Key Points
There are now percutaneous options for several valve legions
The evidence base for percutaneous valve therapy is growing rapidly
Transcatheter aortic valve implantation (TAVI) is becoming established as an effective alternative to surgery in high risk or inoperable patients
Balloon aortic valvuloplasty is enjoying a renaissance in the [TAVI era]
The changing epidemiology of valvular heart disease (VHD) has transformed the clinical profile of patients with this disease.1 It was previously common to see middle-aged females with rheumatic mitral stenosis (MS), but is now more common to see octogenarians with calcific aortic stenosis (AS). This change in patient characteristics has brought challenges for cardiologists and cardiac surgeons alike, mainly because older patients have complex comorbidity. Such patients are often high risk for surgical valve replacement or repair, still widely regarded as the ‘gold standard therapy’ for anatomical correction and treatment of most valve defects. This results in under-treatment of these patients, who are often not even exposed to rigorous assessment by the appropriate specialist teams.1 It is the combination of an unmet need with a desire to find lower risk, perhaps less invasive approaches that has driven the development of percutaneous valve therapy, now an extremely fast-growing area of cardiology. Percutaneous and minimally invasive treatment of VHD presents a very attractive option for this high-risk group.
This article focuses on the percutaneous alternatives to open valve surgery and reviews the techniques which currently offer less invasive alternatives. An attempt is also made to give a perspective on the future direction of this fast-moving field.
The aortic valve
AS is the most common form of VHD, predominantly affecting the elderly. It is usually caused by a degenerative, age-related process of valve calcification/ destruction.1 In our ageing population, AS is an increasingly prevalent condition and well-known to have a poor prognosis with significant morbidity, multiple/prolonged hospital admissions and a marked reduction in quality of life. Once AS becomes symptomatic, life expectancy decreases dramatically.
Balloon aortic valvuloplasty
The technique of balloon aortic valvuloplasty (BAV) was introduced by Alan Cribier in 19862 and is better established as a conventional treatment for congenital AS in children and adolescents.3 The morphology of AS in the acquired form of the disease (in elderly patients) is quite different. The degenerate, calcified stenotic valve is much less predictable when dilated with a balloon and the results therefore more variable. Despite this, the immediate results of BAV in calcific degenerative AS are surprisingly good in terms of symptom relief. In the 1990s, data from large BAV registries showed that, even with the original techniques, BAV is a successful method of increasing aortic valve area and reducing the mean and peak aortic valve gradient.4 Significant improvements in haemodynamics were observed, including an increase in cardiac output and decrease in left ventricular (LV) end-diastolic pressure. Symptomatic benefits were observed at 30 days with 70% of survivors having improvement of at least one functional (NYHA) class. However, restenosis of the valve was frequent, occurring in about 50% of patients within the first few months. As a result, initial enthusiasm for the technique tapered off, particularly when it became apparent that there was no mortality benefit.5 Moreover, the procedure itself was considered high risk and cumbersome, and therefore declined in popularity in the early 1990s.
BAV is now experiencing something of a renaissance since the introduction of transcatheter aortic valve implantation (TAVI) (see below), probably because of a renewed interest in the management of AS and the need to manage a large number and variety of patients within the context of a TAVI programme. The technique has been refined by modern balloons, guidewires and vascular closure devices, while improved imaging techniques have decreased procedural mortality. The introduction of rapid pacing during the procedure has made balloon inflation both more predictable and more effective and has transformed the ease with which the aortic valve can be dilated: rapid pacing (∼ 200 bpm) dramatically reduces cardiac output in the setting of AS, allowing full balloon dilatation without ejection by the heart. The TAVI experience has shown that this is a simple and safe procedure, even in the conscious/sedated patient.
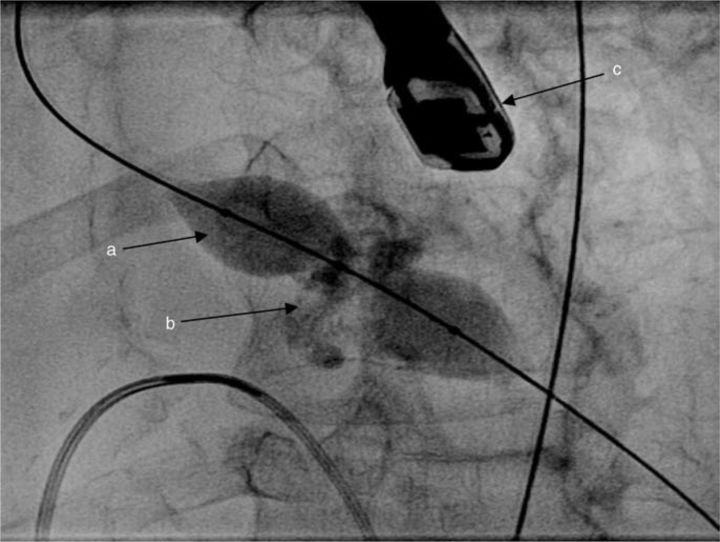
The procedure is usually performed retrogradely via the femoral artery. Extra-stiff guidewires (positioned toward the apex of the LV) allow excellent support for balloon passage across the stenosed valve. A variety of balloons in different sizes are now available, including balloons which ‘dog-bone’ as they inflate to improve stability (Fig 1).
Fig 1.
Aortic balloon valvuloplasty: (a) ‘Nucleus™’ balloon shown with ‘waisting’ at the level of the heavily calcified aortic valve (b) as it inflates. A transoesophageal echocardiography probe is seen (c), although this is not a prerequisite for aortic valvuloplasty.
Complications. Large arterial sheaths (9–14F) are necessary and procedural complications therefore include bleeding and vascular injury (10–20%). The incidence of other complications is low and, rather surprisingly, severe valvular regurgitation is very uncommon (∼ 1%). Procedural mortality has been quoted as 3%, although 30-day mortality can be as high as 14%.4 More recent series have suggested that complication rates and mortality are now significantly lower.6
Indications. The following indications for BAV are accepted:
- As a bridge to definitive treatment (either open aortic valve replacement (AVR) or TAVI) for two reasons:
- severe haemodynamic instability (eg very poor LV function), and
- prior to major non-cardiac surgery (eg cancer surgery when the prognosis or bleeding risk is ill-defined).
As a bridge to new technology (eg those patients whose aortic annulus is too large for the currently available TAVI devices).
Prior to pre-TAVI coronary angioplasty which can often be complex and high risk in the setting of severe AS.
Therapeutic trial, particularly in breathless patients with the combination of severe AS, significant coronary artery disease and severe airways disease.
Palliative: this is more controversial, but some physicians feel that offering 3–6 months of symptomatic benefit to very elderly patients (even when recurrence of symptoms is likely) is worthwhile in some clinical scenarios.
Transcatheter aortic valve implantation
Until recently, the only definitive treatment for AS was conventional (open) surgical AVR, which remains an excellent intervention. However, the novel technique of TAVI has become feasible in recent years. Alan Cribier performed the first antegrade percutaneous aortic valve implantation in 2002, with the valve prosthesis advanced from the venous circulation across the interatrial septum.7 This technique proved technically very demanding and difficult to reproduce, prompting the development of two new approaches: the retrograde (transfemoral) approach and the antegrade (transapical) approach.
Transfemoral approach
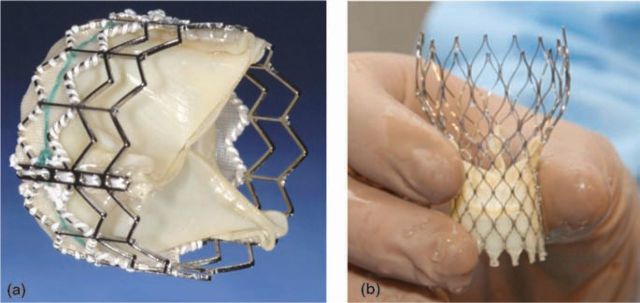
The transfemoral procedure requires 22F or 24F introducer sheaths for implantation of the Edwards-Sapien valve and 18F or 21F for the CoreValve device (Fig 2). The implantation of the aortic valve prosthesis is preceded by BAV to prepare the native valve and facilitate deployment. This is achieved using 20 mm or 23 mm balloons, utilising rapid pacing for optimal balloon expansion. Haemostasis is usually achieved by either surgical repair or suture-mediated closure devices. The apex is securely closed using the pursestring sutures with Teflon reinforcement with brief periods of rapid pacing to facilitate suture closure.
Fig 2.
Edwards Sapien Valve (a) and CoreValve prosthesis (b) prior to loading on to the delivery system ((b) reproduced from Ref 12 with permission from Elsevier).
Early experience of TAVI was analysed in the Registry of Endovascular Critical Aortic Stenosis Treatment (RECAST) trial from Cribier's group.8 Anatomical and procedural success using the transfemoral technique exceeded 90% with a low 30-day mortality (<10%). There was an impressive improvement in aortic valve area and mean aortic gradient which was maintained at follow-up. John Webb's group9 initially reported a valve implantation success rate at 86% with a 30-day mortality of about 12%. These initial results were influenced by a marked learning curve and procedural success increased to 96% when experience was gained.
Transapical approach
The transapical procedure uses the 26F Ascendra delivery system. The largest series of transapical procedures was performed by Walther et al.10 They reported a similar success rate to the transfemoral procedure (>90%) with a very small risk of needing urgent femoro-femoral cardiopulmonary bypass.10 The mortality rate is slightly higher using this approach, probably because transapical TAVI patients have significantly higher risk due to peripheral vascular disease and renal failure, which are markers for a high atheroma burden and worse outcome after the procedure.
Complications
Access site complications are the most common serious adverse outcomes (10–15% according to the device used). New smaller devices will soon be available which should limit vascular damage and improve outcome.11 The risk of embolism from the aortic valve causing stroke is relatively low (3–9%). Atrioventricular block occurs relatively rarely after Edwards- Sapien valve implantation (5–10%). It is more frequent with self-expanding devices (eg CoreValve), with the need for pacemaker implantation in up to 20%.12 Severe aortic regurgitation after valve implantation is very rare, although mild to moderate paravalvular regurgitation with no haemodynamic consequence is frequently observed.
Indications
TAVI is currently indicated in patients with severe symptomatic calcific AS who are deemed unfit for conventional surgical valve replacement based on one of the current risk scoring systems (EuroSCORE or STS Score). Particular comorbidities not adequately reflected by the risk scores, such as porcelain aorta, difficulties with surgical access and severe respiratory disease, are seen as additional indications. The technique has also been used successfully for the treatment of degenerative aortic bioprosthetic valves. It has the potential to revolutionise the treatment of AS but randomised clinical trial (RCT) data are needed, with careful evaluation of longer-term results. Placement of AoRTic traNscathetER valves (PARTNER-IDE) is an RCT comparing standard surgical AVR with TAVI (using the Edwards-Sapien valve) and also optimal medical treatment with TAVI in patients who are deemed inoperable. Recruitment for the study in the US and Canada has completed and one-year results are expected in late 2010.
The mitral valve
Balloon mitral valvuloplasty
The technique of balloon dilatation of the stenosed mitral valve (Fig 3) was first described in 1984 by Inoue and soon adopted into clinical practice, 13 taking over the role of older surgical closed valvotomy procedures. Balloon mitral valvuloplasty (BMV) is now well established as a definitive therapy, but case selection is important and the efficacy and predictability of balloon dilatation depends on the anatomy of the valve.
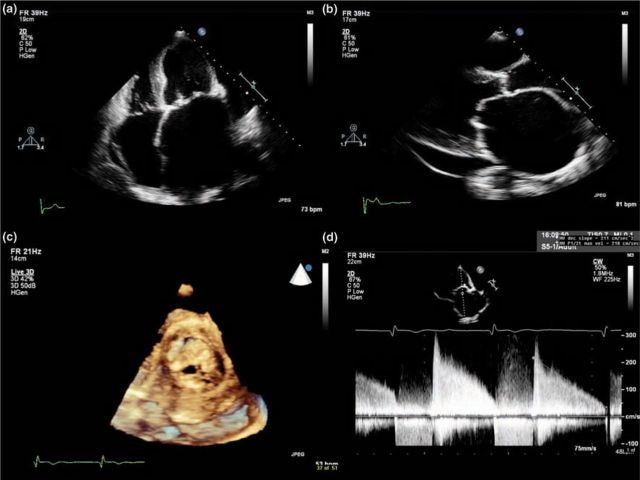
Fig 3.
Typical echocardiographic features of mitral stenosis: limited opening of the valve with thickened and calcified leaflets, severely dilated left atrium, diastolic doming of the anterior mitral valve leaflet, decreased E-F slope of the mitral inflow Doppler tracing, increased mean pressure gradient and small valve area with planimetry: (a) apical four-chamber view; (b) parasternal long axis view; (c) mitral stenosis with 3D echocardiography; (d) mitral valve inflow Doppler tracing.
The procedure is performed via the femoral approach, the much more common antegrade technique requiring a transseptal puncture. The ‘Inoue’ balloon consists of three portions, each with slightly different pressure compliance. As the pressure increases, the distal portion of the balloon inflates first, followed by the proximal portion. The middle part has the least pressure compliance and expands last. This technical feature assists the positioning of the balloon across the mitral valve and secures maximum dilatation at the level of the mitral commissures. Balloon dilatation splits the fused commissures; thus, the response of the valve to this intervention depends not only on the degree of commissural fusion but also on the severity of pre-existing mitral regurgitation (MR), calcification and involvement of the subvalvar apparatus. Detailed transoesophageal assessment also allows the detection of left atrial appendage thrombus which is a contraindication to BMV. The ‘Wilkins score’ has been devised to quantify the suitability of stenosed mitral valves for BMV (Table 1).14 Patients in developing countries with MS present at a younger age and have valves that are generally more suitable for the procedure.
Table 1.
The Wilkins score: anatomical classification and echocardiographic characteristics of mitral stenosis.

Results. The anatomical and procedural results of BMV are usually good, with an immediate success rate exceeding 98%.15 Follow-up restenosis rate varies from 2–20%, with a favourable clinical outcome in 75–99%. The frequency of recurrent problems increases from about five years after intervention and 62% of patients need re-intervention at 8–12 years of follow-up.15 The literature suggests an equal or better success rate than with surgical mitral commissurotomy.16 Moreover, randomised trials comparing BMV with closed commissurotomy suggest that BMV produces better long-term durability.16 Patients with lower Wilkins scores do better and patients who obtain a good immediate haemodynamic response to BMV fare best in the longer term. Recurrence of symptoms is usually due to valvular restenosis; although repeat BMV can be performed, the results are usually not as good.
Complications. Serious complications are relatively uncommon and consist of haemopericardium (0–2%), severe MR (0–19%) and persistent iatrogenic left-right interatrial shunt (0–15%), although the last is usually small and not haemodynamically significant. Risk of embolism from the mitral valve and subsequent stroke is relatively low and does not exceed 2%. The risk of death for most series is less than 0.5%.
Indications. Percutaneous BMV is the treatment of choice in symptomatic patients with haemodynamically significant MS and favourable valve anatomy. BMV is also sometimes indicated if surgery is contraindicated or high risk (eg in pregnancy) even if valve anatomy is not ideal. However, BMV is not suitable for patients with unfavourable anatomy (indicated by a high Wilkins score) or patients with severe coexistent disease of other valves.
Transcatheter mitral valve repair
The progressive refinements of surgical techniques of mitral valve repair and their adoption into routine surgical practice have had a major impact on the management of MR worldwide. Indeed, current guidelines for surgical intervention encourage earlier referral if repair is feasible and surgery is now undertaken in many asymptomatic subjects, with exceedingly low mortality. However, not all patients are suitable candidates for surgery and, encouraged by their successes elsewhere, interventional cardiologists have developed two main techniques for the percutaneous repair of MR over the past five years:
edge-to-edge repair
prosthetic ring annuloplasty.
Edge-to-edge repair. The edge-to-edge technique is akin to the Alfieri surgical procedure, requiring the placement of stitches to oppose the mid portion of both mitral leaflets, thereby creating a double mitral valve orifice (Fig 4).17 The percutaneous procedure is challenging, requiring a transseptal approach and transoesophageal echocardiographic guidance. The EVEREST trial programme has now enrolled 607 patients (September 2009) and results so far have been encouraging. Initial results from the EVEREST II high-risk registry (in patients not considered fit for surgery) suggest a decrease in LV diastolic and systolic volumes in patients receiving the Mitra-Clip™, with a 45% reduction in re-hospitalisation rate (unpublished data). Results of the EVEREST II RCT comparing the Mitra-Clip and surgical valve repair will be available in 2010. Potential limitations of this technique include the inability to address coexistent annular dilatation (thus restricting its use to repair of localised prolapse of the medial portions of either leaflet) and the possibility of leaflet or chordal trauma. These constraints, together with the technical demands of the procedure, may ultimately limit its application.
Fig 4.
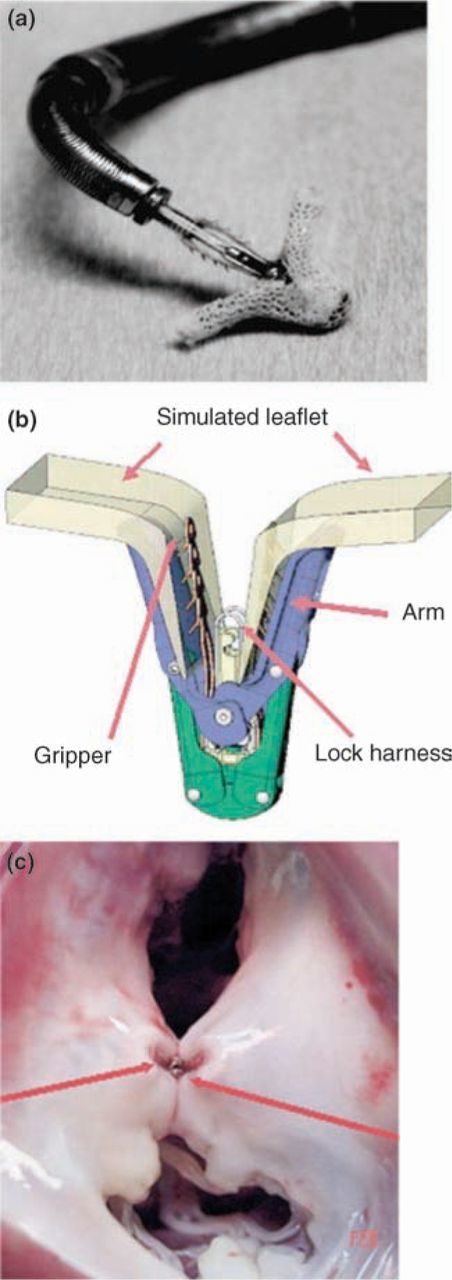
The Evalve mitral clip (Evalve, Inc): (a) clip device on distal tip of triaxial catheter delivery system; (b) illustrated components of clip device grasping mitral valve leaflet; (c) animal pathology specimen of the mitral valve with clip resulting in a double-orifice mitral valve (reproduced from Ref 17 with permission from Elsevier).
Prosthetic ring annuloplasty. Percutaneous prosthetic ring annuloplasty aims to reproduce the effects of surgical ring placement by the delivery of a constraining device within the coronary sinus (Fig 5). Experience derived from electrophysiological procedures suggests easier performance than the edge-to-edge technique. Outcome in animal models and early clinical feasibility studies have been favourable. However, procedural challenges remain, including:
the inconsistent relation of the coronary sinus to the posterior mitral annulus
incomplete annular coverage
the inability to anchor the device in both fibrous trigones
potential damage to the adjacent circumflex coronary artery, and
problems related to annular calcification.
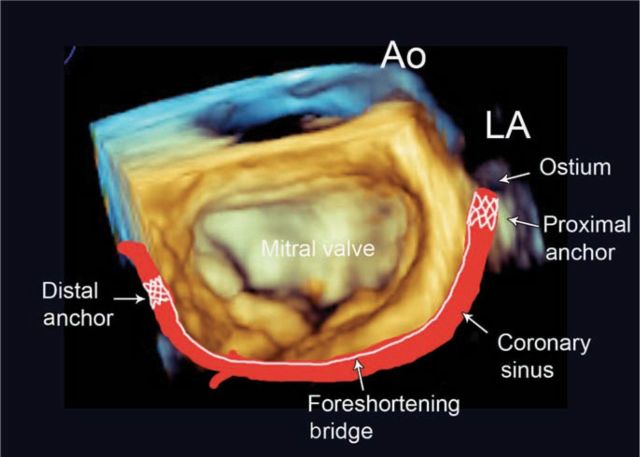
Fig 5.
Schematic derived from a 3D echocardiographic image of the mitral valve, illustrating the location of the coronary sinus and implanted annuloplasty device. Ao = aorta; LA = left ventricle.
Further refinements of the procedure to overcome these issues are being explored and early non-RCTs are ongoing. Ultimately, a combination of the edge-to-edge and annuloplasty techniques may prove useful, though further assessment is awaited.
Transapical valve-in-valve therapy
In certain cases of mitral prosthetic valvular dysfunction, it may be feasible to place a new stented valve inside the mitral bioprosthesis via the cardiac apex. This is a very new technique, demonstrated for the first time in September 2009, and not yet supported by any clinical outcome data.
Overall, it seems unlikely that percutaneous mitral valve repair will be able to match the excellent results of surgical repair in the foreseeable future. Exploration of these evolving techniques against a background of established surgical procedures whilst ensuring patient safety and interest therefore presents an ethical dilemma. In the first instance, experience will remain limited to those patients in whom surgery is contraindicated or presents excessive risk.
The pulmonary valve
Percutaneous balloon valvuloplasty
Pulmonary stenosis accounts for about 8% of all congenital heart disease and can be treated effectively with percutaneous balloon valvuloplasty. This technique is used mainly in children, but immediate and short-term results are good in adults with only a small risk of restenosis at long-term follow up.18 The data from the Valvuloplasty and Angioplasty of Congenital Anomalies Registry (?800 valvuloplasty procedures) show that the incidence of complications is inversely related to age, being substantially higher in infants.18 The procedure is a safe and effective method of lowering pulmonary outflow gradients in the setting of congenital pulmonary stenosis.
Transcatheter pulmonary valve implantation
An emerging therapy for right ventricular outflow dysfunction in congenital heart disease is transcatheter pulmonary valve implantation. Development of a transcatheter percutaneous pulmonary valve was pioneered by the work by Bonhoeffer et al in lambs using a biological valve harvested from the jugular veins of fresh bovine cadavers. The first successful implantation of a pulmonary valve in a patient was performed in 2000.19 The first series of eight patients demonstrated the feasibility and safety of this percutaneous approach. A larger series also showed good results, with the ability to avoid surgical treatment in most cases.20
Current indications. Indications for percutaneous pulmonary valve implantation are currently limited to patients with pulmonary valve stenosis and/or regurgitation in a right ventricle-to-pulmonary artery conduit. Bonhoeffer's group has recently described satisfactory results with this procedure in patients with native pulmonary valve regurgitation (including those with a previous right ventricular outflow tract patch) using pre-stenting techniques.21
The tricuspid valve
Tricuspid stenosis is a rare valve disease, almost always caused by rheumatic fever and coexistent with MS. Severe tricuspid stenosis can also be treated percutaneously with good long-term results and a very low rate of restenosis/regurgitation. Simultaneous percutaneous treatment of rheumatic mitral and tricuspid stenosis can be undertaken if the anatomy is favourable.
Conclusions
Percutaneous treatment of VHD has become a reality. Many procedures are now in mainstream clinical practice and provide good clinical outcomes and supported by a growing evidence base. These techniques are often challenging and the patients frequently have complex comorbidity, making case selection difficult. Sophisticated cardiac imaging is fundamental to both pre-procedural assessment and periprocedural monitoring. The key to success appears to be a multidisciplinary team approach, which should involve cardiac surgeons and imaging specialists as well as interventional cardiologists. This field should not be thought of as a drive to divert patients away from cardiac surgery, but rather one that will involve even closer working between cardiologists and surgeons to provide better patient care.
Techniques (and in turn, outcomes) will improve and this progress will be driven by advancing technology, but accumulation of a robust evidence base along the way remains vital.
References
- 1.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231–43 10.1016/S0195-668X(03)00201-X [DOI] [PubMed] [Google Scholar]
- 2.Cribier A, Savin T, Saoudi N, et al. Percutaneous transluminal valvuloplasty of acquired aortic stenosis in elderly patients: an alternative to valve replacement? Lancet 1986;i:63–7 10.1016/S0140-6736(86)90716-6 [DOI] [PubMed] [Google Scholar]
- 3.Fratz S, Gildein HP, Balling G, et al. Aortic valvuloplasty in paediatric patients substantially postpones the need for aortic valve surgery: a single-centre experience of 188 patients after up to 17.5 years of follow-up. Circulation 2008;117:1201–6 [DOI] [PubMed] [Google Scholar]
- 4.Percutaneous balloon aortic valvuloplasty. Acute and 30-day follow-up results in 674 patients from the NHLBI Balloon Valvuloplasty Registry. Circulation 1991;84:2383–97 10.1161/01.CIR.84.6.2383 [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Mickel MC, Kennedy JW, et al. Three-year outcome after balloon aortic valvuloplasty. Insights into prognosis of valvular aortic stenosis. Circulation 1994;89:642–50 10.1161/01.CIR.89.2.642 [DOI] [PubMed] [Google Scholar]
- 6.Sack S, Kahlert P, Khandanpour S, et al. Revival of an old method with new techniques: balloon aortic valvuloplasty of the calcified aortic stenosis in the elderly. Clin Res Cardiol 2008;97:288–97 10.1007/s00392-008-0650-0 [DOI] [PubMed] [Google Scholar]
- 7.Cribier A, Eltchaninoff H, Bash A, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation 2002;106:3006–8 10.1161/01.CIR.0000047200.36165.B8 [DOI] [PubMed] [Google Scholar]
- 8.Cribier A, Eltchaninoff H, Tron C, et al. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol 2006;47:1214–23 [DOI] [PubMed] [Google Scholar]
- 9.Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755–63 10.1161/CIRCULATIONAHA.107.698258 [DOI] [PubMed] [Google Scholar]
- 10.Walther T, Simon P, Dewey T, et al. Transapical minimally invasive aortic valve implantation: multicentre experience. Circulation 2007;116(11 Suppl)1240–5 10.1161/CIRCULATIONAHA.106.677237 [DOI] [PubMed] [Google Scholar]
- 11.Webb JG, Altwegg L, Masson JB, et al. A new transcatheter aortic valve and percutaneous valve delivery system. J Am Coll Cardiol 2009;53:1855–8 10.1016/j.jacc.2008.07.075 [DOI] [PubMed] [Google Scholar]
- 12.Grube E, Schuler G, Buellesfeld L, et al. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. J Am Coll Cardiol 2007;50:69–76 [DOI] [PubMed] [Google Scholar]
- 13.Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg 1984;87:394–402 [PubMed] [Google Scholar]
- 14.Wilkins GT, Weyman AE, Abascal VM, Block PC, Palacios IF. Percutaneous balloon dilatation of the mitral valve: an analysis of echocardiographic variables related to outcome and the mechanism of dilatation. Br Heart J 1988;60:299–308 10.1136/hrt.60.4.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet 2009;374:1271–83 10.1016/S0140-6736(09)60994-6 [DOI] [PubMed] [Google Scholar]
- 16.Arora R, Nair M, Kalra GS, Nigam M, Khalilullah M. Immediate and long-term results of balloon and surgical closed mitral valvotomy: a randomized comparative study. Am Heart J 1993;125:1091–4 10.1016/0002-8703(93)90118-S [DOI] [PubMed] [Google Scholar]
- 17.Piazza N, Asgar A, Ibrahim R, Bonan R. Transcatheter mitral and pulmonary valve therapy. J Am Coll Cardiol 2009;53:1837–51 10.1016/j.jacc.2008.12.067 [DOI] [PubMed] [Google Scholar]
- 18.McCrindle BW, Kan JS. Long-term results after balloon pulmonary valvuloplasty. Circulation 1991;83:1915–22 10.1161/01.CIR.83.6.1915 [DOI] [PubMed] [Google Scholar]
- 19.Bonhoeffer P, Boudjemline Y, Saliba Z, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 2000;356:1403–5 10.1016/S0140-6736(00)02844-0 [DOI] [PubMed] [Google Scholar]
- 20.Lurz P, Coats L, Khambadkone S, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation 2008;117:1964–72 10.1161/CIRCULATIONAHA.107.735779 [DOI] [PubMed] [Google Scholar]
- 21.Momenah TS, El Oakley R, Al Najashi K, et al. Extended application of percutaneous pulmonary valve implantation. J Am Coll Cardiol 2009;53:1859–63 10.1016/j.jacc.2008.08.061 [DOI] [PubMed] [Google Scholar]