The National Institute for Health and Clinical Excellence (NICE) guideline on the management of type 2 diabetes, prepared by the Royal College of Physicians (RCP),1 was the subject of a conference at the College in June 2008. This report highlights some themes of that meeting, concentrating on where aspects of cardiovascular risk management differ from non-diabetic people, on specific recommendations in regard to microvascular complications, on the guideline's key recommendations, and on recent aspects of blood glucose control. This review does not seek to be comprehensive.
Blood pressure management
Lowering of blood pressure (BP) in type 2 diabetes is associated not just with a reduction in the risk of cardiovascular disease (CVD) but also eye and kidney microvascular complications.2A BP-lowering strategy is thus particularly effective and cost effective in those with diabetes and kidney, eye, or cerebrovascular damage, with interventional thresholds and targets of 130/80 mmHg in these people and 140/80 mmHg in all others. The strategy should include lifestyle advice, medications if lifestyle advice does not achieve targets, and monitoring every one or two months, with intensification of therapy if not to target.
Notably, for type 2 diabetes, there is no general threshold below which BP lowering ceases to be beneficial3; accordingly lower BP if easily attained without side effects is desirable.
Overall, the best evidence for prevention of renal disease and retinopathy is for the renin–angiotensin system blockers (RAS blockers).4–6 Therefore, a generic 24-hour angiotensin-converting enzyme inhibitor (ACEI) should be used first line, with an angiotensin-2 receptor blocker (A2RB) substituted in the event of significant ACEI intolerance, usually a cough, but not if hyperkalaemia or decreased renal function is the problem. In people of Afro-Caribbean descent, either combination ACEI + diuretic therapy or ACEI + calcium-channel blocker (CCB) is recommended first line. A CCB would also be first-line therapy if there is a possibility of a woman becoming pregnant.
Thiazide diuretics and CCBs are recommended as second-line medications, though most patients will need two or more agents to achieve targets. If BP remains above target despite triple therapy, an alpha blocker, a beta blocker or a potassium-sparing diuretic may be added (the last with caution if the individual is already taking an ACEI or an A2RB).
Other aspects of cardiovascular risk protection
The Steno 2 follow-up study has demonstrated that comprehensive CV risk factor treatment, including BP lowering, use of ACEI, glucose lowering, lowering cholesterol and triglyceride, raising high-density lipoprotein (HDL) cholesterol and the use of aspirin, was associated with remarkable absolute reductions in death, CV events, diabetic nephropathy and laser photocoagulation when compared to conventional management.7Numbers needed to treat over 13 years were five to eight in these people with microalbuminuria.
Antiplatelet therapy
Antiplatelet therapy has an established role in the management of people at high risk of CV events and thus, by extrapolation of evidence, in people with diabetes. A post-hoc analysis of 3,866 people with diabetes within a larger study compared aspirin with clopidogrel in the secondary prevention of CVD.8A significant benefit of clopidogrel with a relative risk reduction of 12.4% (absolute 2.1%) was found, alongside a reduction of 37% for re-hospitalisation for any bleeding event (p = 0.031). However, in the absence of useful health-economic analysis, the new guideline chose to follow NICE guidance advising the use of clopidogrel instead of aspirin only in the presence of definite aspirin intolerance (except in the context of acute cardiovascular events and procedures).9The appropriate dose of aspirin to be used in people with type 2 diabetes is unclear. Accordingly the standard 75 mg dose is recommended for:
those ≥50 years old (and BP < 145/90 mmHg)
those <50 years of age with high CV risk (extant CVD, microalbuminuria, metabolic syndrome, family history of CVD, hypertension, smoking).
Cardiovascular risk assessment
Risk engines and charts based on the Framingham study underestimate CV risk in people with diabetes and should not be used. Recent large studies confirm that CV risk for someone with type 2 diabetes is as high as someone without diabetes who has had a myocardial infarction.10Thus in determining therapy for CV risk reduction there is a presumption of high risk without further assessment. However in the unusual situation of an individual not considered to be at high CV risk, the UK Prospective Diabetes Study (UKPDS) risk engine may be used. Although not specifically designed for the purpose, UKPDS risk engine may also be used for educational purposes when discussing risk of CV complications with someone with diabetes.
Control of low-density lipoprotein cholesterol
Given their high risk, there is a general recommendation to start simvastatin (to 40 mg daily) in nearly everyone with type 2 diabetes >40 years old, as well as those under this age judged as particularly CV high risk. Health economic modelling suggests that simvastatin should be increased to 80 mg daily unless total cholesterol level is below 4.0 mmol/l or low-density lipoprotein cholesterol (LDL-C) is below 2.0 mmol/l. This differs, for primary prevention, to the recommendations for non-diabetic people in the co-published NICE lipid guideline. Also different is the recommendation to consider intensifying cholesterol-lowering therapy (with a more effective statin or ezetimibe) if there is existing or newly diagnosed cardiovascular disease or if there is increased albumin excretion rate, also to achieve a total cholesterol level <4.0 mmol/l (assuming HDL-C is not high) or LDL-C <2.0 mmol/l. Statins should not be used if there is a possibility of a woman becoming pregnant, unless the issues have been discussed.
Conference programme.
Key recommendations
Educational interventions
The core recommendation to offer structured education to everyone around the time of their diagnosis echoes that of the earlier NICE technology appraisal. However annual reinforcement and review are now required. In line with the recommendations of a national working party it is a requirement that the programme should be evidence based, have a structured curriculum that is theory-driven, written, evidence-based and resource-effective. Group education programmes are the preferred option and cultural, linguistic, cognitive, and literacy needs should be met.
Nutritional management
As the evidence suggests that lifestyle management can have profound effects on control of blood glucose and other CV risk factors, nutritional advice is given some prominence even though it is largely uncontroversial. Dietary advice should be provided in a form sensitive to individual needs, culture and beliefs, be sensitive to willingness to change, and cognizant of effects on quality of life. Dietetic consultation should be provided to all newly diagnosed people and reviewed annually, using the skills of someone with specific expertise in the field. The advice itself in general follows that of healthy balanced eating with encouragement of high-fibre, low glycaemic index sources of carbohydrate and oily fish, and control of the intake of foods containing free sugars and saturated and trans fatty acids. For inpatients with diabetes, a meal planning system that provides consistency in the carbohydrate content of meals is recommended.
Self-monitoring and blood glucose targets
Self-monitoring of plasma glucose is a contentious issue, being expensive and badly studied. It should not be seen as a standalone therapeutic intervention. On the basis of some large observational studies it is recommended that it should be generally available only in the context of a self-management programme, with proper aims and annual assessment of utility. Other indications include insulin or sulfonylurea therapy to provide information on hypoglycaemia, for assessment of alterations of blood glucose control resulting from changes in medications or lifestyle changes, and during intercurrent illness. Self-monitoring may also be useful when HbA1c monitoring is invalid because of disturbed erythrocyte turnover or abnormal haemoglobin type. HbA1c otherwise remains the cornerstone of clinical monitoring of blood glucose control, performed every six months where control is stable, or every two to four months when therapies have been changed. While the guidelines espouse a central target of <6.5% HbA1c, this should be individualised by agreement, and may often then be higher for people on insulin therapy for whom hypoglycaemia is a problem.
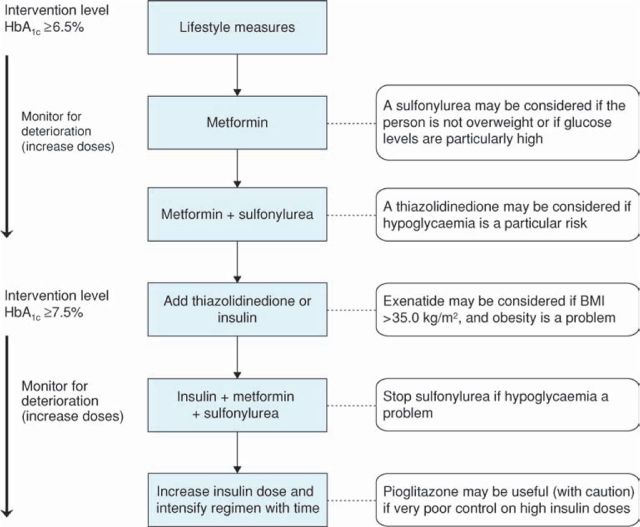
Glucose control interventions (Figure 1)
The recommendations as regards metformin (as usual first-line therapy) or sulfonylurea are little changed over the prior NICE guideline, and similar to some international guidelines. Of note a sulfonylurea may be considered for first-line use where a rapid response to therapy is required. Thiazolidinediones can be used as second-line agents if hypoglycaemia on sulfonylureas is a problem, including in respect of employment. While these medications are generally used at or above an HbA1c of 6.5%, insulin or thiazolidinediones are recommended once HbA1c rises above 7.5%. This reflects evidence on the glucose-reducing properties of insulin therapy, and the calculation that thiazolidinediones only approach cost-effectiveness when compared with the overall expense of insulin therapy. The new GLP-1 mimetic exenatide also has a limited role at this point, in those with an obesity problem (and body mass index >35 kg/m2), and with continuation only if response in HbA1c and body weight.
Fig 1.
Algorithm for the management of blood glucose control in people with type 2 diabetes. BMI = body mass index. Adapted from Reference 1.
When starting basal insulin therapy, metformin and the sulfonylurea should be continued (and acarbose, if used). Stop sulfonylurea if hypoglycaemia occurs.
Consider combining pioglitazone with insulin therapy if there was a marked glucose-lowering response to the thiazolidinedione previously in those on high-dose insulin therapy in whom blood glucose is inadequately controlled. Caution the person to discontinue pioglitazone if clinically significant fluid retention develops.
The recommendations are to usually start insulin treatment with human neutral protamine Hagedorn (NPH) insulin. However a long-acting basal insulin may be considered in some circumstances such as special risk from hypoglycaemia, and where twice daily injection is problematic. Biphasic human insulin (premix) may be used if HbA1c >9.0% on oral agents, and biphasic analogues in case of hypoglycaemia with human premixes or for people with marked post-prandial glucose excursions.
Diabetic kidney and eye damage
Management of diabetic kidney disease
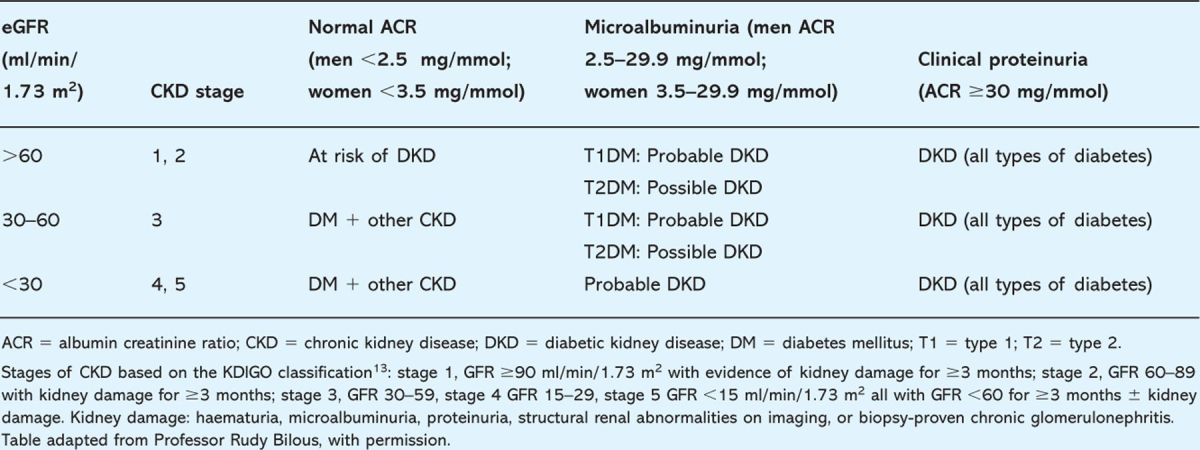
The diagnosis of diabetic kidney disease (DKD) is classically based on increased albumin excretion rate (‘microalbuminuria’). However, it is recognised that significant renal impairment can occur in the presence of normal albumin excretion (Table 1). Annual estimation of albumin:creatinine ratio (ACR) on a first-void morning urine sample is recommended, in the absence of proteinuria/urinary tract infection (UTI), to be confirmed within three to four months if raised. Estimated glomerular filtration rate (eGFR), using the four-variable Modification of Diet in Renal Disease (MDRD) equation, should also be calculated annually.
Table 1.
The likelihood of diabetic kidney disease based on estimated glomerular filtration rate (eGFR) and degree of albuminuria and proteinuria.
Other types of renal disease occur in people with diabetes; absence of retinopathy, nephrotic-range proteinuria, haematuria, rapid progression, and more severe hypertension are among the factors which should alert the diabetes team to this.
Management in all with confirmed raised ACR (>2.5 mg/mmol for men, >3.5 mg/mmol for women) is with an ACEI titrated to the full dose (again an A2RB if intolerance). If BP is > 130/80 mmHg it should be treated to below that level. Again, informed discussion prior to commencing ACEI is essential in women with a possibility of becoming pregnant.
Referral criteria for specialist renal care would include suspicion of non-diabetic kidney disease, a decline in eGFR of > 15% during the first two months of RAS blockade, progressive fall in eGFR once below 30 ml/min/1.73 m2, and problematic BP control.
Prevention of diabetic eye damage
At diagnosis, almost 30% of people with diabetes have background retinopathy which increases as the duration of diabetes increases.11The National Screening Programme for Diabetic Retinopathy is a quality-assured retinal screening programme that recommends an annual assessment of visual acuity and digital retinal photography with mydriasis by trained staff at, or around the time of, diagnosis and then structured eye surveillance annually (or earlier review or referral to an ophthalmologist if indicated).12Furthermore, links with ophthalmology services and with clinical care are essential to ensure that the findings of retinal screening are made available to both the specialist and the generalist looking after the patient.
Conclusion
The new guidelines provide a welcome update to those published in 2002. The emphasis on discussion and agreement with the individual person with diabetes is also welcome, as is that on structured programmes for education, and clarification of the role of self-monitoring. Differences in blood pressure and blood lipid management from those who do not have diabetes are significant. The update in 2009 on new glucose-lowering therapies will add to the changes espoused in the current guideline to clinical practice.
Disclaimer
The views expressed in this article are those of the authors, and do not seek in any way to represent those of the guideline development group, NICE or the RCP.
References
- 1.National Collaborating Centre for Chronic Conditions. Type 2 diabetes: national clinical guideline for management in primary and secondary care (update). London: Royal College of Physicians, 2008. [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–13. 10.1136/bmj.317.7160.703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler AI, Stratton IM, Neil HA. et al Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000; 321:412–9. 10.1136/bmj.321.7258.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strippoli GF, Craig M, Craig JC. Antihypertensive agents for preventing diabetic kidney disease. Cochrane Database Syst Rev 2005:CD004136. [DOI] [PubMed]
- 5.Lewis EJ, Hunsicker LG, Clarke WR. et al Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–60. [DOI] [PubMed] [Google Scholar]
- 6.Chaturvedi N. Modulation of the renin-angiotensin system and retinopathy. Heart 2000; 84(Suppl 1): i29–31. 10.1136/heart.84.suppl_1.i29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358:580–91. 10.1056/NEJMoa0706245 [DOI] [PubMed] [Google Scholar]
- 8.Bhatt DL, Marso SP, Hirsch AT. et al Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol 2002; 90:625–8. 10.1016/S0002-9149(02)02567-5 [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Clinical Excellence. Clopidogrel and modified-release dipyridamole in the prevention of occlusive vascular events. London: NICE, 2005. [Google Scholar]
- 10.Schramm TK, Gislason GH, Kober L. et al Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008; 117:1945–54. 10.1161/CIRCULATIONAHA.107.720847 [DOI] [PubMed] [Google Scholar]
- 11.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984; 102:527–32. 10.1001/archopht.1984.01040030405011 [DOI] [PubMed] [Google Scholar]
- 12.UK National Screening Committee. Essential Elements in Developing a Diabetic Retinopathy Screening Programme. Workbook 4 2007:1–78. [Google Scholar]
- 13.Levey AS, Eckardt KU, Tsukamoto Y. et al Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67:2089–100. 10.1111/j.1523-1755.2005.00365.x [DOI] [PubMed] [Google Scholar]