Pemphigus is an uncommon autoimmune blistering disease, mediated by antibodies directed against desmosomal adhesion proteins (most particularly desmogleins 1 and 3) that are responsible for maintaining integrity of the epidermis. This article will discuss its epidemiology, pathogenesis, clinical variants and approaches to treatment.
Epidemiology
Robust epidemiologic data on pemphigus incidence are scarce but a recent study from the UK suggests an incidence of 0.68 cases per 100,000 person years.1Incidence varies in different parts of the world, being more common in the Near and Middle East than in Western Europe and North America. Those with Jewish ancestry also seem to have a particularly high incidence.2All ages can be affected by pemphigus, though it is most common in middle age, and both sexes are affected equally. Several studies on the effect of smoking on pemphigus seem to suggest that, at least in some populations, it has a protective effect.3
A rare endemic form of pemphigus foliaceus (fogo selvagem) has been identified in parts of Brazil and in North Africa.4The cause remains to be identified, but cases seem to cluster in rural villages around rivers and resolve when patients move away from the endemic areas. It has been suggested that the disease may be transmitted by insects living in the area.
Before the advent of systemic steroid therapy pemphigus was often a life-threatening disease. Morbidity is now more often a result of therapy than related to the underlying disorder. Disease duration varies widely, though remission rarely occurs in less than three years. Some patients require ongoing therapy for over 30 years.
Genetics
The genetics of pemphigus are complex.5,6 Familial cases are uncommon but there are strong associations with certain genes in the MHC complex on chromosome 6, some of which vary according to the population studied. Thus, in Jewish populations DRB1*0402 is highly overexpressed whereas this is less common in Western Europeans. Conversely, the DRB1*1401 allele is highly overexpressed in European and Japanese groups.6Interestingly, genetic polymorphisms have recently been identified in the desmoglein 3 antibody target gene that associate with the disease and are in epistasis with the MHC associations.7
Pathogenesis
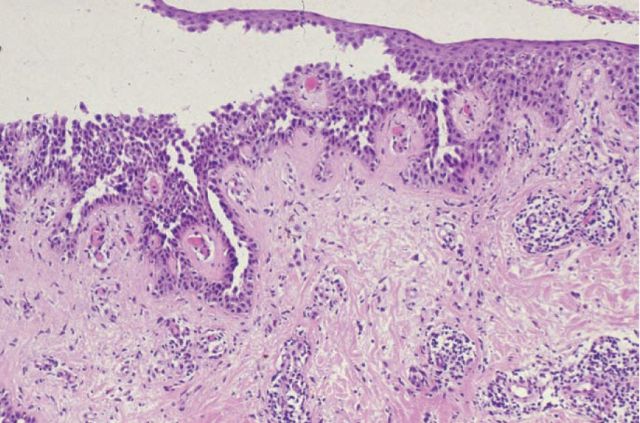
The primary pathogenic event in all forms of pemphigus is acantholysis, the separation of epidermal keratinocytes from each other. This leads to blister formation within the epidermis and is a key histological diagnostic feature of the disease (Fig 1).
Fig 1.
Histopathology of pemphigus vulgaris. There is rounding up and separation of keratinocytes from each other, resulting in intra-epithelial blister formation.
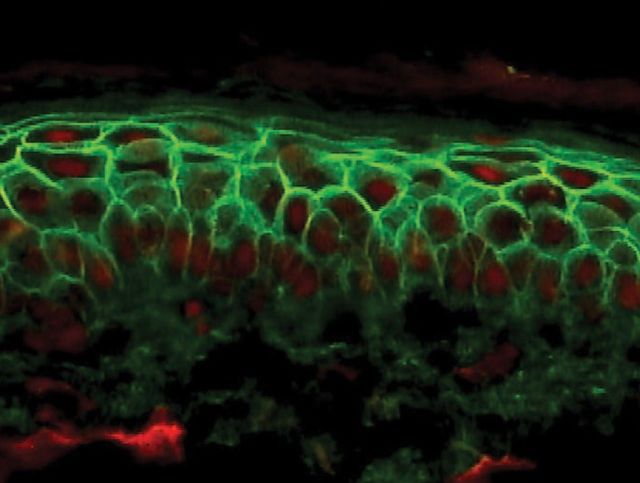
The immunopathology of pemphigus is increasingly well understood.8,9 Antibodies develop against various elements of the desmosomes, particularly desmogleins 1 and 3. Desmoglein 1 is found particularly in the superficial layers of the epidermis; antibodies against this protein alone result in pemphigus foliaceus. Desmoglein 3 is more widely distributed in the lower parts of the epidermis and in mucosal epithelia. Thus, antibodies against this protein are associated with pemphigus vulgaris, typically presenting with mucosal ulceration. Tissue-bound antibodies (generally immunoglobulin (Ig) G but rarely IgA) and complement can be detected by direct immunofluorescence of skin biopsy tissue (Fig 2). Circulating antibodies are detected by indirect immunofluorescence using skin or mucosal tissue substrates. More recently, specific ELISAs have become available for measurement of individual antidesmoglein antibody levels.10,11
Fig 2.
Pemphigus vulgaris direct immunofluorescence shows deposition of IgG in the intercellular spaces between epidermal keratinocytes.
A number of lines of evidence indicate that these antibodies are truly pathogenic. Titres of antibodies tend to correlate with severity of disease and injection of antibodies into neonatal mice leads to acantholytic blistering.8However, the exact mechanisms by which the antibodies lead to blister formation remain to be clarified. Steric hindrance plays a role but is not the full explanation.12–14 In addition, a number of non-desmoglein antibodies have been detected in patients with pemphigus vulgaris, including those against E-cadherin15 and the acetylcholine receptor.16Exactly how these develop and the nature of their significance is unclear, though genetically modified mice immunised with recombinant desmogleins develop a range of circulating antibodies. Such epitope spreading may be a common feature of the immune response to desmosomal antigens.17
Clinical features
Pemphigus occurs in a number of clinical forms, each characterised by a distinct profile of autoantibodies.
Pemphigus vulgaris
This form is associated with the presence of IgG antibodies against desmoglein 3, with or without antidesmoglein 1 antibodies. Patients with both antibodies tend to have more severe or active disease;11 desmoglein 1 antibodies tend to decrease more rapidly on treatment than desmoglein 3 antibodies.
Pemphigus vulgaris typically presents with oral mucosal ulceration, subsequently followed by the development of superficial blisters and erosions affecting the trunk, face, scalp and proximal limbs (Fig 3). Because the blister forms within the epidermis it is fragile and breaks easily, so the tense blisters characteristic of subepidermal blistering conditions are rarely seen. The eyes can occasionally be affected, particularly when antidesmoglein 3 antibody levels are high. Mucosal involvement can also spread to affect the pharynx, oesophagus and genitalia.
Fig 3.
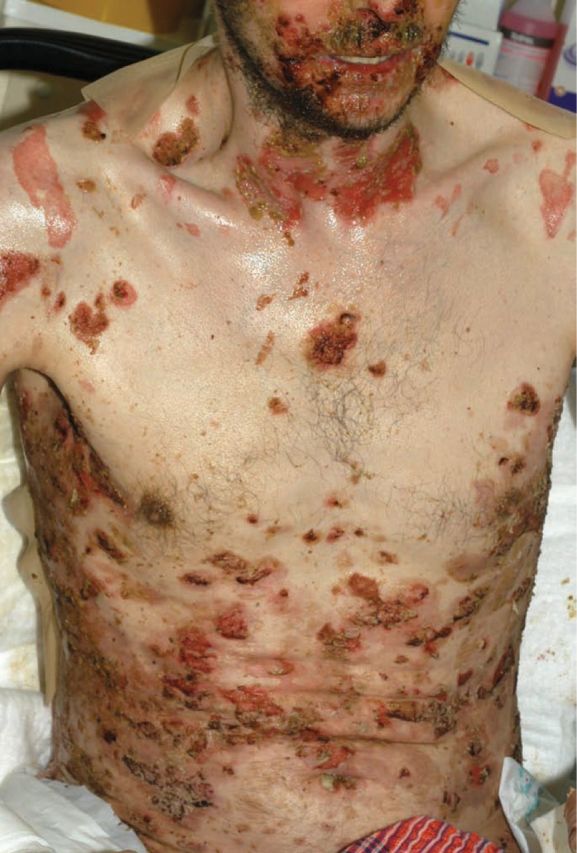
Severe pemphigus vulgaris showing widespread flaccid blisters and erosions on the trunk.
Pemphigus vegetans
A variant of pemphigus vulgaris, pemphigus vegetans, is associated with ‘heaped up’ vegetating lesions in the flexures. It can be seen in partially treated pemphigus and perhaps represents an exaggerated healing response of the eroded and blistered skin.
Pemphigus foliaceus
Pemphigus foliaceus is characterised by the presence of antidesmoglein 1 antibodies alone. Acantholysis and blister formation occur superficially within the epidermis, resulting in superficial erosions typically with an inpointing peripheral scale. As in pemphigus vulgaris, lesions tend to occur on the face, trunk and proximal limbs. Mucosal lesions are not seen.
Pemphigus erythematosus
A rare form is pemphigus erythematosus (Senear-Usher syndrome). It particularly affects the face and is perhaps best considered as a variant of pemphigus foliaceus. Direct immunofluorescence demonstrates intercellular IgG and complement deposition as in other forms of pemphigus. Indirect immunofluorescence is frequently positive. However, there is also basement membrane deposition of immunoreactants (as in lupus erythematosus) and many patients are antinuclear antibody positive. In some patients there is a possible drug cause while in others additional autoimmune diseases have been noted, particularly myasthenia gravis.
Key points
Pemphigus is an important autoimmune blistering skin disease caused by the development of antibodies directed against desmosomal adhesion proteins, most notably desmogleins 1 and 3
Blistering in pemphigus occurs within the epidermis resulting in flaccid blisters and erosions. The characteristic histopathological finding is acantholysis (rounding up and separation of keratinocytes from each other)
There are a number of different clinical forms of pemphigus, each characterised by a distinct autoantibody profile
Treatment of pemphigus with systemic steroids and cytotoxic immunosuppressants can be highly effective and allow patients to return to a normal life
Paraneoplastic pemphigus
The first report of paraneoplastic pemphigus was in 1990 and it has since been recognised in many patients.18,19 It is most commonly associated with haematological malignancy and thymoma but has also been seen in association with some solid tumours. It is characterised by a broad immunological attack on the epidermis, often with initial lichenoid features. Patients develop a range of autoantibodies to desmogleins and other desmosomal proteins including desmocollins and desmoplakins. Direct immunofluorescence demonstrates intercellular and basement membrane zone deposition of IgG and complement. Indirect immunofluorescence is positive on a wide variety of substrates including transitional epithelia (which lack desmogleins and thus reflect the presence of antiplakin antibodies). In addition to widespread cutaneous erosions and blistering there is frequently severe mucosal involvement. The condition improves with treatment of the underlying malignancy.
Immunoglobulin A pemphigus
An uncommon variant of the disease is IgA pemphigus in which IgA rather than IgG is deposited in the epidermis. In addition to desmoglein antibodies, some patients have antibodies directed against other desmosomal proteins such as the desmocollins. Clinical manifestations of IgA pemphigus vary, tending to form rather superficial pus-filled bullae, and there is crossover with sub-corneal pustular dermatosis (Sneddon-Wilkinson disease).
Treatment
All patients with pemphigus require attention to topical therapy, particularly in the early stages of the disease. Appropriate wound care will make the patient more comfortable and decrease the risk of infection. Topical or intralesional steroids are of limited benefit in widespread disease but can be useful for treatment of persisting small lesions in areas such as the scalp.
Systemic steroid therapy
The mainstay of treatment for all forms of pemphigus is systemic steroids. Usually a prednisolone dose of 0.5–1 mg/kg is used in the first instance, gradually reduced as the patient responds. Intermittent oral or intravenous (iv) high-dose steroids are of little benefit as sole adjunctive therapy,20 though this approach forms part of the pulsed steroid/cyclophosphamide regimen discussed below. Careful consideration needs to be given to detecting and managing potential adverse effects of such high-dose steroid therapy. Weight gain, hypertension, infection and osteoporosis are all very real risks.
Immunosuppression
Almost all patients will require some kind of steroid sparing immunosup-pressant. Azathioprine (2–3 mg/kg), mycophenolate mofetil (1.5–3 g daily) and cyclophosphamide (see below) are all widely used. There is some evidence to suggest that mycophenolate mofetil is better tolerated and achieves remission in more patients than azathioprine.21However it is significantly more expensive and current practice in our clinic is to use azathioprine initially (assuming that thiopurine methyltransferase (TPMT) activity is within the normal range), switching to mycophenolate mofetil only if the drug is not tolerated or there is an inadequate response.
Cyclophosphamide
Cyclophosphamide can be a highly useful drug for the treatment of pemphigus. It is generally used in a monthly pulsed infusion regimen together with methylprednisolone. Our current practice is to use cyclophosphamide 500 mg by iv infusion once monthly, together with methylprednisolone 1 g daily for three days. In addition, patients receive cyclophosphamide 50 mg daily and prednisolone, tapered according to disease response. This regimen requires close monitoring but it can be highly effective in patients whose disease has failed to respond to steroids and azathioprine/mycophenolate alone. A concern however is the risk of permanent gonadal failure in both men and women and patients must be appropriately counselled and selected carefully. In men, sperm can be stored. Cyclophosphamide is usually inappropriate for women of child-bearing age.
Monoclonal antibody therapy
Anti-B-cell monoclonal antibody therapy has recently been shown to be highly effective in the treatment of pemphigus including paraneoplastic disease.22–24 B-lymphocytes express the CD20 molecule and can be selectively removed by the use of anti-CD20 therapeutic antibodies such as rituximab. Several recent studies have demonstrated the efficacy of this approach; some patients have long lasting remission after a single course of treatment. As with all treatments discussed so far, infection is a real risk following rituximab therapy and needs to be carefully watched for. There have been reports of overwhelming infection in some patients.25However, such treatments offer a highly selective approach to the disease and will undoubtedly become more widely used in the future.
Other approaches
A wide variety of other drugs has been used for the management of pemphigus, including gold, ciclosporin, dapsone, methotrexate and intravenous immunoglobulin (lg). Immunoadsorption has also shown promise in some small studies.
Conclusions
Pemphigus represents an antibody mediated cluster of diseases in which the target antigens are well understood. While previously fatal, modern therapeutic strategies can lead to reductions in circulating antibody levels and marked clinical improvement, allowing most patients to live a normal life. New monospecific drugs such as rituximab are providing exciting additional insights into the immunology of the disease.
References
- 1.Langan SM, Smeeth L, Hubbard R. et al Bullous pemphigoid and pemphigus vulgaris - incidence and mortality in the UK: population based cohort study. BMJ 2008;337:a180. 10.1136/bmj.a180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simon DG, Krutchkoff D, Kaslow RA, Zarbo R. Pemphigus in Hartford County, Connecticut, from 1972 to 1977. Arch Dermatol 1980; 116:1035–7. [PubMed] [Google Scholar]
- 3.Brenner S, Tur E, Shapiro J. et al Pemphigus vulgaris: environmental factors. Occupational, behavioral, medical, and qualitative food frequency questionnaire. Int J Dermatol 2001; 40:562–9. 10.1046/j.1365-4362.2001.01266.x [DOI] [PubMed] [Google Scholar]
- 4.Aoki V, Millikan RC, Rivitti EA. et al. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem). J Investig Dermatol Symp Proc 2004; 9:34–40. 10.1111/j.1087-0024.2004.00833.x [DOI] [PubMed] [Google Scholar]
- 5.Gazit E, Loewenthal R. The immuno-genetics of pemphigus vulgaris. Autoimmun Rev 2005; 4:16–20. [DOI] [PubMed] [Google Scholar]
- 6.Tron F, Gilbert D, Joly P. et al Immunogenetics of pemphigus: an update. Autoimmunity 2006; 39:531–9. 10.1080/08916930600971497 [DOI] [PubMed] [Google Scholar]
- 7.Capon F, Bharkhada J, Cochrane NE. et al Evidence of an association between desmoglein 3 haplotypes and pemphigus vulgaris. Br J Dermatol 2006; 154:67–71. 10.1111/j.1365-2133.2005.06882.x [DOI] [PubMed] [Google Scholar]
- 8.Amagai M. Pemphigus vulgaris and its active disease mouse model. Curr Dir Autoimmun 2008; 10:167–81. 10.1159/000131453 [DOI] [PubMed] [Google Scholar]
- 9.Gniadecki R. Desmoglein autoimmunity in the pathogenesis of pemphigus. Autoimmunity 2006; 39:541–7. 10.1080/08916930600971505 [DOI] [PubMed] [Google Scholar]
- 10.Harman KE, Gratian MJ, Seed PT. et al Diagnosis of pemphigus by ELISA: a critical evaluation of two ELISAs for the detection of antibodies to the major pemphigus antigens, desmoglein 1 and 3. Clin Exp Dermatol 2000; 25:236–40. 10.1046/j.1365-2230.2000.00624.x [DOI] [PubMed] [Google Scholar]
- 11.Harman KE, Seed PT, Gratian MJ. et al The severity of cutaneous and oral pemphigus is related to desmoglein 1 and 3 antibody levels. Br J Dermatol 2001; 144:775–80. 10.1046/j.1365-2133.2001.04132.x [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci 2007; 48:1–14. 10.1016/j.jdermsci.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 13.Chernyavsky AI, Arredondo J, Kitajima Y, Sato-Nagai M, Grando SA. Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J Biol Chem 2007; 282:13804–12. 10.1074/jbc.M611365200 [DOI] [PubMed] [Google Scholar]
- 14.Waschke J, Bruggeman P, Baumgartner W, Zillikens D, Drenckhahn D. Pemphigus foliaceus IgG causes dissociation of desmoglein 1-containing junctions without blocking desmoglein 1 transinteraction. J Clin Invest 2005; 115:3157–65. 10.1172/JCI23475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelista F, Dasher DA, Diaz LA, Prisayanh PS, Li N. E-cadherin is an additional immunological target for pemphigus autoantibodies. J Invest Dermatol 2008; 128:1710–8. 10.1038/sj.jid.5701260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vu TN, Lee TX, Ndoye A. et al The patho-physiological significance of nondesmoglein targets of pemphigus autoimmunity. Development of antibodies against ker-atinocyte cholinergic receptors in patients with pemphigus vulgaris and pemphigus foliaceus. Arch Dermatol 1998; 134:971–80. [DOI] [PubMed] [Google Scholar]
- 17.Mouquet H, Drouot L, Charlionnet R. et al Proteomic analysis of the autoantibody response following immunization with a single autoantigen. Proteomics 2006; 6:4829–37. 10.1002/pmic.200600257 [DOI] [PubMed] [Google Scholar]
- 18.Anhalt GJ. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc 2004; 9:29–33. [DOI] [PubMed] [Google Scholar]
- 19.Anhalt GJ, Kim SC, Stanley JR. et al Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990; 323:1729–35. 10.1016/0020-7292(91)90751-P [DOI] [PubMed] [Google Scholar]
- 20.Mentink LF, Mackenzie MW, Toth GG. et al Randomized controlled trial of adjuvant oral dexamethasone pulse therapy in pemphigus vulgaris: PEMPULS trial. Arch Dermatol 2006; 142:570–6. 10.1001/archderm.142.5.570 [DOI] [PubMed] [Google Scholar]
- 21.Beissert S, Werfel T, Frieling U. et al A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of pemphigus. Arch Dermatol 2006; 142:1447–54. 10.1001/archderm.142.11.1447 [DOI] [PubMed] [Google Scholar]
- 22.Ahmed AR, Spigelman Z, Cavacini LA, Posner MR. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med 2006; 355:1772–9. 10.1056/NEJMoa062930 [DOI] [PubMed] [Google Scholar]
- 23.Joly P, Mouquet H, Roujeau JC. et al A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med 2007; 357:545–52. 10.1056/NEJMoa067752 [DOI] [PubMed] [Google Scholar]
- 24.Barnadas M, Roe E, Brunet S. et al Therapy of paraneoplastic pemphigus with rituximab: a case report and review of literature. J Eur Acad Dermatol Venereol 2006; 20:69–74. 10.1111/j.1468-3083.2005.01345.x [DOI] [PubMed] [Google Scholar]
- 25.Schmidt E, Seitz CS, Benoit S, Bröcker EB, Groebeler M. Rituximab in autoimmune bullous diseases: mixed responses and adverse effects. Br J Dermatol 2007; 156:352–6. 10.1111/j.1365-2133.2006.07646.x [DOI] [PubMed] [Google Scholar]