Abstract
Background
Health behaviour change is an important component of management for patients with chronic kidney disease (CKD); however, the optimal method to promote health behaviour change for self-management of CKD is unknown. The See Kidney Disease (SeeKD) targeted screening programme screened Canadians at risk for CKD and promoted health behaviour change through individual counselling and goal setting.
Objectives
The objectives of this study are to determine the effectiveness of individual counselling sessions for eliciting behaviour change and to describe participant characteristics associated with behaviour change.
Design
This is a cross-sectional, descriptive study.
Setting
The study setting is the National SeeKD targeted screening programme.
Patients
The participants are all ‘at risk’ patients who were screened for CKD and returned a follow-up health behaviour survey (n = 1129).
Measurements
Health behaviour change was defined as a self-reported change in lifestyle, including dietary changes or medication adherence.
Methods
An individual counselling session was provided to participants by allied healthcare professionals to promote health behaviour change. A survey was mailed to all participants at risk of CKD within 2–4 weeks following the screening event to determine if behaviour changes had been initiated. Descriptive statistics were used to describe respondent characteristics and self-reported behaviour change following screening events. Results were stratified by estimated glomerular filtration rate (eGFR) (< 60 and ≥ 60 mL/min/1.73 m2). Log binomial regression analysis was used to determine the predictors of behaviour change.
Results
Of the 1129 respondents, the majority (89.8 %) reported making a health behaviour change after the screening event. Respondents who were overweight (body mass index [BMI] 25–29.9 kg/m2) or obese (BMI ≥ 30.0 kg/m2) were more likely to report a behaviour change (prevalence rate ratio (PRR) 0.66, 95 % confidence interval (CI) 0.44–0.99 and PRR 0.49, 95 % CI 0.30–0.80, respectively). Further, participants with a prior intent to change their behaviour were more likely to make a behaviour change (PRR 0.58, 95 % CI 0.35–0.96). Results did not vary by eGFR category.
Limitations
We are unable to determine the effectiveness of the behaviour change intervention given the lack of a control group. Potential response bias and social desirability bias must also be considered when interpreting the study findings.
Conclusions
Individual counselling and goal setting provided at screening events may stimulate behaviour change amongst individuals at risk for CKD. However, further research is required to determine if this behaviour change is sustained and the impact on CKD progression and outcomes.
Keywords: Chronic kidney disease, Behaviour change intervention, Counselling
Abrégé
Mise en contexte
Les changements dans les habitudes de vie sont une composante majeure dans la prise en charge des patients atteints d’insuffisance rénale chronique (IRC). Malgré cela, la méthode pour promouvoir efficacement ces changements auprès de cette clientèle particulière n’est pas connue. Le programme de dépistage précoce et ciblé See Kidney Disease (SeeKD) a permis d’identifier les Canadiens à risque de développer une IRC. Ce programme a aussi servi à promouvoir l’adoption de changements d’habitudes bénéfiques pour la santé, par le biais de consultations individuelles et par l’établissement d’objectifs.
Objectifs de l’étude
Cette étude avait pour objectif de mesurer l’efficacité de séances de consultation individuelle offertes aux patients en vue de susciter des changements comportementaux. L’étude visait également à établir les caractéristiques des patients associées à ces changements de comportement.
Cadre de l’étude
Il s’agit d’une étude transversale descriptive qui s’est tenue dans le cadre du programme national de dépistage ciblé SeeKD.
Participants
La cohorte était constituée de tous les patients identifiés « à risque de développer une IRC » par le programme SeeKD, et ayant retourné le questionnaire de suivi au sujet des changements dans leurs habitudes de vie, soit un total de 1129 participants.
Mesures
Une séance de consultation individuelle ayant pour but de promouvoir l’intégration de nouvelles d’habitudes de vie a été offerte aux participants par les professionnels de la santé à la suite de l’activité de dépistage. Entre deux et quatre semaines plus tard, les participants ont également reçu un questionnaire par la poste à l’aide duquel on a pu vérifier s’ils avaient entamé les changements de comportement proposés. Des statistiques descriptives ont été utilisées pour établir les caractéristiques des répondants ainsi que les changements de comportement que ces derniers ont rapporté avoir adoptés à la suite de l’activité de dépistage. Les résultats ont été stratifiés en deux groupes selon les valeurs de DFGe des participants (< 60 mL/min/1.73 m2 et ≥ 60 mL/min/1.73 m2). L’analyse par régression logistique binomiale a été utilisée pour identifier les indicateurs de changement de comportement chez les patients.
Résultats
La grande majorité (89,8 %) des 1129 participants ont rapporté avoir adopté de nouveaux comportements en matière de santé après avoir été déclarés à risque de développer une IRC. Les répondants souffrant d’embonpoint (indice de masse corporelle [IMC] entre 25 et 29,9 kg/m2) ou obèses (IMC ≥ 30,0 kg/m2) se sont avérés plus ouverts à l’idée d’adopter de nouveaux comportements en regard de leur santé (rapport du taux de prévalence [RTP] : 0,66 ; intervalle de confiance à 95 % [I.C. à 95 %] : entre 0,44 et 0,99 et RTP : 0,49 ; I.C. à 95 % : entre 0,30 et 0,80 respectivement). Par ailleurs, les répondants qui avaient déjà l’intention d’adopter de nouveaux comportements avant même d’être dépistés ont été les plus enclins à le faire (RTP : 0,58 ; I.C. à 95 % : entre 0,35 et 0,96). Aucune variation significative de ces résultats n’a été observée selon le DFGe.
Limites de l’étude
Nous n’avons pu déterminer avec précision l’efficacité des changements de comportement adoptés en raison de l’absence d’un groupe contrôle. De plus, un biais dû aux réponses des participants ou par désirabilité sociale est à considérer dans l’interprétation des résultats.
Conclusions
L’établissement d’objectifs ainsi que le counselling individuel fourni à la suite de l’activité de dépistage pourraient stimuler l’adoption de nouvelles habitudes de vie chez les patients à risque de développer une IRC. Toutefois, des recherches supplémentaires sont requises afin de déterminer si ces changements de comportement sont maintenus par les patients et s’ils ont une réelle influence sur le pronostic de la maladie.
What was known before
Health behaviour change is an important aspect for the management of patients with chronic kidney disease (CKD).
What this adds
Individual counselling and goal setting provided at the screening events may stimulate behaviour change amongst individuals at risk for CKD. However, participants who were identified as having lower eGFR (< 60 mL/min/1.73 m2) were not more likely to change their behaviour given their recent diagnosis at the screening event. Further research is required to determine if this behaviour change is sustained and the impact on CKD progression and outcomes.
Background
CKD is associated with an increased risk for cardiovascular disease and concomitant chronic illness [1, 2]. Progression to end-stage renal disease (ESRD) has traditionally been considered the most serious complication of CKD [3] given its association with high morbidity and mortality [4, 5]. However, the majority of patients with CKD die prematurely from CKD-related complications before progressing to ESRD [6, 7]. Consequently, compliance with chronic disease management such as blood pressure control [8, 9], glycaemic control [10–12], and use of statins [13] is critical to slowing the progression to ESRD, preventing vascular-related adverse outcomes and reducing the risk of premature mortality [14]. In addition to the use of medications, management of chronic medical conditions including CKD requires lifestyle (behaviour) changes. This relates to the transformation or modification of behaviours by addressing knowledge, attitudes, and practices. Promoting behaviour change, through improving patient motivation and health knowledge, has been identified as a key component of chronic disease management given the known association between poor health behaviours and adverse clinical outcomes [15, 16].
Michie et al. [17] identified three core components to behaviour change: capability, motivation, and opportunity. While educational interventions build capability for behaviour change [18–20], research suggests that healthcare professionals play an important role in providing motivation and opportunity for behaviour change [21]. Specifically, individual counselling has been identified as a potentially effective intervention to improve health behaviour change within various chronic conditions (diabetes and hypertension) [22, 23]. Although evidence is limited in CKD, behaviour change interventions have focused on overall health and lifestyle changes (namely diet modification). Though not specific to CKD management, these interventions which aim to improve overall quality of life and slow kidney progression have shown promise in reducing CKD-related symptoms and complications [24]. However, given the heterogeneous interventions published and a paucity of evidence, the optimal method to elicit behaviour change within the CKD population remains unknown [25].
The Kidney Foundation of Canada launched the See Kidney Disease (SeeKD) targeted screening programme for Canadians at risk of CKD to promote early detection of CKD and to improve health knowledge in CKD management through individual counselling and goal setting provided at the screening events. We sought to determine the effectiveness of the individual counselling sessions for eliciting behaviour change amongst participants and to describe the participant characteristics associated with self-reported behaviour change.
Methods
The Kidney Foundation of Canada conducted the SeeKD targeted screening programme from 2011 to 2014 and recruited 6329 individuals across nine Canadian provinces, of whom 5194 were determined to be ‘at risk’ and subsequently screened for CKD. Eligible participants were adults 18 years of age and older who provided informed consent. Details of the SeeKD programme and methodology have previously been reported [26]. In brief, all participants that attended a screening event were surveyed to gather baseline sociodemographic characteristics, risk factors for CKD, knowledge of kidney disease, current health behaviours, and to determine those at risk of CKD. At risk of CKD was defined as having at least one of the following self-reported risk factors: diagnosed diabetes, diagnosed high blood pressure, existing kidney problems, family history of kidney disease, member of a high-risk ethnic population, current vascular disease, and currently using tobacco products. Only participants determined to be at risk of CKD were screened using point-of-care creatinine measurements (to calculate an estimated glomerular filtration rate (eGFR) and determine those with eGFR < 60 mL/min/1.73 m2). Participants were informed about the results, and if necessary, and with participant consent, results were forwarded to their family physician to arrange additional testing and follow-up. All surveys and educational documents were translated into the participant’s language of preference by the Kidney Foundation of Canada.
Immediately following kidney-specific testing, an individual counselling and goal setting session was provided to each participant determined to be at risk of CKD, with the goal of promoting health behaviour change amongst participants. Each one-on-one counselling session lasted approximately 20 min and was delivered by a registered nurse, pharmacist, or dietician specializing in kidney disease (Appendix 1). These counselling sessions provided educational information about CKD and its management and provided the participants with specific strategies tailored to their needs based on clinical measures taken during the screening and participants’ responses to the pre-screening survey. In the pre-screening survey, participants answered questions about their health knowledge of CKD (e.g. ‘Which of the following are risk factors for kidney disease?’), their motivation to participate in the screening event (e.g. ‘What made you participate in the SeeKD screening event today?’), and intent to change their health behaviours (e.g. ‘Are you planning to make any changes to improve your health?’ and ‘If you could change a health behaviour which one or two would be most important?’). We categorized intent to change behaviour as no intent to change any health behaviours, an expressed intent to change health behaviours, and preliminary health behaviour changes recently started.
Approximately 2–4 weeks after the SeeKD screening events, a follow-up survey was mailed to participants who received the individual counselling session and provided consent to be contacted. The post-screening survey sought to determine whether participants had begun to make health behaviour changes as recommended through the individual counselling sessions. The primary outcome of ‘health behaviour change’ was defined as a self-reported positive response to the post-screening question ‘Have you made any changes to improve your health in the past two weeks?’ Participants could choose more than one response from a predetermined list of health behaviour changes which were broadly categorized into common themes including the following: dietary changes (e.g. reducing fat or salt intake or adhering to Canada’s food guide); improving adherence to recommendations and prescriptions from healthcare providers (e.g. taking medications as prescribed, monitoring blood pressure or sugars, or routine visits to physician); reducing health-risk behaviours (e.g. quitting smoking or reducing alcohol intake); and daily lifestyle changes (increasing daily activity, reducing stress, or weight loss). Responses from participants who chose ‘other’ and indicated a specific health behaviour change were manually coded into the binary behaviour change variable during data cleaning. The response of ‘no health behaviour change’ was determined if the participant did not choose any of the suggested behaviour changes on the predetermined list or if they selected other and indicated they had not made any behaviour changes following the SeeKD screening event.
Analysis
Descriptive statistics were used to characterize participants that responded to both the pre- and post-screening surveys. These characteristics include sociodemographic (age, sex), clinical characteristics (eGFR, BMI), self-reported risk factors for CKD, self-reported motivation to participate in screening, health knowledge of risk factors for CKD, and self-reported behaviour change. Specifically, age was categorized as ≤ 49, 50–64, and ≥ 65 years and BMI was categorized as ≤ 24.9, 25–29.9, and ≥ 30 kg/m2. Motivation to participate was reported in four groups (no specified motivation, concerned for personal health status, influenced by external sources, and recruitment efforts) while self-reported health knowledge and behaviour change was reported as dichotomous (yes/no) variables. Participant characteristics were also compared amongst those that did and did not respond to the post-survey to determine whether these groups differed systematically. Descriptive statistics were reported using numbers and proportions for categorical variables and means with standard deviations (SD) for normally distributed continuous variables.
We fit multivariable log binomial regression models to determine the prevalence rate ratios (PRRs) for characteristics associated with the primary outcome of health behaviour change. Selection of characteristics to include within our regression models was a balance between factors previously associated with health behaviour change amongst chronic disease populations [18–25] and those available within the patient survey. Given that the prevalence of self-reported health behaviour change was very high (89.8 %), we modeled the outcome of no behaviour change. The interpretation of a negative outcome (no behaviour change) is difficult. For example, a PRR of < 1.0 translates to a participant being less likely to make no behaviour change (alternatively stated, more likely to make a behaviour change). Consequently, we interpret the PRR and 95 % confidence intervals (CIs) in terms of a positive self-reported health behaviour change in our Results and Discussion sections.
We constructed models and tested variables for inclusion (using p < 0.05) that had been identified a priori as being potentially associated with the outcome. These candidate predictors of behaviour change were considered on the basis of previous literature and clinical relevance. Variables that were independent predictors of behaviour change through bivariate analysis, along with age and sex, were then used to create a full model. Backward elimination was used to create the most parsimonious model. Model fit was assessed using the Bayesian information criterion (BIC) where the model with the lowest BIC is preferred.
Regression analysis using eGFR category (< 60 or ≥60 mL/min/1.73 m2) as a potential effect modifier was attempted, but the model did not converge due to a small sample size. Consequently, a stratified analysis was conducted to determine if characteristics related to health behaviour change varied by eGFR category. Specifically, we hypothesized that participants who were identified as having lower eGFR (< 60 mL/min/1.73 m2) may be more likely to change their behaviour given their recent diagnosis at the screening event. Variables independently associated with the outcome of health behaviour change, determined through log binomial regression, were stratified by eGFR category. Results were compared using Pearson’s chi-square tests for proportions, the Wilcoxon rank-sum test for multi-level categorical variables, and t tests for continuous variables. Finally, we conducted a sensitivity analysis excluding participants who self-reported having kidney problems amongst those with an eGFR of < 60 mL/min/1.73 m2 to determine the potential influence on participant characteristics and whether those with a new diagnosis of eGFR < 60 mL/min/1.73 m2 were more motivated to change their behaviour than those with more longstanding kidney disease. No imputation methods were used to account for the small proportion of patients with missing data (n = 7). Rather, footnotes were included below all descriptive analyses where denominators were influenced by missing data. Further, all patients with missing data were excluded from the regression models.
The SeeKD targeted screening programme obtained research ethics board approval from Health Canada. Ethics approval for analysis was also obtained from the Conjoint Health Research Ethics Board at the University of Calgary. All statistical analyses were conducted using Stata, version 12 [27].
Results
Overall, 5194 participants of the SeeKD programme were screened for CKD, of whom the majority (84.6 %) consented to receiving a post-screening follow-up survey, and 26 % responded (Fig. 1). The majority of the 1129 participants who responded were females (70.1 %) with a mean age of 63.8 years and were overweight or obese (33.3 and 27.9 %, respectively) (Table 1). Approximately, one in five (20.6 %) respondents had an eGFR of < 60 mL/min/1.73 m2 and the most common self-reported risk factors for CKD were hypertension (45.5 %) and member of a high-risk ethnic population (45.1 %). The majority of respondents were aware of at least one risk factor for CKD (health knowledge, 90.1 %), and their predominant motivation for participating in the screening events was a personal concern for health status (54.7 %).
Fig. 1.
Participant flow chart
Table 1.
Participant characteristics amongst respondents to post-screening survey
| Respondents (N = 1129a) | |
|---|---|
| Gender, male, n (%) | 337 (29.9) |
| Age (years), mean (SD) | 63.8 (14.3) |
| Age (years), n (%) | |
| ≤ 49 | 183 (16.3) |
| 50–64 | 345 (30.8) |
| ≥ 65 | 594 (52.9) |
| eGFR < 60 mL/min/1.73 m2, n (%) | 208 (20.6) |
| Self-reported behaviour change, n (%) | 1014 (89.8) |
| Motivation for participating, n (%) | |
| Concern for personal health status | 618 (54.7) |
| Influence from external source | 227 (20.1) |
| Recruitment efforts | 361 (32.0) |
| None | 110 (9.7) |
| Self-reported risk factors, n (%) | |
| Diagnosed diabetes | 274 (24.3) |
| Diagnosed hypertension | 524 (45.5) |
| Problems with kidneys | 156 (13.8) |
| High-risk ethnic groups | 509 (45.1) |
| Vascular disease | 268 (23.7) |
| Family history of kidney problems | 166 (14.7) |
| Smoking or tobacco use | 128 (11.3) |
| Knowledge of risk factors for CKD, n (%) | |
| Yes | 1017 (90.1) |
| No | 110 (9.7) |
| Body mass index, n (%) | |
| Normal/underweight (≤ 24.9 kg/m2) | 385 (34.1) |
| Overweight (25.0–29.9 kg/m2) | 376 (33.3) |
| Obese (≥ 30.0 kg/m2) | 315 (27.9) |
SD standard deviation, CKD chronic kidney disease, eGFR estimated glomerular filtration rate
aDenominators vary for each variable depending on the number of participants with complete data available
When comparing individuals who responded to the post-screening survey to those who did not, we found that a higher proportion of respondents were females (70.1 vs. 65.6 %), older (mean age 63.8 years vs. 56.5 years), and had a BMI in the normal or underweight category (BMI ≤ 24.9) (34.1 vs. 31.1 %) (Appendix 2). Further, more respondents self-reported hypertension (45.5 vs. 36.3 %, respectively), although non-respondents were more likely to be members of high-risk ethnic groups. Finally, respondents were more likely to be aware of the risk factors for CKD (health knowledge) than non-respondents (90.1 vs. 87.7 %, respectively).
The majority (89.8 %) of participants self-reported a health behaviour change in the post-screening survey. Amongst those who reported making a health behaviour change, most people indicated making dietary changes (79.9 %), improving their adherence to recommendations provided by their healthcare providers (65.7 %), and making daily lifestyle changes (75.8 %). A small proportion (6.4 %) of respondents indicated quitting smoking, chewing tobacco, or reducing alcohol intake as their health behaviour change (Table 2).
Table 2.
Proportion of respondents who self-reported a behaviour change, by category of change
| Categories of behaviour change | Respondentsa (N = 1014) |
|---|---|
| Dietary, n (%) | 810 (79.9) |
| Improving adherence, n (%) | 666 (65.7) |
| Reducing risk behaviours, n (%) | 65 (6.4) |
| Daily lifestyle, n (%) | 769 (75.8) |
aProportions do not total to 100 % as respondents may chose more than one category
We identified four significant predictors of behaviour change (Fig. 2). Individuals classified as overweight (BMI 25.0–29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) were more likely to make a behaviour change (PRR 0.66, 95 % CI 0.44–0.99 and PRR 0.49, 95 % CI 0.30–0.80) as compared to those with a normal or underweight BMI (≤ 24.9 kg/m2). Further, participants unaware of the risk factors for CKD were less likely (PRR 1.75, 95 % CI 1.07–2.87) to make a behaviour change. Conversely, respondents who reported no particular motivation to participate in the screening events were more likely (PRR 0.44, 95 % CI 0.22–0.88) to make a behaviour change following the screening event. Finally, individuals who indicated intent to make health behaviour changes during the pre-screening survey were more likely to self-report making a behaviour change (PRR 0.58, 95 % CI 0.35–0.96) and those who said they had initiated preliminary behaviour changes were more likely to continue to make health behaviour changes (PRR 0.45, 95 % CI 0.29–0.68).
Fig. 2.
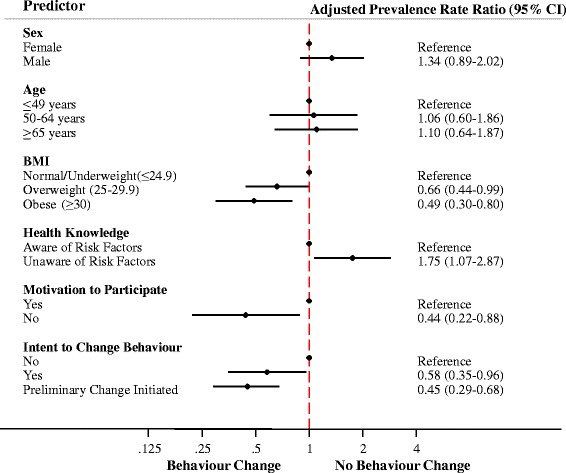
Adjusted prevalence rate ratio (PRR) for the association between participant characteristics and likelihood of behaviour change
Within our stratified analysis, the proportion of participants who self-reported a behaviour change was similar amongst those with eGFR < 60 and ≥ 60 mL/min/1.73 m2 for most patient characteristics (Fig. 3). However, a significantly higher proportion of females with eGFR < 60 mL/min/1.73 m2 (79 vs. 66 %, respectively (p < 0.05)) and individuals over 65 years old with eGFR < 60 mL/min/1.73 m2 (78 vs. 46 %, respectively (p < 0.05) reported self-reported behaviour change as compared to the participants with eGFR ≥ 60 mL/min/1.73 m2. Finally, results were similar in a sensitivity analysis excluding the 156 participants who self-reported having kidney problems and had an eGFR of <60 mL/min/1.73 m2.
Fig. 3.
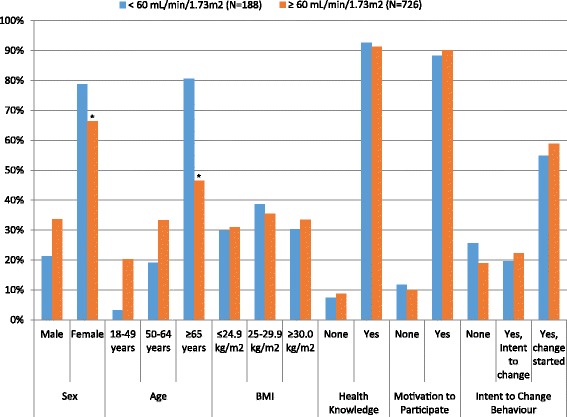
Proportion of participants who self-reported behaviour change by participant characteristics and eGFR category. *denotes statistically significant differences in proportions
Discussion
In this national targeted screening programme to identify patients with unrecognized CKD, we found that individual counselling and goal setting may be an effective strategy to elicit a self-reported health behaviour change. We were also able to identify specific subgroups that could be targeted for this type of intervention. Specifically, participants unaware of the risk factors for CKD (limited health knowledge) were less likely to make a health behaviour change. However, individuals who were clinically overweight or obese, those with no self-identified motivation to participate in the screening event, and those who indicated an intent to change their behaviour were more likely to report a health behaviour change. Results were similar for patients with eGFRs of < 60 and ≥ 60 mL/min/1.73 m2 except for age and gender, where a higher proportion of women over 65 years of age with eGFR < 60 mL/min/1.73 m2 made a behaviour change as compared to their eGFR ≥ 60 mL/min/1.73 m2 counterparts.
Behaviour change interventions aim to promote healthy lifestyles and improve the uptake and optimal use of effective clinical services using a ‘coordinated set of activities designed to change specified behaviour patterns [17].’ Unfortunately, a combination of paucity of evidence, heterogeneous interventions, and poor reporting [28] leads to difficulty ascertaining the effectiveness of behaviour change interventions within CKD populations [25]. Although multifaceted educational interventions used to support behaviour change [21] have been shown to be effective in lowering blood pressure, improving blood sugars, and increasing health knowledge for various chronic conditions (diabetes and hypertension) [18], research to date has only shown effectiveness in improving knowledge [19] and prompting belief changes [20] within CKD. Given the difficulty in designing effective behaviour change interventions [17], recent evidence suggests that these interventions should be tailored to the individual and their disease trajectory [21, 29]. In fact, an individualised nutritional counselling intervention reported significant reductions in self-reported symptoms and problems associated with kidney disease in a pre-dialysis CKD population which shows promise for individual counselling in CKD [24]. Further research is required to understand the use of behaviour change interventions for patients with CKD. Our study highlights the use of individual counselling and goal setting to promote behaviour change following a targeted screening clinic.
Our results suggest that individual counselling and goal setting provided during targeted screening may be effective in eliciting behaviour change in certain groups of participants. We found that participants who were overweight or obese were more likely to change their behaviour, which could be attributed to a realization of poor health status at the screening event. In fact, recent evidence suggests goal setting is associated with weight reduction in patients with diabetes [30]. However, further research is required to determine if these interventions result in sustained long-term behaviour change.
Overall, the SeeKD individual counselling and goal setting intervention provided knowledge and skills on risk factors for kidney disease and prevention strategies (capability), external factors to prompt behaviour (opportunity), as well as some motivation (habitual or emotional processes to direct behaviour) to participants; all which differentially affected participant groups. For example, participants with an intent to change likely required opportunity and additional motivation, while those who had begun preliminary changes were simply reinforced to continue their behaviour change, thus highlighting pre-existing motivation in both groups. This is consistent with Proshaska and DiClemente’s model on the stages of behaviour change [31]. Participants with an intent to change are in the ‘preparation’ stage, while those who had begun preliminary changes would be in the beginning of the ‘action’ stage [31, 32].
Further, participants with no health knowledge of CKD (unaware of risk factors) were less likely to make a behaviour change. While this group may have low health literacy, which is associated with poor health outcomes and poor use of healthcare services [33], we cannot overlook the potential confounding effect of socioeconomic status [34]. Unfortunately, this information was not collected at the screening events. Finally, participants with no self-identified motivation to participate may be generally unaware of their personal health status but given the knowledge and skills, accompanied by externally derived motivation, are able to leverage the opportunity to make a behaviour change.
Our work suggests that future screening programmes may consider using individual counselling as a component of a health behaviour change intervention, but perhaps a different intervention is necessary when targeting individuals with low health knowledge. In general, counselling sessions should first identify the specific behaviour(s) for change and, using a behaviour framework [35], design an intervention focused on improving the uptake of knowledge and skills and simultaneously increasing motivation and empowerment [36] in order to improve the extent of behaviour change and engage those less likely to change. Given that many of the commitments to change fell within the ‘soft’ categories (e.g. dietary and lifestyle modifications), additional work is required to identify strategies aimed at more risky health behaviours such as smoking and alcohol consumption.
Though it is difficult to determine whether counselling sessions provided at a screening event would be similar to what is currently provided by physicians/nurses/pharmacists during clinic visits, it is likely that clinical discussions are more ad hoc and variable. Using a standardized tool that focuses on the needs of the patient may be promising but requires further research and evaluation before implementation in clinical practice. Further, the collection of additional participant information related to tolerability and satisfaction may also be important considerations in the adoption of such an intervention within a clinical setting.
Consideration should be given to limitations of the SeeKD screening programme when interpreting these results. As all participants screened for CKD were provided with this relatively short intervention (~20-min individual counselling session) and given the lack of a control group, we are unable to determine the true effectiveness of the behaviour change intervention. There may also be volunteer bias as participants self-selected to participate and may be systematically different from those who did not participate [37]. This is evident by the respondent characteristics, where the majority of participants were older females who participated due to a personal concern for their health. Follow-up bias is also of concern as survey respondents differed from the original study population. These potential selection biases may limit generalizability of the study population to the Canadian population at risk for CKD. Social desirability bias, a type of reporting bias whereby participants have a tendency to present a favourable image of themselves (e.g. overreport behaviour change) is of particular concern given the high proportion of participants who self-reported a behaviour change (89.8 %). However, this unrealistic positive response rate may also be driven by questionnaire design where participants did not explicitly have the option of stating no behaviour change.
Conclusions
In this national survey of participants with risk factors for CKD, we found that the use of individual counselling and goal setting may be an effective intervention for stimulating behaviour change. This study highlights the importance of targeting specific groups with behaviour change interventions for optimal uptake. However, the current findings should be interpreted with caution given the study limitations. Despite the high rate of reported behaviour change amongst participants, future research is required to determine the key components of individual counselling as a behaviour change intervention, particularly within CKD populations.
Abbreviations
BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; PRR, prevalence rate ratio; SD, standard deviation; SeeKD, See Kidney Disease targeted screening programme
Acknowledgements
The authors thank the many Kidney Foundation of Canada staff, volunteers, nurses, and pharmacists who helped make the SeeKD programme possible.
Funding
The See Kidney Disease targeted screening programme was supported by the Kidney Foundation of Canada through a partnership with Canadian National Railway. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. LG is supported by a strategic training in health research award from the Knowledge Translation Canada, PR is supported by the Roy and Vi Baay Chair assistant professorship, SS is supported by a Canadian Institutes of Health Research operating grant, BM is supported by the Svare Professorship in Health Economics, and BH is supported by the Roy and Vi Baay Chair in Kidney Research. The Interdisciplinary Chronic Disease Collaboration is funded by the Alberta Innovates Health Solutions-Collaborative Research Innovation Opportunity (CRIO) Team Grants Programme.
Availability of data and materials
The datasets supporting the conclusions of this article are intellectual property of the Kidney Foundation of Canada and are not publically available.
Authors’ contributions
LG conducted the data cleaning, analysis, and drafting of the manuscript. LG, PR, and BH participated in the interpretation of the results, and all authors have been involved in critically revising the manuscript for important intellectual content. All authors agree to be accountable for all aspects of the work and ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethical approval and consent to participate
The SeeKD targeted screening programme obtained research ethics board approval from the Health Canada. Ethics approval for analysis was also obtained from the Conjoint Health Research Ethics Board at the University of Calgary.
Appendix 1
Behaviour change intervention
The behaviour change intervention, defined as an individual counselling and goal setting session conducted at each of the SeeKD screening events, followed the SeeKD protocol developed by the Kidney Foundation of Canada. The behaviour change intervention was carried out by a registered nurse, a pharmacist, or a dietician with experience in CKD, who tailored recommendations and strategies for behaviour change to each participant based on their risk factors and clinical measurements documented on their health data form. These sessions were allotted 20 min for discussion of kidney disease and how the participant may reduce their risk of developing CKD and CKD-related complications. Each screening event included a private counselling area. Brochures on kidney disease, its risk factors and prevention, generated by the Kidney Foundation of Canada, as well as copies of the Canada’s Food Guide and Canada Fitness Guide, were also provided to participants. These documents were offered in Chinese, Punjabi, French, and English in screening events across Canada. However, some regions (Ontario) were able to translate the brochures into the participants’ language of preference (i.e. Vietnamese, Korean, and Cambodian).
Appendix 2
Table 3.
Comparison of respondents to non-respondents
| Respondents (N = 1129a) | Non-respondents (N = 3265a) | |
|---|---|---|
| Gender, male, n (%) | 337 (29.9) | 1119 (34.4) |
| Age (years), mean (SD) | 63.8 (14.3) | 56.5 (15.4) |
| CKD (eGFR < 60 mL/min/1.73 m2), n (%) | 208 (20.6) | 509 (17.3) |
| Motivation for participating, n (%) | ||
| Concern for personal health status | 618 (54.7) | 1761 (53.9) |
| Influence from external source | 227 (20.1) | 803 (24.6) |
| Recruitment efforts | 361 (32.0) | 789 (24.2) |
| None | 110 (9.7) | 424 (13.0) |
| Self-reported risk factors, n (%) | ||
| Diagnosed diabetes | 274 (24.3) | 693 (21.2) |
| Diagnosed hypertension | 524 (45.5) | 1186 (36.3) |
| Problems with kidneys | 156 (13.8) | 422 (12.9) |
| High-risk ethnic groups | 509 (45.1) | 2026 (62.1) |
| Vascular disease | 268 (23.7) | 614 (18.8) |
| Family history of kidney problems | 166 (14.7) | 454 (13.9) |
| Smoking or tobacco use | 128 (11.3) | 541 (16.6) |
| Knowledge of risk factors for CKD, n (%) | ||
| Yes | 1017 (90.1) | 2862 (87.7) |
| No | 110 (9.7) | 403 (12.3) |
| Body mass index, n (%) | ||
| Normal/underweight (≤ 24.9 kg/m2) | 385 (34.1) | 1015 (31.1) |
| Overweight (25.0–29.9 kg/m2) | 376 (33.3) | 1057 (32.4) |
| Obese (≥ 30.0 kg/m2) | 315 (27.9) | 1070 (32.8) |
SD standard deviation, CKD chronic kidney disease
aDenominator varied for each variable depending on the number of participants with complete data available
Contributor Information
Lauren Galbraith, Email: lgalbrai@ucalgary.ca.
Brenda Hemmelgarn, Email: Brenda.Hemmelgarn@albertahealthservices.ca.
Braden Manns, Email: Braden.Manns@albertahealthservices.ca.
Susan Samuel, Email: S.Samuel@albertahealthservices.ca.
Joanne Kappel, Email: jekappel@sasktel.net.
Nadine Valk, Email: NValk@champlainpalliative.ca.
Paul Ronksley, Email: peronksl@ucalgary.ca.
References
- 1.Tonelli M, Wiebe N, Culleton B, House A. Rabbat C, Fok M, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–47. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 2.Stevens LA, Li S, Wang C, Huang C, Becker BN, Bomback AS, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: results from the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2010;55:S23–33. doi: 10.1053/j.ajkd.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Group KDIGOKCW KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [PubMed] [Google Scholar]
- 4.Kucirka LM, Grams ME, Lessler J, Hall EC, James N, Massie AB, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306:620–6. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osthus TB, von der Lippe N, Ribu L, Rustoen T, Leivestad T, Dammen T, et al. Health-related quality of life and all-cause mortality in patients with diabetes on dialysis. BMC Nephrol. 2012;13:78. doi: 10.1186/1471-2369-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–85. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–95. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 8.Bolignano D, Palmer SC, Navaneethan SD, Strippoli GF. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2014;4:CD007004. doi: 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Lv J, Perkovic V, Foote CV, Craig ME, Craig JC, Strippoli GF. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev. 2012;12:CD004136. doi: 10.1002/14651858.CD004136.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oomichi T, Emoto M, Tabata T, Morioka T, Tsujimoto Y, Tahara H, et al. Impact of glycemic control on survival of diabetic patients on chronic regular hemodialysis: a 7-year observational study. Diab Care. 2006;29:1496–500. doi: 10.2337/dc05-1887. [DOI] [PubMed] [Google Scholar]
- 11.Ishimura E, Okuno S, Kono K, Fujino-Kato Y, Maeno Y, Kagitani S, et al. Glycemic control and survival of diabetic hemodialysis patients—importance of lower hemoglobin A1C levels. Diabetes Res Clin Pract. 2009;83:320–6. doi: 10.1016/j.diabres.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 12.Agrawal V, Giri C, Solomon RJ. The effects of glucose-lowering therapies on diabetic kidney disease. Curr Diabetes Rev. 2015;11:191–200. doi: 10.2174/1573399811666150331160534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, et al. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;5:CD007784. doi: 10.1002/14651858.CD007784.pub2. [DOI] [PubMed] [Google Scholar]
- 14.James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296–309. doi: 10.1016/S0140-6736(09)62004-3. [DOI] [PubMed] [Google Scholar]
- 15.Hibbard JH, Mahoney ER, Stock R, Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–63. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 17.Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427–38. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 19.Mason J, Khunti K, Stone M, Farooqi A, Carr S. Educational interventions in kidney disease care: a systematic review of randomized trials. Am J Kidney Dis. 2008;51:933–51. doi: 10.1053/j.ajkd.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Li T, Wu HM, Wang F, Huang CQ, Yang M, Dong BR, et al. Education programmes for people with diabetic kidney disease. Cochrane Database Syst Rev. 2011;6:CD007374. doi: 10.1002/14651858.CD007374.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Walker R, James H, Burns A. Adhering to behaviour change in older pre-dialysis populations—what do patients think? A qualitative study. J Ren Care. 2012;38:34–42. doi: 10.1111/j.1755-6686.2012.00262.x. [DOI] [PubMed] [Google Scholar]
- 22.Cremin I, Nyamukapa C, Sherr L, Hallett TB, Chawira G, Cauchemez S, et al. Patterns of self-reported behaviour change associated with receiving voluntary counselling and testing in a longitudinal study from Manicaland, Zimbabwe. AIDS Behav. 2010;14:708–15. doi: 10.1007/s10461-009-9592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. BMJ. 1999;319:943–7. doi: 10.1136/bmj.319.7215.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell KL, Ash S, Bauer JD. The impact of nutrition intervention on quality of life in pre-dialysis chronic kidney disease patients. Clin Nutr. 2008;27:537–44. doi: 10.1016/j.clnu.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Bonner A, Havas K, Douglas C, Thepha T, Bennett P, Clark R. Self-management programmes in stages 1-4 chronic kidney disease: a literature review. J Ren Care. 2014;40:194–204. doi: 10.1111/jorc.12058. [DOI] [PubMed] [Google Scholar]
- 26.Galbraith L, Ronksley P, Barnieh L, Kappel J, Manns B, Samuel S, Jun M, Weaver R, Valk N, Hemmelgarn B. The See Kidney Disease (SeeKD) targeted screening program for CKD. Clin J Am Soc Nephrol. 2016;11:964–74. doi: 10.2215/CJN.11961115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.StataCorp . Stata statistical software: release 12. College Station: StataCorp LP; 2011. [Google Scholar]
- 28.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS—practical systematic review of self-management upport for long-term conditions. Southampton: Health Services and Delivery Research (NIHR) Journals Library; 2014. [PubMed] [Google Scholar]
- 30.Hankonen N, Sutton S, Prevost AT, Simmons RK, Griffin SJ, Kinmonth AL, et al. Which behavior change techniques are associated with changes in physical activity, diet and body mass index in people with recently diagnosed diabetes? Ann Behav Med. 2015;49:7–17. doi: 10.1007/s12160-014-9624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51:390–5. doi: 10.1037/0022-006X.51.3.390. [DOI] [PubMed] [Google Scholar]
- 32.Johnson SS, Driskell MM, Johnson JL, Dyment SJ, Prochaska JO, Prochaska JM, et al. Transtheoretical model intervention for adherence to lipid-lowering drugs. Dis Manag. 2006;9:102–14. doi: 10.1089/dis.2006.9.102. [DOI] [PubMed] [Google Scholar]
- 33.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Int Med. 2011;155:97–107. doi: 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- 34.Stewart DW, Reitzel LR, Correa-Fernandez V, Cano MA, Adams CE, Cao Y, et al. Social support mediates the association of health literacy and depression among racially/ethnically diverse smokers with low socioeconomic status. J Behav Med. 2014;37:1169–79. doi: 10.1007/s10865-014-9566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michie S, Abraham C. Interventions to change health behaviours: evidence-based or evidence-inspired? Psychol Health. 2004;19:29–49. doi: 10.1080/0887044031000141199. [DOI] [Google Scholar]
- 36.Camerini L, Schulz PJ, Nakamoto K. Differential effects of health knowledge and health empowerment over patients’ self-management and health outcomes: a cross-sectional evaluation. Patient Educ Couns. 2012;89:337–44. doi: 10.1016/j.pec.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Oleckno WA. Epidemiology: concepts and methods. Long Grove: Waveland Press, Inc.; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are intellectual property of the Kidney Foundation of Canada and are not publically available.


