Introduction
Inhibition of the androgen receptor (AR) represents the most important therapeutic target in prostate cancer. Although AR is expressed in 77% of all breast cancers (BCs), even more than estrogen receptors (ERs), its role in BC growth and progression remains indefinite [Guedj et al. 2012]. AR expression is associated with somewhat more indolent BC [Lehmann et al. 2011; Liedtke et al. 2008; Cochrane et al. 2014]. The drug development pipeline of AR-targeted therapeutics in prostate cancer is facilitating the evaluation of AR signaling inhibition in triple-negative breast cancer (TNBC): including bicalutamide, a nonsteroidal partial agonist; enzalutamide, an inhibitor of nuclear localization of AR; and VT-464, a dual inhibitor of CYP17 and AR. Given the controversy in the role of AR, other ongoing or completed trials are testing dehydroepiandrosterone (DHEA) or 4-OH testosterone (see Table 1).
Table 1.
Clinical trials of AR therapies in breast cancer.
| ClinicalTrials.gov identifier | Phase | Patient population | Treatment |
|---|---|---|---|
| NCT01889238 | II | Advanced AR+ TNBC | Enza |
| NCT02457910 | I/II | Postmenopausal AR+, metastatic TNBC | Enza +/− taselisib |
| NCT02348281 | II | Postmenopausal AR+, metastatic TNBC | Bicalutamide |
| NCT02067741 | I | Postmenopausal metastatic or locally advanced, endocrine responsive-Her2- and TN-AR+ | CR1447 (4-OH-testosterone) |
| NCT02000375 | II | Postmenopausal pretreated metastatic, AR+ | DHEA |
| NCT02000375 | II | Postmenopausal pretreated metastatic, AR+ | DHEA |
| NCT02368691 | II | Advanced AR+ TNBC | GTx-024 |
| NCT02580448 | I/II | Advanced AR+ TNBC; ER+/Her2- BC | VT-464 |
| NCT02605486 | II | Advanced AR+ BC | Bicalutamide + palbociclib |
| NCT02689427 | IIB | Advanced AR+ BC | Taxol +/− enzalutamide |
AR, androgen receptor; BC, breast cancer; DHEA,; ER, estrogen receptors; Her2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer; VT,
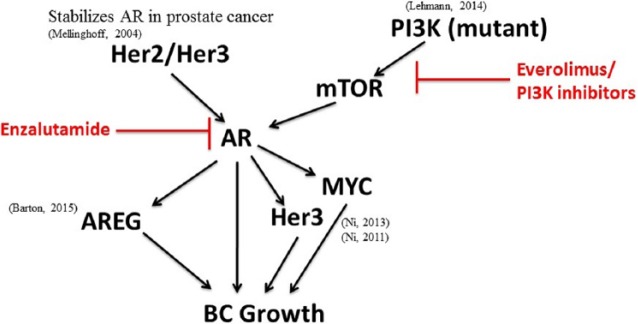
Figure 1.
AR signaling integration in TNBC.
Targeted therapies are highlighted in red. AR, androgen receptor; AREG, amphiregulin; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; MYC, myelocytomatosis viral oncogene homolog; Her2, human epidermal growth factor receptor 2; Her3, human epidermal growth factor receptor 3; BC, breast cancer.
Preclinical justification for anti-androgen therapies in breast cancer
Gene expression profiling of BC suggests a significant functional role for AR in multiple subtypes of BC [Guedj et al. 2012; Lehmann et al. 2011]. While AR is expressed to varying degrees across all BC subtypes, preclinical modeling suggests that its functional role in disease progression is subtype-specific. Gene expression profiling of TNBC has revealed a number of potential subtypes within TNBC, including basal-like 1, basal-like 2, immunomodulatory, mesenchymal-like, mesenchymal stem-like, and luminal AR (LAR) [Lehmann et al. 2011], although these subtypes do not yet dictate individualized treatment with specific targeted agents to date. Although ER expression is absent, the LAR subtype is characterized by AR signaling with a gene expression pattern similar to luminal BC. Patients with LAR tumors are more slowly growing when metastatic, however they have decreased relapse-free survival in the adjuvant setting relative to other TNBC subtypes [Cochrane et al. 2014], perhaps due to lower chemotherapy sensitivity. LAR cell line models are sensitive to the AR partial antagonist bicalutamide [Lehmann et al. 2011], and are even more sensitive to the next-generation AR inhibitor enzalutamide [Cochrane et al. 2014].
AR is expressed in 12–55% of cases of TNBC [Barton et al. 2015; Collins et al. 2011; Gucalp et al. 2013; Thike et al. 2014; Traina et al. 2015]. Some of the variability in frequency of expression between studies is due to different anti-AR antibodies used and to different assay cutoffs (1% versus 10%). Preclinically, BC expressing as little as 1% AR may respond to enzalutamide, although higher levels may be associated with greater response [Barton et al. 2015]. Optimal assay for response to AR inhibitors in clinic is as yet unknown. Although the LAR subtype of TNBC is AR enriched, other TNBC subtypes also express AR, and have responded to AR inhibition using preclinical models [Barton et al. 2015]. In TNBC models, AR appears to regulate amphiregulin (AREG), an epidermal growth factor receptor (EGFR) ligand, which when secreted could potentially support even AR negative tumor cells [Barton et al. 2015].
Phosphoinositide 3-kinase (PI3K3) activation through loss of phosphatase and tensin homolog (PTEN) or mutation of PIK3CA is common in TNBC [Shah et al. 2012; Kriegsmann et al. 2014], and is associated with increased AR levels in BC [Gonzalez-Angulo et al. 2009]. The combination of bicalutamide and the PI3K inhibitors pictilisib and apitolisib showed additive efficacy in PI3K-mutant TNBC cells in vitro and in vivo [Lehmann et al. 2014]. Enzalutamide plus everolimus appeared to be synergistic in multiple in vitro preclinical models of BC, including TNBC [Gordon et al. 2014].
Clinical trials of anti-AR therapies in TNBC
Promising preclinical modeling of AR inhibition in TNBC has led to evaluation in the clinic. Interim results suggest that enzalutamide in particular provides significant clinical benefit for AR+ TNBC. A summary of trials is listed in Table 1.
Of 424 patients with ER/progesterone receptor (PR) negative metastatic breast cancer eligible for testing were screened by immunohistochemistry (IHC) for AR using a Dako antibody (AR441), 51 (12%) had >10% AR staining in archived tissues. Ultimately 26 patients with advanced AR+ TNBC (four had ER/PR 1–10%) were enrolled into a phase II trial of bicalutamide 150 mg po daily run by Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, USA) and the Translational Breast Cancer Research Consortium (TBCRC). The patients had a median age of 66 years, performance status (PS) of 0, and a median of 1 (0–8) prior lines of chemotherapy for metastatic disease. Median progression-free survival (PFS) was 12 weeks (95% CI: 11, 23). A total of five patients (ER 0–3%, PR negative) had stable disease with a clinical benefit rate (CBR) at 24 weeks of 19% (95% CI: 7, 39), including one patient on therapy for 57+ months [Gucalp et al. 2013]. No partial responses (PRs) or complete responses (CRs) were observed. The most common possibly drug-related toxicities included grade 1/2 fatigue, hot flashes, limb edema, and transaminitis.
A phase II trial of single-agent enzalutamide in advanced AR+ TNBC has been completed [Traina et al. 2015]. In this trial, AR positivity was defined as at least 1% nuclear staining by IHC (using a Ventana antibody). Patients with advanced AR+ TNBC with any number of prior therapies were eligible. Because of a possible risk for seizures with enzalutamide, no brain metastases were allowed. The primary endpoint was CBR at 16 weeks. The study was designed as a Simon two-stage trial powered to have an 85% power to detect a true CBR16 of ⩽8% versus ⩾20% with a 1-sided alpha of 5%. Of 165 patients screened, 118 (72%) (intent-to-treat (ITT) population) were AR+, of whom 89 had AR staining ⩾10%. Of the patients with AR IHC ⩾ 10% and who had a post-baseline tumor assessment, 75 patients constituted the ‘evaluable population’. Median age was 57 years and median prior therapy for advanced TNBC was 1 (0–8). At the time of presentation at the 2015 ASCO meeting, 11 (9%) were still on treatment. The CBR16 was 25% (95% CI: 17, 33), CBR24 20% (95% CI: 14, 29), CR/PR 6%, and median PFS 13 weeks for the ITT patient population. Most of the benefit was concentrated in the ‘evaluable population’. The predominant toxicities were fatigue (40% (5% G3)), nausea (32%), decreased appetite (19%). Grade 3 toxicities were observed in 10%. From 178 tissues (AR+ in 140, and AR- in 38), 521 genes were significantly different between the AR+ and AR- tissues. A proprietary androgen-driven gene signature called PREDICT AR was created from gene expression profiling, and patients whose tumors were positive for this signature had increased progression-free survival compared to those without an androgen-driven gene signature (32 versus 9 weeks).
Phase Ib/II trials of enzalutamide with or without the PI3K inhibitor taselisib, and VT-464 in TNBC are ongoing, with results not yet reported (ClinicalTrials.gov identifiers: NCT02457910, NCT02580448). VT-464 is a combination inhibitor of AR as well as CYP17, and therefore it dramatically decreases the ligands estradiol and testosterone, without requiring steroid replacement.
Conclusion
The role of the androgen receptor in BC biology remains controversial, but based on preclinical studies and new clinical trials, inhibition of AR signaling appears to be a viable therapeutic target. At this point, there is some evidence that AR inhibition has clinical benefit in TNBC (some more prolonged stable disease, occasional CR/PR), however the CBR at 16 and 24 weeks may be confounded by the observation that AR+ TNBC (particular with luminal gene patterns) may be more indolent biologically. The correlation between potential benefit and AR expression by IHC is not strong, although the study by Traina and colleagues would suggest that benefit may be more likely in tumors with >10% AR nuclear expression [Traina et al. 2015]. The PREDICT AR test might enhance the signal because it includes gene expression downstream of AR, and thus would indicate tumors in which the AR pathway is activated. However, because this test is proprietary, it is not possible to analyze this further.
AR inhibition alone is well tolerated and may be useful to patients with TNBC, as the toxicity is significantly less than that of chemotherapy. However, it is likely that AR inhibition will be combined with other agents. Preclinical data would support combinations with paclitaxel and other chemotherapy agents [Gordon et al. 2014], combination with mTOR inhibitors [Gordon et al. 2014], combination with EGFR and other ErbB inhibitors [Barton et al. 2015], combination with PIK3 inhibitors [Kriegsmann et al. 2014], and combinations with anti-PDL1 antibodies [Tung et al. 2015]. Randomized trials will be needed to establish the clinical utility of AR inhibitors. Validated predictive biomarkers will be critical to select appropriate patients for AR inhibition.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. DOD BCRP Clinical Translational Award BC120183 to JKR and ADE.
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
Contributor Information
Valerie N. Barton, Department of Pathology, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Michael A. Gordon, Department of Pathology, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Jennifer K. Richer, Department of Pathology, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO, USA
Anthony Elias, Division of Medical Oncology, University of Colorado School of Medicine, Anschutz Medical Campus, ACP 5310, MS 8117 1665 Aurora Court, Aurora, CO 80045, USA.
References
- Barton V., D’Amato N., Gordon M., Lind H., Spoelstra N., Babbs B., et al. (2015) Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther 14: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane D., Bernales S., Jacobsen B., Cittelly D., Howe E., D’Amato N., et al. (2014) Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res 16: R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L., Cole K., Marotti J., Hu R., Schnitt S., Tamimi R. (2011) Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Modern Pathol 24: 924–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Angulo A., Stemke-Hale K., Palla S., Carey M., Agarwal R., Meric-Berstam F., et al. (2009) Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res 15: 2472–2478. [DOI] [PubMed] [Google Scholar]
- Gordon M., D’Amato N., Gu H., Wong D., Elias A., Richer J. (2014) Targeting multiple pathways in breast cancer: Androgen receptor, HER2, and mTOR. Cancer Res 75: P6-03-07. [Google Scholar]
- Gucalp A., Tolaney S., Isakoff S., Ingle J., Liu M., Carey L., et al. (2013) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res 19: 5505–5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj M., Marisa L., de Reynies A., Orsetti B., Schiappa R., Bibeau F., et al. (2012) A refined molecular taxonomy of breast cancer. Oncogene 31: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsmann M., Endris V., Wolf T., Pfarr N., Stenzinger A., Loibl S., et al. (2014) Mutational profiles in triple-negative breast cancer defined by ultradeep multigene sequencing show high rates of PI3K pathway alterations and clinically relevant entity subgroup specific differences. Oncotarget 5: 9952–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann B., Bauer J., Chen X., Sanders M., Chakravarthy A., Shyr Y., et al. (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 121: 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann B., Bauer J., Schafer J., Pendleton C., Tang L., Johnson K., et al. (2014) PIK3CA mutations in androgen receptor-positive triple-negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res 16: 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke C., Mazouni C., Hess K., André F., Tordai A., Mejia J., et al. (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26: 1275–1281. [DOI] [PubMed] [Google Scholar]
- Mellinghoff I., Vivanco I., Kwon A., Tran C., Wongvipat J., Sawyers C. (2004) HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6: 517–527. [DOI] [PubMed] [Google Scholar]
- Ni M., Chen Y., Fei T., Li D., Lim E., Liu X., et al. (2013) Amplitude modulation of androgen signaling by c-MYC. Genes Dev 27: 734–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M., Chen Y., Lim E., Wimberly H., Bailey S., Imai Y., et al. (2011) Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 20: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S., Roth A., Goya R., Oloumi A., Ha G., Zhao Y., et al. (2012) The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thike A., Yong-Zheng Chong L., Cheok P., Li H., Wai-Cheong Yip G., Huat Bay B., et al. (2014) Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Modern Pathol 27: 352–360. [DOI] [PubMed] [Google Scholar]
- Traina T., Miller K., Yardley D., O’Shaughnessy J., Cortes J., Awada A., et al. (2015) Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). J Clin Oncol 33: 1003. [Google Scholar]
- Tung N., Garber J., Torous V., Hacker M., Freeman G., Rodig S., et al. (2015) Prevalence and predictors of androgen receptor (AR) and programmed death-ligand 1 (PD-L1) expression in BRCA1-associated and sporadic triple-negative breast cancer (TNBC). J Clin Oncol 33: 1005. [DOI] [PMC free article] [PubMed] [Google Scholar]