Abstract
Toxic shock syndrome (TSS) represents a fascinating example of immune activation caused by infection resulting in a dramatic and challenging clinical syndrome. TSS is commonly associated with tampon use and still causes significant morbidity and mortality in young healthy women. A misconception is that TSS presents with a skin rash and only occurs in women and children; however, it can occur in males and can present without skin changes. TSS presents initially as a febrile illness and within a few hours can progress to severe hypotension and multiple organ failure (MOF). Staphylococcus aureus and group A beta haemolytic streptococcus (GABHS) can secrete toxins from a small or hidden focus of infection and hence blood culture and sensitivity (C+S) tests can be negative, thereby making diagnosing this condition challenging. Clindamycin is superior to penicillin in the treatment of this condition and significantly decreases the mortality rate in TSS. However, there is also an important role for intravenous immunoglobulins (IVIG). Early intensive care unit (ICU) as well as surgical team involvement (in selected cases) is required to avoid mortality which may approach 70%.
KEYWORDS : Toxic shock syndrome (TSS), group A beta haemolytic streptococcus (GABHS), Staphylococcus aureus, necrotising fasciitis, multiple organ failure, intravenous immunoglobulins
Presentation
A 45-year-old female patient presented to the accident and emergency department (A&E) having been generally unwell for the past 12 hours. She had begun to develop rigors. Her past medical history was of birdshot chorioretinopathy, an autoimmune disease causing posterior uveitis, for which she was taking prednisolone and mycophenolate mofetil. She denied any preceding flu-like illness or respiratory, urinary or gastrointestinal symptoms. She had neither headaches nor neck stiffness. She worked as a teaching assistant and was a mother to two children who were well. She did not consume alcohol, had never smoked and had no recent travel history.
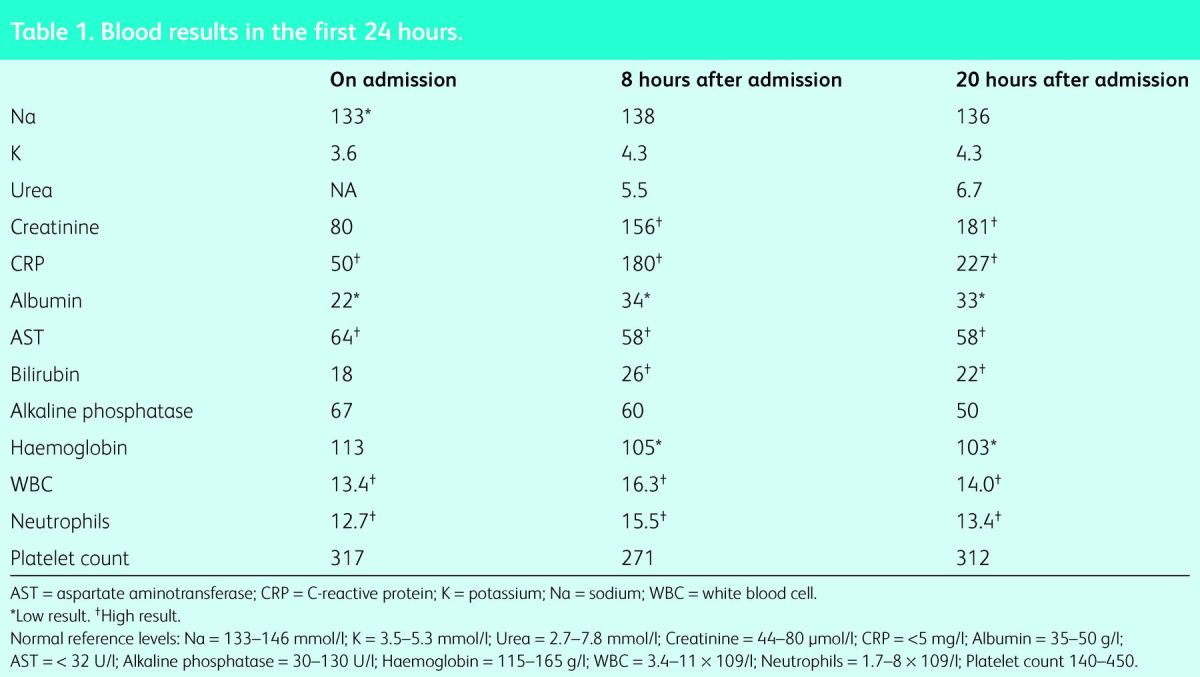
On examination she had a temperature of 39.7°C and tachycardia at 110 beats per minute. However, her blood pressure (BP), oxygen saturations and respiratory rate were within normal limits and she was fully alert and orientated. Cardiorespiratory and abdominal examination was normal. There were no signs of skin rash, joint swelling or stigmata of infective endocarditis. An oral examination revealed good oral hygiene, but mild pharyngitis was noted. Electrocardiography (ECG) showed sinus tachycardia and chest X-ray was unremarkable. A urine dipstick test only revealed a trace of blood and was negative for nitrites and leucocytes. Bloods on admission showed venous lactate of 1 mmol/l (blood results are shown in Table 1). Initially this gave an impression of sepsis with unknown origin (in view of fever on a background of an immunosuppressed status). The treatment plan included intravenous (IV) crystalloid fluids, Tazocin (broad spectrum antibiotic) after blood cultures were taken, doubling the dose of prednisolone and omitting the mycophenolate mofetil, pending the results of the septic screen.
Table 1.
Blood results in the first 24 hours.
However, 2 hours later it was noted the patient appeared to be drowsy, but responsive and obeying commands. Another hour later the patient was given a computed tomography (CT) scan of the head following further concerns about her progressive drowsiness. This was reported as normal and a lumbar puncture was planned to rule out meningoencephalitis. Following the CT scan, she became profoundly hypotensive with a systolic BP of 60–70 mmHg. Given the amount of fluid resuscitation needed, a catheter was inserted by nursing staff who reported that there was a tampon present with some green discharge. This was removed and sent for culture and sensitivity (C+S) testing. The patient reported that the tampon had been inserted less than 12 hours prior to admission. A diagnosis of toxic shock syndrome was made and the patient was transferred to the intensive care unit (ICU).
Following advice from the microbiology department the patient was started on clindamycin and meropenem treatment on arrival at ICU, and Tazocin was discontinued. Noradrenaline was commenced; however, it soon became clear that the patient had developed vasoplegia secondary to severe sepsis. Adrenaline and vasopressin were subsequently commenced with good effect. Intravenous immunoglobulin (IVIG) was also started. Despite vasopressors and aggressive fluid resuscitation the patient remained oliguric and became progressively more acidotic. Continuous veno-venous haemofiltration (CVVHF) was initiated. After 3 days on intensive care she was weaned off the CVVHF and vasopressor support, and transferred to ward care. Four days later she was discharged home from the ward, with advice from the gynaecologist to not use tampons again. A high vaginal swab subsequently grew Staphylococcus aureus, while two sets of blood C+S tests were negative.
Discussion
Toxic shock syndrome (TSS) is an acute systemic illness associated with infection by strains of S aureus which produce toxic shock syndrome toxins (TSST-1) or streptococcus pyogenes M types I and III – mainly group A beta haemolytic streptococcus (GABHS)-producing streptococcal pyogenic exotoxin A (SPEA). TSST-1 and SPEA are ‘super-antigens’ that interact with antigen-presenting cells (APCs) and T Cells to induce T Cell proliferation and massive cytokine production including tumour necrosis factor (TNF)-α, TNF-β, interleukin (IL)-1, IL-2 and IL-6, causing severe shock1–2.
S aureus infection can be divided into menstrual and non-menstrual cases (ie postmenopausal women, men and children). Menstrual cases are associated with vaginal colonisation with a toxigenic strain of S aureus-producing TSST-1 followed by penetration of a sufficient concentration of TSST-1 across the epithelium, aided by abrasion from barrier contraception or tampon use. The absence of, or depression in, titres of neutralising antibody to the toxin, as in an immunosuppresed host, can enhance the severity of TSS.1–3
The focus of infection in non-menstrual cases of S aureus TSS can be pharyngitis, burns, cellulitis or even simple skin abrasion.1–3 GABHS TSS occurs more commonly in patients who are intravenous drug users, have had surgical implants and potentially in those with simple blunt trauma. It is enhanced in an immunosuppressed patient including those with diabetes, alcoholism and steroid use.4
Clinical presentation of S aureus TSS is usually an abrupt onset of fever (ie a temperature of >38.9°C), vomiting, diarrhoea and abdominal pain, macular erythroderma involving palms and soles with desquamation 1–2 weeks later, mucous membrane hyperaemia (vaginal, oesophageal and conjunctival), sore throat, myalgia, headache and photophobia.4 It is worth mentioning that our patient had not complained of gastrointestinal tract (GIT) disturbance, nor had a skin rash. Hypotension with multiple organ failure (MOF) occurs usually within 72 hours with an estimated mortality rate of 5%.5 GABHS TSS differs in that it is usually present with flu-like symptoms and febrile illness, but GIT disturbance is much less likely and rash occurs in only 10% of cases.6
Infective myositis and necrotising fasciitis occurs in 70% of patients as GABHS usually arises from a deep-seated area of the skin or after minor blunt trauma.7 Hypotension and MOF develops within 4–8 hours in 95% of patients with a mortality rate of up to 70%.6–10
Investigations
Clinical suspicion is the most important step, as there may be no clues. Common laboratory findings include abnormal liver function tests with hypoalbuminemia, acute renal failure, low platelet count and raised creatinine kinase.14
Skin, vaginal and pharyngeal swabs (according to the focus of infection) can confirm the source of infection. Blood C+S may be positive and, in cases of GABHS TSS, antistreptolysin O titre (ASOT) may be detected.14,15
Failure to treat the infection with penicillin is common. Clindamycin is superior to penicillin because of its potency in suppressing bacterial toxin synthesis as well as facilitating phagocytosis. Clindamycin also suppresses lipopolysaccharide-induced monocyte synthesis of TNF-α.16
Intravenous immunoglobulin (IVIG) therapy reduces T cell production of pro-inflammatory cytokines, and significantly increases the plasma neutralising activity against super-antigens, thereby resulting in improved survival in many TSS studies.16,17 IVIG therapy has also demonstrated an anti-TSST-1 effect and significantly improved survival in cases of S aureus TSS.18
Identification of potentially implicated foreign bodies – especially tampons, surgical drainage and irrigation or excision of infected sites such as implants or necrotizing fasciitis – is of high priority, and input from surgical teams can be essential.
Our case emphasises the importance of maintaining a high level of suspicion for TSS in patients who are at high risk of the condition (eg menstruating ladies using tampons, patients who are immunosuppressed, and patients who have trauma, burns, cellulitis, pharyngitis or implants) and who are presenting with a febrile illness and signs of shock or hypoperfusion. It is important to take a formal and accurate menstrual and sexual history in selected cases.
Fluid resuscitation and intravenous antibiotics after blood C+S is essential and early ICU involvement for possible vasopressors, haemofiltration and IVIG use is required. Surgical excision of any focus of infection may result in a significant decrease in morbidity and mortality.
Key points.
Menstrual and sexual history including tampon or barrier use is vital
Toxic shock syndrome (TSS) should be suspected in immunosuppressed patients as well as in menstruating ladies using tampons who present with febrile illness with no obvious source of infection
Hypotension and multiple organ failure occur within hours and can be resistant to aggressive fluid and antibiotic therapy
Infective myositis and necrotising fasciitis can occur in 70% of group A beta haemolytic streptococcus (GABHS) TSS cases
Blood culture and sensitivity can be negative in TSS
Clindamycin is superior to penicillin in upper case and there is a role for intravenous immunoglobulins
References
- 1.Brosnahan AJ, Schlievert PM. Gram-positive bacterial superantigen outside-in signalling causes toxic shock syndrome. FEBS J 2011;278:4649–67. 10.1111/j.1742-4658.2011.08151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuda Y1, Kato H, Ono E, et al. Diagnosis of toxic shock syndrome by two different systems; clinical criteria and monitoring of TSST-1-reactive T cells. Microbiol Immunol 2008;52:513–21. 10.1111/j.1348-0421.2008.00071.x [DOI] [PubMed] [Google Scholar]
- 3.Hackett SP, Stevens DL. Streptococcal toxic shock syndrome: synthesis of tumour necrosis factor and interleukin-1 by monocytes stimulated with pyogenic exotoxin A and streptolysin O. J Infect Dis 1992;165:879–85. 10.1093/infdis/165.5.879 [DOI] [PubMed] [Google Scholar]
- 4.Dellaripa PF. Toxic shock syndrome. Journal of Intensive Care Medicine 2000;15:314–20. 10.1046/j.1525-1489.2000.00314.x [DOI] [Google Scholar]
- 5.Hajjeh RA, Reingold A, Weil A, et al. Toxic shock syndrome in the United States: surveillance update, 1979–1996. Emerg Infect Dis 1999;5:807–10. 10.3201/eid0506.990611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens DL, Tanner MH, Winship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989;321:1–7. 10.1056/NEJM198907063210101 [DOI] [PubMed] [Google Scholar]
- 7.Stevens DL. Invasive group A streptococcus infections. Clin Infect Dis 1992;14:2–11. 10.1093/clinids/14.1.2 [DOI] [PubMed] [Google Scholar]
- 8.Holm SE, Norrby A, Bergholm AM, Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden 1988–1989. J Infect Dis 1992;166:31–37. 10.1093/infdis/166.1.31 [DOI] [PubMed] [Google Scholar]
- 9.Stegmayr B, Björck S, Holm S, et al. Septic shock induced by group A streptococcal infections: clinical and therapeutic aspects. Scand J Infect Dis 1992;24:589–97. 10.3109/00365549209054644 [DOI] [PubMed] [Google Scholar]
- 10.Demers B, Simor AE, Vellend H, et al. Severe invasive group A streptococcus infections in Ontario, Canada: 1987–1991. Clin Infect Dis 1993;16:792–800. 10.1093/clind/16.6.792 [DOI] [PubMed] [Google Scholar]
- 11.Barnham MR, Weightman NC, Anderson AW, Tanna A. Streptococcal toxic shock syndrome: a description of 14 cases from North Yorkshire, UK. Clin Microbiol Infect 2002;8:174–81. 10.1046/j.1469-0691.2002.00396.x [DOI] [PubMed] [Google Scholar]
- 12.Todd J, Fishaut M, Kapral F, Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet 1978;2:1116–8. 10.1016/S0140-6736(78)92274-2 [DOI] [PubMed] [Google Scholar]
- 13.Lowy FD. Staphylococcus aureus infections. N Eng J Med 1998;339:520–32. 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 14.Chapnick EK, Gradon JD, Lutwick LI, et al. Streptococcal toxic shock syndrome due to noninvasive pharyngitis. Clin Infect Dis 1992;14:1074–7. 10.1093/clinids/14.5.1074 [DOI] [PubMed] [Google Scholar]
- 15.Parsonnet J, Hansmann MA, Delaney ML, et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol 2005;43:4628–34. 10.1128/JCM.43.9.4628-4634.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korman TM, Boers A, Gooding TM, et al. Fatal case of toxic shock-like syndrome due to group C streptococcus associated with superantigen exotoxin. J Clin Microbiol 2004;42:2866–9. 10.1128/JCM.42.6.2866-2869.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Darenberg J, Ihendyane N, Sjölin J. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:333–40. 10.1086/376630 [DOI] [PubMed] [Google Scholar]
- 18.Melish ME, Frogner K, Hirata S, et al. Use of IVIG for therapy in the rabbit model of TSS [Abstract]. Clin Res 1987;35:220. [Google Scholar]


