Abstract
Obesity represents one of the biggest public health challenges facing us today. Urbanisation, sedentary lifestyles and the availability of inexpensive, highly palatable foods have promoted the increasing prevalence of obesity over the past 30 years. However, some people gain weight more easily than others, and there is strong evidence that, within a given environment, this variance in body weight is influenced by genetic factors. This article discusses how genetic studies have contributed to our understanding of the mechanisms involved in the regulation of body weight. We now understand that weight is regulated by neural mechanisms that regulate appetite and energy expenditure and that disruption of these pathways can result in severe obesity in some patients. These studies provide a framework for investigating patients and ultimately may guide the development of more rational, targeted therapies for genetically susceptible individuals with severe obesity.
KEYWORDS : Appetite, genetics, hypothalamus, leptin, obesity
Introduction
Since their inception in 1639, the Goulstonian Lectures have frequently addressed the clinical challenges of the time. Unsurprisingly, yellow fever and febrile contagion (1806) and the ‘nature and affinities of tubercle’ (1867) occupied physicians for much of the nineteenth century. Given the impact of obesity and associated disorders such as type 2 diabetes, it is perhaps appropriate therefore, that obesity was the focus of my lecture in 2013.
Obesity as a disorder of energy homeostasis
In its simplest terms, obesity arises when there is an imbalance between energy intake and energy expenditure. Evidence clearly shows that both increases in energy intake and reductions in energy expenditure during physical activity have driven increases in the mean body mass index (BMI) seen in many countries over the past 30 years.1 However, the interaction between an individual's genetic predisposition and their environment influences where they lie within the population's BMI distribution. Family, twin and adoption studies suggest that the heritability of body weight ranges between 40% and 70%. Genetic influences seem to be more potent at the extremes of the BMI distribution and in people from some ethnic groups who seem to be particularly susceptible to developing obesity (eg Polynesians). Given the estimated heritability of BMI, genetic approaches can be a useful tool to investigate the mechanisms involved in weight regulation and how those mechanisms are disrupted to contribute to obesity. Working with colleagues in Cambridge and with many collaborators around the world, we have discovered several genes whose disruption leads to severe obesity that begins in childhood (Fig 1). The study of these patients and the molecular and physiological mechanisms that link genetic changes to obesity and associated clinical phenotypes have informed our understanding of the systems that are involved in the regulation of body weight and the coupling of changes in energy balance to changes in neuroendocrine axes, immunity and other parameters.
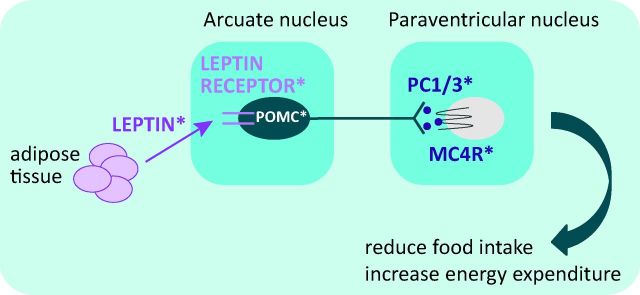
Fig 1.
Schematic of the hypothalamic leptin-melanocortin pathway. MC4R = melanocortin 4 receptor; PC1/3 = prohormoneconvertase 1/3; POMC = pro-opiomelanocortin. *Molecules disrupted by genetic mutations found in severely obese patients.
Hypothalamic pathways involved in energy balance
Mammals will make coordinated adjustments to their food intake or energy expenditure in response to food restriction and overfeeding to defend a set point for body weight.2 Experiments with lesions in animals established the key role of brain regions, including the hypothalamus, in the regulation of body weight, and these findings were supported by clinical reports of patients with tumours involving hypothalamo-pituitary structures associated with food-seeking behaviour and obesity.3 Parabiosis experiments in inbred strains of mice with severe obesity, such as ob/ob and db/db mice, suggested the existence of a circulating factor that regulated weight.4 Identification of this hormone, leptin, through positional cloning of the ob gene, and the finding that this gene was mutated in severely obese ob/ob mice5 paved the way for the identification of the neural circuits that control energy homeostasis.
Leptin – a critical regulator of energy balance
Leptin is an adipocyte-derived secreted hormone, circulating levels of which correlate closely with fat mass.6 Leptin's physiological role is primarily to signal in response to nutritional depletion, in that fasting results in a decrease in leptin levels, which triggers a series of changes in energy intake, energy expenditure and neuroendocrine function in order to maintain energy homeostasis.7 Many of the physiological effects of leptin are mediated through hypothalamic neurones that express the signalling form of the leptin receptor.8 Primary leptin-responsive neurones in the hypothalamus project to second-order neurones in the hypothalamus and other brain regions that express the melanocortin 4 receptor (MC4R). These hypothalamic pathways interact with other neural systems to coordinate appetite and modulate efferent signals to the periphery, regulating intermediary metabolism and energy expenditure.9
Mutations in the leptin pathway cause severe human obesity
We showed that homozygous mutations in the genes that encode the hormone leptin result in severe obesity from a young age.10,11 The key clinical features in affected patients are an intense drive to eat (hyperphagia) and aggressive behaviour when food is denied. Patients also exhibit impaired satiety, with food-seeking behaviour evident soon after the end of a meal. Although the study of neural mechanisms in detail has obvious challenges in humans, functional neuroimaging allows for whole-brain evaluation of the neural response to food-related stimuli. Functional magnetic resonance imaging (fMRI) studies have provided clear evidence that the brain's control of eating is not confined to the hypothalamus and brainstem but involves a complex array of subcortical and cortical regions. Based on the clinical observation that patients with leptin deficiency like all foods and display emotional responses to food, we hypothesised that leptin may play a broader role in mediating aspects of eating behaviour, such as the rewarding properties of food. Using fMRI, we found that leptin-deficient patients had marked activation of the ventral striatum – an area associated with pleasure and reward – in response to the visual presentation of food vs non-food items; even images of very bland foods were rewarding and elicited striatal activation in these patients compared with healthy volunteers.12
Mutations in the gene that encodes the leptin receptor result in a very similar phenotype characterised by hyperphagia and severe early-onset obesity.13,14 Leptin and leptin receptor deficiency are associated with hypogonadotropic hypogonadism and a failure of normal pubertal development. However, some evidence indicates delayed but spontaneous onset of menses in some leptin receptor-deficient adults.15 It is plausible that the excess adipose tissue mass in these patients leads to the production of sufficient oestrogen (due to the action of aromatase) to result in uterine development and irregular menses in the absence of fully developed secondary sexual characteristics. Leptin may exert these effects on the reproductive system through a number of molecules, including kisspeptin, which signals through GPR54 to modify the release of gonadotrophin-releasing hormone.
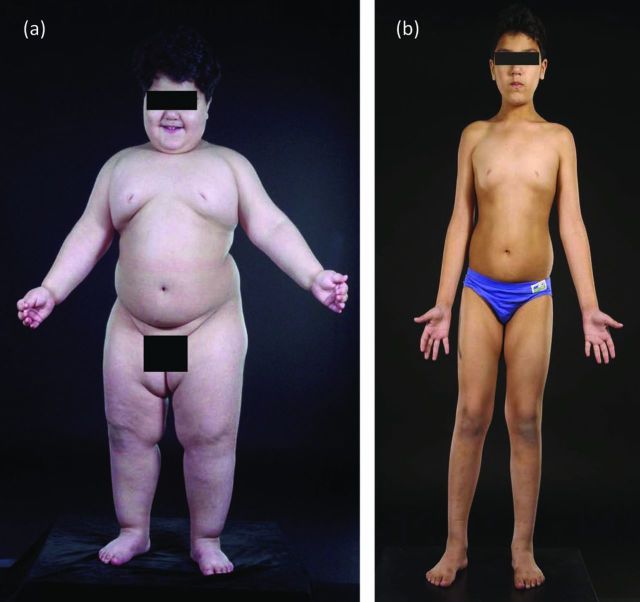
Although congenital leptin deficiency is rare, we were able to show that it is entirely treatable using daily subcutaneous injections of recombinant human leptin, with beneficial effects on the degree of hyperphagia, reversal of the immune defects and infection risk seen in leptin-deficient patients, and permissive effects on the appropriate development of puberty (Fig 2).11,16 Such treatment is currently available on a named-patient basis. We showed that the major effect of leptin administration is on food intake, with normalisation of hyperphagia, enhanced satiety and normalisation of the striatal response to food images. We were unable to demonstrate a major effect of leptin on basal metabolic rate (BMR) or free-living energy expenditure, but, as weight loss by other means is associated with a decrease in BMR, the fact that energy expenditure did not fall in leptin-deficient subjects is notable. Administration of leptin permits progression of appropriately timed pubertal development, which suggests that leptin is a permissive factor for the development of puberty in humans.
Fig 2.
Severe obesity due to congenital leptin deficiency is treatable. A 3-year-old patient with leptin deficiency (a) before and (b) after treatment with recombinant human leptin.
Central melanocortin pathways
In further studies in patients with severe early-onset obesity, we identified mutations in a number of genes involved in pathways that regulate appetite downstream of leptin. Leptin stimulates the expression of pro-opiomelanocortin (POMC) in primary neurones located in the arcuate nucleus of the hypothalamus. Pro-opiomelanocortin is extensively modified post-translationally to generate the melanocortin peptides that activate the melanocortin receptors to modulate diverse functions in the central nervous system, adrenal gland and skin. The melanocortins are agonists at melanocortin receptors and suppress food intake. In addition, leptin inhibits orexigenic pathways mediated by neurones expressing the melanocortin antagonist Agouti-related peptide (AgRP) and neuropeptide Y (NPY), which can suppress expression of POMC. These two sets of primary leptin-responsive neurones project to second order neurones expressing MC4R.17
We and others have reported that heterozygous mutations in MC4R are found in 2–3% of children seen in obesity clinics and up to 5% of patients with severe early-onset obesity, making MC4R deficiency the most common genetic form of severe obesity. Functionally significant mutations in MC4R are found at a frequency of about one in 1,000 in the general population in the UK, which makes MC4R deficiency one of the most common highly penetrant genetic diseases.18 Functionally significant mutations of the MC4R gene are inherited in a codominant manner, with variable penetrance and expression in heterozygous carriers. As a result of this growing body of information, assessment of the MC4R gene is increasingly seen as a necessary part of the clinical evaluation of a severely obese child.19
Carriers of the MC4R gene mutation are objectively hyperphagic, but the degree of hyperphagia is not as severe as that seen in leptin deficiency. By studying a large number of patients with different mutations of the MC4R gene, we found that the severity of receptor dysfunction seen in in vitro assays predicted food intake at a test meal, which suggests that signalling through this pathway is a major mechanism for the regulation of appetite.20 As such, a number of drugs that target MC4R are being developed for the potential treatment of severe obesity, initially focusing on this group of patients.21
We found that MC4R-deficient patients have a lower prevalence of hypertension and lower systolic and diastolic blood pressures compared with equally obese volunteers.22 We hypothesised that the lower blood pressures seen in MC4R-deficient humans may be explained by altered activation of the sympathetic nervous system. Information about central autonomic regulation can be inferred from the response of end organs such as the heart by the measurement of heart rate variability, which is a widely accepted non-invasive tool that has been validated against more direct pharmacological measurements of sympathetic and parasympathetic activation. We found that sleeping heart rate (mediated predominantly by parasympathetic activation) was similar in MC4R-deficient subjects and controls. The increase in heart rate on waking (driven predominantly by sympathetic nervous system activation) was reduced in MC4R-deficient subjects. We found that urinary excretion of noradrenaline was markedly reduced in MC4R deficiency, which would be consistent with a predominant effect of central MC4Rs on impaired release of noradrenaline from nerve terminals rather than on catecholamines released from the adrenal medulla. These findings established the importance of MC4R-mediated signalling in the regulation of blood pressure and suggested that melanocortinergic circuits play a key role in mediating the link between changes in weight and changes in blood pressure.
Conclusions
Obesity is one of the major public health threats of our time, and a number of approaches will need to be employed to tackle the health burden at a population level. However, it is important not to lose sight of the fact that some individuals are particularly susceptible to developing severe obesity. Future strategies to treat and support this group of patients, whose numbers are increasing, will need to consider the major biological influences on the drive to eat and how heritable differences between individuals influence their risk of obesity. Our work has emphasised the need to recognise and characterise the heterogeneity of obesity and to define subgroups of patients at risk of different metabolic and cardiovascular complications, who may benefit from targeted preventative and therapeutic strategies.
Acknowledgements
I would like to thank the many colleagues and collaborators who have contributed to this work, and particularly the physicians who have referred patients with severe obesity, without whom this work would not have been possible. I would also like to thank the patients and their families for active involvement in many years of research for the benefit of others. Additional papers and resources can be found at www.goos.org.uk.
References
- 1.Friedman JM. Obesity in the new millennium. Nature 2000;404:632–4. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc London 1953;140:578–96. 10.1098/rspb.1953.0009 [DOI] [PubMed] [Google Scholar]
- 3.Anand BK, Brobeck JR. Hypothalamic control of food intake in rats and cats. Yale J Biol Med 1951;24:123–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman DL, Hummel KP. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol 1969;217:1298–304. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425–32. 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 6.Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995;1:1155–61. 10.1038/nm1195-1155 [DOI] [PubMed] [Google Scholar]
- 7.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature 1996;382:250–2. 10.1038/382250a0 [DOI] [PubMed] [Google Scholar]
- 8.Elmquist JK, Bjørbaek C, Ahima RS, et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 1998;395:535–47. 10.1002/(SICI)1096-9861(19980615)395:43.0.CO;2-2 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz MW, Woods SC, Porte D, Jr, et al. Central nervous system control of food intake. Nature 2000;404:661–71. [DOI] [PubMed] [Google Scholar]
- 10.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 1997;387:903–8. 10.1038/43185 [DOI] [PubMed] [Google Scholar]
- 11.Farooqi IS, Jebb SA, Langmack G, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 1999;341:879–84. 10.1056/NEJM199909163411204 [DOI] [PubMed] [Google Scholar]
- 12.Farooqi IS, Bullmore E, Keogh J, et al. Leptin regulates striatal regions and human eating behavior. Science 2007;317:1355. 10.1126/science.1144599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 1998;392:398–401. 10.1038/32911 [DOI] [PubMed] [Google Scholar]
- 14.Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 2007;356:237–47. 10.1056/NEJMoa063988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 1999;84:3686–95. 10.1210/jcem.84.10.5999 [DOI] [PubMed] [Google Scholar]
- 16.Farooqi IS, Matarese G, Lord GM, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Invest 2002;110:1093–103. 10.1172/JCI0215693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997;88:131–41. 10.1016/S0092-8674(00)81865-6 [DOI] [PubMed] [Google Scholar]
- 18.Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 2000;106:271–9. 10.1172/JCI9397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farooqi IS. The severely obese patient – a genetic work-up. Nat Clin Pract Endocrinol Metab 2006;2:172–7. 10.1038/ncpendmet0137 [DOI] [PubMed] [Google Scholar]
- 20.Farooqi IS, Keogh JM, Yeo GS, et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med 2003;348:1085–95. 10.1056/NEJMoa022050 [DOI] [PubMed] [Google Scholar]
- 21.René P, Le Gouill C, Pogozheva ID, et al. Pharmacological chaperones restore function to MC4R mutants responsible for severe early-onset obesity. J Pharmacol Exp Ther 2010;335:520–32. 10.1124/jpet.110.172098 [DOI] [PubMed] [Google Scholar]
- 22.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 2009;360:44–52. 10.1056/NEJMoa0803085 [DOI] [PubMed] [Google Scholar]