et al. (2011) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- Van-Cutsem E., Lang I., D’haens G., Moiseyenko V., Zaluski J., Folprecht G., et al. (2008) KRAS status and efficacy in the first-line treatment of patients with metastatic colorectal cancer (mCRC) treated with FOLFIRI with or without cetuximab: the CRYSTAL experience. J Clin Oncol 26: abstr 2. [Google Scholar]
- Van-Cutsem E., Nordlinger B., Cervantes A. (2010a) Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 21: v93–v97. [DOI] [PubMed] [Google Scholar]
- Van-Cutsem E., Nowacki M., Lang I., Cascinu S., Shchepotin I., Maurel J., et al. (2007) Randomized phase III study of irinotecan and 5-FU/FA with or without cetuximab in the first-line treatment of patients with metastatic colorectal cancer (mCRC): The CRYSTAL trial. J Clin Oncol 25: 4000. [Google Scholar]
- Van-Cutsem E., Peeters M., Siena S., Humblet Y., Hendlisz A., Neyns B., et al. (2010b) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25: 1658–1664. [DOI] [PubMed] [Google Scholar]
- Van-Cutsem E., Tabernero J., Lakomy R., Prenen H., Prausová J., Macarulla T., et al. (2012) Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 30: 3499–3506. [DOI] [PubMed] [Google Scholar]
- Vaughn C., Zobell S., Furtado L., Baker C., Samowitz W. (2011) Frequency of KRAS, BRAF, and NRAS mutations in colorectal cancer. Genes Chromosomes Cancer 50: 307–312. [DOI] [PubMed] [Google Scholar]
- Vecchione L., Jacobs B., Normanno N., Ciardiello F., Tejpar S. (2011) EGFR-targeted therapy. Exp Cell Res 317: 2765–2771. [DOI] [PubMed] [Google Scholar]
- Vincenzi B., Zoccoli A., Pantano F., Venditti O., Galluzzo S. (2010) Cetuximab: from bench to bedside. Curr Cancer Drug Targets 10: 80–95. [DOI] [PubMed] [Google Scholar]
- Wan P., Garnett M., Roe S., Lee S., Niculescu-Duvaz D., Good V., et al. (2004) Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116: 855–867. [DOI] [PubMed] [Google Scholar]
- Wang T., Lockhart A. (2012) Aflibercept in the treatment of metastatic colorectal cancer. Clin Med Insights Oncol 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R., Staschke K. (2010) How do tumours adapt to nutrient stress? EMBO J 29: 1946–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S., Dumas J., Adnane L., Lynch M., Carter C., Schütz G., et al. (2011) Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129: 245–255. [DOI] [PubMed] [Google Scholar]
- Winau F., Westphal O., Winau R. (2004) Paul Ehrlich – in search of the magic bullet. Microbes Infect 6: 786–789. [DOI] [PubMed] [Google Scholar]
- Wood L., Parsons D., Jones S., Lin J., Sjöblom T., Leary R., et al. (2007). The genomic landscapes of human breast and colorectal cancers. Science 318: 1108–1113. [DOI] [PubMed] [Google Scholar]
- Workman P., Aboagye E., Chung Y., Griffiths J., Hart R., Leach M., et al. (2006) Minimally invasive pharmacokinetic and pharmacodynamic technologies in hypothesis-testing clinical trials of innovative therapies. J Natl Cancer Inst 98:580–598. [DOI] [PubMed] [Google Scholar]
- Wu S., Gan Y., Wang X., Liu J., Li M., Tang Y. (2013) PIK3CA mutation is associated with poor survival among patients with metastatic colorectal cancer following anti-EGFR monoclonal antibody therapy: a meta-analysis. J Cancer Res Clin Oncol 139: 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G., Davis S., Gale N., Rudge J., Wiegand S., Holash J. (2000) Vascular-specific growth factors and blood vessel formation. Nature 407: 242–248. [DOI] [PubMed] [Google Scholar]
- Yang J., Haworth L., Sherry R. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y., Sliwkowski M. (2001) Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2: 127–137. [DOI] [PubMed] [Google Scholar]
- Yasui W., Sumiyoshi H., Hata J., Kameda T., Ochiai A., Ito H., et al. (1988) Expression of epidermal growth factor receptor in human gastric and colonic carcinomas. Cancer Res 48: 137–141. [PubMed] [Google Scholar]
- Yau T., Chan P., Ng K., Chok S., Cheung T., Fan S., et al. (2009) Phase II open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population. Cancer 115: 428–436. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yang P., Gray N. (2009) Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 9: 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Gaspard J., Chung D. (2001) Regulation of vascular endothelial growth factor by the Wnt and K-Ras pathways in colonic neoplasia. Cancer Res 61: 6050–6054. [PubMed] [Google Scholar]