Introduction
Gastro-oesophageal reflux disease (GORD) is a very common under-diagnosed condition resulting in a significant impairment in quality of life. A recent population-based study demonstrated the prevalence of reflux symptoms to be 44% with 24% of individuals experiencing symptoms for two days or more per week. The prevalence of oesophagitis and Barrett's oesophagus was 12% and 1.3% respectively, irrespective of symptoms.1 Thirty-three per cent of individuals with oesophagitis and 46% with Barrett's oesophagus were asymptomatic. Severe GORD can lead to potentially avoidable complications including severe oesophagitis with scarring and stricture formation, Barrett's oesophagus and adenocarcinoma. This overview describes the pathophysiology, presentation, investigation and treatment of GORD.
Definition of gastro-oesophageal reflux disease
The Montreal classification defined GORD as ‘a condition that develops when the reflux of stomach contents causes troublesome symptoms and/or complications’.2
The acid component of the gastric refluxate is responsible for most of the symptoms and pathology associated with GORD, however, other components, such as bile, may also contribute (known as non-acid reflux). Quantification of reflux can be achieved either by measuring acid exposure to the distal oesophagus (pH studies) or movement of liquid in the distal oesophagus (impedance studies). Combined impedance and pH measurement characterises all acid and non-acid reflux episodes.
Non-erosive reflux disease
Nearly 70% of patients with heartburn do not have evidence of erosive changes on endoscopy. Of these, a proportion have increased acid reflux on 24-hour pH monitoring and are classified as having non-erosive reflux disease (NERD).3
Functional heartburn
A proportion of patients describe typical reflux symptoms without evidence of reflux. Clouse and colleagues have described this condition as ‘functional heartburn’.4 The Rome II definition of functional heartburn is ‘an episodic burning in the absence of pathologic gastroesophageal reflux, motility disorders and any structural explanations’. It is likely that acid exposure is not the major cause of symptoms in patients with functional heartburn and, as such, the pathophysiology remains obscure.
Reflux oesophagitis
Reflux oesophagitis is defined as reflux causing inflammation or ulceration of the oesophagus. Attempts have been made to classify the extent of damage, of which the Los Angeles classification is now the most commonly used (Table 1).5
Table 1.
Los Angeles (LA) classification of oesophagitis.
Pathophysiology
Gastro-oesophageal reflux disease results from disruption of the anti-reflux barrier, which is comprised of the internal lower oesophageal sphincter (LOS), a 3–4 cm region of circular muscle in the distal oesophagus and externally, the crural diaphragm. Other factors contributing towards the magnitude of reflux include oesophageal clearance and neutralisation mechanisms.
Relaxation of the LOS and crura are normal physiological processes occurring during swallowing and also during gas venting. Relaxations not initiated by a swallow are known as transient lower oesophageal relaxations (TLOSRs). Abnormal TLOSRs have a greater cross-sectional area at the gastro-oesophageal junction resulting in the reflux of gastric fluid in addition to gas.6 TLOSRs probably account for 90% of reflux episodes.
The TLOSR reflex is initiated by tension receptors in the stomach and mediated by a vagovagal pathway via the brainstem leading to simultaneous relaxation of the crura, LOS and inhibition of peristalsis.7 These responses are inhibited by gammaaminobutyric acid-B (GABA-B) receptor agonists, and thus may constitute a future therapeutic target. Hiatus hernias appear to increase the magnitude of reflux during TLOSRs.
Transient lower oesophageal relaxations appear to be less significant in more severe reflux oesophagitis. A hypotensive LOS, which allows high pressure gradients across the diaphragm, is probably responsible for more severe oesophagitis.8 Reflux occurs more frequently when the pressure in the LOS is <10 mm Hg, and free reflux only occurs if the LOS pressure is <4 mm Hg.9,10
Factors which relax the LOS, such as caffeine, fat, smoking, drugs (calcium channel antagonists and nitrates) and gastric distention, will increase the likelihood of reflux.
The association of increased body mass index and GORD remains unclear.11,12 A recent study failed to demonstrate an increased risk of reflux symptoms or oesophagitis in obese individuals.1
Acid in the distal oesophagus has been shown to be neutralised by saliva. Therefore any processes reducing saliva production results in a delay in acid neutralisation. Nocturnal reflux episodes are prolonged due to depressed salivation.
Acid is cleared by oesophageal peristalsis. Therefore impaired distal oesophageal peristalsis results in prolonged acid exposure to acid reflux episodes. This is apparent in both hiatus hernias and ulcerative oesophagitis where there is peristaltic dysfunction.9,13
A proportion of patients present with typical symptoms of GORD with normal 24-hour pH studies and endoscopic findings (functional heartburn). These patients have an elevated symptomatic response to acid exposure or oesophageal balloon distension.14 The mechanism behind the symptoms is not well understood, but is likely to be multi-factorial, including oesophageal distention with refluxate, spasm and visceral hyperalgesia.
Pathogenic factors in gastro-oesophageal reflux disease
Pathogenic factors include:
lower oesophageal sphincter and crural function (hiatus hernia)
transient lower oesophageal sphincter relaxations
oesophageal clearance mechanisms (oesophageal motility, saliva)
refluxate (gastric acid, bile, enzymes)
mucosal response to refluxate
drugs affecting anti-reflux barrier (nitrates, calcium antagonists)
diet (fat, caffeine).
Clinical presentation of gastro-oesophageal reflux disease
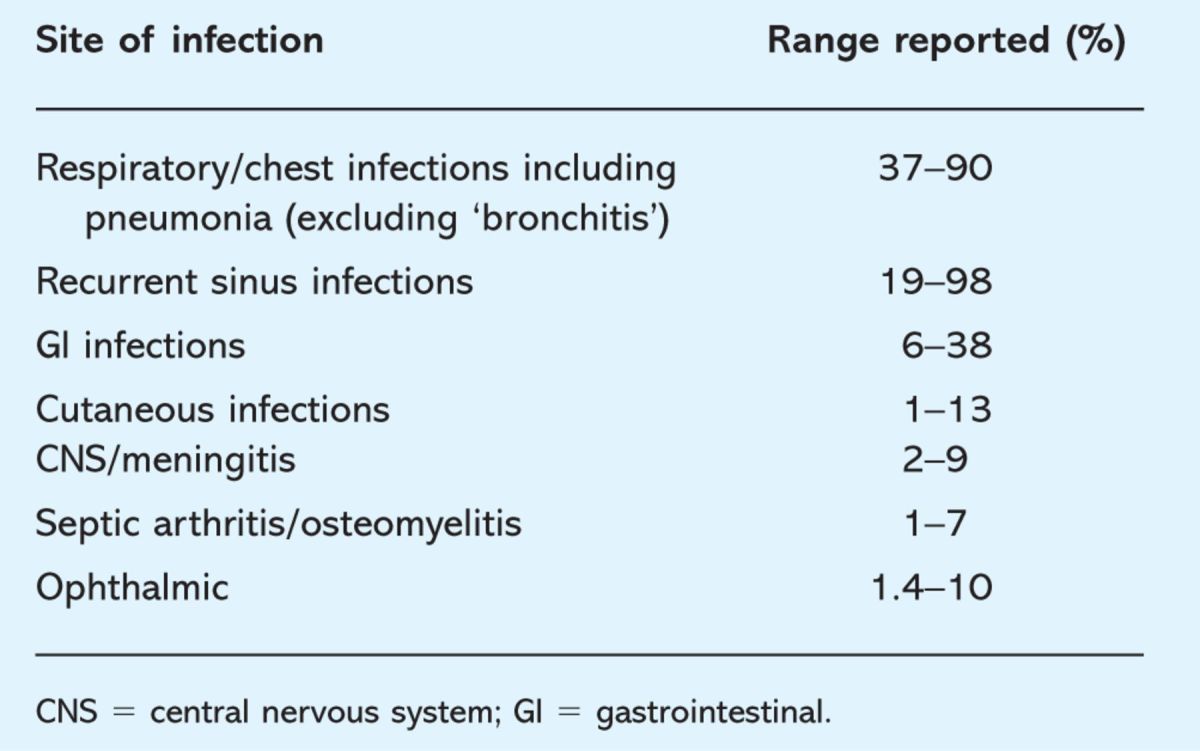
The most common symptoms attributed to reflux include heartburn, regurgitation, belching, epigastric pain, chest pain, dysphagia and acid brash. Less frequent symptoms include odynophagia, globus, nausea and pharyngeal symptoms. Symptoms of GORD, however, can be highly variable.
Diagnosis of GORD can be difficult. For example the presence of heartburn is not entirely predictive of acid reflux. Furthermore, only 5–10% of episodes of acid reflux produce heartburn.15 Moreover, the positive predictive value of heartburn is poor as a predictor of endoscopic oesophagitis, although the negative predictive value is slightly better.16,17
Investigations
Most patients with typical symptoms of GORD do not require investigation and will respond to a trial of proton-pump inhibitor (PPI). High-dose PPI (40 mg omeprazole twice daily for one week) has a sensitivity of 80% as a diagnostic test for GORD.18,19
Oesophago-gastro-duodenoscopy
Patients of any age presenting with typical reflux symptoms with the following alarm symptoms should have an urgent endoscopy:
dysphagia
unintentional weight loss
gastrointestinal bleeding
anaemia
persistent vomiting.
Endoscopy should also be performed before oesophageal pH studies. Endoscopy for patients presenting with heartburn is not otherwise indicated. Moreover, a negative endoscopy does not exclude NERD.3
Screening endoscopy for the diagnosis of Barrett's oesophagus in patients of any age with a history of chronic heartburn cannot be recommended, as the absolute risk of adenocarcinoma is less than 1:1,000.20 Furthermore, 40% of patients who develop adenocarcinoma in association with Barrett's oesophagus do not have a history of heartburn.21
Oesophageal ambulatory pH studies
Acid exposure of the distal oesophagus can be measured either by placing a nasooesophageal probe 5 cm above the physiological LOS or by placing a wireless capsule probe endoscopically. Measurements of pH can be taken for either 24 or 48 hours. Proton-pump inhibitors or histaminereceptor antagonists (H2A) should be stopped one week before the procedure. Normal ranges are seen in Table 2 although they may vary between laboratories.22,23 Acid exposure above these values is considered to be pathological.
Table 2.
Normal ranges for oesophageal pH studies.
Key points
Gastro-oesophageal reflux disease affects up to 44% of the population
The prevalence of Barrett's oesophagus on routine endoscopy is 1.3%
Endoscopy is not indicated for reflux symptoms without alarm symptoms
Ambulatory pH studies are indicated for reflux symptoms not responding to proton-pump inhibitors
The indications for pH studies are listed in Table 3.24 Patients who have oesophagitis demonstrated on endoscopy do not need pH studies for diagnostic purposes. Patients who have refractory symptoms to a trial of PPI may have inadequate acid suppression.25 This can be demonstrated by pH studies obtained while the patient is taking PPIs. It is reasonable as an alternative, however, to escalate PPI dose in these patients.
Table 3.
Indications for ambulatory oesophageal pH studies.
Oesophageal manometry
Oesophageal manometry does not influence the management of GORD except in a few circumstances. The procedure may be useful where oesophageal motility abnormalities are suspected such as achalasia. Manometry is also required to correctly place the pH probe 5 cm above the LOS. Some groups advocate manometry prior to anti-reflux surgery, although studies suggest this very rarely changes outcome or helps tailor surgery.26
Multichannel intraluminal impedance
Impedance testing measures changes in electrical resistance within the oesophagus. Resistance (impedance) increases if the oesophagus is empty and decreases with ionic fluid. Therefore, if impedance is measured at multiple levels, the direction and duration of refluxate can be determined. Furthermore, if combined with pH studies, non-acid and acid reflux episodes can be determined and the relevance of each reflux episode can be determined by the association with symptoms. Multichannel intraluminal impedance may therefore be useful for the investigation of PPI-resistant reflux symptoms although its role has not yet been completely defined.
Treatment
The aim of GORD treatment is to effectively control symptoms and prevent GORD-associated complications. As with many conditions, adopting a stepped approach to treatment helps tailor disease severity to treatment regimen.
Most studies have addressed the effects of drugs on either the healing and/or symptom relief of oesophagitis or NERD. Broadly, the responses are similar for both groups, although the benefit over placebo is generally greater in patients with endoscopy proven oesophagitis.
Lifestyle changes
Simple manoeuvres may have a marked effect on symptoms. These are outlined below:
dietary changes. Some substances influence oesophageal physiology favouring increased acid reflux. These include fat, caffeine and alcohol
avoiding late meals. Acid reflux episodes are prolonged when asleep as a result of both gravity, and also reduced peristalsis and acid clearance. Nocturnal reflux can therefore be minimised by consuming small meals long before sleep
although the association of obesity and GORD is unclear, there does appear to be an association with oesophageal adenocarcinoma and many other well-known diseases.11 On this basis weight loss is suggested as part of GORD management.
Antacids/alginate combinations
Antacids consist of calcium carbonate, magnesium and aluminum salts in various compounds or combinations. The effect of antacids is due to partial neutralisation of gastric hydrochloric acid and inhibition of the proteolytic enzyme, pepsin. Alginate mechanism of action is due to the formation of a gel in the presence of gastric acid. Alginate-based raftforming formulations usually contain sodium or potassium bicarbonate; in the presence of gastric acid, the bicarbonate is converted to carbon dioxide, which becomes entrapped within the gel precipitate, converting it into a foam which floats on the surface of the gastric contents, much like a raft on water. Both in vitro and in vivo studies have demonstrated that alginate-based rafts can entrap carbon dioxide, as well as antacid components contained in some formulations, thus providing a relatively pH-neutral barrier.
Antacids and alginates have been shown to improve reflux symptoms, however, they do not heal oesophagitis.27 They are indicated for very mild symptoms, where step-up treatment is not necessary. There is no role for antacids/alginates in the maintenance of GORD.
Inhibitors of acid secretion
Proton-pump inhibitors are prodrugs, which are activated in the acidic canalicular space of the parietal cell, inhibiting the final pathway of acid secretion and suppress acid production for several days. Histamine-2-receptor antagonists (H2A) reversibly bind H2 gastric parietal cell receptors.
Both H2As and PPIs have been shown to effectively heal and prevent relapse of oesophagitis, although PPIs have been shown to be superior to both H2As and prokinetic agents.28
Healing occurred in 22% of patients on placebo, 39% on H2A (number needed to treat (NNT) = 6) and 76% on a PPI (NNT = 2).29 The efficacy of PPIs is dose dependant and mucosal healing has been shown to be dependent upon the proportion of time the intra-gastric pH is >4. The dose effect of H2As, however, is constant. The superiority of PPIs over H2As has also been proven for the treatment of NERD patients.30 At equivalent doses, all the PPIs appear to be equally efficacious.
In general, H2As and PPIs should be prescribed for patients with symptoms not well controlled on lifestyle changes or antacids/alginates (Table 4).
Table 4.
Effects of long-term use of proton-pump inhibitors (PPIs).

Patients not responding to proton-pump inhibitors
Patients with typical reflux symptoms, who do not respond to initial courses of PPIs fall into one of the following groups:
patients with an alternative diagnosis
patients with inadequate acid suppression
patients with functional heartburn.
The effect of food on the bioavailability of PPIs is variable. However, to maximise the individual response, PPIs should be taken 30 minutes before a meal.
If symptom control is not achieved after four weeks, we suggest extending PPI therapy to two months. Healing rates improve from 68% to 84% after four and eight weeks respectively at standard dose.29
A proportion of patients fail to adequately increase the gastric pH >4 with standard-dose PPIs. Doubling the PPI dose does appear to have an additional effect, although this is small, with an absolute increase of 5% (NNT = 19).29 In patients with more severe oesophagitis (LA grade C and D) the effect of doubling PPI dose is more marked, with an absolute increase in healing rates of 20%.31
There is some evidence that a proportion of patients experience nocturnal reflux symptoms associated with nocturnal acid breakthrough, even with twice daily PPI. The addition of an H2A before sleep may reduce nocturnal symptoms in this group, however, the benefit may be limited by tachyphylaxis.32,33
Prokinetic agents
Correction of the anti-reflux barrier is the most obvious therapeutic target for the treatment of GORD, however, existing drugs have not shown promise. Little data exist for domperidone in the treatment of GORD. GABA-B agonists may be potential targets for future developments.
Surgery
The rationale behind anti-reflux surgery is to reverse the physiological LOS abnormalities associated with gastro-oesophageal reflux. This is achieved by lengthening and increasing the proportion of intra-abdominal LOS, which results in a higher LOS resting pressure. The most common technique is the Nissen fundoplication, where the gastric fundus is wrapped 360° around the distal oesophagus.
There is no statistical difference between outcomes of medical therapy (PPI or H2A) and surgery for GORD. Given that there is a recognised postoperative mortality associated with surgery of up to 0.4%, this is only recommended if patients with proven GORD do not respond to medical therapy, do not wish to take medication or have GORD-associated respiratory symptoms not responding to PPI. The most common complications of Nissen fundoplication include dysphagia and gas-bloat syndrome which have been described in up to 50% of patients. Furthermore, after a median of five years, up to 50% of patients after surgery require medication to control symptoms.34
Summary
Gastro-oesophageal reflux disease results from impaired function of the LOS and acid clearance of the distal oesophagus. Most patients do not require investigation and respond either to lifestyle changes, antacid/alginates, H2A, PPI or a combination of these treatments. Surgery is only rarely indicated.
References
- 1.Zagari RM, Fuccio L, Wallander MA. et al Gastro-oesophageal reflux symptoms, oesophagitis and Barrett's oesophagus in the general opoulation: the Loiano-Monghidoro study. Gut 2008;57:1354–9. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900–20. 10.1111/j.1572-0241.2006.00630.x [DOI] [PubMed] [Google Scholar]
- 3.Jones RH, Hungrin ADS, Phillips J. Gastroesophageal reflux disease in primary care in Europe: clinical presentation and endoscopic findings. Eur J Gen Pract 1995;1:149–54. [Google Scholar]
- 4.Clouse RE, Richter JE, Heading RC, Janssens J, Wilson JA. Functional esophageal disorders. Gut 2006;45 (Suppl 2):II31–6. 10.1136/gut.45.2008.ii31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong D, Bennett JR, Blum AL. et al The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996;111:85–92. 10.1053/gast.1996.v111.pm8698230 [DOI] [PubMed] [Google Scholar]
- 6.Pandolfino JE, Shi G, Trueworthy B, Kahrilas PJ. Esophagogastric junction opening during relaxation distinguishes nonhernia reflux patients, hernia patients, and normal subjects. Gastroenterology 2003;125:1018–24. 10.1016/S0016-5085(03)01210-1 [DOI] [PubMed] [Google Scholar]
- 7.Blackshaw LA. New insights in the neural regulation of the lower oesophageal sphincter. Eur Rev Med Pharmacol Sci 2008;12(Suppl 1): 33–9. [PubMed] [Google Scholar]
- 8.Barham CP, Gotley DC, Mills A, Alderson D. Precipitating causes of acid reflux episodes in ambulant patients with gastro-oesophageal reflux disease. Gut 1995;36:505–10. 10.1136/gut.36.4.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Dodds WJ, Hogan WJ. et al Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology 1986;91:897–904. [DOI] [PubMed] [Google Scholar]
- 10.Dodds WJ, Dent J, Hogan WJ. et al Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med 1982;307:1547–52. 10.1056/NEJM198212163072503 [DOI] [PubMed] [Google Scholar]
- 11.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med 2005;143:199–211. [DOI] [PubMed] [Google Scholar]
- 12.Pandolfino JE, El-Serag HB, Zhang Q. et al Obesity: a challenge to esophagogastric junction integrity. Gastroenterology 2006;130:639–49. 10.1053/j.gastro.2005.12.016 [DOI] [PubMed] [Google Scholar]
- 13.Johnson LF. 24-hour pH monitoring in the study of gastroesophageal reflux. J Clin Gastroenterol 1980;2:387. 10.1097/00004836-198012000-00016 [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Stanley S, Robinson M, Earnest DL, Greenwood-Van Meerveld B, Miner P. Esophageal hypersensitivity may be a major cause of heartburn. Am J Gastroenterol 1999;94:628–31. [DOI] [PubMed] [Google Scholar]
- 15.Breumelhof R, Smout AJ. The symptom sensitivity index: a valuable additional parameter in 24-hour esophageal pH recording. Am J Gastroenterol 1991;86:160–4. [PubMed] [Google Scholar]
- 16.Adang RP, Ambergen AW, Talmon JL. et al The discriminative value of patient characteristics and dyspeptic symptoms for upper gastrointestinal endoscopic findings: a study on the clinical presentation of 1,147 patients. Digestion 1996;57:118–34. 10.1159/000201325 [DOI] [PubMed] [Google Scholar]
- 17.Hansen JM, Bytzer P, Schaffalitzky De Muckadell OB. Management of dyspeptic patients in primary care. Value of the unaided clinical diagnosis and of dyspepsia subgrouping. Scand J Gastroenterol 1998;33:799–805. [DOI] [PubMed] [Google Scholar]
- 18.Schindlbeck NE, Klauser AG, Voderholzer WA, Muller-Lissner SA. Empiric therapy for gastroesophageal reflux disease. Arch Intern Med 1995;155:1808–12. [PubMed] [Google Scholar]
- 19.Johnsson F, Hatlebakk JG, Klintenberg AC. et al One-week esomeprazole treatment: an effective confirmatory test in patients with suspected gastroesophageal reflux disease. Scand J Gastroenterol 2003;38:354–9. [PubMed] [Google Scholar]
- 20.Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: clinical applications. JAMA 2002;287:1982–6. 10.1001/jama.287.15.1982 [DOI] [PubMed] [Google Scholar]
- 21.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825–31. 10.1056/NEJM199903183401101 [DOI] [PubMed] [Google Scholar]
- 22.Galmiche JP, Scarpignato C. Esophageal pH monitoring. In: Functional evaluation in esophageal disease. Front Gastrointest Res 1994;22:71. [Google Scholar]
- 23.Richter JE, Bradley LA, DeMeester TR, Wu WC. Normal 24-hr ambulatory esophageal pH values. Influence of study center, pH electrode, age, and gender. Dig Dis Sci 1992;37:849–56. [DOI] [PubMed] [Google Scholar]
- 24.Bodger K, Trudgill N. Guidelines for oesophageal manometry and pH monitoring. London:BSG, 2006. [Google Scholar]
- 25.Leite LP, Johnston BT, Just RJ, Castell DO. Persistent acid secretion during omeprazole therapy: a study of gastric acid profiles in patients demonstrating failure of omeprazole therapy. Am J Gastroenterol 1996;91:1527–31. [PubMed] [Google Scholar]
- 26.Fibbe C, Layer P, Keller J. et al Esophageal motility in reflux disease before and after fundoplication: a prospective, randomized, clinical, and manometric study. Gastroenterology 2001;121:5–14. 10.1053/gast.2001.25486 [DOI] [PubMed] [Google Scholar]
- 27.Stanciu C, Bennett JR. Alginate/antacid in the reduction of gastro-oessophageal reflux. Lancet 1974;I:109–111. [DOI] [PubMed] [Google Scholar]
- 28.Armbrecht U, Abucar A, Hameeteman W, Schneider A, Stockbrugger RW. Treatment of reflux oesophagitis of moderate and severe grade with ranitidine or pantoprazole – comparison of 24-hour intragastric and oesophageal pH. Aliment Pharmacol Ther 1997;11:959–65. 10.1046/j.1365-2036.1997.00195.x [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Clinical Excellence. Dyspepsia: managing dyspepsia in adults in primary care. London: NICE, 2004. [Google Scholar]
- 30.Richter JE, Campbell DR, Kahrilas PJ, Huang B, Fludas C. Lansoprazole compared with ranitidine for the treatment of nonerosive gastroesophageal reflux disease. Arch Intern Med 2000;160:1803–9. 10.1001/archinte.160.12.1803 [DOI] [PubMed] [Google Scholar]
- 31.Richter JE, Kahrilas PJ, Sontag SJ. et al Comparing lansoprazole and omeprazole in onset of heartburn relief: results of a randomized, controlled trial in erosive esophagitis patients. Gastroenterology 2001;96:3089–98. [DOI] [PubMed] [Google Scholar]
- 32.Peghini PL, Katz PO, Bracy NA, Castell DO. Nocturnal recovery of gastric acid secretion with twice-daily dosing of proton pump inhibitors. Am J Gastroenterol 1998;93:763–7. [DOI] [PubMed] [Google Scholar]
- 33.Fackler WK, Ours TM, Vaezi MF, Richter JE. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002;122:625–32. 10.1053/gast.2002.31876 [DOI] [PubMed] [Google Scholar]
- 34.Dominitz JA, Dire CA, Billingsley KG, Todd-Stenberg JA. Complications and antireflux medication use after antireflux surgery. Clin Gastroenterol Hepatol 2006;4:299–305. 10.1016/j.cgh.2005.12.019 [DOI] [PubMed] [Google Scholar]


