ABSTRACT
Multidrug-resistant and extensively drug-resistant tuberculosis are recent global health issues, which makes tuberculosis – after the success of short course treatment during the second half of the last century – a major health challenge. Globalisation, health inequalities, competing economic interests and political instability contribute substantially to the spread of drug-resistant strains, which are associated with high rates of morbidity and mortality. Issues such as increasing transmission of drug-resistant strains, poor diagnostic coverage and a lengthy, toxic treatment need to be overcome by innovative approaches to tuberculosis control, prevention, diagnostics and treatment. This review addresses recent developments and future concepts.
KEYWORDS: : Drug-resistant, tuberculosis, resistance testing, anti-tuberculosis drugs, tuberculosis control
Introduction
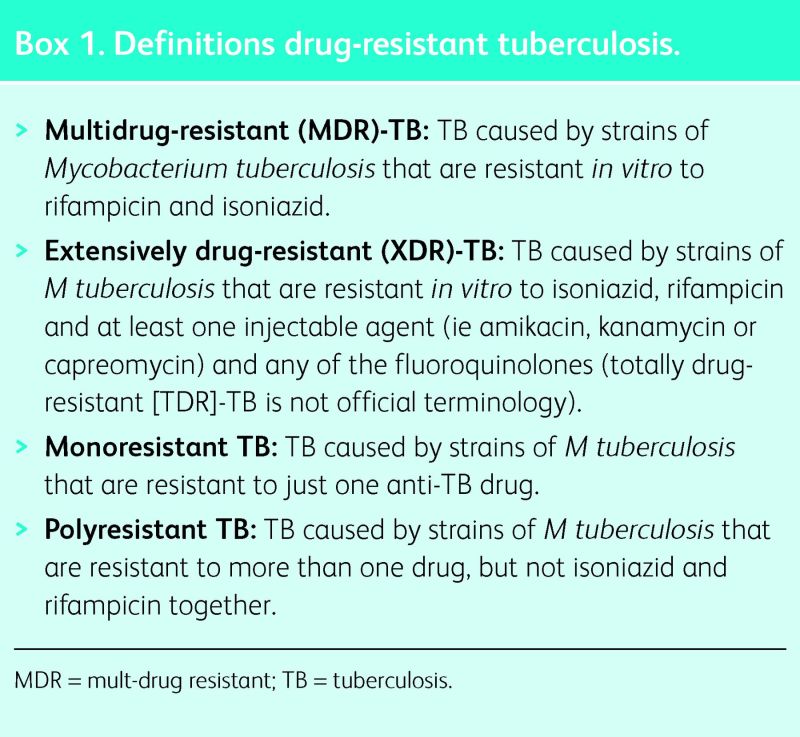
Tuberculosis (TB) was declared a global emergency by the World Health Organization (WHO) in 1994. The incidence of TB is diminishing globally by approximately 2% each year and TB-related mortality decreased by 45% between 1995 and 2012; nonetheless, in 2012, 8.6 million people developed the disease and 1.3 million died from it. Despite the fall in incidence and mortality, multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB are presenting enormous new challenges in the management and control of this disease (Box 1). The WHO estimated there were 450,000 incident cases of drug-resistant TB and 170,000 drug-resistant TB-related deaths in 2012 worldwide.1
Unfortunately, less than 25% of the estimated cases of MDR-TB are detected due to insufficient drug resistance testing. Currently, among notified cases globally, 3.6% of new cases and 20% of retreatment cases represent drug-resistant TB. There are strong regional variations. In Belarus in 2012, for instance, 32.2% of new cases and 75.6% of retreatment patients had MDR-TB, within an overall estimated TB incidence of 70 per 100,000 people.2 By contrast, in South Africa in 2012, 1.8% of new cases and 6.7% of retreatment patients had MDR-TB within an overall estimated TB incidence of 1,000 per 100,000 people.1 The precise global trends of drug-resistant TB incidence and prevalence are currently ill-defined because of poor surveillance and detection methods.
Worldwide in 2012, only 77,000 patients with MDR-TB started treatment, representing only 17% of the total estimated cases. Of the cohort of patients with MDR-TB who started treatment in 2010, only 48% had a successful treatment outcome, reflecting high rates of either loss to follow up and/or mortality.1 In 2012, 78,000 MDR-TB cases were estimated to have occurred in the European region, as defined by the WHO, but only approximately 30,000 patients were detected and even fewer started treatment.3
XDR-TB has now been detected in 92 countries and, on average, 9.6% of MDR-TB cases are also XDR-TB cases.1 Recently, there have been publications on totally drug resistant TB (TDR-TB).4 Such patients were resistant to all second-line anti-TB medication tested. However, TDR-TB is not officially recognised nomenclature and, therefore, should not currently be used as official terminology.5
The definitions of MDR-TB and XDR-TB relate intimately to the outcome of treatment. Using individual patient data from cohort studies, Falzon et al reported a successful treatment outcome for 64% of patients with MDR-TB, but only 40% of patients with XDR-TB.6 These compare with old data suggesting a spontaneous TB cure rate of approximately 30%.7
Such data highlight current challenges regarding drug-resistant TB care, with poor case detection and treatment outcome. The increasing proportion of MDR-TB among new TB cases, as seen for instance in Belarus and China, demonstrates that MDR-TB strains are being actively transmitted from person to person with increasing frequency.3,8 Paradoxically, based on old data for susceptible TB, it can be argued that, from a public health perspective, poor treatment programmes are worse than no TB control at all.9,10
Recent developments in tuberculosis diagnostics and resistance testing
Microscopy and culture are still the basis of TB diagnostics. In 2010, the WHO endorsed Xpert®MTB/Rif (Cephaid, Sunnyvale, USA), a PCR-based diagnostic tool. This tool detects both Mycobacterium tuberculosis complex DNA and rifampicin resistance-associated mutations, adding a new dimension to the diagnosis of TB, particularly in low-resource settings. The cartridge-based test has a turn-around time of approximately 2 h and does not require a biosafety-level laboratory. The WHO recommends the use of the test in patients who are human immunodeficiency virus (HIV) positive and in cases when resistance is suspected. It is also recommended in smear-negative cases in which suspicion of TB remains.11 The simplicity of the test enables its use outside reference laboratories, improving patient access to TB diagnostics and rapid drug-susceptibility testing (DST).
Box 1. Definitions drug-resistant tuberculosis.
In a recent Cochrane meta-analysis, the test had a sensitivity of 88% and a specificity of 93% for diagnosing TB when used as an initial test, replacing microscopy. As an add-on test in cases with negative smear microscopy, the test yielded 67% sensitivity and 98% specificity. Rifampicin resistance was detected with 94% sensitivity and 98% specificity.12 The major advantage of this test for TB control is the time required—less than 1 day to detect both M tuberculosis and rifampicin resistance. By contrast, in the first multicentre implementation study, it took a median 20 days using line-probe assays (a DNA strip test that enables simultaneous molecular identification of TB and the most common genetic mutations causing resistance to rifampicin and isoniazid – see below) and a median 106 days using conventional DST to detect resistance.13 In low-incidence countries, the positive predictive value of Xpert®MTB/Rif is low and, therefore, the false positive rate is higher;14 thus, a confirmatory culture-based DST is always required.
Although Xpert®MTB/Rif and line-probe assays are important steps, their accuracy, rapidity, affordability and simplicity still do not meet criteria for a real point-of-care test.15 One key criterion for such a test is that it is able to deliver results during a single healthcare contact, that is, within 3 h. Providing an accurate diagnosis and initiating treatment at the same health consultation would reduce the number of patients lost to follow up. The desperate need for such a test, including resistance testing, is stressed by the fact that less than 25% of the estimated MDR-TB cases are currently detected.1
Line-probe assays are used to test resistance to rifampicin and isoniazid. Hain Genotype®MTBDRplus (Hain, Nehren, Germany), a WHO-endorsed test, can, in its latest version, detect resistance to rifampicin and isoniazid in both smear-positive and smear-negative (culture positive) samples with high accuracy. An early evaluation study on smear-negative samples indicated 90.7% sensitivity and 96% specificity for rifampicin resistance, and 93.5% sensitivity and 82.3% specificity for isoniazid resistance.16 The use of the assay is technically more demanding and has a longer turn-around time compared with Xpert®MTB/Rif, but is a good alternative test for isoniazid and rifampicin resistance in reference laboratories.
Line-probe assays provide the only currently available molecular routine test to detect resistance to fluoroquinolones, injectable drugs and ethambutol (Genotype®MTBDRsl). This test has predominantly been evaluated in culture specimens. A new version of the test to be used directly with sputum samples is currently under evaluation. The test can currently only be recommended as a ‘rule in’ test for resistance to fluoroquinolones and injectables.17 A positive result should be confirmed by phenotypic DST. However, the test is not endorsed by the WHO and results should be interpreted with caution.18
Other new diagnostic tests have become available, but do not serve the goals outlined above. A urine dipstick test (Determine® TB-LAM; Alere, Waltham, MA, USA), checking for lipoarabinomannan (LAM) antigen to detect M tuberculosis, only works well in patients with CD4 count below 50 cells/μl (with 66.7% sensitivity and >98% specificity).19 Newer approaches to point-of-care testing, such as new nucleic acid amplification technologies and the use of volatile organic compounds, are under development and have been discussed elsewhere.15
It is recommended that any patient with risk factors for drug resistance, such as migration from a high-incidence country, contact with M/XDR-TB cases, and previous TB treatment, should undergo a molecular resistance test before the initiation of TB treatment to avoid treatment with ineffective regimens.18
Current M/XDR-TB treatment recommendations
The latest guidelines for the treatment of drug-resistant TB were developed by the WHO in 2011 (Tables 1 and 2),20 based on the retrospective analysis of 9,153 patient records from individuals with resistance to at least isoniazid and rifampicin, from 32 observational studies investigating the impact of type, number of drugs and duration of treatment on outcome.21 The overall treatment success in this cohort was 54%. The study showed that the likelihood of treatment success is highest using at least four in vitro susceptible drugs in the intensive phase of treatment and at least three during the continuation phase for patients who had not received second-line drugs previously. The study also highlighted the importance of later-generation fluoroquinolones for treatment success and the potential benefit of ethionamide and prothionamide in drug-resistant TB. The greatest chance for treatment success was with an intensive phase (including an injectable in the regimen) for 7–8.5 months, and a total treatment duration of 25–27 months. The result of this study clearly underlines the enormous challenges of such treatment. The current best guidelines for management of drug-resistant TB are based on retrospective, observational data, where barely half of the patients successfully completed treatment.
Table 1.
Second-line anti-tuberculosis drugs. Reproduced with permission from WHO (2011).20
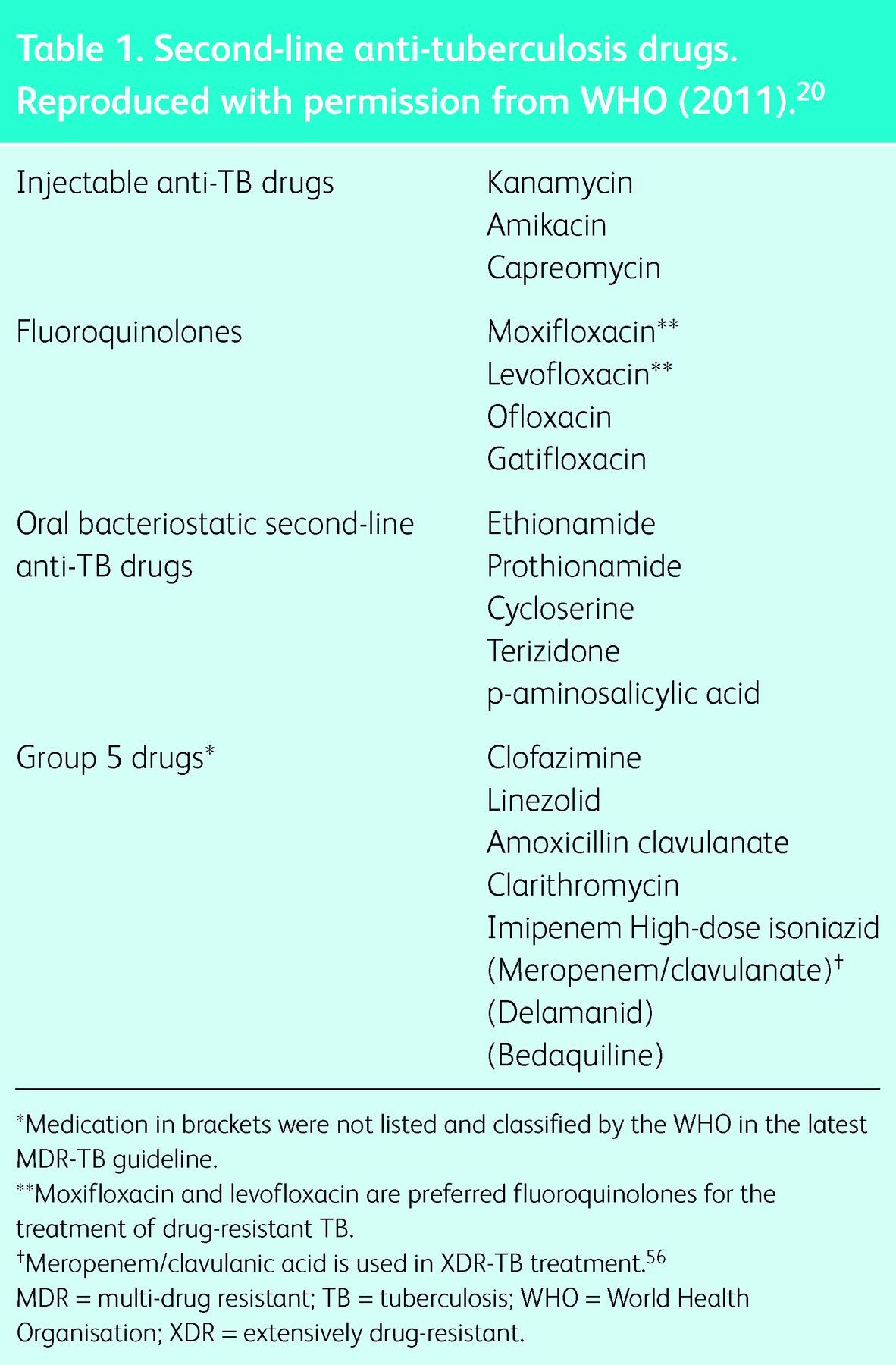
Table 2.
Initial treatment recommendations for drug-resistant tuberculosis.18,20
The same data set was analysed to identify treatment successes for patients with MDR-TB plus additional resistance to injectables (56%), fluoroquinolones (48%) or both (40%). The greatest chance of treatment success for XDR-TB was found with at least six active drugs during the intensive phase and four during the continuation phase, the intensive phase lasting 6.6–9.0 months and the total treatment duration for 21.1–25.0 months.6
In an innovative and widely discussed study, van Deun et al showed higher treatment success rates with a shorter regimen.22 In Bangladesh, where most patients have been second-line drug naive, patients were assigned to six standardised regimens. The subsequent regimens were changed in their composition based on treatment success. The most effective treatment regimen tested required a minimum of 9 months of treatment with gatifloxacin, clofazimine, ethambutol and pyrazinamide, and was supplemented by prothionamide, kanamycin and high-dose isoniazid during the first 4 months. In total, 87.9% of patients had a relapse-free cure. Major adverse events were scarce. The study demonstrated the benefit of the later-generation fluoroquinolone gatifloxacin over ofloxacin for remaining free of adverse outcome (hazard ratio 0.39; 95% confidence interval 0.25–0.59). It does appear that later-generation fluoroquinolones are crucial for treatment success.6,23,24 However, based on the results of the above study, the WHO issued a policy statement that, for the moment, this so-called ‘short-course regimen’ should only be used after appropriate ethics review within an externally monitored operational research context.25
Furthermore, increasing resistance to second-line drugs poses additional challenges to diagnostics and treatment of drug-resistant TB. The Preserving Effective TB Treatment Study (PETTS) demonstrated resistance to at least one second-line drug in 43.7% of 1,278 patients with MDR-TB from seven countries. Of these patients, 20.0% were resistant to at least one second-line injectable drug and 12.9% to at least one fluoroquinolone.26 Unpublished data from the author confirmed this trend in Europe. Given that resistance to these key drugs reduces further treatment success, such developments are relevant and worrying for the future management of drug-resistant TB.6
New drugs and innovations in M/XDR-TB therapy
There is consensus that only a completely new approach to drug-resistant TB treatment, using a short-course, oral, non-toxic regimen will successfully control the disease.27 Some progress in this direction is reflected in the pipeline for new TB drugs, which was almost completely empty a decade ago.28 Rifampicin was licenced in 1964, but from then until December 2012, no new anti-TB drugs were registered worldwide. Then, bedaquiline (Situro®), a diarylquinoline, was approved under conditional licensing by the US Federal Drug Administration (FDA). The European Medicines Agency (EMA) gave a conditional licencing recommendation for delamanid (Deltyba®), a nitroimidazole, in November 2013, and to bedaquiline in December 2013. Even though both drugs received only conditional licencing, these are landmark events in the history of anti-TB drug development. Although licensing was based on phase IIb studies,29–31 a phase III trial with delamanid is under way (clinical trials identifier: NCT01424670) and has completed recruitment, whereas a phase III trial with bedaquiline is pending (clinical trials identifier: NCT01600963).
Box 2. Requirements for a new drug-resistant tuberculosis regimen.27
If adjustment is required in a drug-resistant TB regimen, at least two drugs should be changed simultaneously to avoid the development of immediate resistance to a single new drug added to a failing regimen. Unfortunately, there are no clinical data on the use of delamanid and bedaquiline together, and neither bedaquiline nor delamanid have been used in trials together with moxifloxacin,30,31 the potentially most potent fluoroquinolone in TB treatment. Furthermore, although both these new drugs were generally tolerated well, cardiac toxicity and QT-interval prolongation remain a problem.
The optimal use of these new drugs in MDR-TB regimens remains unclear. The WHO issued interim guidance, based on a low level of evidence. Bedaquiline should be used in cases when a regimen with four active drugs, excluding pyrazinamide, cannot be designed. Alternatively, the drug can be used in patients who have MDR-TB and additional resistance to any fluoroquinolone. It was stressed that bedaquiline should not be used for longer than 6 months and regular electrocardiography (ECG) testing is essential to detect pathological QT- interval prolongation.32
Innovative approaches to the development of a shortened, less toxic regimen for drug-resistant TB are mandatory. A group of influential experts in the field of drug-resistant TB recently defined detailed requirements for a new regimen (Box 2).27
The Standardised Treatment Regimen of Anti-tuberculosis Drugs for Patients with MDR-TB (STREAM) trial is testing a regimen similar to the one used by van Deun et al in Bangladesh,22 replacing gatifloxacin with high-dose moxifloxacin (www.control-trials.com: SRCTN78372190). The TB Alliance is testing various new regimens containing nitroimidazole PA 824, moxifloxacin, bedaquiline and pyrazinamide, assessing 8-week culture conversion rates (clinical trials identifier: NCT01498419).
The annual Treatment Action Group (TAG) pipeline report gives an excellent overview of ongoing trials (www.pipelinereport.org). In addition, the Working Group on New TB drugs provides on its website (www.newtbdrugs.org) a concise overview of new anti-TB drugs and an updated pipeline.
The potential to use high-dose later-generation fluoroquinolones (ie moxifloxacin 800 mg and levofloxacin 1,000 mg), which are more bactericidal and would potentially contribute to shortened treatment, is currently under discussion. However, there is a lack of safety data for long-term treatment and particular concerns about cardiac toxicity.33
Several recent studies confirmed the antimycobacterial activity of linezolid, which is currently the preferred class 5 drug.18 A clinical trial in South Korea showed its beneficial effects in patients failing XDR-TB treatment.34 A meta-analysis showed 93% culture conversion, using individualised regimens with linezolid. Of the patients, 58.9% experienced adverse events and 68.4% had major adverse events. Neuropathies and cytopenias were the most frequent complications.35 The optimal dosing is not yet known, although there is agreement on a maximum daily dose of 600 mg.18
Surgical treatment of MDR-TB might be an option and should be considered in selected cases with limited lesions and poor potential for cure otherwise.36 However, no randomised data are available for guidance on patient selection and optimal timing for surgery.
TB therapeutic drug monitoring (TDM) has been proposed as a means to optimise the outcome. So far, data on TDM in TB treatment are scarce, cost is high and adequate know-how exists only from a few centres.37 Currently, a methodology using dry blood-spot tests has been established to make TDM more accessible,38 but critical data on pharmacokinetics and pharmacodynamics of anti-TB drugs remain unknown.
M/XDR-TB in children
TB diagnostics and treatment in children poses particular challenges. Data on the management of MDR-TB in children are scarce. Children acquire drug-resistant TB typically from an adult index case. Treatment should be tailored according to the DST of the index case if a DST from the patient is not available. A recent systematic review and meta-analysis of MDR-TB treatment in children identified eight studies with 315 children. Despite a huge variation in treatment approaches, the pooled estimate of treatment success was 81.7% and the mortality 5.9%.39 These data highlight that it is possible to treat children successfully with treatment strategies similar to those for adults.40 There is no better evidence on the optimal treatment for children available.41 A crucial issue is the adequate provision and dosing of second-line anti-TB drugs to children because most medications are not manufactured for paediatric use.42
Children with a latent infection and under the age of 5 years are at the highest risk to progress to active TB and would benefit from chemoprophylaxis. However, strategies and recommendations vary widely. A study from Cape Town with ofloxacin, ethambutol and high-dose isoniazid recently showed safety and efficacy,43 but comparative trials are urgently required. The Sentinel Project on Paediatric Drug-Resistant Tuberculosis recommends chemoprophylaxis for children aged under 5 years and those who are HIV positive.40
M/XDR-TB in patients co-infected with HIV
XDR-TB came to the attention of the world when extremely high mortality rates in patients co-infected with HIV were observed in South Africa.44 All patients with M/XDR-TB need to be tested for HIV. If positive, antiretroviral therapy (ART) should be started according to current guidelines.20
Smear-negative TB is more common in patients who are HIV positive.45 Xpert®MTB/Rif has a reasonable sensitivity in smear-negative cases.12 To obtain a timely diagnosis of TB and rifampicin resistance, Xpert®MTB/Rif is the recommended initial test for the diagnosis of TB in patients with HIV. TB treatment should follow the guidelines for the management of MDR-TB. The WHO recommends starting ART within 8 weeks of starting M/XDR-TB treatment. Based on the evidence of drug-susceptible TB, it might be assumed that patients with advanced immunosuppression (CD4 count <50 cells/μl) should start ART within 2 weeks.46–48
Current shortcomings in drug-resistant TB control
A rapid, accurate diagnosis, resistance testing and subsequent initiation of treatment are most crucial for the success of any TB control effort. Only 57% of the 4.6 million notified new TB cases in 2012 have been confirmed bacteriologically. Despite strong efforts between 2009 and 2012, the percentage of new TB cases in which DST was performed worldwide increased only from 4% to 5%, and in retreatment cases from 6% to 9%. Such a low testing rate makes it impossible adequately to detect and control drug-resistant TB.1
Treatment scale-up is limited by the paucity of trained staff, second-line drugs, facilities for treatment and monitoring, and by weaknesses in the coordination of programmatic TB management.49 A major impediment to treatment scale-up is the cost of treatment and availability of drugs. A recent report quoted the cost of a standard MDR-TB treatment regimen, based on the 2011 WHO guidelines and using WHO prequalified drugs suppliers, of between US$2,909 and US$4,014, using capreomycin, moxifloxacin, ethionamide, pyrazinamide and cycloserine. Replacing capreomycin with kanamycin and moxifloxacin with levofloxacin reduced the cost by about US$1,500.42 Diel et al recently estimated the overall cost for standard treatment of TB as Ä10.282, MDR-TB as Ä57.213 and XDR-TB as Ä170.712 in the 15 old European Union (EU) states. These figures illustrate the economic burden of the disease.50 Notably, linezolid has shown beneficial effects in M/XDR-TB,34 but even in high-burden M/XDR-TB countries such as South Africa, the drugs costs US$68 per tablet, making such treatment prohibitively expensive.42
The prevention of TB transmission and disease is particularly crucial in high-incidence settings. Two recent studies demonstrated the limitation of interventions, such as enhanced case-finding and isoniazid prophylaxis, in areas with high rates of HIV/TB co-infection. The ZAMSTAR cluster-randomised trial tested enhanced case-finding strategies and household interventions. Household interventions might have had an effect in reducing TB incidence, although this was not statistically significant, whereas enhanced case finding did not.51 Unfortunately, the Thibela TB study showed no effect of mass screening and prophylaxis with isoniazid on TB incidence in South African gold-miners.52 Early antiretroviral therapy reduces the risk of active TB and earlier initiation of ART is an important measure to prevent active TB and its transmission to patients who are HIV positive.53 Ambulatory treatment approaches during the intensive phase of treatment are successful and recommended for treatment of drug-resistant TB, that is, to reduce rates of nosocomial transmission.20,54 TB infection control remains a crucial topic. Rapid diagnosis and accurate treatment are most important to reduce transmission.18
Currently, there are no controlled studies on preventive chemotherapy in M/XDR-TB. The European Center of Disease Control (ECDC) issued a guidance document on this topic. There are two strategies: (1) use preventive chemotherapy in patients with latent TB infection, based on the principles proven in drug-sensitive TB; and (2) explain the risk to the patient and carefully observe them to detect TB early.55 Expert consensus recommends, after careful risk assessment, preventive treatment with a fluoroquinolone (or another bactericidal drug) together with prothionamide, ethionamide, pyrazinamide or ethambutol, depending on the DST of the source case. The optimal length of treatment is not known. Evidence of efficacy exists for treatment with isoniazid for drug-sensitive TB for 9 months. This might also be a good approach for drug-resistant TB.18
Conclusion
Of the estimated 450,000 new cases of MDR-TB worldwide, almost 10% are XDR-TB. Poor diagnostic and treatment coverage globally, social and economic instability in many high-burden countries, and the presumed increasing transmission of drug-resistant strains8 are making the battle against drug-resistant TB one of the most important and relevant health challenges of the 21st century. Besides the topics described in this article, a consorted effort to develop an effective vaccine against TB should be one of the foremost priorities of health research.
References
- 1.World Health Organization Global Tuberculosis Report. Geneva: WHO, 2013. [Google Scholar]
- 2.Skrahina A, Hurevich H, Zalutskaya A, et al. Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull WHO 2013;91:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization European Center of Disease Control: Tuberculosis surveillance and monitoring in Europe. Geneva: WHO, 2013. [Google Scholar]
- 4.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. Totally drug-resistant tuberculosis in India. Clin Infect Dis 2012;54:579–81. 10.1093/cid/cir889 [DOI] [PubMed] [Google Scholar]
- 5.Udwadia ZF, Amale RA, Ajbani KK, Rodrigues CS. Nomenclature of drug-resistant tuberculosis. Lancet Infect Dis 2013;13:917. 10.1016/S1473-3099(13)70291-3 [DOI] [PubMed] [Google Scholar]
- 6.Falzon D, Gandhi N, Migliori GB, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J 2013;42:156–68. 10.1183/09031936.00134712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Tuberculosis Institute Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull WHO 1974;51:473–88. [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant -tuberculosis in China. N Engl J Med 2012;366:2161–70. 10.1056/NEJMoa1108789 [DOI] [PubMed] [Google Scholar]
- 9.Chiang CY, Van Deun A, Enarson DA. A poor drug-resistant -tuberculosis programme is worse than no programme: time for a change. Int J Tuberc Lung Dis 2013;17:714–8. 10.5588/ijtld.12.0989 [DOI] [PubMed] [Google Scholar]
- 10.Grzybowski S, Enarson D. Results in pulmonary tuberculosis patients under various treatment program conditions. Bull Int Union Tuberc 1978;53:70–5. [PubMed] [Google Scholar]
- 11.World Health Organization Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF System. Geneva; WHO, 2011. [PubMed] [Google Scholar]
- 12.Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2013;1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495–505. 10.1016/S0140-6736(11)60438-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rie A, Mellet K, John MA, et al. False-positive rifampicin resistance on Xpert(R) MTB/RIF: case report and clinical implications. Int J Tuberc Lung Dis 2012;16:206–8. 10.5588/ijtld.11.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dheda K, Ruhwald M, Theron G, et al. Point-of-care diagnosis of tuberculosis: past, present and future. Respirology 2013;18:217–32. 10.1111/resp.12022 [DOI] [PubMed] [Google Scholar]
- 16.Crudu V, Stratan E, Romancenco E, et al. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J Clin Microbiol 2012;50:1264–9. 10.1128/JCM.05903-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miotto P, Cabibbe AM, Mantegani P, et al. GenoType MTBDRsl performance on clinical samples with diverse genetic background. Eur Respir J 2012;40:690–8. 10.1183/09031936.00164111 [DOI] [PubMed] [Google Scholar]
- 18.Lange C, Abubakar I, Alffenaar JW. et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J 2014. doi: 10.1183/09031936.00188313 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis 2012;12:201–9. 10.1016/S1473-3099(11)70251-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. Geneva: WHO, 2011. http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf [Accessed 13 May 2014]. [PubMed] [Google Scholar]
- 21.Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLOS Med 2012;9:e1001300. 10.1371/journal.pmed.1001300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Deun A, Kya Jai Maug A, Halim MA, et al. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2010;182:684–92. 10.1164/rccm.201001-0077OC [DOI] [PubMed] [Google Scholar]
- 23.Johnson JL, Hadad DJ, Boom WH, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis 2006;10:605–12. [PubMed] [Google Scholar]
- 24.Jacobson KR, Tierney DB, Jeon CY, et al. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2010;51:6–14. 10.1086/653115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization The use of short course regimen for drug resistant tuberculosis. Geneva: WHO, 2012. [Google Scholar]
- 26.Dalton T, Cegielski P, Akksilp S, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 2012;380:1406–17. 10.1016/S0140-6736(12)60734-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brigden G, Nyang’wa BT, du Cros P, et al. Principles for designing future regimens for multidrug-resistant tuberculosis. Bull WHO 2014;92:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Working Group on New TB Drugs , 2014. www.newtbdrugs.org/pipeline-discovery.php [Accessed 28 March 2014]. [Google Scholar]
- 29.Diacon AH, Donald PR, Pym A, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob Agents Chemother 2012;56:3271–6. 10.1128/AAC.06126-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med 2009;360:2397–405. 10.1056/NEJMoa0808427 [DOI] [PubMed] [Google Scholar]
- 31.Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 2012;366:2151–60. 10.1056/NEJMoa1112433 [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization The use of bedaquiline for the treatment of drug resistant tuberculosis. Geneva: WHO, 2013. [Google Scholar]
- 33.Yew WW, Nuermberger E. High-dose fluoroquinolones in short-course regimens for treatment of MDR-TB: the way forward? Int J Tuberc Lung Dis 2013;17:853–4. 10.5588/ijtld.13.0301 [DOI] [PubMed] [Google Scholar]
- 34.Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med 2012;367:1508–18. 10.1056/NEJMoa1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sotgiu G, Centis R, D’Ambrosio L, et al. Efficacy, safety and -tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur Respir J 2012;40:1430–42. 10.1183/09031936.00022912 [DOI] [PubMed] [Google Scholar]
- 36.Marrone MT, Venkataramanan V, Goodman M, et al. Surgical -interventions for drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis 2013;17:6–16. 10.5588/ijtld.12.0198 [DOI] [PubMed] [Google Scholar]
- 37.Srivastava S, Peloquin CA, Sotgiu G, Migliori GB. Therapeutic drug management: is it the future of multidrug-resistant tuberculosis treatment? Eur Respir J 2013;42:1449–53. 10.1183/09031936.00073213 [DOI] [PubMed] [Google Scholar]
- 38.Vu DH, Bolhuis MS, Koster RA, et al. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 2012;56:5758–63. 10.1128/AAC.01054-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ettehad D, Schaaf HS, Seddon JA, et al. Treatment outcomes for children with multidrug-resistant tuberculosis: a systematic review and meta-analysis. Lancet Inf Dis 2012;12:449–56. 10.1016/S1473-3099(12)70033-6 [DOI] [PubMed] [Google Scholar]
- 40.The Sentinel Project for Paediatric Drug-Resistant Tuberculosis Management of drug resistant tuberculosis in children. A field guide. Boston: The Sentinel Project for Paediatric Drug-Resistant Tuberculosis, 2012. [Google Scholar]
- 41.Seddon JA, Furin JJ, Gale M, et al. Caring for children with drug-resistant tuberculosis: practice-based recommendations. Am J Respir Crit Care Med 2012;186:953–64. 10.1164/rccm.201206-1001CI [DOI] [PubMed] [Google Scholar]
- 42.International Union against Tuberculosis and Lung Disease, Medicines sans Frontieres Tuberculosis drugs under the microscope, 3rd ed. Geneva: International Union against Tuberculosis and Lung Disease; Medicines sans Frontieres, 2013. [Google Scholar]
- 43.Seddon JA, Hesseling AC, Finlayson H, et al. Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 2013;57:1676–84. 10.1093/cid/cit655 [DOI] [PubMed] [Google Scholar]
- 44.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575–80. 10.1016/S0140-6736(06)69573-1 [DOI] [PubMed] [Google Scholar]
- 45.Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, et al. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int J Tuberc Lung Dis 2010;14:1295–302. [PMC free article] [PubMed] [Google Scholar]
- 46.Karim Abdool SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011;365:1492–501. 10.1056/NEJMoa1014181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011;365:1482–91. 10.1056/NEJMoa1013607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011;365:1471–81. 10.1056/NEJMoa1013911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiang CY, Van Weezenbeek C, Mori T, Enarson DA. Challenges to the global control of tuberculosis. Respirology 2013;18:596–604. 10.1111/resp.12067 [DOI] [PubMed] [Google Scholar]
- 50.Diel R, Vandeputte J, de Vries G, et al. Costs of tuberculosis disease in the European Union: a systematic analysis and cost calculation. Eur Respir J 2014;43:554–65. 10.1183/09031936.00079413 [DOI] [PubMed] [Google Scholar]
- 51.Ayles H, Muyoyeta M, Du Toit E, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet 2013;382:1183–94. 10.1016/S0140-6736(13)61131-9 [DOI] [PubMed] [Google Scholar]
- 52.Churchyard GJ, Fielding KL, Lewis JJ, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med 2014;370:301–10. 10.1056/NEJMoa1214289 [DOI] [PubMed] [Google Scholar]
- 53.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral therapy for prevention of tuberculosis in adults with HIV: a systematic review and meta-analysis. PLOS Med 2012;9:e1001270. 10.1371/journal.pmed.1001270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isaakidis P, Cox HS, Varghese B, et al. Ambulatory multi-drug resistant tuberculosis treatment outcomes in a cohort of HIV-infected patients in a slum setting in Mumbai, India. PLOS ONE 2011;6:e28066. 10.1371/journal.pone.0028066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.European Center of Disease Preventation and Control Management of contacts of MDR TB and XDR TB patients. Stockholm: ECDC, 2012. [Google Scholar]
- 56.De Lorenzo S, Alffenaar JW, Sotgiu G, et al. Efficacy and safety of meropenem-clavulanate added to linezolid-containing regimens in the treatment of MDR-/XDR-TB. Eur Respir J 2013;41:1386–92. 10.1183/09031936.00124312 [DOI] [PubMed] [Google Scholar]